Abstract
Objective
To evaluate the feasibility, acceptability, and preliminary efficacy of a mind–body intervention for moderate to severe primary dysmenorrhea (PD).
Design
Open trial (single arm).
Setting
Academic medical school.
Subjects
A total of 20 young adult women with moderate to severe primary dysmenorrhea were included across four separate intervention groups.
Methods
All participants received five 90-minute sessions of a mind–body intervention and completed self-report measures of menstrual pain, depression, anxiety, somatization, and pain catastrophizing at baseline, post-treatment, and at one-, two-, three-, and 12-month follow-up. Self-report of medication use and use of skills learned during the intervention were also collected at all follow-up points.
Results
Participants reported significantly lower menstrual pain over time compared with baseline. No changes in anxiety, depression, or somatization were observed, although pain catastrophizing improved over time. Changes in menstrual pain were not associated with changes in medication use or reported use of skills.
Conclusions
A mind–body intervention is a promising nondrug intervention for primary dysmenorrhea, and future research should focus on testing the intervention further as part of a randomized clinical trial.
Keywords: Menstrual Pain, Alternative Medicine, Catastrophizing, Cognitive Behavior Therapy, Pain Management, Psychology
Introduction
Menstrual pain without an identifiable medical cause, known as primary dysmenorrhea (PD), is the leading cause of school and work absences [1–5], and 20–25% of young women with PD report experiencing significantly impaired functioning because of their symptoms [6–9]. Despite this high prevalence and significant impact, there is a paucity of research on effective interventions for girls and young women with PD [10].
Firstline treatments for PD typically involve oral contraceptive pills or nonsteroidal anti-inflammatory drugs. Although generally considered efficacious, the failure rate for these medications is still relatively high (18–25%) [11–14]. A recent review concluded that there is only limited evidence for pain improvement with either low- or medium-dose estrogen oral contraceptive pills [15]. Another comprehensive review found that nonsteroidal anti-inflammatory drugs were generally effective for treating PD, although these medications carry a significant risk of adverse effects [16]. Furthermore, even women who demonstrate an adequate response to treatment with nonsteroidal anti-inflammatory drugs often have substantial residual pain [17].
These challenges have led to the investigation of nondrug, nonsurgical interventions for PD. Studies evaluating transcranial electrical nerve stimulation, hypnotherapy, exercise, acupuncture and acupressure, dietary changes, supplements, and herbal remedies have produced limited findings and suffered from methodological flaws, such as lack of control groups [10,18,19]. Behavioral interventions such as exercise, relaxation, and pain management training are based on the biopsychosocial model of pain, which posits that biological, psychological, and environmental factors influence the pain experience [20]. The few studies to date examining the efficacy of behavioral interventions have produced mixed results. Generally, there appears to be some support for pain management training for reducing menstrual pain [21] and less support for biofeedback and relaxation training [22–25]. However, it is difficult to draw any strong conclusions about these findings because of significant methodological issues, such as small sample size, lack of control group or randomization, inconsistent timing of interventions during the menstrual cycle, lack of blinding procedures, and inconsistent outcome measures [26]. In addition, these nonspecific coping-based approaches may fail to target specific mechanisms that may be contributing to ongoing cyclical pain in girls and women with PD.
We believe that the development and evaluation of a structured mind–body intervention rooted in evidence-based, empirically supported interventions for pain may provide relief for girls and young women suffering from PD. Effectively treating girls with PD has the potential to address mechanisms that may lead to more chronic pain conditions later in life [27].
Method
Design
The current pilot study used a pre–post within-subjects design (single-arm open trial) to evaluate the feasibility, acceptability, and preliminary efficacy of a cognitive–behavioral, mind–body intervention aimed at reducing menstrual pain and improving functioning in young adult women with moderate to severe PD.
Participants
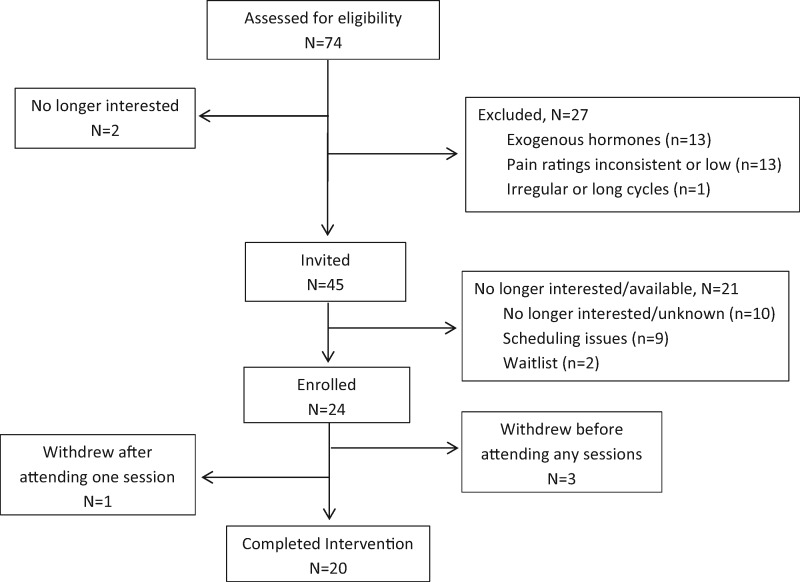
Some participants were recruited via a series of mass e-mails sent to college students; others had previously participated in other studies and given permission to be contacted for future studies. Seventy-four potential participants were screened for eligibility via telephone. Two individuals (2.7% of total screened) were not interested in participating, and 27 (36.5% of total screened) were excluded as a result of use of exogenous hormones in the previous three months (N = 13), menstrual pain ratings not meeting inclusion criteria (N = 13), and irregular or long cycle lengths (N = 1). Of the 45 eligible individuals who were invited to participate, 21 (46.7%) declined participation because of lack of interest or availability. This study employed a staged consent process during which additional information was provided to the participant as they progressed through the recruitment and enrollment process (e.g., basic information was provided in study advertisements and was expanded on during the phone screen). Therefore, participants may have expressed interest initially after reading the recruitment material but no longer desired to participate after completing the phone screen. Because participants could decline to participate at any time, we asked one probing question to ascertain the reasons for discontinuing but did not probe further after the participants’ initial replies. Some explanations that were provided by participants include being too busy, not feeling like she could commit to the time commitment, jobs/classes that conflicted with group session times, etc. Recruitment was conducted before each cohort, and enrollment in the upcoming cohort was conducted until the maximum group size of eight participants was reached or until the group sessions started. Participants who expressed interest and completed the phone screen but were not available for the planned session times were contacted again before subsequent cohorts to check on their availability and interest. Twenty-four participants enrolled in the study. Four participants enrolled but subsequently withdrew—three before attending any of the group sessions and one after attending one group session. See Figure 1 for a flowchart depicting study enrollment. These four participants cited scheduling conflicts, with group session times as the reason for being unable to continue participation.
Figure 1.
Flowchart of study participation.
Inclusion criteria were 1) self-reported menstrual cycle averaging 24–32 days; 2) must have at least moderate to severe menstrual pain, as indicated by a menstrual pain rating of ≥6/10 on the numerical rating scale (NRS; see below) for at least the previous three menstrual cycles before participation; and 3) must provide written informed consent. Participants were excluded for 1) use of oral contraceptives or any exogenous hormones in the previous three months; 2) presence of persistent pelvic pain throughout the menstrual cycle (indicating a more chronic pelvic pain condition); 3) diagnosis of an underlying medical cause for dysmenorrhea symptoms; 4) minimal or moderate menstrual pain (NRS rating ≤ 4/10) during any of the three previous cycles before study participation; 5) daily use of opioids (participants using other analgesics were included); and 6) developmental delay, autism, or significant anatomic impairment with the potential to preclude understanding of study procedures or treatment.
Procedure
Respondents who contacted the study were scheduled for a telephone screen to review eligibility criteria. Eligible participants were then scheduled for an intake session to sign informed consent and completed a packet of questionnaires (PRE). Participants were compensated $25 for this session. Following the group, all participants completed another packet of questionnaires (POST) and a brief, qualitative interview to assess the utility and acceptability of the intervention and were compensated $75. Four follow-up assessments were conducted online at one, two, three, and 12 months postintervention (FU-1, FU-2, FU-3, and FU-12). Participants were compensated $10 for completion of each of these assessments.
The treatment for this study was limited to five sessions conducted over the course of six weeks, primarily to enhance participant retention, as well as to ensure that all participants experienced one menstrual cycle over the course of treatment. Each session was scheduled weekly, with the exception of Sessions 4 and 5, which were spaced two weeks apart. This schedule allowed for consolidation and practicing of skills learned. Participants were required to attend at least three of the five scheduled sessions or they would be removed from the study. This was determined based on research on the efficacy of brief interventions. Extant data have supported the use of brief mindfulness interventions (once per week for four weeks or less) for acute and chronic pain [28,29], stress [30], and depression [31], with a recent meta-analysis supporting the use of brief mindfulness-based interventions across a variety of conditions [32]. Additionally, single-session pain catastrophizing interventions are now being developed and have shown promise for low back pain [33,34]. All study procedures were approved by the UCLA Institutional Review Board (IRB).
Intervention
This treatment is based on existing mind–body and cognitive–behavioral treatments that have been developed for treating adults with other pain problems [34–37]. All treatment sessions were audio-recorded to monitor adherence. The treatment was structured as follows: Session 1: Introduction to group therapy procedures and members, psychoeducation about the menstrual cycle and pain, introduction to mindfulness; Session 2: Introduction to automatic pain thoughts and de-catastrophizing, mindfulness skills; Session 3: De-catastrophizing automatic pain thoughts; Session 4: Identifying and applying coping skills, progressive muscle relaxation; Session 5: Feedback on use of coping skills, review of material learned, anticipating future challenges. All group sessions were led by LAP and facilitated by LCS.
Measures
Feasibility and Acceptability
Feasibility was defined as at least 75% of enrolled participants completing the program (i.e., attending three or more of the five sessions). Acceptability was determined by the credibility factor of the Credibility Expectancy Questionnaire (CEQ) [38], which was administered at the end of the first group session (which introduced the rationale and empirical support for the intervention) in each cohort and was completed anonymously. The credibility factor consists of three questions assessing acceptability of the outlined treatment program. Answer choices range from 1 = not at all logical/useful/confident to 9 = very logical/useful/confident. Item scores are summed to create the factor score, which ranges from 3 to 27.
Menstrual Pain
A 0–10 numerical rating scale (0 = no pain, 10 = worst pain possible) assessed participants’ retrospective accounts of the overall level of menstrual pain without analgesic medication during the first two days of the most recent menstrual period (LMP-2days).
Depression, anxiety, and somatization was assessed using the three subscales of the Brief Symptom Inventory–18-Item Version (BSI-18) [39].
Pain catastrophizing was measured using the Pain Catastrophizing Scale (PCS) [40], a well-validated 13-item questionnaire that asks participants to indicate the degree to which they have different thoughts when experiencing pain.
Pain Medication Use
Participants retrospectively reported their use of medication for pain during their most recent period and, if used, which medication they took, the dose, and how often/for how long they took the medication. The study coordinator then calculated the total number of pills taken, and these values were cross-checked by the first author, who reviewed the open-ended data as reported by participants and verified the coordinator’s categorization of the dosage and usage frequency.
Skill Use
Participants’ use of learned skills was assessed by a questionnaire developed for this study. Participants endorsed which of the three sets of skills (mindfulness, de-catastrophizing, coping skills) they had used during their most recent period. A composite variable for skill use was calculated by adding the number of skills used across the four follow-up time periods (i.e., 3 skills × 4 time points, range = 0–12), hereafter referred to as skill-months.
Treatment Fidelity
Treatment fidelity was not formally assessed by an independent rater as part of this pilot study. However, the lead therapist and group facilitator communicated following each session to confirm that all primary topics were addressed and there were no significant deviations from the protocol.
Statistical Analysis
Baseline characteristics were compared using a Student t test or Wilcoxon test to compare mean or median of continuous variables, respectively; the chi-square test of association was performed for categorical variables. Generalized linear mixed models (GLMMs) with a constant symmetry (CS) covariance structure were used to model the change in pain over time. Change in LMP-2days, skill use, and medication use were examined in a mixed model in which LMP-2days was the outcome variable and skill use, medication use, LMP-2days at baseline, and time were included as predictors.
All tests were two-sided, and P values <0.05 were considered statistically significant. The analyses were performed using SAS 9.4 (SAS Institute, Inc., Cary, NC, USA).
Results
Given the nature of this feasibility study, no a priori sample size was calculated. We estimated that a total of 18 participants (six in each of the three cohorts) would provide sufficient data on feasibility and acceptability for this open trial, and we exceeded this target sample size, with 20 participants completing the intervention. Demographic variables are presented in Table 1. There were no significant differences in self-reported average menstrual pain without medication between the cohorts.
Table 1.
Demographics and menstrual characteristics
| Enrolled (N = 24) | Completers (N = 20) | |
|---|---|---|
| Age, mean (SD), y | 20.8 (2.1) | 20.9 (2.2) |
| Ethnicity, No. (%) | ||
| Hispanic/Latino | 12 (50.0) | 11 (55.0) |
| Non-Hispanic/Non-Latino | 12 (50.0) | 9 (45.0) |
| Race, No. (%) | ||
| White | 7 (29.2) | 6 (30.0) |
| African American | 1 (4.2) | 0 (0.0) |
| Asian | 7 (29.2) | 6 (30.0) |
| American Indian or Alaska Native | 1 (4.2) | 1 (5.0) |
| Multiracial | 2 (8.3) | 2 (10.0) |
| Does not identify with any of the choices | 6 (25.0) | 5 (25.0) |
| Menstrual pain NRS,* mean (SD) | 7.3 (1.4) | 7.5 (1.4) |
| Age at menarche,* mean (SD) | 11.7 (1.3) | 11.7 (1.3) |
Missing for three noncompleters (N = 21).
NRS = numerical rating scale.
Four separate cohorts were conducted; cohort size ranged from three to eight participants (three, eight, five, and four in Cohorts 1–4, respectively). Seventy-five percent of participants (N = 15) attended all five sessions; 20% of participants (N = 4) attended four of the five sessions; 5% of participants (N = 1) attended three of the five sessions.
Feasibility and Acceptability
Twenty of the 24 enrolled participants (83.3%) completed the intervention. All four participants who did not complete the intervention dropped out either before any group sessions or after the first group session. All dropouts were due to scheduling difficulties (e.g., internship conflicted with group session times, etc.). No participants were excluded from analyses or removed from the study due to low attendance. The average CEQ Credibility Factor score (SD) was 20.43 (2.4).
Menstrual Pain
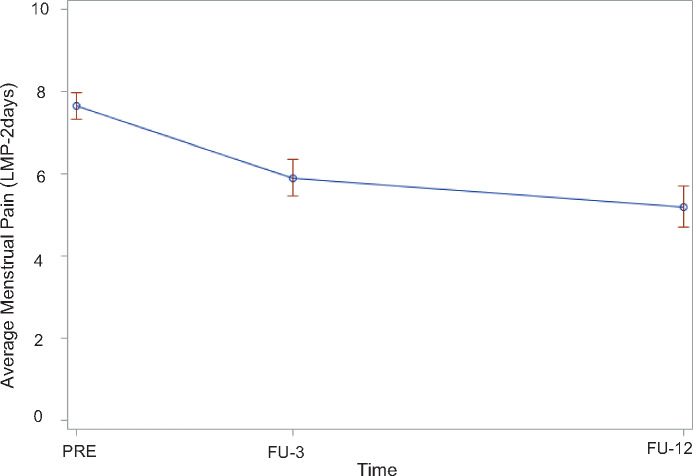
As shown in Table 2 and Figure 2, participants demonstrated significantly lower LMP-2days over time. There was no significant interaction between time and skill use during the past month and decrease in LMP-2days over time. Additionally, there was no significant interaction between time and medication use and decrease in LMP-2days over time.
Table 2.
Mixed-model results for changes in menstrual pain (LMP-2days) from pretreatment and skill use over time
| Variable | Regression Coefficient (95% CI) | P Value |
|---|---|---|
| LMP-2days | 0.57 (0.13 to 1.02) | 0.015 |
| Time, mo | –0.11 (–0.18 to –0.04) | 0.003 |
| Sum of No. of skills used in past mo | –0.04 (–0.36 to 0.28) | 0.794 |
| No. of pills taken during LMP | 0.11 (–0.02 to 0.24) | 0.086 |
CI = confidence interval; LMP = last menstrual period; LMP-2days = average menstrual pain rating during the first two days of the last menstrual period. Bolded numbers indicate findings were statistically significant.
Figure 2.
Change in menstrual pain ratings over time.
Skill Use
The mean skill-months (SD, range) was 6.85 (2.3, 3–12). Frequency of skill use across time points ranged from 25% to 60% for mindfulness, 30% to 60% for de-catastrophizing, 70% to 100% for coping skills, and 85% to 100% for use of any skill (Table 3).
Table 3.
Frequencies of reported skill use during prior menses
| Time Point | Mindfulness | De-catastrophizing | Coping Skills | Any Skill |
|---|---|---|---|---|
| FU-1 | 12 (60.0) | 12 (60.0) | 17 (85.0) | 20 (100.0) |
| FU-2 | 5 (25.0) | 12 (60.0) | 20 (100.0) | 20 (100.0) |
| FU-3 | 5 (25.0) | 8 (40.0) | 18 (90.0) | 18 (90.0) |
| FU-12 | 8 (40.0) | 6 (30.0) | 14 (70.0) | 17 (85.0) |
Data are presented as No. (%).
FU-1 = one-month follow-up; FU-2 = two-month follow-up; FU-3 = three-month follow-up; FU-12 = 12-month follow-up.
Psychological Measures
There were no significant changes in BSI-18 subscales or total scores at any time point. However, there was a significant decrease in PCS total scores over time (Table 4).
Table 4.
Results of mixed models for changes in psychological measures from pretreatment over time
| Dependent Covariate | Predictor | Regression Coefficient (95% CI) | P Value |
|---|---|---|---|
| BSI–somatization | Time | –0.06 (–0.20 to 0.09) | 0.443 |
| BSI–somatization | 0.57 (0.36 to 0.79) | <0.001 | |
| BSI–depression | Time | 0.04 (–0.09 to 0.16) | 0.535 |
| BSI–depression | 0.72 (0.47 to 0.97) | <0.001 | |
| BSI–anxiety | Time | 0.03 (–0.09 to 0.15) | 0.602 |
| BSI–anxiety | 0.79 (0.57 to 1.02) | <0.001 | |
| BSI–global severity index | Time | 0.01 (–0.30 to 0.33) | 0.933 |
| BSI–global severity index | 0.70 (0.50 to 0.89) | <0.001 | |
| PCS–total score | Time | –0.26 (–0.49 to –0.03) | 0.025 |
| PCS–total score | 0.63 (0.31 to 0.94) | <0.001 |
Each psychological measure was modeled in a separate mixed model, and all models were adjusted for baseline value.
BSI = Brief Symptom Inventory; CI = confidence interval; PCS = Pain Catastrophizing Scale. Bolded numbers indicate findings were statistically significant.
Discussion
The current study evaluated the feasibility, acceptability, and initial efficacy of a brief cognitive–behavioral, mind–body intervention in young adult women with moderate to severe menstrual pain. We hypothesized that participants would find the intervention acceptable and feasible and that participants in the group would report significantly lower menstrual pain ratings over the follow-up period. Additionally, we hypothesized that objective indicators of pain severity, such as medication use, would be significantly lower over follow-up as compared with baseline. We anticipated that usage of all three skills taught during the intervention would be associated with less menstrual pain and that measures of anxiety, depression, somatization, and pain catastrophizing would all also show improvement over the follow-up period.
Results demonstrated that the intervention was both feasible and acceptable, suggesting that this nondrug treatment may be a valuable alternative or adjunctive treatment to pharmacological approaches. Limited research exists on nonpharmacological approaches for menstrual pain [26]. A relatively substantial body of research exists on the use of herbal medicine and acupuncture, with some suggested benefits for menstrual pain [41]; however, these nonpharmacological approaches are not easily accessed by many women. The mind–body intervention used in this study is both brief and skills-based, which allows for potentially easier dissemination as well as the development of skills that can be used long term. Only one previous study has delivered a similar intervention for PD using pain management skills; it involved four individual intervention sessions, two hours each, delivered weekly [21]. However, attrition was very high, as only 16 of the 24 participants who enrolled in the study completed the post-treatment evaluation. Additionally, the intervention in part used a procedure known as “systematic desensitization” [42], which is based on the theory of “counterconditioning” whereby fearful stimuli (or, in this case, painful stimuli) are paired with relaxation strategies (e.g., deep breathing). Subsequent research has not provided support for the efficacy of interventions based on counterconditioning, which may have led to less improvement and greater rates of attrition in that particular study. Our data suggest that a group intervention with targeted cognitive–behavioral skills may be more feasible and acceptable for young women.
Participants reported significantly lower levels of menstrual pain over the course of study participation; however, neither skill use nor medication use was associated with decreased pain over the follow-up period. This contradicts previous research suggesting that active behavioral interventions such as biofeedback and pain management training are associated with significant reductions in menstrual pain following treatment and at follow-up (see [26] for a review). It is possible that skill use was overall relatively low, and therefore we did not see much change over the follow-up period. Although we did not find that skill use was related to pain outcomes, our data do support the notion that the improvement in menstrual pain was not related to simply taking additional pain medication, which provides support for the intervention’s impact on menstrual pain in this population.
Of note, menstrual pain NRS ratings decreased by only approximately two points, and it is questionable whether this change actually translates to clinical improvement. A large study in 2001 was able to begin to address this question by comparing multiple clinical trials for pain that included both pain NRS ratings (0–10) and patient global impressions of change [43]. Patients who endorsed being “much improved” or “very much improved” were considered to have demonstrated clinically meaningful change. Generally, this corresponded to a two-point decrease on the 0–10 NRS scale. Based on this study, guidelines were established supporting the two-point difference as clinically significant [44]. Although many additional factors must be considered when assessing clinical change, the reduction of two points in the current study suggests that the change was meaningful. However, as there are no studies that we are aware of that have examined the clinical impact of NRS change in menstrual pain specifically, it is not clear if the same criteria apply. Future research should include additional measures of quality of life and interference to better capture overall clinical improvement.
There were no changes in anxiety, depression, or somatization during the course of study participation; however, the data did show improvements in pain catastrophizing over time. This suggests that decreases in pain catastrophizing may be a mechanism of effective treatment, despite no reported change in the specific use of the skill of de-catastrophizing. To our knowledge, no other studies evaluating interventions for PD have measured these psychological constructs, so it is difficult to compare our findings with what has been already established. These findings are clinically significant; providers should consider targeting pain catastrophizing in their treatments, as it appears to be a potential mechanism in the experience of menstrual pain. At the same time, de-catastrophizing, specifically, may not be the best way to reduce pain catastrophizing. Instead, providers can help patients focus on other specific skills that facilitate this cognitive change. Indeed, significant reductions in pain catastrophizing are common outcomes when pain has improved as the result of an intervention (e.g., [45]).
This study is not without limitations. Primarily, no control condition was employed as part of this open trial design, so it is not possible to conclude that the improvement in menstrual pain was due to the intervention. The finding that pain catastrophizing improved over time is not evidence of a causal relationship, so of course it is also possible that other variables, including normal variability in menstrual pain, affected menstrual pain ratings. Additionally, the sample used included primarily young adult, college-aged women, so it is not clear whether these results are generalizable to other populations. This intervention was relatively short, so it is also possible that a longer intervention may have resulted in additional benefits with regard to pain reduction, decreased pain catastrophizing, and improvement on other measures. Self-selection bias may have also resulted in a study sample that was open to or perhaps already using alternative approaches to pain management. Although our data suggest that women are eager to participate in studies of nondrug approaches, it is possible that this group intervention may not be acceptable for other populations.
Conclusions
Complementary and alternative medicines such as herbal remedies and acupuncture have demonstrated some benefits reported for menstrual pain [26,41,46], but virtually no behavioral approaches have been developed for this population. Mind–body interventions may be more accessible and therefore acceptable to girls and women with menstrual pain. The mind–body intervention used in this study is both brief and skills-based, which allows for potentially easier dissemination as well as development of skills that can be used long term. The results of this study demonstrate the potential utility of mind–body interventions for menstrual pain, as well as the need for more rigorous evaluations of behavioral approaches for this cyclical pain condition. Based on the results of this study, future studies should continue to evaluate behavioral interventions for PD in randomized controlled trials. As with many other pain populations, girls and women with PD may significantly benefit from a nondrug approach. If determined to be efficacious, exploring mechanisms of symptom improvement, specifically pain catastrophizing, may help elucidate the complex factors contributing to girls’ and women’s experience of menstrual pain. These data may reduce suffering and will potentially lead to more personalized medicine approaches for this common and disabling pain condition.
Funding sources: This research was supported by grants from the National Institute of Child Health and Human Development (K23HD077042; PI: Laura A. Payne), UCLA Children’s Discovery and Innovation Institute (Seed Grant Award; PI: Laura A. Payne), and National Center for Advancing Translational Sciences UCLA Clinical and Translational Science Institute (KL2TR000122; PI: Steve Dubinett).
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of interest: The authors report no conflicts of interest.
References
- 1. Davis AR, Westhoff CL.. Primary dysmenorrhea in adolescent girls and treatment with oral contraceptives. J Pediatr Adolesc Gynecol 2001;14(1):3–8. [DOI] [PubMed] [Google Scholar]
- 2. Grandi G, Ferrari S, Xholli A, et al. Prevalence of menstrual pain in young women: What is dysmenorrhea? J Pain Res 2012;5:169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Iacovides S, Avidon I, Baker FC.. What we know about primary dysmenorrhea today: A critical review. Hum Reprod Update 2015;21(6):762–78. [DOI] [PubMed] [Google Scholar]
- 4. Klein JR, Litt IF.. Epidemiology of adolescent dysmenorrhea. Pediatrics 1981;68(5):661–4. [PubMed] [Google Scholar]
- 5. Proctor M, Farquhar C.. Diagnosis and management of dysmenorrhoea. BMJ 2006;332(7550):1134–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sundell G, Milsom I, Andersch B.. Factors influencing the prevalence and severity of dysmenorrhoea in young women. Br J Obstet Gynaecol 1990;97(7):588–94. [DOI] [PubMed] [Google Scholar]
- 7. De Sanctis V, Bernasconi S, Bianchin L, et al. Onset of menstrual cycle and menses features among secondary school girls in Italy: A questionnaire study on 3,783 students. Indian J Endocrinol Metab 2014;18(Suppl 1):S84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gunn HM, Tsai MC, McRae A, Steinbeck KS.. Menstrual patterns in the first gynecological year: A systematic review. J Pediatr Adolesc Gynecol 2018;31(6):557–65.e6. [DOI] [PubMed] [Google Scholar]
- 9. Righarts A, Osborne L, Connor J, Gillett W.. The prevalence and potential determinants of dysmenorrhoea and other pelvic pain in women: A prospective study. BJOG 2018;125(12):1532–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Khan KS, Champaneria R, Latthe PM.. How effective are non-drug, non-surgical treatments for primary dysmenorrhoea? BMJ 2012;344:e3011. [DOI] [PubMed] [Google Scholar]
- 11. Dawood MY. Dysmenorrhea. J Reprod Med 1985;30(3):154–67. [PubMed] [Google Scholar]
- 12. Henzl MR. Dysmenorrhea: Achievements and challenges. Sex Med Today 1985;9:8–12. [Google Scholar]
- 13. O'Connell K, Davis AR, Westhoff C.. Self-treatment patterns among adolescent girls with dysmenorrhea. J Pediatr Adolesc Gynecol 2006;19(4):285–9. [DOI] [PubMed] [Google Scholar]
- 14. Oladosu FA, Tu FF, Hellman KM.. Nonsteroidal antiinflammatory drug resistance in dysmenorrhea: Epidemiology, causes, and treatment. Am J Obstet Gynecol 2018;218(4):390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wong CL, Farquhar C, Roberts H, Proctor M.. Oral contraceptive pill for primary dysmenorrhoea. Cochrane Database Syst Rev 2009;4:CD002120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marjoribanks J, Ayeleke RO, Farquhar C, Proctor M.. Nonsteroidal anti-inflammatory drugs for dysmenorrhoea. Cochrane Database Syst Rev 2015;7:CD001751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Iacovides S, Baker FC, Avidon I.. The 24-h progression of menstrual pain in women with primary dysmenorrhea when given diclofenac potassium: A randomized, double-blinded, placebo-controlled crossover study. Arch Gynecol Obstet 2014;289(5):993–1002. [DOI] [PubMed] [Google Scholar]
- 18. Denney DR, Gerrard M.. Behavioral treatments of primary dysmenorrhea: A review. Behav Res Ther 1981;19(4):303–12. [DOI] [PubMed] [Google Scholar]
- 19. Park KS, Park KI, Hwang DS, Lee JM, Jang JB, Lee CH.. A review of in vitro and in vivo studies on the efficacy of herbal medicines for primary dysmenorrhea. Evid Based Complement Alternat Med 2014;2014:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC.. The biopsychosocial approach to chronic pain: Scientific advances and future directions. Psychol Bull 2007;133(4):581–624. [DOI] [PubMed] [Google Scholar]
- 21. Quillen MA, Denney DR.. Self-control of dysmenorrheic symptoms through pain management training. J Behav Ther Exp Psychiatry 1982;13(2):123–30. [DOI] [PubMed] [Google Scholar]
- 22. Amodei N, Nelson RO, Jarrett RB, Sigmon S.. Psychological treatments of dysmenorrhea: Differential effectiveness for spasmodics and congestives. J Behav Ther Exp Psychiatry 1987;18(2):95–103. [DOI] [PubMed] [Google Scholar]
- 23. Bennink CD, Hulst LL, Benthem JA.. The effects of EMG biofeedback and relaxation training on primary dysmenorrhea. J Behav Med 1982;5(3):329–41. [DOI] [PubMed] [Google Scholar]
- 24. Chesney MA, Tasto DL.. The effectiveness of behavior modification with spasmodic and congestive dysmenorrhea. Behav Res Ther 1975;13(4):245–53. [DOI] [PubMed] [Google Scholar]
- 25. Hart AD, Mathisen KS, Prater JS.. A comparison of skin temperature and EMG training for primary dysmenorrhea. Biofeedback Self Regul 1981;6(3):367–73. [DOI] [PubMed] [Google Scholar]
- 26. Proctor ML, Murphy PA, Pattison HM, Suckling J, Farquhar CM.. Behavioural interventions for primary and secondary dysmenorrhoea. Cochrane Database Syst Rev 2007;3:CD002248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hardi G, Evans S, Craigie M.. A possible link between dysmenorrhoea and the development of chronic pelvic pain. Aust N Z J Obstet Gynaecol 2014;54(6):593–6. [DOI] [PubMed] [Google Scholar]
- 28. McClintock AS, McCarrick SM, Garland EL, Zeidan F, Zgierska AE.. Brief mindfulness-based interventions for acute and chronic pain: A systematic review. J Altern Complement Med 2019;25(3):265–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Miller-Matero LR, Coleman JP, Smith-Mason CE, Moore DA, Marszalek D, Ahmendani BK.. A brief mindfulness intervention for medically hospitalized patients with acute pain: A pilot randomized clinical trial. Pain Med. 2019;20(11):2149–54. [DOI] [PubMed] [Google Scholar]
- 30. Shearer A, Hunt M, Chowdhury M, Nicol L.. Effects of a brief mindfulness intervention on student stress and heart rate variability. Int J Stress Manag 2016;23(2):232–54. [Google Scholar]
- 31. Sass SM, Early LM, Long L, Burke A, Gwinn D, Miller P.. A brief mindfulness intervention reduces depression, increases nonjudgment, and speeds processing of emotional and neutral stimuli. Mental Health Prev 2019;13:58–67. [Google Scholar]
- 32. Howarth A, Smith JG, Perkins-Porras L, Ussher M.. Effects of brief mindfulness-based interventions on health-related outcomes: A systematic review. Mindfulness 2019;10(10):1957–68. [Google Scholar]
- 33. Darnall BD, Ziadni MS, Roy A, et al. Comparative Efficacy and Mechanisms of a Single-Session Pain Psychology Class in Chronic Low Back Pain: Study Protocol for a Randomized Controlled Trial. Trials. 2018;19(1):165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Darnall BD, Sturgeon JA, Kao MC, Hah JM, Mackey SC.. From catastrophizing to recovery: A pilot study of a single-session treatment for pain catastrophizing. J Pain Res 2014;7:219–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Thorn BE, Boothby JL, Sullivan M.. Targeted treatment of catastrophizing for the management of chronic pain. Cognitive Behav Pract 2002;9(2):127–38. [Google Scholar]
- 36. Thorn BE, Pence LB, Ward LC, et al. A randomized clinical trial of targeted cognitive behavioral treatment to reduce catastrophizing in chronic headache sufferers. J Pain 2007;8(12):938–49. [DOI] [PubMed] [Google Scholar]
- 37. Bergeron S, Binik YM, Khalife S, et al. A randomized comparison of group cognitive–behavioral therapy, surface electromyographic biofeedback, and vestibulectomy in the treatment of dyspareunia resulting from vulvar vestibulitis. Pain 2001;91(3):297–306. [DOI] [PubMed] [Google Scholar]
- 38. Devilly GJ, Borkovec TD.. Psychometric properties of the credibility/expectancy questionnaire. J Behav Ther Exp Psychiatry 2000;31(2):73–86. [DOI] [PubMed] [Google Scholar]
- 39. Derogatis LR. Brief Symptom Inventory (BSI) 18 Administration, Scoring, and Procedures Manual. Minneapolis, MN: NCS Pearson, Inc; 2001. [Google Scholar]
- 40. Sullivan MJL, Bishop SR, Pivik J.. The Pain Catastrophizing Scale: Development and validation. Psychol Assess 1995;7(4):524–32. [Google Scholar]
- 41. Leem J, Jo J, Kwon CY, Lee H, Park KS, Lee JM.. Herbal medicine (Hyeolbuchukeo-tang or Xuefu Zhuyu decoction) for treating primary dysmenorrhea: A systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 2019;98(5):e14170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wolpe J. Psychotherapy by Reciprocal Inhibition. Palo Alto, CA: Stanford University Press; 1958. [Google Scholar]
- 43. Farrar JT, Young JP Jr, LaMoreaux L, Werth JL, Poole RM.. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001;94(2):149–58. [DOI] [PubMed] [Google Scholar]
- 44. Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain 2008;9(2):105–21. [DOI] [PubMed] [Google Scholar]
- 45. Vowles KE, McCracken LM, Eccleston C.. Processes of change in treatment for chronic pain: The contributions of pain, acceptance, and catastrophizing. Eur J Pain 2007;11(7):779–87. [DOI] [PubMed] [Google Scholar]
- 46. Kannan P, Chapple CM, Miller D, Claydon-Mueller L, Baxter GD.. Effectiveness of a treadmill-based aerobic exercise intervention on pain, daily functioning, and quality of life in women with primary dysmenorrhea: A randomized controlled trial. Contemp Clin Trials 2019;81:80–6. [DOI] [PubMed] [Google Scholar]