Abstract
We conducted a survey of pediatric infectious diseases providers in the Emerging Infections Network regarding the workup and treatment of children withStaphylococcus aureus bacteremia (SAB). We found significant practice variation in the management of children with SAB. These findings emphasize the need for further research to guide best practices.
Keywords: bacteremia, children, Staphylococcus aureus
Staphylococcus aureus is a leading cause of bacteremia in children; however, optimal management of these patients remains unclear [1,2]. The Infectious Diseases Society of America (IDSA) guidelines for the treatment of methicillin-resistantS aureus (MRSA) bacteremia recommend vancomycin therapy for up to 6 weeks, depending on the source and clinical response [3]. Guidelines for the treatment of methicillin- susceptibleS aureus (MSSA) bacteremia recommend nafcillin as first-line therapy, with cefazolin as an alternative [4].
Despite these recommendations, the application of broad guidelines to specific scenarios encountered in the management ofS aureus bacteremia (SAB) in children remains difficult. Recent literature has challenged long-held notions regarding optimal management of SAB associated with osteoarticular infections [5,6]; however, there remains a paucity of evidence to guide management in several other common scenarios including the following: nonpersistent SAB without a source, prolonged MRSA bacteremia, and central-line associated SAB. Given the complexity in the management of children with SAB, we sought to describe practice patterns and clinical decision making among pediatric infectious diseases (PID) providers.
METHODS
From January to February 2017, we distributed a 7-question, electronic, vignette-based survey (Supplementary Data,https://ein.idsociety.org/surveys/survey/100/) of clinical scenarios related to the management of SAB in children to PID providers in the Emerging Infections Network of the Infectious Diseases Society of America (IDSA EIN).
The survey questions explored diagnostic and treatment practices of providers for patients with osteomyelitis-associated SAB, nonpersistent SAB (<48 h), persistent MRSA bacteremia, peripherally inserted central catheter (PICC)-associated thrombosis with SAB, and parenteral treatment of choice for MSSA bacteremia.
Demographic information regarding practice region, experience, and employment was collected by EIN membership profile. Similar to previous EIN surveys, the response rate was calculated from PID EIN members who had ever responded to a survey [7,8]. Descriptive statistics were calculated as percentages for each response category.
RESULTS
Two hundred twenty-three of 334 (67%) clinicians with a PID practice responded from the United States and Canada, most (37%) having 5–14 years of clinical experience, and the majority practicing at a university hospital (62%). Fourteen (6%) providers do not manage children with SAB and were excluded from the analysis.
Osteomyelitis-AssociatedStaphylococcus aureus Bacteremia
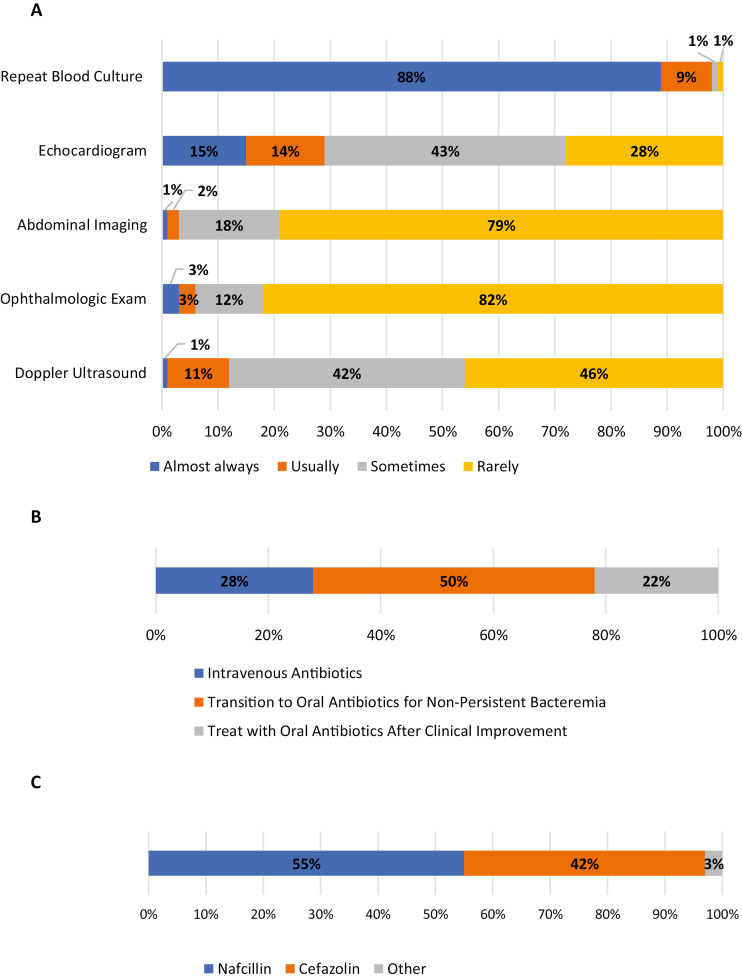
For children with osteomyelitis-associated SAB showing clinical improvement shortly after initiation of antibiotics, almost all (97%) providers routinely repeat blood cultures until negative, whereas a minority routinely image the heart (29%), abdomen (3%), retina (6%), or vasculature (eg, Doppler ultrasound, 12%) (Figure 1A).Figure 1B shows the various treatment approaches taken by pediatric providers, with half (50%) treating intravenously only for those with persistent bacteremia (>48–72 hours), whereas 28% treat almost all patients with 10–14 days of intravenous (IV) antibiotics and 22% treat with oral antibiotics once they have clinically improved (ie, no minimum duration of parenteral therapy). For those with MSSA bacteremia, nafcillin and cefazolin are used similarly (Figure 1C).
Figure 1.
Practice patterns among providers for the workup and treatment of osteomyelitis-associatedStaphylococcus aureus bacteremia (SAB). (A) Diagnostic workup for a child with osteomyelitis-associated SAB that is improving shortly after antibiotic initiation. Almost all providers repeat blood cultures until negative; however, the minority will routinely look for other foci of infection. (B) For the treatment of osteomyelitis-associated SAB, 50% of providers will switch to an oral regimen for nonpersistent bacteremia. Twenty-eight percent and 22% will treat with all intravenous antibiotics or treat with oral antibiotics after clinical improvement, respectively. (C) For the treatment methicillin-susceptibleStaphylococcus aureus bacteremia with osteomyelitis, 55% of clinicians use nafcillin, 42% use cefazolin, and 3% use an alternative agent.
NonpersistentStaphylococcus aureus Bacteremia
For febrile, well appearing children with only 1S aureus blood culture, no clear source of infection, and subsequent negative cultures before antibiotics, 54% of providers treat with oral antibiotics after empiric vancomycin; 25% complete therapy with IV antibiotics, and 17% discontinue antibiotics, assuming thatS aureus is a contaminant. A small proportion (4%) of providers chose an alternative management strategy, including evaluating for a source of infection (3%).
Persistent Methicillin-ResistantStaphylococcus aureus Bacteremia
For the treatment of children with persistent MRSA bacteremia despite adequate vancomycin levels, 37% continue vancomycin and add a second agent, most commonly rifampin. A minority (12%) continue vancomycin monotherapy, whereas 43% change to other agents, most commonly daptomycin; 8% of providers chose an alternative management strategy.
Peripherally Inserted Central Catheter-Associated Thrombus WithStaphylococcus aureus Bacteremia
There was no consensus on the duration of IV therapy for children with PICC-associated thrombus and bacteremia; 34% treated for 2 weeks, 37% treated for 4 weeks, 18% treated for 6 weeks, and 11% chose an alternative treatment strategy. The majority (71%) recommend anticoagulation.
DISCUSSION
This study provides insights on the variation in the management of children withS aureus bacteremia. Notably, we found very little agreement in (1) the optimal route of antibiotics for children with osteomyelitis-associated SAB and nonpersistent SAB, (2) choice of antibiotic for MSSA bacteremia, (3) antibiotic approach in children with persistent MRSA bacteremia on vancomycin, and (4) optimal duration of antibiotics for PICC-associated thrombus and SAB.
These results highlight the complexity in caring for children with SAB and current gaps in consensus guidelines for the optimal management of these patients. Clinicians often encounter scenarios with little data to support treatment decisions, or they are forced to extrapolate data from adult studies. In children with SAB, however, the mortality, pathophysiology, and antibiotic pharmacokinetics are quite different than adults [3,9–11]. The IDSA guidelines for MRSA bacteremia recommend echocardiography for all adult patients, whereas in children this is only recommended for those with congenital heart disease, persistent bacteremia (>48–72 hours), or clinical findings suggestive of endocarditis. Our results reflect this recommendation because less than one third of providers routinely obtain an echocardiogram in a clinically improving child with SAB (Figure 1A). Notably, for a vignette describing bacteremia in a child with significant atopic dermatitis, 17% of respondents chose to discontinue antibiotics, assuming thatS aureus was a contaminant—a recommendation at odds with the Committee on Infectious Diseases recommendation that “S. aureus almost never is a contaminant when isolated from a blood culture” [12]. Although it is certainly biologically plausible that a positive blood culture forS aureus could be a result of skin contamination, particularly in atopic dermatitis with or without cutaneousS aureus infection, there is historic precedent to treat these children with a short course of parenteral antibiotics. Whether this is suitable in practice is thus far unstudied and merits further discussion in subsequent guideline revisions.
The optimal route of antibiotics for children with SAB is a scenario in which practice variation is high and is an area of ongoing research among PID specialists. There is a growing body of evidence, particularly in bacteremic osteoarticular infections, that an early switch to oral antibiotics is safe and does not increase the risk of treatment failure [5,6]; however, many clinicians remain hesitant to adopt this practice due to the invasive nature of the infection and uncertainty of absorption/penetration of some oral antibiotics. This study illuminates the need for ongoing investigation in this area.
The implications of practice variation in the management of children with SAB are numerous, including unnecessary diagnostic testing (eg, echocardiogram, visceral imaging), invasive procedures (eg, PICC line insertion), and prolonged antibiotic exposure. Moreover, this variation leads to increased healthcare costs and risk of adverse effects. Identifying areas in which these variations exist provides an opportunity to improve the value of healthcare delivery and reduce costs without compromising patient care.
CONCLUSIONS
Our study has certain limitations. The response rate, although acceptable for this type of survey, may lead to sampling bias, and it may not be generalizable to all PID providers. However, there was an equal distribution of responders throughout most regions of the United States, which may limit this bias. In addition, responses to vignette-based questions may be subject to response bias, and we recognize that vignettes may only approximate what is actually done in clinical practice. Finally, the anonymity of the survey is designed to allow responders to answer questions as accurately as possible; however, survey answers may not accurately reflect true practice patterns. Nevertheless, this study highlights the substantial variation in the management of children with SAB, can inform guidelines, and should guide our next steps in evaluating best practice patterns for this challenging pathogen.
Supplementary Data
Supplementary materials are available atJournal of the Pediatric Infectious Diseases Society online (http://jpids.oxfordjournals.org).
Notes
Acknowledgments. None.
Financial support. This publication was supported by the Cooperative Agreement Number 1 U50 CK000477, funded by the Centers for Disease Control and Prevention. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention or the Department of Health and Human Services.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Irwin AD,Drew RJ,Marshall P,et al. . Etiology of childhood bacteremia and timely antibiotics administration in the emergency department.Pediatrics 2015;135:635–42. [DOI] [PubMed] [Google Scholar]
- 2. Cobos-Carrascosa E,Soler-Palacín P,Nieves Larrosa M,et al. . Staphylococcus aureus bacteremia in children: changes during eighteen years.Pediatr Infect Dis J 2015;34:1329–34. [DOI] [PubMed] [Google Scholar]
- 3. Liu C,Bayer A,Cosgrove SE,et al. . Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistantStaphylococcus aureus infections in adults and children.Clin Infect Dis 2011;52:e18–55. [DOI] [PubMed] [Google Scholar]
- 4. Baddour LM,Wilson WR,Bayer AS. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America.Circulation 2005;111:e394–434. [DOI] [PubMed] [Google Scholar]
- 5. Pääkkönen M,Kallio PE,Kallio MJ,Peltola H. Does bacteremia associated with bone and joint infections necessitate prolonged parenteral antimicrobial therapy?J Pediatric Infect Dis Soc 2015;4:174–7. [DOI] [PubMed] [Google Scholar]
- 6. McNeil JC,Kaplan SL,Vallejo JG. The influence of the route of antibiotic administration, methicillin susceptibility, vancomycin duration and serum trough concentration on outcomes of pediatricStaphylococcus aureus bacteremic osteoarticular infection.Pediatr Infect Dis J 2017;36:572–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Banerjee R,Beekmann SE,Doby EH,et al. . Outpatient parenteral antimicrobial therapy practices among pediatric infectious diseases consultants: results of an Emerging Infections Network Survey.J Pediatric Infect Dis Soc 2014;3:85–8. [DOI] [PubMed] [Google Scholar]
- 8. Cruz AT,Hersh AL,Starke JR,et al. . Controversies in tuberculous infection among pediatric infectious disease specialists in North America.Int J Tuberc Lung Dis 2016;20:1463–8. [DOI] [PubMed] [Google Scholar]
- 9. Hamdy RF,Hsu AJ,Stockmann C,et al. . Epidemiology of methicillin-resistantStaphylococcus aureus bacteremia in children.Pediatrics 2017;139:e20170183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Klieger SB,Vendetti ND,Fisher BT,Gerber JS. Staphylococcus aureus bacteremia in hospitalized children: incidence and outcomes.Infect Control Hosp Epidemiol 2015;36:603–5. [DOI] [PubMed] [Google Scholar]
- 11. Goldman JL,Harrison CJ,Myers AL,et al. . No evidence of vancomycin minimal inhibitory concentration creep or heteroresistance identified in pediatricStaphylococcus aureus blood isolates.Pediatr Infect Dis J 2014;33:216–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Staphylococcal infections. In:Kimberlin DW. (ed).Red Book: 2015 Report of the Committee on Infectious Diseases, 30 Edition.Elk Grove Village, IL:American Academy of Pediatrics;2015: pp715–32. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.