Summary
Champiat and colleagues suggest that a small subset of patients on PD1/PDL1 inhibitors at their center appear to exhibit hyperprogression of disease. This commentary goes over some limitations in their preliminary analysis, a possible mechanism to explain the phenomenon, and a means by which other investigators can attempt to validate and further characterize these results.
In this issue of Clinical Cancer Research, Champiat and colleagues seek to ask the question: could some patients actually be harmed via accelerated progression induced by one of the most promising and revolutionary therapies to be tested in the field of oncology to date? [1] In terms of the sheer number of histologies, the breadth of activity seen with inhibitors of programmed death 1 (PD1) and programmed death ligand 1 (PDL1) is beyond that of nearly any other class of targeted anticancer therapy available thus far. If there is a potential harm of accelerated progression induced by the therapies, this must be assessed and characterized, and potentially mitigated, as quickly as possible.
The investigators ask a question which goes beyond the usual benefit-risk ratio assessment. More commonly, medical oncologists have focused on the risk of immune-related adverse events in patients treated with PD1/PDL1 inhibitors to determine if the therapy is appropriate for their patients. As is well known, the same mechanisms that allow for immune-mediated tumor destruction can also lead to immune-related adverse events. Based on several anecdotal observations of patients whose disease appeared to grow much more aggressively after PD1/PDL1 inhibitor therapy, Champiat et al. raise the question of whether a small subset of patients could actually have tumor growth accelerated when given PD1/PDL1-targeting agents. The accompanying article is provocative, but it is important to point out three significant limitations to the analysis as well as one potential mechanism.
In reviewing the cases of patients on clinical trials with PD1/PDL1 inhibitors and examining in particular those whose disease progressed, Champiat suggests that a subset of patients with disease progression have a course that is more deleterious than they might have had with other therapies, or even in the absence of therapy. In oncology drug development, patients with disease progression are typically removed from study treatment and not followed further, other than for endpoints such as overall survival. The field’s standard for determining progression on clinical trials, the RECIST criteria, has been criticizing for failing to capture the minority of patients treated with immunotherapy agents who experience an apparent disease progression, which is then followed by a response.[2] The evidence for these false progression events, termed pseudo-progression, has led to numerous patients staying on PD1/PDL1 inhibitor therapy longer than they may have otherwise. Various methods of re-evaluating standardized response criteria, such as RECIST, have long been underway, but the success of immunotherapy has enhanced this debate in order to capture evidence of these rare patterns of response to therapy. Most clinical trials of immune checkpoint inhibitors are now designed to include confirmation of progression, lest an individual with the potential for benefit lose the chance just because of the appearance of a new lesion or a stubborn tumor’s initial growth and failure to regress.
Champiat estimates that hyperprogression may occur in at least 9% of cases overall, and the investigators characterize this phenomenon as disease which dramatically progression outpaces the expected rate of growth in the absence of PD1/PDL1 inhibitors based primarily on evidence from prior imaging scans. Since several patients progressed clinically prior to an imaging assessment, the number of hyperprogression events in their patient cohort could have been significantly higher.
The first limitation to this analysis is the relatively few patients evaluated. A cutpoint for hyperprogression would be difficult to obtain in the best of circumstances, but Champiat is starting from a rather small base of 131 patients and only 12 which were deemed “hyperprogressors”. In order to truly evaluate this question, more patients and more centers will need to contribute scans to such an effort. As PD1 and PDL1 inhibitors are now approved in the United States and much of the industrialized world, the potential source of patient images can come from patients treated as standard of care, who have authorized consent for the use of their imaging scans for this purpose.
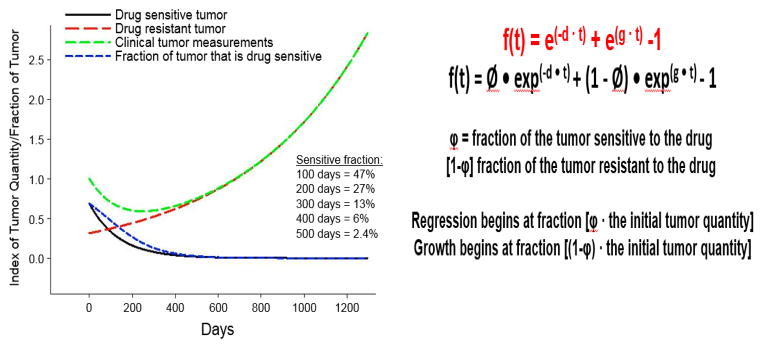
A second limitation involves the use of an unvalidated measure to assess tumor growth. Champiat and colleagues are using the tumor growth rate as a measure for either response or hyperprogression. This is not a standardized method for assessing response, but the reasoning behind the use of a tumor growth rate is sound. Various groups, including those led by Charles Ferte and Jean-Charles Soria, co-authors on the discussed study, and another group involving Antonio Tito Fojo, Julia Wilkerson, and Wilfred Stein, have been examining tumor growth rates as an alternative means of assessing the response or failure of various therapies.[3–5] These methods of assessment are not yet accepted by the broader oncology community. They involve mathematical formulae to determine the potential trajectory of a given tumor’s growth or regression in the presence of a given therapy (See Figure 1). While from the analysis, the investigators seem to impugn the PD1/PDL1 inhibitors as the cause of hyperprogression, there is no benchmarking or historical control to help one determine if this is typical for patients with a given histology. Perhaps chemotherapy also leads to a certain proportion of patients with a rapid clinical deterioration and corresponding hyperprogression of measured lesions on imaging scans? Or, could this occur in some patients who receive no therapy, implying that the rapid progression seen in patients in Champiat’s analysis is not actually occurring in response to PD1/PDL1 inhibitors, but is rather due to intrinsic cancer biology or a resistance to therapy? Even so, however, some type of mathematical evaluation of tumor growth would be necessary. The discrete category of progressive disease employed by RECIST is simply too broad a category to capture this subtlety.
Figure 1.
Panel A: In the tumor growth rate model developed by Stein and Fojo, tumors are composed of fractions of cells which are either sensitive or resistant to a drug being studied. As a result, an initial regression of a tumor may be transient if tumor cells resistant to the therapy continue to grow and divide. The effect seen on an imaging scan or by a biomarker can be represented by the green line in Panel A. Tumor growth continues after an initial regression as the proportion of drug sensitive tumor cells (represented by the blue line) decreases and tumor growth continues. Panel B: Equations used to derive tumor growth rate model. The constant d represents the rate of cell decay, and the constant g represents the rate of tumor growth. Panel A adapted from Burotto M et al. (2014) Continuing a Cancer Treatment Despite Tumor Growth May Be Valuable: Sunitinib in Renal Cell Carcinoma as Example. PLOS ONE 9(5): e96316. doi:10.1371/journal.pone.0096316.
The third limitation is the most problematic, but not necessarily resolvable at this point. Assuming that their tumor growth rate-based analysis has uncovered a certain subset of patients that are suffering more from their cancers as a result of the PD1/PDL1 inhibitor therapy, what is the mechanism of this purported effect? Is this mere resistance or an actual acceleration? As the authors note, this cannot be answered in the absence of biopsy specimens from patients who are experiencing hyper-progression and comparing these with ordinary progressors or patients with responses to PD1/PDL1 inhibitors. Groups led by Padmanee Sharma and Antoni Ribas have implicated the interferon pathway as being significant in areas of primary and secondary resistance to immune checkpoint blockade.[6, 7] Sharma’s work is particularly significant, as she was able to show that a greater burden of mutations in the interferon pathway in the tumors analyzed was associated with a lower rate of response to CTLA4 inhibition. Ribas and colleagues have suggested, in a small number of patients who lost an initial response to PD1 inhibition, that mutations in the interferon pathway may have led to the emergence of resistance, and more recently, the same group implicated JAK1/2 mutations as a possible mechanism of primary resistance to PD1-blocking therapies.[8]
The data outlined above suggest a potential mechanism of resistance, but they are not likely sufficient to explain the hyper-progressive nature that may have been seen in Champiat’s cohort. Perhaps, one possibility is seen in the world of infectious disease. The PD1/PDL1 pathway is significant in cancer immunology as well as in the immune response to infectious disease. While the immune response to virally-infected cells is often enhanced in the presence of PD1/PDL1 blockade, mycobacterial infections are somewhat different. In tuberculosis-infected mouse models, PD1 blockade appears to worsen the infection, by driving CD4 T cell-derived gamma-interferon production. Infections are so severe that most models with mice infected with tuberculosis who are also given PD1 inhibitors have an onset of uncontrolled mycobacterial infection that is more rapid and fatal than the inhibition of any other immune-related pathway tested in their model, similar to the outcomes seen in Champiat’s hyper-progressing patients.[9]
This is mere speculation, but Champiat’s analysis certainly opens the door to the possibility that a subset of tumors may have a similar gamma-interferon-driven growth that is enhanced in the presence of PD1/PDL1 inhibition. There is much to learn before making any definitive conclusions, but the first step should be the confirmation of the imaging results. The US National Cancer Institute sponsors an effort known as The Cancer Imaging Archive (TCIA). This data warehouse allows investigators to offer collections of images of patients with a particular treatment modality and anatomical site for analysis by the broader oncology community. To date, these are over 400 collections of imaging datasets available online, but none of them involve patients who have been treated by immune checkpoint inhibitors of any kind.
Researchers in medicine, like all physicians and healthcare providers, have an obligation best exemplified by the Latin phrase, primum non nocere, meaning first do no harm. In the West, this has been passed down to us from the days of Hippocrates, but the concept has been broadly accepted as the foundation for the ethics of the modern healthcare. PD1/PDL1 inhibitors represent a major advance, but they are not without cost. We now have the tools to at least help identify what that cost may be. In order to make more meaningful advances, we will need to see what harm these and other therapies may be doing to an overlooked portion of the patient population, those with primary resistance and potentially, even hyper-progression. The Cancer Imaging Archive and researchers around the world are waiting for these datasets to help answer these and other important questions in the emerging world of cancer immunotherapy. If a problem of hyper-progression exists, we now have the tools to identify, characterize and possibly even solve it. If not now, when?
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Champiat S, et al. Hyperprogressive disease (HPD) is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clinical Cancer Research. 2016 doi: 10.1158/1078-0432.CCR-16-1741. [DOI] [PubMed] [Google Scholar]
- 2.Hodi FS, et al. Evaluation of Immune-Related Response Criteria and RECIST v1.1 in Patients With Advanced Melanoma Treated With Pembrolizumab. Journal of Clinical Oncology. 2016;34(13):1510–1517. doi: 10.1200/JCO.2015.64.0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blagoev KB, et al. Therapies with Diverse Mechanisms of Action Kill Cells by a Similar Exponential Process in Advanced Cancers. Cancer Research. 2014;74(17):4653–4662. doi: 10.1158/0008-5472.CAN-14-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferté C, et al. Tumor Growth Rate Is an Early Indicator of Antitumor Drug Activity in Phase I Clinical Trials. Clinical Cancer Research. 2014;20(1):246–252. doi: 10.1158/1078-0432.CCR-13-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilkerson J, et al. Estimation of tumour regression and growth rates during treatment in patients with advanced prostate cancer: a retrospective analysis. The Lancet Oncology. doi: 10.1016/S1470-2045(16)30633-7. [DOI] [PubMed] [Google Scholar]
- 6.Gao J, et al. Loss of IFN-γ Pathway Genes in Tumor Cells as a Mechanism of Resistance to Anti-CTLA-4 Therapy. Cell. 2016;167(2):397–404.e9. doi: 10.1016/j.cell.2016.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zaretsky JM, et al. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. New England Journal of Medicine. 2016;375(9):819–829. doi: 10.1056/NEJMoa1604958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shin DS, et al. Primary Resistance to PD-1 Blockade Mediated by JAK½ Mutations. Cancer Discovery. 2016 doi: 10.1158/2159-8290.CD-16-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakai S, et al. CD4 T Cell-Derived IFN-γ Plays a Minimal Role in Control of Pulmonary Mycobacterium tuberculosis Infection and Must Be Actively Repressed by PD-1 to Prevent Lethal Disease. PLOS Pathogens. 2016;12(5):e1005667. doi: 10.1371/journal.ppat.1005667. [DOI] [PMC free article] [PubMed] [Google Scholar]