Abstract
Due to the well-established therapeutic benefit of boosting anti-tumor responses through blockade of the T cell inhibitory receptor PD-1, it has been proposed that PD-1 blockade could be also useful in infectious disease settings, including Mycobacterium tuberculosis (Mtb) infection. However, in pre-clinical models Mtb-infected PD-1−/− mice mount exaggerated Th1 responses that drive lethal immunopathology. Multiple cases of tuberculosis during PD-1 blockade have been observed in cancer patients, but in humans little is understood about Mtb-specific immune responses during checkpoint blockade-associated tuberculosis. Here we report two more cases. We describe a patient who succumbed to disseminated tuberculosis after PD-1 blockade for treatment of nasopharyngeal carcinoma, and we examine Mtb-specific immune responses in a Merkel cell carcinoma patient who developed checkpoint blockade-associated tuberculosis and was successfully treated for the infection. Following anti-PD-1 administration, interferon-γ-producing Mtb-specific CD4 T cells became more prevalent in the blood, and a tuberculoma developed a few months thereafter. Mtb-specific Th17 cells, CD8 T cells, regulatory T cells, and antibody abundance did not change prior to appearance of the granuloma. These results are consistent with the murine model data, and suggest that boosting Th1 function with PD-1 blockade may increase the risk or severity of tuberculosis in humans.
INTRODUCTION
The therapeutic benefits of enhancing T cell function by blockade of co-inhibitory receptor Programmed Cell Death-1 (PD-1) and one of its ligands, Programmed Cell Death Ligand 1 (PD-L1) are now well established for multiple malignancies. Targeting the PD-1 pathway for treatment of chronic infectious diseases is also an area of active development (1). However, whereas PD-1 blockade unleashes beneficial tumor-specific T cell responses during cancer immunotherapy, increasing pathogen-specific T cell function through PD-1 blockade can either enhance control of infection or drive lethal immunopathology, depending on the timing of PD-1 blockade after infection and the particular microbe present (2). Given the long duration of standard treatment regimens for tuberculosis (TB) and increasing prevalence of drug-resistant strains, host-directed therapies that prove particularly useful in TB (3, 4) are desirable. Human Mycobacterium tuberculosis (Mtb)-specific CD4 T cells express PD-1 during active TB, and levels of PD-1 on Mtb-specific T cells decrease after successful TB treatment (5, 6). Accordingly, PD-1 blockade has been suggested as a host-directed therapy for TB (7), but it is not clear if PD-1 blockade would be beneficial or detrimental in human TB.
Pre-clinical data in mice suggest that boosting Type 1 helper T cell (Th1) responses by targeting PD-1 may be detrimental during Mtb infection. PD-1 knockout (PD-1−/−) mice are hyper-susceptible to Mtb infection, developing large necrotic lesions with high bacterial loads and succumbing faster than even T cell-deficient mice (8–10). The inability of PD-1−/− mice to control Mtb infection is due to increased Mtb-specific CD4 T cell responses, as early mortality in PD-1−/− mice is prevented by CD4 T cell depletion (9). Moreover, it has been shown that over-production of interferon (IFN)-γ by CD4 T cells drives early mortality in Mtb infected PD-1−/− mice (11). Thus, although IFN-γ-producing Th1 cells are required for host resistance to mycobacteria, their enhanced activity in the absence of PD-1 counter-intuitively exacerbates TB in mice. The mechanisms of IFN-γ-driven disease exacerbation, however, are not yet defined.
Several cases of TB following checkpoint blockade have recently been reported (12–16), and the atezolizumab (anti-PD-L1) product label indicates cases of mycobacterial infections were also observed on trial. Therefore, there is a growing body of evidence suggesting that PD-1 might play a similar role in suppressing immunopathologic T cell responses in human TB. However, there is no information on Mtb-specific immune responses in individuals developing checkpoint blockade-associated TB.
Here we report two cases of TB in individuals receiving anti-PD-1 monoclonal antibodies as cancer treatment. In one patient with nasopharyngeal carcinoma (NPC), PD-1 blockade was followed by the rapid development of disseminated TB that was eventually fatal. In the second patient being treated with anti-PD-1 for Merkel cell carcinoma (MCC), pulmonary TB reactivation was much milder and was successfully treated with anti-TB therapy. Here we characterize the peripheral adaptive immune response and the bacteria isolated from the pulmonary lesion following PD-1 blockade of the MCC patient.
RESULTS
Lethal disseminated TB following PD-1 blockade in patient with NPC
A 59-year-old male with metastatic NPC enrolled in a clinical trial evaluating an anti-PD-1 therapy, nivolumab, at a dose of 3 mg per kg every 2 weeks in May 2016 (17) (Fig. 1A). Prior to study enrollment, no testing for latent TB infection was performed. The patient was HIV negative, but he had emigrated from Vietnam, a country highly endemic for TB, more than a decade before.
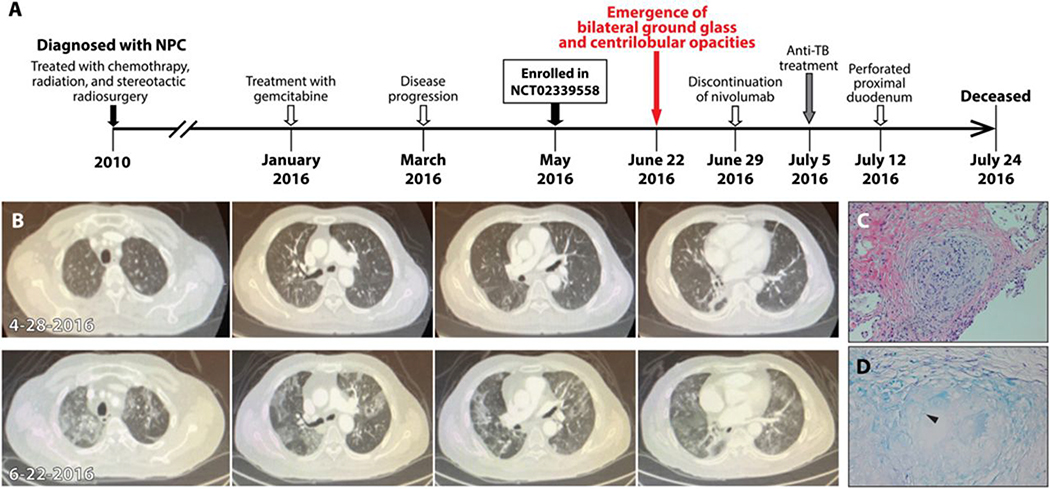
Figure 1. Development of tuberculosis in a patient treated with nivolumab for nasopharyngeal carcinoma.
(A) The timeline of therapy and disease status of a patient with nasopharyngeal carcinoma (NPC). (B) Chest CT images of a patient 5 days prior to the initiation of PD-1 blockade (4–28-2016, upper) and ~8 weeks later (6–22-2016, lower). (C) The hematoxylin and eosin stain of transbronchial biopsy of the right lung nodule. (D) The result of acid-fast stain of the lung biopsy. Arrow head shows an acid-fast bacterium (AFB) in the granuloma.
In June 2016, after 3 cycles of anti-PD-1 therapy, computed tomography (CT) scanning showed an interval development of ground glass and centrilobular nodular opacities throughout both lungs (Fig. 1C) which were not present from scanning two months prior (Fig. 1B). Other known sites of NPC remained stable or had decreased in size. The patient underwent transbronchial biopsy to obtain fragments of his lung’s right middle lobe at the site of the nodular infiltrate. Pathology revealed a single non-necrotizing epithelioid granuloma that stained positively for acid-fast bacilli (AFB) (Fig. 1, C and D). Sputum samples were positive for Mtb DNA by PCR at this time, and AFB were found in sputum and rectum samples. The patient was diagnosed with disseminated TB. The patient denied TB symptoms including shortness of breath, cough, fevers, chills, night sweats or weight loss. He denied any history of TB or sick contacts and did not recall previous TB testing by purified protein derivative (PPD).
The patient stopped treatment with nivolumab in June 2016 after 3 cycles soon after the development of bilateral ground glass opacities was found. He underwent a bronchoscopy, bronchoalveolar lavage (BAL) and biopsy, to evaluate the cause of the new opacities. Five days later, the patient presented to the hospital with lethargy, confusion and hypoxemia at which time the culture from his BAL specimen grew Mtb that was resistant to streptomycin but susceptible to other 1st line drugs. The patient was initiated on an anti-TB medication regimen of rifampin 600 mg per day, isoniazid 300 mg per day, ethambutol 1200 mg per day, pyrazinamide 1500 mg per day and pyridoxine 25 mg per day on 7/5/2016. The patient developed profound diarrhea. Cultures of stool grew Mtb. On 7/12/2016, air was found on routine chest X-ray, and CT scan of his abdomen found moderate intraperitoneal air consistent with perforation of the proximal duodenum. Due to co-morbidities and high operative risk, the surgery team recommended medical management including gastric tube decompression, proton pump inhibition, and bowel rest. Because the patient was on strict bowel rest, his oral anti-TB medication regimen was changed to parenteral treatment. This consisted of streptomycin 15 mg per kg IM per day, rifampin 600 mg IV per day, moxifloxacin 400 mg IV per day, and linezolid 600 mg IV every 12 hours. A goals-of-care discussion with the family resulted in a change in his resuscitative strategy to avoid intubation, chest compressions, and pressor medications. A sputum sample obtained on 7/22/16 was positive for AFB. No other samples were obtained thereafter.
Despite ongoing anti-mycobacterial therapy, the patient’s condition progressively worsened. The patient had increased work of breathing requiring higher doses of supplemental oxygen, remained persistently febrile, and became hypotensive requiring normal saline boluses. The patient passed away in July 2016.
Pulmonary TB following PD-1 blockade in patient with MCC
An 83-year-old male with metastatic MCC enrolled in a clinical trial evaluating an anti-PD-1 therapy, pembrolizumab, at a dose of 2 mg per kg in June 2015 (18) (Fig. 2A). Prior to study enrollment, no testing for latent TB infection was performed. The patient is an HIV negative Caucasian from Florida, and there were no other co-morbidities associated with increased TB risk apart from his age. In December 2015, after 11 cycles of anti-PD-1 therapy, CT scanning showed a new right lower lobe pulmonary nodule measuring 1.1 × 1.6 cm (Fig. 2B). Other known sites of MCC remained stable or had decreased in size. The patient underwent video-assisted thoracic surgery to obtain a right wedge excision of the nodule in January 2016. Pathology revealed necrotizing granuloma that stained positively for AFB (Fig. 2, C and D). No Mtb DNA in the nodule was detectable by PCR, and three tests for acid fast organisms in sputum samples were also negative. The patient denied classic TB symptoms, any history of TB or sick contacts, and did not recall previous TB testing by PPD. He had not traveled outside the country in the past year, but did report a distant travel history to Europe, China, South America, and the Caribbean.
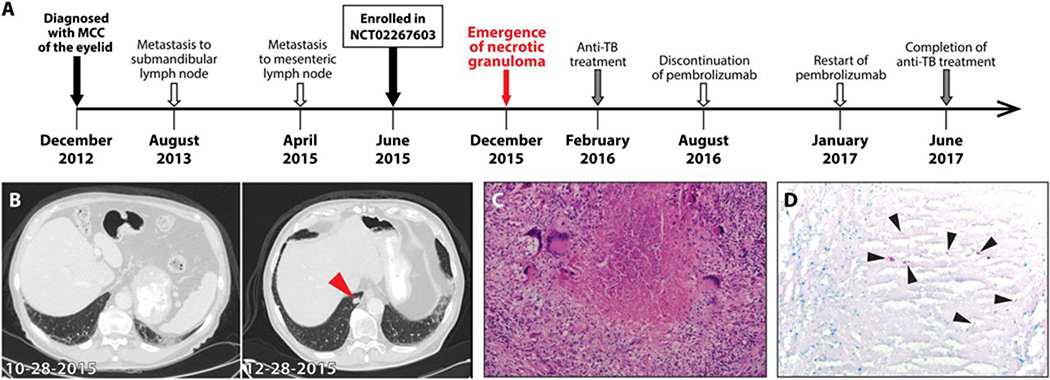
Figure 2. Development of tuberculosis in a patient treated with pembrolizumab for Merkel cell carcinoma.
(A) The timeline of therapy and disease status of a patient with Merkel cell carcinoma (MCC). (B) The chest CT images of a patient ~5 months after initiation of PD-1 blockade (10–28-2015, left) and ~2 months later (12–28-2015, right). Arrow head indicates necrotizing nodule in the right lower lobe. (C) The hematoxylin and eosin stain of the excised lung nodule with necrotic center surrounded by multinucleated giant cells. (D) The result of acid-fast stain of the lung nodule. Arrow heads denote AFB in the granuloma.
The patient continued on treatment per protocol and received his 12th cycle of pembrolizumab in January 2016. In February 2016, the culture from his lung specimen grew Mtb that was pan-susceptible to antibiotics. Although not performed prior to immunotherapy, following detection of Mtb by culture, the patient was found to have a positive IFN-γ release assay (IGRA) test, which detects the presence of T cell responses to Mtb (unstimulated negative control (Nil): 0.1 IU/ml, Mitogen stimulated positive control-Nil: >10 IU/ml, Mtb antigen stimulation-Nil: 1.59 IU/ml). The patient was initiated on an anti-TB medication regimen of rifampin 600 mg per day, isoniazid 300 mg per day, ethambutol 1200 mg per day, pyrazinamide 1500 mg per day and pyridoxine 25 mg per day. Sputum collection from 2/9/2016 to 2/12/2016 was found to be negative for Mtb by AFB smear, PCR screening and bacterial culture. The patient initially proved unable to tolerate anti-TB medications in the setting of pembrolizumab. The patient was hospitalized due to fever, confusion, and elevated liver enzymes, but no additional infectious etiology was found to explain his signs and symptoms. The patient was taken off pembrolizumab treatment in August 2016 with CT imaging showing a continued partial response.
Following discontinuation of pembrolizumab, the patient tolerated an anti-TB regimen of isoniazid and rifabutin until he again developed elevated liver enzymes. Thus, he was changed to levofloxacin and rifabutin, which was well-tolerated. Unfortunately, the patient developed a 6cm x 7cm duodenal metastatic mass, which on biopsy revealed MCC. Given the patient’s progressive disease, pembrolizumab infusions were restarted in January 2017 with resultant marked reduction in the duodenal mass by April 2017. The patient completed 9 months of TB therapy on June 2017 without evidence of recurrence and as of January 2018 has returned to his previous physical baseline of being able to perform a daily level of moderate physical activity (Fig. 2A).
Increases in circulating Mtb-specific Th1 cells after PD-1 blockade preceding granuloma formation
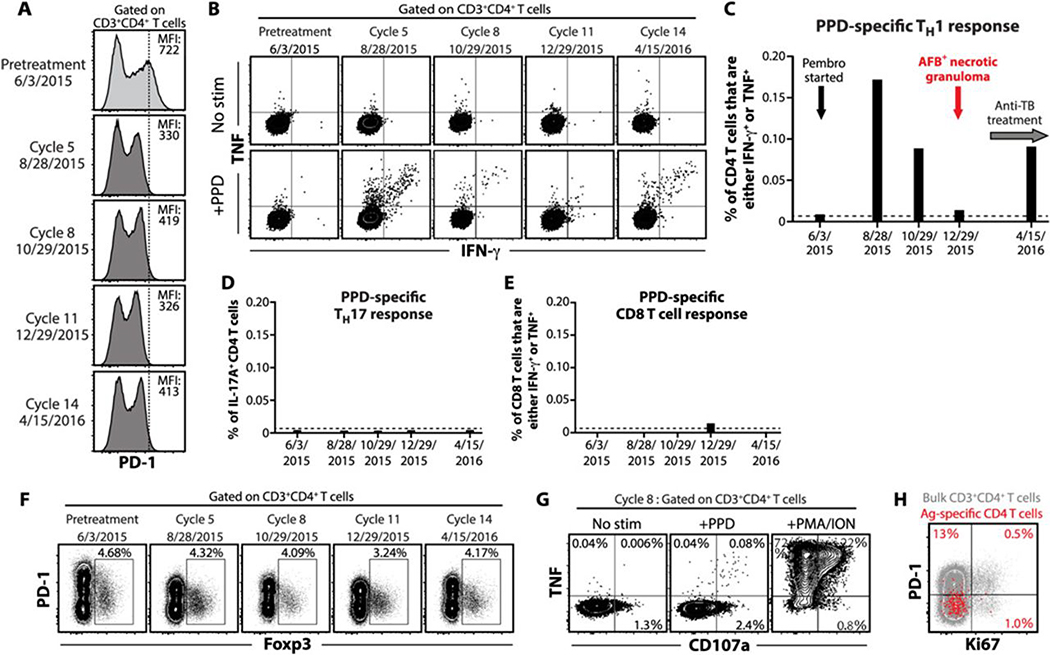
Based on the murine model data we speculated that PD-1 blockade in the MCC patient would lead to enhanced Mtb-specific Th1 responses (9, 11). Cryopreserved peripheral blood mononuclear cells (PBMCs) obtained immediately prior to treatment with pembrolizumab and at treatment cycles 5, 8, 11, and 14 were analyzed. PD-1 staining intensity decreased but was still detectable following administration of pembrolizumab (Fig. 3A). We measured mycobacteria-specific T cell responses by stimulating PBMCs with PPD and performing intracellular cytokine staining. Prior to treatment, there were no detectable IFN-γ or tumor necrosis factor (TNF) producing PPD-specific CD4 T cells. However, approximately 3 months after the first dose of pembrolizumab, we detected a spike in PPD-specific Th1 cells, and antigen-specific Th1 cells were detected at all time points post-treatment (Fig. 3, B and 3C).
Figure 3. Mtb-specific T cell responses in a patient with MCC after pembrolizumab treatment.
(A) PD-1 expression on CD4 T cells in PBMCs from a patient at baseline and serial samples taken throughout disease course. The vertical line indicates the peak intensities of PD-1 staining on CD4 T cells before PD-1 blockade. (B) Flow cytometry plots of CD4 T cells in PBMCs from the patient with MCC showing intracellular cytokine staining for IFN-γ and TNF after a 6-hour stimulation with PPD. Summary graphs are shown of PPD-specific Th1 (C), Th17 (D) and CD8 T cell (E) responses in PBMCs from the patient after PD-1 blockade. The dashed lines depict the baseline frequency of IFN-γ or TNF-producing CD4 T cells prior to the initiation of PD-1 blockade. (F) The frequency of Foxp3+ regulatory CD4 T cells in PBMCs from the patient throughout disease course. (G) Flow cytometry plots of CD4 T cells showing TNF and CD107a expression after 6-hour stimulation with PPD or phorbol myristate acetate and ionomycin (PMA/ION). (H) The expression of PD-1 and Ki67 on bulk (gray) or PPD-specific (red) CD4 T cells in PBMCs from the patient after 5 cycles of PD-1 blockade. Overlaid PPD-specific CD4 T cells are gated on TNF+ IFN-γ+ cells following stimulation. Data were obtained from each sample at different points after PD-1 blockade (n=1 per time point).
To further examine immune alterations associated with the development of TB after PD-1 blockade in this case, we measured other T cell populations. In certain settings, interleukin-17A (IL-17A) has been shown to be pathogenic in Mtb infected mice (19), but PPD-specific Th17 cells, which are defined by their production of IL-17A, were not detected in this patient at any time point (Fig. 3D). CD8 T cells are the principal antitumor effector cells boosted by PD-1 blockade during cancer immunotherapy (20). In contrast, mycobacteria-specific CD8 T cells were not detected following PD-1 blockade (Fig. 3E). Foxp3+ regulatory T cells express PD-1 and have been implicated in regulation of Mtb-specific immune responses (21), so we asked if TB after PD-1 blockade was associated with expansion of this immunosuppressive cell type. We found that pembrolizumab treatment was not associated with changes in the frequency of Foxp3+ CD4 T cells in the peripheral blood of this individual (Fig. 3F). Effector T cells can sometimes transiently upregulate Foxp3 after stimulation, so these data also indicate that PD-1 blockade was also not associated with a significant upregulation of Foxp3 by effector T cells. In summary, TB in this patient was not associated with an increase in Th17 cells, CD8 T cells or increases in Foxp3+ CD4 T cells.
Although we were unable to detect PPD-specific cytotoxic CD8 T cell responses in this individual, we asked if the Mtb-specific CD4 T cells displayed the ability to degranulate by measuring the appearance of CD107 on the cell surface after stimulation. Interestingly, we found that PPD-specific Th1 cells present at cycle 8 just prior to the development of the pulmonary lesion displayed the ability to degranulate based on the appearance of CD107a on the cell surface after antigen stimulation (Fig. 3G). Although we were not able to directly measure killing, these data indicate that the Mtb-specific CD4 T cells that expanded following PD-1 blockade were potentially cytotoxic.
PD-1 was not detected on Mtb-specific CD4 T cells from peripheral blood (Fig. 3H), which reflects either interference of PD-1 staining by pembrolizumab, or alternatively the anatomical site sampled, as PD-1 expression is greatly increased on CD4 T cells in the lungs compared to the peripheral blood in mice and non-human primates (9, 22, 23). Ki67, a marker of proliferating cells, was only found in ~1% of the PPD-specific CD4 T cells at cycle 5 (Fig. 3H), suggesting that the first time point available for analysis may have been already past the peak CD4 T cell proliferative response.
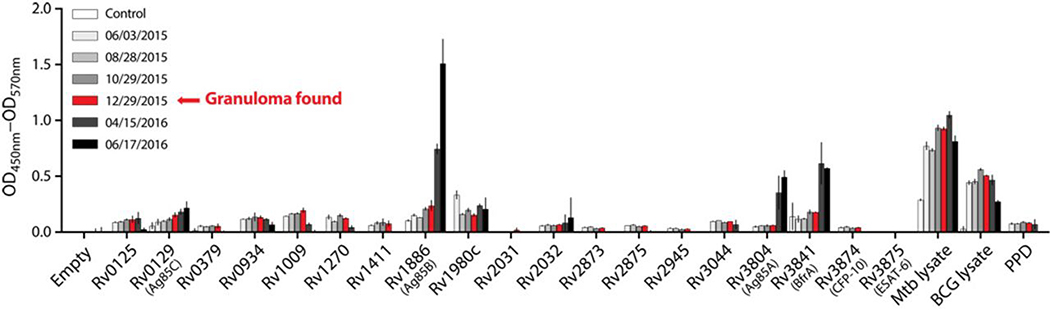
Recently, there has been renewed interest in the role of antibodies in TB (24), so we next tested if PD-1 blockade affected Mtb-specific antibody responses. Serum concentrations of Mtb-specific IgG changed minimally or not at all in the interval between pre-treatment and appearance of pulmonary tuberculoma (Fig. 4). However, after anti-TB chemotherapy was initiated, antibody responses to Rv3804 (Ag85A), Rv1886 (Ag85B), and Rv3841 (BrfA) rose sharply. Therefore, while antibody responses against some Mtb proteins did increase after pembrolizumab treatment, they did so months after the TB-related adverse event, so it is unlikely that they played a role in the development of TB.
Figure 4. Mtb-specific antibody responses in a patient with MCC after pembrolizumab treatment.
IgG against mycobacterial antigens measured in sera from the MCC patient at different points after PD-1 blockade. Error bars depict ±SEM of duplicate wells.
No evidence of recent local transmission of the Mtb strain isolated from the MCC patient
The Mtb strain isolated from the MCC patient’s lung nodule was identified as Euro-American lineage 4 (spoligotype (777776777760601) and MIRU-VTNR pattern (224221153323)), which causes the majority of TB in North America and Europe but relatively few cases in other regions of the world (25). There were only 12 other cases of TB in the United States due to infection with this specific bacterial genotype between 12/2013 and 12/2016. In this patient’s home state of Florida, there were only three other cases between 2007 and 2016. The most recent previous case was ~11 months prior to development of the tuberculoma in the patient reported here, and all were in counties several hundred miles away. Moreover, in the same time period, there were no reported cases of TB due to this genotype of bacteria in the state of Georgia, where the patient was treated with pembrolizumab for MCC. Therefore, the case of TB in this individual was neither temporally nor geographically associated with a cluster of transmission. Taken together, the patient’s risk factors, such as age, travel history, lack of contact with TB patients, along with the lack of evidence for local transmission of this bacterial genotype, collectively suggest that TB in this patient was likely due to a pre-existing latent Mtb infection.
DISCUSSION
In this study, we provide two case reports of checkpoint blockade-associated TB. One individual developed disseminated fatal disease after PD-1 blockade, whereas the other presented with a single pulmonary lesion and was successfully treated for TB. We were able to characterize the adaptive immune response in the patient who survived. In this individual, PD-1 blockade was followed by expansion of circulating Mtb-specific Th1 cells, but not Mtb-specific Th17 cells, CD8 T cells or increased antibody responses. The increase in Th1 cells was followed by the development of a necrotic tuberculoma in the lung that contained AFB. These results are consistent with data from the murine model of Mtb infection that predict a potential exacerbation of TB upon PD-1 blockade.
In mice, PD-1 deficiency boosts Mtb-specific Th1 cell expansion and effector functions, but rather than leading to enhanced bacterial control, the increased production of IFN-γ by CD4 T cells drives large necrotic lesions and leads to accelerated death of the host (9, 11). No long-term PD-1 antibody blockade studies have been performed in Mtb-infected animals, however short-term blockade of the PD-1 pathway (i.e. ~2 weeks) with monoclonal antibodies in Mtb-infected mice results in neither a therapeutic nor detrimental outcome (26, 27). Therefore, it is not yet clear if exacerbation of TB in the setting of the PD-1−/− mouse model may be an accurate predictor of detrimental outcome following long-term blockade with monoclonal antibodies. The cases reported here suggest the PD-1−/− mouse data may be generalizable. Nevertheless, these results suggest that PD-1 blockade in Mtb-infected humans can lead to Mtb-specific Th1 cell-mediated pathology. These results have implications for the development of host-directed therapies for TB as well as the clinical management of individuals receiving checkpoint inhibitors for cancer.
There are several limitations to our study. The MCC patient’s age was in itself a predisposing factor for TB, as it is thought that the decline in immunity with age may allow for the reactivation of latent Mtb infection. However, the Mtb-specific Th1 response was boosted following PD-1 blockade, suggesting that it is unlikely that this was TB reactivation due to declining immunity associated with immunosenescence. We should also point out that the use of PPD as a stimulation antigen may have limited our ability to detect Mtb-specific CD8 T cells, as whole protein antigens may preferentially stimulate CD4 T cells. However, in mice it has been shown that CD8 T cells have very little contribution to the pathology observed in Mtb-infected PD-1−/− mice (9). Lastly, validation of these results with kinetic analysis of Mtb-specific T cell responses in additional cases of checkpoint blockade-associated tuberculosis as well as analysis of T cell responses from the airways or lungs from these individuals will be required to support the hypothesis that boosting T cell responses through PD-1 blockade exacerbated tuberculosis.
Mtb infection is the leading cause of death due to a single infectious agent despite the availability of chemotherapy (28). There is great need for the development of improved therapies for both drug resistant and susceptible Mtb infection (4). The success of PD-1 blockade in cancer immunotherapy has led to persistent interest in treating human TB with PD-1 blockade to boost Mtb-specific T cell function, despite murine model data demonstrating extreme susceptibility of Mtb-infected PD-1−/− animals. These data support exercising caution when treating TB with pro-inflammatory strategies in general, but PD-1 blockade in particular. It is worth noting that animal data suggest that not all mycobacterial infections may be exacerbated by PD-1 deficiency, as BCG is more readily cleared in PD-1−/− mice (29). Moreover, not all checkpoint-targeting therapies are likely to result in exacerbated Mtb infection, as TIM-3−/− mice control virulent Mtb infection better than wild-type animals (30).
As PD-1 blockade becomes more globally deployed in TB endemic regions, it is possible that TB-related adverse events in cancer immunotherapy may increase. Unlike immunosuppressive biologic therapies utilized for treatment of autoimmune disease, very few investigational protocols incorporating anti-PD-1 or anti-PD-L1 therapy require TB pre-screening. These cases highlight that treatment with anti-PD-1 in a patient with latent or active TB may have deleterious outcomes as suggested by murine model experiments. Our study suggests that TB screening prior to checkpoint therapy may be warranted.
MATERIALS AND METHODS
Study design
In recently reported studies of PD-1 blockade for the treatment of NPC (17) (Clinicaltrials.gov ID: NCT02339558) and MCC (18) (Clinicaltrials.gov ID: NCT02267603) two participants developed TB as an infection-associated adverse event. The objectives of this study were (i) to evaluate disease progression in the both patients after anti-PD-1 treatments and (ii) to identify changes to the Mtb-specific immune responses that were associated with the development of TB in the survived patient. No data were excluded from this study. Written informed consent was obtained from the patient before conducting the study. The protocol was approved by an institutional review board at each participating center. Primary data are reported in table S1.
Flow cytometry and antibody assay
For intracellular cytokine staining, cryopreserved PBMCs were stimulated with 10 μg/ml PPD (Statens Serum Institut, Copenhagen, Denmark) or phorbol myristate acetate and ionomycin (eBioscience) for 6 hours in the presence of anti-CD107a (BioLegend), brefeldin A and monensin (eBioscience). Cells were stained with various combinations of fluorochrome-labeled antibodies and Fixable Viability Dye eFluor 780 (purchased from BioLegend or eBioscience). All samples were acquired by LSRFortessa flow cytometer (BD Biosciences) and analyzed with FlowJo software (Tree Star).
Antibody abundance was quantitated by the ELISA. 384-well high binding plates (Corning) were coated with Mtb protein antigens (31) at 2 μg/ml, or PPD, BCG lysate, and Mtb lysate at 6 μg/ml. Serum samples (1:500 dilution) were incubated overnight at 4°C. Plates were washed, followed by the addition of recombinant Protein-G HRP and incubated for 1 hour at room temperature. Plates were washed and developed with SureBlue TMB Peroxidase Substrate (KPL Inc.) followed by 1N H2SO4. Optical densities (OD) were read at 450 nm and 570 nm. Antibody levels were calculated by (OD450 nm - OD570 nm) subtracted from control wells of antigen and HRP alone.
Bacteriological analysis
Sputum samples underwent decontamination with N-acetyl-L-cysteine (NALC)-sodium hydroxide and AFB stains were performed using modified Kinyoun preparation. Following decontamination, the specimens were plated onto Middlebrook 7H11 agar and Lowenstein-Jensen slant culture, and inoculated on BD BACTEC MGIT tubes (BD Biosciences), which were incubated in the BD BACTEC MGIT automated mycobacterial detection system. After positivity on the instrument, the AccuProbe Culture Identification test (Hologic) identified the isolate as Mtb. Drug susceptibility assays were performed by the Georgia Public Health Laboratory for the MCC patient or ARUP Laboratories for the NPC patient.
Genotyping of Mtb isolate
The MCC patient’s Mtb isolate was submitted for spoligotype and mycobacterial interspersed repetitive units-variable number tandem repeat (MIRU-VTNR) pattern genotypic analysis as has been required per required CDC protocols since 2004 (32). The genotypic pattern was compared with the patterns of all other Mtb specimens collected in the United States utilizing the TB Genotyping Information Management System (TB-GIMS) database (33).
Supplementary Material
Acknowledgments
We thank the patients and their families for participating this study. We are grateful to Drs. Howard Streicher and Brigette Buig-Yue Ma for their support in the clinical trials. Merck provided pembrolizumab for the clinical portion of the trial via a collaborative research and development agreement (CRADA) with the NCI for the patient treated for Merkel Cell Cancer on NCT02267603, and a small amount of pembrolizumab was provided to DLB and SS for the correlative experiments shown via a materials transfer agreement between Merck and the NIH. Bristol-Myers Squibb provided nivolumab through a CRADA with the NCI for the patient treated for nasopharyngeal cancer on NCT02339558.
Funding: Supported by the following grants from the National Cancer Institute: 1U01CA154967 (MAC, SPF, LL), K24CA139052 (PTN), R01CA162522 (PTN), and UM1CA186717 (JJN). In addition, the NIH/NCI Cancer Center Support Grant in Seattle (P30 CA015704 – SPF, LL, MAC, PTN), the Norris Cancer Center Support Grant (P30 CA014089 – JJN) and the Winship Cancer Institute Cancer Center Support Grant (P30-CA138292 – RRK) supported this work. DLB and SS are supported by the Intramural Research Program of NIAID/NIH.
Footnotes
Competing interests: D.L.B. has received royalties from patents held relating to PD-1 blockade.
Data and materials availability: All data associated with this study are present in the paper or Supplemental Materials.
REFERENCES AND NOTES
- 1.Wykes MN, Lewin SR, Immune checkpoint blockade in infectious diseases. Nat Rev Immunol 18, 91–104 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R, Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439, 682–687 (2006). [DOI] [PubMed] [Google Scholar]
- 3.Zumla A, Rao M, Dodoo E, Maeurer M, Potential of immunomodulatory agents as adjunct host-directed therapies for multidrug-resistant tuberculosis. BMC Med 14, 89 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wallis RS, Hafner R, Advancing host-directed therapy for tuberculosis. Nat Rev Immunol 15, 255–263 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Saharia KK, Petrovas C, Ferrando-Martinez S, Leal M, Luque R, Ive P, Luetkemeyer A, Havlir D, Koup RA, Tuberculosis Therapy Modifies the Cytokine Profile, Maturation State, and Expression of Inhibitory Molecules on Mycobacterium tuberculosis-Specific CD4+ T-Cells. PLoS One 11, e0158262 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Day CL, Abrahams DA, Bunjun R, Stone L, de Kock M, Walzl G, Wilkinson RJ, Burgers WA, Hanekom WA, PD-1 Expression on Mycobacterium tuberculosis-Specific CD4 T Cells Is Associated With Bacterial Load in Human Tuberculosis. Front Immunol 9, 1995 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rao M, Valentini D, Dodoo E, Zumla A, Maeurer M, Anti-PD-1/PD-L1 therapy for infectious diseases: learning from the cancer paradigm. Int J Infect Dis 56, 221–228 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Lazar-Molnar E, Chen B, Sweeney KA, Wang EJ, Liu W, Lin J, Porcelli SA, Almo SC, Nathenson SG, Jacobs WR Jr., Programmed death-1 (PD-1)-deficient mice are extraordinarily sensitive to tuberculosis. Proc Natl Acad Sci U S A 107, 13402–13407 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barber DL, Mayer-Barber KD, Feng CG, Sharpe AH, Sher A, CD4 T cells promote rather than control tuberculosis in the absence of PD-1-mediated inhibition. J Immunol 186, 1598–1607 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tousif S, Singh Y, Prasad DV, Sharma P, Van Kaer L, Das G, T cells from Programmed Death-1 deficient mice respond poorly to Mycobacterium tuberculosis infection. PLoS One 6, e19864 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakai S, Kauffman KD, Sallin MA, Sharpe AH, Young HA, Ganusov VV, Barber DL, CD4 T Cell-Derived IFN-gamma Plays a Minimal Role in Control of Pulmonary Mycobacterium tuberculosis Infection and Must Be Actively Repressed by PD-1 to Prevent Lethal Disease. PLoS Pathog 12, e1005667 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JJ, Chan A, Tang T, Tuberculosis reactivation in a patient receiving anti-programmed death-1 (PD-1) inhibitor for relapsed Hodgkin’s lymphoma. Acta Oncol 55, 519–520 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Fujita K, Terashima T, Mio T, Anti-PD1 Antibody Treatment and the Development of Acute Pulmonary Tuberculosis. J Thorac Oncol 11, 2238–2240 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Chu YC, Fang KC, Chen HC, Yeh YC, Tseng CE, Chou TY, Lai CL, Pericardial Tamponade Caused by a Hypersensitivity Response to Tuberculosis Reactivation after Anti-PD-1 Treatment in a Patient with Advanced Pulmonary Adenocarcinoma. J Thorac Oncol 12, e111–e114 (2017). [DOI] [PubMed] [Google Scholar]
- 15.Picchi H, Mateus C, Chouaid C, Besse B, Marabelle A, Michot JM, Champiat S, Voisin AL, Lambotte O, Infectious complications associated with the use of immune checkpoint inhibitors in oncology: reactivation of tuberculosis after anti PD-1 treatment. Clin Microbiol Infect 24, 216–218 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Takata S, Koh G, Han Y, Yoshida H, Shiroyama T, Takada H, Masuhiro K, Nasu S, Morita S, Tanaka A, Hashimoto S, Uriu K, Suzuki H, Tamura Y, Okamoto N, Nagai T, Hirashima T, Paradoxical response in a patient with non-small cell lung cancer who received nivolumab followed by anti-Mycobacterium tuberculosis agents. J Infect Chemother (2018) 10.1016/j.jiac.2018.06.016. [DOI] [PubMed] [Google Scholar]
- 17.Ma BBY, Lim WT, Goh BC, Hui EP, Lo KW, Pettinger A, Foster NR, Riess JW, Agulnik M, Chang AYC, Chopra A, Kish JA, Chung CH, Adkins DR, Cullen KJ, Gitlitz BJ, Lim DW, To KF, Chan KCA, Lo YMD, King AD, Erlichman C, Yin J, Costello BA, Chan ATC, Antitumor Activity of Nivolumab in Recurrent and Metastatic Nasopharyngeal Carcinoma: An International, Multicenter Study of the Mayo Clinic Phase 2 Consortium (NCI-9742). J Clin Oncol 36, 1412–1418 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nghiem PT, Bhatia S, Lipson EJ, Kudchadkar RR, Miller NJ, Annamalai L, Berry S, Chartash EK, Daud A, Fling SP, Friedlander PA, Kluger HM, Kohrt HE, Lundgren L, Margolin K, Mitchell A, Olencki T, Pardoll DM, Reddy SA, Shantha EM, Sharfman WH, Sharon E, Shemanski LR, Shinohara MM, Sunshine JC, Taube JM, Thompson JA, Townson SM, Yearley JH, Topalian SL, Cheever MA, PD-1 Blockade with Pembrolizumab in Advanced Merkel-Cell Carcinoma. N Engl J Med 374, 2542–2552 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cruz A, Fraga AG, Fountain JJ, Rangel-Moreno J, Torrado E, Saraiva M, Pereira DR, Randall TD, Pedrosa J, Cooper AM, Castro AG, Pathological role of interleukin 17 in mice subjected to repeated BCG vaccination after infection with Mycobacterium tuberculosis. J Exp Med 207, 1609–1616 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ribas A, Wolchok JD, Cancer immunotherapy using checkpoint blockade. Science 359, 1350–1355 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shafiani S, Dinh C, Ertelt JM, Moguche AO, Siddiqui I, Smigiel KS, Sharma P, Campbell DJ, Way SS, Urdahl KB, Pathogen-specific Treg cells expand early during mycobacterium tuberculosis infection but are later eliminated in response to Interleukin-12. Immunity 38, 1261–1270 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakai S, Kauffman KD, Schenkel JM, McBerry CC, Mayer-Barber KD, Masopust D, Barber DL, Cutting edge: control of Mycobacterium tuberculosis infection by a subset of lung parenchyma-homing CD4 T cells. J Immunol 192, 2965–2969 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kauffman KD, Sallin MA, Sakai S, Kamenyeva O, Kabat J, Weiner D, Sutphin M, Schimel D, Via L, Barry CE 3rd, Wilder-Kofie T, Moore I, Moore R, Barber DL, Defective positioning in granulomas but not lung-homing limits CD4 T-cell interactions with Mycobacterium tuberculosis-infected macrophages in rhesus macaques. Mucosal Immunol 11, 462–473 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li H, Javid B, Antibodies and tuberculosis: finally coming of age? Nat Rev Immunol 18, 591–596 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Gagneux S, Small PM, Global phylogeography of Mycobacterium tuberculosis and implications for tuberculosis product development. Lancet Infect Dis 7, 328–337 (2007). [DOI] [PubMed] [Google Scholar]
- 26.Reiley WW, Shafiani S, Wittmer ST, Tucker-Heard G, Moon JJ, Jenkins MK, Urdahl KB, Winslow GM, Woodland DL, Distinct functions of antigen-specific CD4 T cells during murine Mycobacterium tuberculosis infection. Proc Natl Acad Sci U S A 107, 19408–19413 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Einarsdottir T, Lockhart E, Flynn JL, Cytotoxicity and secretion of gamma interferon are carried out by distinct CD8 T cells during Mycobacterium tuberculosis infection. Infect Immun 77, 4621–4630 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.WHO, “Global Tuberculosis Report 2017,” (Geneva, Switzerland: ). [Google Scholar]
- 29.Sakai S, Kawamura I, Okazaki T, Tsuchiya K, Uchiyama R, Mitsuyama M, PD-1-PD-L1 pathway impairs T(h)1 immune response in the late stage of infection with Mycobacterium bovis bacillus Calmette-Guerin. Int Immunol 22, 915–925 (2010). [DOI] [PubMed] [Google Scholar]
- 30.Jayaraman P, Jacques MK, Zhu C, Steblenko KM, Stowell BL, Madi A, Anderson AC, Kuchroo VK, Behar SM, TIM3 Mediates T Cell Exhaustion during Mycobacterium tuberculosis Infection. PLoS Pathog 12, e1005490 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ireton GC, Greenwald R, Liang H, Esfandiari J, Lyashchenko KP, Reed SG, Identification of Mycobacterium tuberculosis antigens of high serodiagnostic value. Clin Vaccine Immunol 17, 1539–1547 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National TB Controllers Association, CDC, Guide to the Application of Genotyping to Tuberculosis Prevention and Control. (2004).
- 33.Ghosh S, Moonan PK, Cowan L, Grant J, Kammerer S, Navin TR, Tuberculosis genotyping information management system: enhancing tuberculosis surveillance in the United States. Infect Genet Evol 12, 782–788 (2012). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.