SECTION 1
Optimal use of conventional drugs in the treatment of ulcerative colitis
Treatment strategy in ulcerative colitis (UC) is based on disease severity, extent (proctitis, left colon involvement, and extensive disease), and pattern (frequent relapsing, course, response to previous treatment, disease side effects, and extraintestinal involvement). Age at the onset of the disease and disease duration are also important. Severe disease may require inpatient treatment, whereas mild and moderate diseases may be treated on an outpatient basis. In UC, disease remission is associated with the resolution of clinical symptoms (diarrhea and rectal bleeding) and mucosal healing (resolution of inflammation and ulceration in endoscopy).
Treatment in mild or moderate ulcerative proctitis
First-line treatment of mild or moderate UC includes symptomatic remission induction with rectally administered 5-aminosalicylic acid (ASA) (mesalazine) 1 g daily. Foam or enema formulations of mesalazine (mesalamine) may be used; however, mesalazine suppositories show better rectal distribution and are better tolerated than the other formulations. Topical forms of mesalazine are more effective than topical steroids (1). Combination therapy of topical and systemic mesalazine is associated with higher rates of clinical, endoscopic, and histological remission than monotherapy (2). Mesalazine suppositories at a dose of 1 g daily may induce clinical remission within 2 weeks in 64% of patients with proctitis and induce endoscopic remission within 4 weeks in 84% of patients (3,4). Topical mesalazine is more effective than oral mesalazine in the treatment of proctitis (5). Combination treatment may be used if required. Rectal mesalazine at a dose of >1 g/day does not provide additional benefits.
Treatment in mild to moderate UC (of any extent)
Oral 5-ASA preparations at doses of 2–4.8 g daily are the first-line treatment to induce complete remission induction in UC of any extent other than proctitis. Compliance with daily doses of orally administered 5-ASA is important in the maintenance of disease control. Combination therapy with oral and rectal 5-ASA preparations is a more effective alternative first-line treatment for inducing complete remission. In placebo-controlled studies, the rates of clinical remission and endoscopic mucosal healing after 8 weeks of treatment with oral multi-matrix mesalazine were found to be 40% and 32%, respectively (6). The rates of clinical remission and endoscopic mucosal healing after 8 weeks of combination treatment with oral 5-ASA 4 g daily and topical 5-ASA 1 g daily were found to be better than those of oral treatment alone (7,8). Although 5-ASA is not more effective than sulfasalazine (SASP), its medication tolerance is better. SASP should be preferred in patients with Crohn’s disease (CD) associated with arthropathy. Adherence to daily doses of oral 5-ASA therapy is important for disease control; however, long-term adherence to oral preparations is poor, and an adherence <80% increases the risk for exacerbations; it has been shown that adherence might not improve, even with once daily doses (9).
Novel multi-matrix system formulation of budesonide provides the release of the drug throughout the colon, and its safety and efficacy have been demonstrated in mild to moderate UC (10). When compared with placebo, budesonide MMX administered for >8 weeks at a dose of 9 mg was found to be significantly more efficient in inducing clinical and endoscopic remission. Budesonide MMX can be used instead of conventional steroid therapy in patients with mild to moderate UC who have been unresponsive to optimized treatment with steroid (11).
Oral corticosteroids (CSs) are the second-line treatment for inducing remission in mild to moderate refractory, active UC. Meta-analysis has demonstrated that conventional CSs are significantly more efficient in inducing remission than placebo (12). Although the optimal dose of systemic steroids has not been settled in UC, meta-analysis has failed to show any evidence of additional benefits of steroids at doses >60 mg daily. A consensus has been achieved on a dose range of 40–60 mg daily (13). The optimal initial dose of prednisolone has been determined as 40 mg. Adverse effects are more prevalent with higher doses; however, additional therapeutic benefit from higher doses is limited (14). Oral prednisolone is used in a tapering regimen for 8 weeks. It is recommended to taper 5 mg prednisolone/week. Prednisolone therapy for <3 weeks has been associated with frequent relapses (15).
Maintenance of remission in UC (patients who have entered into remission with 5-ASA)
The 2-month relapse rates were found to be 41% with oral mesalazine and 58% with placebo in studies on the maintenance of clinical and endoscopic remission in UC (16). As with the induction of remission, higher maintenance doses (≥2 g daily) are more effective (17). Topical mesalazine administered ≥3 times weekly has been proven to be effective in maintaining clinical and endoscopic remission of distal colitis (18). Although long-term rectal treatment is effective, studies have demonstrated that treatment with oral preparations alone has been preferred in 80% of patients (19). However, combination treatment with oral and topical preparations are more efficient than either oral or rectal treatment alone in maintaining remission; therefore, combination treatment may be considered to avoid to switch immunomodulatory agents or biologics in these patients (18).
Treatment in moderate to severe UC
CSs are the first-line treatment for inducing remission in moderate to severe UC. In moderate UC, steroids are given for 1–2 weeks at a dose of 40–60 mg daily. Steroids are tapered every 5 days or week and discontinued at the end of 8 weeks of treatment. Methylprednisolone administered intravenously at a dose of 60 mg daily may be effective in the treatment of severe, active UC. Although higher doses are not more effective, lower doses are less effective. Intravenous (iv) push administration is as effective as IV infusions. Maximum response to iv steroids is obtained within 3 days. Prolonged treatment for >7–10 days has no additional effect on the outcome. IV cyclosporine (CsA) monotherapy is an alternative treatment option in patients who have developed serious side effects associated with steroids. CsA given intravenously at a dose of 2–4 mg/kg daily has been found to be as effective as methylprednisolone administered at a dose of 40 mg daily (20). Every patient should receive adequate IV fluids and low molecular weight heparin for thromboprophylaxis. Electrolyte imbalances should be restored, and anemia should be treated.
CSs are not recommended for maintenance of remission. Azathioprine (AZA) is indicated for the maintenance of remission. In a meta-analysis, AZA has been proven to be more effective in preventing UC relapses than placebo (21). AZA is more effective than 5-ASA in maintaining clinical and endoscopic remission in steroid-dependent UC (22). The optimum dose of AZA is found to be 2 mg/kg. Steroid-free remission rates provided by AZA in steroid-dependent patients at months 12, 24, and 36 were found to be 55%, 52%, and 45%, respectively (23). In an observational study lasting for 30 years, overall remission rate was found to be 58%, whereas the rates were found to be 87.5% at month 6 and 62% at year 5 in 346 patients who were treated with AZA. However, therapeutic outcomes are also associated with how well the treatment is tolerated and relapses occur at an average of 18 months after the discontinuation of AZA treatment (24). In a recent retrospective study, the 3-year relapse rate was found to be 36% in patients in long-lasting remission. Extensive UC or active disease at discontinuation or short-term treatment with AZA has been found to be risk factors for relapse, in particular (25).
SECTION 2
Optimal use of conventional drugs in the treatment of CD
Treatment recommendations in CD are based on the location, severity of the disease, disease-related complications, and prognosis. Therapeutic approaches are personalized on the basis of symptomatic response to medical interventions and medication tolerance. Medical treatment of CD is usually divided into two parts, which are induction of remission and maintenance of remission. Other therapeutic targets include prevention of disease complications, such as strictures and fistulae. Disease activity, location, and behavior should be considered during disease management planning, and the management plan should always be discussed with the patients. Drugs used in the conventional treatment of CD include 5-ASA, CSs, and immunomodulatory agents.
5-ASA
5-ASA drugs include SASP, mesalazine, olsalazine, and balsalazide. SASP and mesalazine are available in Turkey. Oral and topical (suppositories, enema, and foam) formulations of mesalazine are also available in Turkey.
Indications
5-ASA is a topical anti-inflammatory agent that is effective in the colonic lumen. Although the use of 5-ASA is well-established in the treatment of UC and justified by evidence-based criteria, its use in CD has not been established yet. Oral mesalazine has not been proven to be more effective than placebo in inducing remission and ensuring mucosal healing in active CD (26–28).
SASP is formulated as a combination of 5-ASA and sulfapyridine. 5-ASA is responsible for the anti-inflammatory effect of this drug, whereas sulfapyridine is the transporter of this drug, ensuring the release of the drug into the colon. SASP (at doses of 3–6 g daily) is effective in the treatment of mild to moderate colonic CD and/or in resolving symptoms associated with ileocolonic CD; however, SASP is not effective in the treatment of patients with isolated small bowel disease. SASP has not been proven to be more effective than placebo in inducing mucosal healing in patients with CD (26). Eudragit-coated mesalazine has been reported to be effective in ileocolic or colonic disease at a dose of 3.2 g daily (29). Ethylcellulose-coated mesalazine has been reported to be effective in ileitis, ileocolitis, and colitis at a dose of 4 g daily (30). As a result, mesalazine has become a popular treatment for mild disease with a limited toxicity. However, systemic analyses of clinical study data and meta-analyses have failed to show any clinically significant improvement with ASA preparations versus placebo.
Based on the data reported to date, 5-ASA is not recommended in maintaining drug-induced remission (31).
Clinical practice recommendations
Based on the available evidence, both experts and consensus-based guidelines recommend the use of high-dose SASP (3–6 g daily) in CD, only in patients with limited disease. SASP should be used short-term, whereas active disease beyond 16 weeks of treatment should be considered as treatment failure. SASP is ineffective as maintenance treatment following drug-/surgery-induced remission (mesalazine may have a limited role in the latter). Furthermore, SASP may play a role in the management of patients with arthropathy associated with CD. However, a recent review of the available evidence indicated that benefit from SASP is limited to certain patients with peripheral arthropathy and early ankylosing spondylitis (i.e., patients with higher erythrocyte sedimentation rate (ESR) or active disease).
Contraindications
History of drug hypersensitivity or any side effects associated with the drug
Kidney impairment
Side effects of ASA preparations
Side effects associated with SASP may be seen in 10%–45% of patients in a dose-dependent manner, but serious idiosyncratic reactions may occur (32). Mesalazine intolerance is very rare, and serious side effects are rare (33) (Table 1).
Table 1.
Side effects associated with sulfasalazine and aminosalicylic acid.
| Common (>10%) | Rare (1%–10%) | Very rare (<1%) | |
|---|---|---|---|
| Sulfasalazine | Nausea | Abdominal pain | Hepatitis |
| Male infertility | Hemolytic anemia | Pneumonia | |
| Headache | Leukopenia | Neutropenia | |
| Rash | Thrombocytopenia | Pancreatitis Agranulocytosis |
|
| Aminosalicylic acid | Watery diarrhea | Pancreatitis | Pneumonia |
| Abdominal pain | Activation of colitis | Pericarditis | |
| Headache | Fever | Nephritis | |
| Nausea | Rash | Thrombocytopenia |
Monitoring
Although side effects are quite rare, a 6-month monitoring including complete blood count (CBC), urinalysis, liver tests, and kidney function tests (urea and creatinine) is recommended. The frequency and parameters of monitoring may be personalized according to comorbidities and concurrent medications.
The use of ASA in pregnant and breastfeeding women has been reported to be safe based on the IBHD and ECCO guidelines (34,35).
Corticosteroids
Indications
CSs (prednisone and methylprednisolone) are primarily used in active CD. Conventional CSs are effective in easing sign and symptoms and inducing remission in moderate to severe CD.
Steroids (e.g., hydrocortisone and 6-methylprednisolone) may be used intravenously in patients with more severe disease or in those who present with more severe acute manifestations; the superiority of IV administration over the oral route has not been proven. The main advantage of the IV route is to allow the administration of the drug in patients not tolerating oral intake.
Conventional CSs are not effective in providing mucosal healing. These may act as a “bridge” in providing symptom control and clinical remission in active disease until the onset of action of immunomodulatory agents.
Systemic CSs are ineffective as maintenance treatment of CD.
Controlled, ileal-release budesonide can provide short-term relief from mild to moderate CD symptoms in patients with disease limited to the ileum and right colon, although budesonide is not as effective as oral CSs, such as prednisone. Budesonide is a pH-dependent, ileal-release oral CS with high topical activity and low systemic bioavailability (~10%–20%). It has been proven to be effective in the treatment of mild to moderate ileocecal CD in randomized, placebo-controlled studies (34,35). It should be preferred over prednisolone when the extent and activity of the disease are appropriate for treatment with budesonide (36,37).
CSs play a role in the development of perforating complications (abscess and fistulas) and are contraindicated in patients who exhibit such symptoms.
Dosage
The initial dose of prednisone is 40 mg daily, and most patients respond to prednisone at this dose. In the treatment of active CD, the starting dose of prednisone varies from 40 to 60 mg daily, and the starting dose of methylprednisolone varies from 32 to 48 mg daily. These doses are maintained for 2–3 weeks at least, and the dose is tapered 5 mg/week until the weekly dose is reduced to 20 mg and then the dose is tapered 2.5–5.0 mg/week. This period should not exceed 3 months. Oral prednisone or other oral steroid formulation doses >60 mg are not recommended. There are limited comparative studies on different steroid-tapering regimens in the treatment of CD. However, it is recommended to taper off CSs upon discontinuation.
The risk for side effects associated with budesonide can be lowered by limiting treatment duration to 24 weeks and tapering off budesonide upon discontinuation after the first 3 months. The initial dose is 9 mg. The dose should be reduced to 6 mg after the first 3 months and discontinued within 6 months of treatment at most. Longer treatment durations have no effect on maintaining remission (12).
Contraindications
Contraindications include systemic fungal infections, herpes simplex keratitis, varicella infections, other uncontrolled systemic infections, and uncontrolled diabetes mellitus. The benefit-harm balance should be taken into consideration in patients with osteoporosis and in those who have a history of osteoporotic fractures.
Side effects
Three types of side effects can be defined. Although less severe than those associated with prednisolone in intensity, steroid side effects may occur with budesonide with similar or lower frequency. Immediate side effects associated with supraphysiological doses used to induce remission in active CD include cosmetic effects (acne, moon face, edema, and striae), sleep and mood disorders, dyspepsia, and glucose intolerance. Side effects associated with prolonged use (usually >12 weeks, but it may be <12 weeks) include posterior subcapsular cataract, osteoporosis, osteonecrosis of the femoral head, myopathy, and susceptibility to infections.
Budesonide is less likely to decrease bone mineral density than prednisolone (in a randomized study, the mean decrease in 272 patients >2 years was found to be −1.04% vs. 3.04%, p=0.0084) (38).
Withdrawal side effects include acute adrenal insufficiency (due to abrupt discontinuation), pseudo-rheumatoid syndrome (myalgia, fatigue, and arthralgia associated with manifestations similar to the recurrence of CD), or increased intracranial pressure. Therefore, CSs should be carefully used in CD.
Monitoring
Any metabolic disorders including blood glucose levels should be monitored in patients receiving CSs. The risk for developing hypertension should be taken into consideration, and a salt-free diet should be recommended. Vitamin D and calcium prophylaxis are recommended to prevent osteoporosis for treatment durations >6 weeks.
IV glucocorticoid support is required before surgery as the adrenocortical axis may be suppressed in patients who use systemic steroids at doses >20 mg.
Live vaccines should not be administered to patients who have received 20 mg prednisolone or equivalent/day. Vaccination is considered safe 1 month after the discontinuation of the medication.
Glucocorticoids are rated as pregnancy category C. They can be used to treat active disease during pregnancy; however, side effect should be taken into consideration and shared with the patient. The use of higher systemic doses during pregnancy may result in an increased risk for cleft lip/palate, infectious diseases in pregnancy, and premature births (12,35). Maternal use of steroids appears to be safe during lactation.
Purine analogues
Over the last few decades, the introduction of thiopurines (TPs) into clinical use has been a cornerstone in the treatment of CD (Figure 1) (39). Both AZA and 6-mercaptopurine (6-MP) are effective in inducing remission off-steroid therapy in patients with steroid-resistant or steroid-dependent CD.
Figure 1.
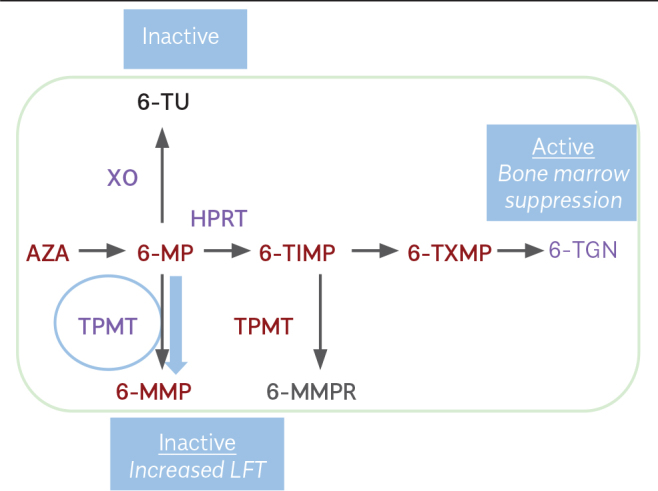
Thiopurine pharmacology
Indications
AZA has not been found to be superior over placebo in inducing remission and should not be used for this purpose. However, these treatments are effective in maintaining remission in CD and fistulizing CD and in preventing postoperative relapses (34,38,40). Therapeutic response to TP occurs 12–17 weeks after the onset of the treatment, and it has been shown that this period is required to include thioguanines (TGNs) into the DNA. A Cochrane analysis on the prevention of postoperative recurrences reported that TPs significantly decreased clinical recurrences (relative risk (RR) 0.59, 95% confidence interval (CI) 0.38–0.92, number needed to treat (NNT) 7) and severe endoscopic recurrences (RR 0.64, 95% CI 0.44–0.92, NNT 4) compared with placebo, and they are more effective than mesalamine (41).
Side effects
The use of TP in inflammatory bowel disease (IBD) over a period of 60 years has provided a wide and very long-term safety profile. In general, the medication is well tolerated; however, side effects leading to discontinuation may occur in 10%–18% of patients.
AZA may lead to a variety of side effects from nausea to myelosuppression (in TP methyltransferase (TPMT) polymorphism). Most side effects may be divided into dose-independent and dose-dependent groups. Drug-induced reactions tend to occur as hypersensitivity, allergy-like reactions within a couple of weeks after the administration of the first dose. Pancreatitis, fever, arthralgia, gastrointestinal disorders, and rash are common symptoms in drug-induced reactions.
Dose-dependent reactions are associated with metabolite formation including leukopenia, cholestatic jaundice, rare bacterial infections, hepatitis, nausea, and myelosuppression that frequently occur as late side effects in long-term treatment. Hepatotoxicity occurs in 10%–17% of patients and may be related to increasing 6-methylmercaptopurine riboside concentrations. Concurrent therapy with allopurinol normalizes liver enzyme levels by shifting the TP metabolism to the 6-TGN metabolic pathway. Dose-dependent side effects generally disappear when the dose is reduced. Drug-related reactions persist until the discontinuation of the drug.
All immunomodulatory treatments increase the risk for infections. TPs cause a tendency to viral infections in particular. In case of acute infection, TP treatment is discontinued until recovery from infection and then resumed.
Myeloproliferative disorders observed in patients treated with TPs are associated with Epstein-Barr virus (EBV).
TPs increase the risk for non-melanoma skin cancers, urinary tract cancers, non-Hodgkin lymphoma, hepatosplenic T cell lymphoma, and primary lymphoproliferative intestinal disorders (40). 18.2% of patients over the age of 50 years who have been treated with TPs are at risk for developing malignancy. This rate has been found to be 3.8% in patients under the age of 50 years (p=0.0008). Treatment duration >4 years has been found to be associated with an increased risk for skin cancers.
TP—before starting treatment
-
Risk factors for TP toxicity should be assessed
Age of 65 years
EBV naive, young, and particularly male patients
Suspicious skin lesions
Pathology of the uterine cervix
All vaccinations should be completed, if applicable
-
Laboratory tests
Hepatitis B virus (HBV), hepatitis C virus (HCV), human immunodeficiency virus (HIV), EBV, varicella zoster virus (VZV)
CBC, liver function tests (LFTs), creatinine, Papanicolaou test
TPMT (if accessible)
-
Radiology
Chest X-ray
TP treatment—induction and maintenance
-
Empirical dose strategy
Initial dose: 50 mg AZA or 25 mg MP
Slow dose increase (every 2 weeks)—until achieving the dose calculated per body weight kg
Follow-up: CBC, LFTs every week during the first month and then every 2 weeks for 2–3 months
-
TPMT dosing strategy
TPMT is started at doses appropriate for disease activity
Follow-up: CBC, LFTs every 2 weeks for 1–3 months
-
Maintenance
How long should treatment duration be?
Discontinuation of TP treatment may be considered when objective signs of inflammation disappear (42)
High rates of relapse have been reported from studies on the discontinuation of the drug
Discontinuation of the drug should be considered on a patient basis and discussed with the patient
Which patients can stop taking the drug?
-
In patients who have taken the drug for at least 4 years, discontinuation may be considered after taking into account:
The age of the patient
Depth of remission (clinical, serological, endoscopic)
Comorbidities
Risk for malignancy and infections associated with long-term treatment
Close monitoring is required in patients who discontinue the drug (40)
A number of clinical studies, meta-analyses, and consensus notes have suggested that the use of TPs is safe during pregnancy and breastfeeding. The rates of spontaneous abortion, prematurity, low birth weight, congenital abnormalities, and neonatal adverse outcomes observed in pregnant women on TP treatment were not higher than those in women with IBD who do not receive any medication or treated with other drugs. Guidelines report that it can be used during pregnancy or breastfeeding (34,35).
Methotrexate
Methotrexate (MTX) may be used in a way similar to TP. In a controlled study, 141 steroid-dependent patients with active CD were randomized to receive either MTX intramuscular (im) injections at a dose of 25 mg weekly or placebo. Prednisolone was tapered (20 mg at baseline) simultaneously over 3 months. In the MTX group, more patients could be withdrawn from steroids compared with the placebo group (39% vs. 19%, p=0.025) (43). The efficacy was confirmed in a review (44).
Same indications as TP are applicable for MTX. However, MTX is still reserved to patients with active or relapsing CD, non-responsive or intolerant to TPs, or anti-tumor necrosis factor (TNF) agents.
Dosage and follow-up
In contrast to rheumatoid arthritis, doses <15 mg weekly are ineffective in active CD, and standard induction dose is 25 mg weekly. In CD, MTX should be initiated via im or subcutaneous routes. Simultaneous folic acid support is recommended.
CBCs and LFTs should be performed within 4 weeks after the initiation of the treatment and then with longer intervals. The same warnings as for the follow-up of TP treatment are applicable for the follow-up of MTX. Patients should remain on the follow-up of a specialist for long-term. The duration of treatment is predicted to exceed 1 year (42–44).
Before starting treatment
CBC, creatinine concentration, serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase, albumin, bilirubin levels
Hepatitis B and C and HIV serology
Pregnancy test
Chest X-ray
Monitoring toxicity during treatment
CBC and creatinine concentration every week in the beginning and every 2–3 months after the discontinuation of the treatment.
Serum ALT, AST, and albumin levels every 4–6 weeks
Liver biopsy
If more than half of regularly monitored AST levels are >2-folds of the upper limit of normal
In case of a progressive increase in serum levels of liver enzyme activity
Indications of MTX discontinuation
Clinically significant liver disease
Fibrosis or cirrhosis in the histological examination of liver biopsy sample
Side effects
Immediate MTX toxicity is primarily gastrointestinal (nausea, vomiting, and stomatitis) and may be limited by initiating folic acid at a dose of 5 mg 2 or 3 days after the administration of MTX.
Although leukopenia, hepatotoxicity, hypersensitivity pneumonia, and opportunistic infections have been reported, these are not common.
Major long-term concerns are kidney impairment, hepatotoxicity, and pneumonia.
MTX should not be used by both sexes within at least 6 months prior to conception. It is contraindicated during pregnancy, and pregnancy should be deferred until 6 months after the discontinuation of the treatment.
MTX is contraindicated if glomerular filtration rate is <50 mL/min.
SECTION 3
To do’s before biological therapies are initiated in patients with IBD
Biological therapies have radically changed our approach to the management of IBD over the last two decades. Anti-TNF (infliximab, adalimumab, and certolizumab pegol) and anti-integrin (vedolizumab (VDZ)) molecules have been approved as second- or third-line treatment of IBD in Turkey. The use of biologics has a moderate effect on disease progress with an acceptable increase in the risk for side effects. Safety concerns include risks for infections, reactivation of latent infections (e.g., fungal infection and granulomatous infection), malignancies, and autoimmune and neurological side effects. The use of a routine checklist before starting biological therapy will decrease the potential risk for side effects.
Counseling before therapy
A detailed medical history and a comprehensive evaluation of the disease type and activity are keys for establishing therapeutic indications and contraindications (Table 2). A thorough discussion with patients about the potential benefits and risks of biological therapies is also very important prior to initiating IBD treatment. Concomitant use of steroids and immunomodulatory agents is common among patients with IBD who need biologics. Unfortunately, concomitant use of other immunosuppressive drugs increases the risk for side effects, such as infections, and physicians must be aware of current medications (45). It is also important to set realistic goals for therapeutic success and advise patients about the early signs (e.g., fever, persistent cough, pain, and rash) of side effects. Patients with IBD on biologics should be able to contact their physicians or nurses whenever they feel that something is not normal about their health condition. A clear communication is essential. Before starting treatment, patients need to undergo a series of diagnostic tests and vaccinations to decrease the potential side effects (Table 2).
Table 2.
Checklist before starting biologics.
Medical history check
|
Blood tests
|
Immunizations
|
Others
|
Tuberculosis screening
It is well known that anti-TNF therapy increases the risk for flare-ups in latent tuberculosis (TB) (46). TB prophylaxis with isoniazid dramatically reduces this risk. A history of latent or active TB infection in the patient or his/her family is of paramount importance. A thorough evaluation of the patient’s TB status including chest X-ray and preferably IGRA (interferon (IFN)-c release assay), such as QuantiFERON or TB spot tests, must be performed. If chest X-ray shows images compatible with TB, the patient should be referred to a pulmonary specialist.
IGRA tests might not be available in all clinics. In that case, TST (PPD) tests could be used, but IGRA test is more sensitive and specific. TST may be false positive due to prior BCG (Bacillus Calmette-Guerin) vaccination, and almost all members of the Turkish population are BCG-vaccinated. A TST (PPD) ≤5 mm must be considered positive. If the IGRA test or TST test is positive, then prophylactic isoniazid must be started and continued for 9 months. If the result of IGRA test is indeterminate, a second test must be performed. If the second test is negative, biologic treatment can be started. However, if it is still indeterminate or positive, prophylactic treatment is recommended for 1 month prior to anti-TNF therapy (47). Only in case of emergency, both isoniazid and an anti-TNF agent can be started simultaneously. It is important to keep in mind that the concomitant use of steroids or other immunosuppressive drugs may lead to false negative results. Biologic treatments are associated with a risk for latent TB reactivation, but proper screening before initiating treatment and prophylaxis along with regular controls can decrease the risk effectively.
Hepatitis B
Hepatitis B reactivation might be associated with significant morbidity and mortality in patients receiving anti-TNF therapy. Hepatitis B virus status should be determined before starting biological therapy in patients with IBD. HbsAg, anti-HBs, and anti-HBc IgG must be checked. All patients who tested negative for anti-HBs (≥10 U) should be vaccinated. It is important to remember that the rate of response to hepatitis B vaccination may be low among patients with IBD (48–49). This could be due to impaired immune response to infectious agents in these patients, malnutrition, and use of other immunosuppressive drugs (50). An accelerated vaccination protocol with hepatitis B vaccine in double doses at months 0, 1, and 2 has been shown to increase response rates (51). Patients who tested positive for HbsAg need prophylaxis with oral nucleos(t)ide analogues regardless of their active viral status 1–3 weeks before anti-TNF treatment Prophylactic treatment must be maintained for 12 months at least after the discontinuation of biologics (52). Patients who tested positive for anti-HBc/negative for HbsAg may also show reactivation during anti-TNF treatment, but it is less common. Guidelines do not recommend prophylaxis for these patients and advise monitoring of HBV DNA and LFT levels during treatment (47).
Hepatitis C
Fortunately, concurrent hepatitis C infection in patients with IBD is uncommon.
The use of anti-TNF agents appears to be relatively safe in patients with chronic hepatitis C infection. However, anti-HCV should be checked before initiating anti-TNF treatment. If anti-HCV is positive, HCV-RNA status should be addressed, and appropriate therapy should be started accordingly (47). If compensated liver disease is present, anti-TNF agents should be used with caution, and the benefit/risk ratio should be considered. Anti-TNF agents are contraindicated in decompensated cirrhosis due to the high risk of potentially fatal infections (53).
HIV infection
Although TNF increases viral replication in HIV infection, biologics may also increase the risk of infection as a result of impaired immune function due to HIV infection. Therefore, the use of biologics should be carefully considered on the basis of risks versus benefits, and patients should also be monitored very carefully during the treatment period (54).
Malignancy
Before starting anti-TNF treatment, every patient should undergo screening tests for cancer, appropriate to their age/gender according to the local guidelines. Although the risk for cutaneous malignant melanoma is increased 1.32-fold in those treated with anti-TNF agents, guidelines emphasize that there is not enough evidence that monotherapy with anti-TNF drugs increases the overall risk of malignancy in patients with IBD (55–56). However, regarding the risk of lymphoma, data are conflicting. In a meta-analysis, a 3-fold increased risk of NHL was found in patients who were exposed to an immunomodulatory agent previously, whereas data obtained from observational studies and registries did not show an increased risk in patients exposed to an anti-TNF agent alone. In addition, in recent reports, there was an increase in the rare hepatosplenic T cell lymphoma cases in young male patients with IBD treated with AZA in combination with an anti-TNF agent (57). It is important to keep in mind that the Turkish Health Ministry reimbursement policy permits only step up treatments in IBD. Therefore, most of our patients have already been exposed to immunomodulatory agents, and close follow-up is essential. Patients with a history of cancer and requiring biologic therapies present another challenge to physicians who treat patients with IBD. If the patient has completed oncologic treatment within 2 years, 5-aminosalicylates, CSs, antibiotics, nutritional therapy, and surgery should form the foundation of IBD treatment if applicable. The administration of conventional IS or an anti-TNF agent should be decided on a case-by-case basis by the oncologist and the patient in patients with refractory IBD. MTX could be an alternative to TPs and anti-TNF drugs. However, if necessary, anti-TNF drugs could be used after explaining the risk and benefits to the patient, with close collaboration with the oncologist (58).
Immunization
Immunization of patients with IBD before starting immunosuppressive treatments can prevent serious infectious complications and must be in the checklist of physicians treating IBD (Table 3). As a rule, live attenuated vaccines (measles, mumps, rubella, polio, yellow fever, varicella, BCG, and oral typhoid) must be strictly avoided in patients receiving immunosuppressive therapy. The varicella vaccine should be considered in patients with no history of chickenpox or shingles, no prior immunization but with a negative serology for VZV. Since varicella vaccine is a live vaccine, it should be avoided in patients receiving immunomodulatory treatment. Patients should receive varicella vaccine according to a two-dose vaccination schedule at least 4 weeks before starting immunosuppression.
Table 3.
Contraindications to anti-TNF therapy.
|
It is well known that inactivated vaccines are safe in immunocompromised patients. Inactivated vaccines against influenza and pneumococcal infections are recommended annually. Intranasal influenza vaccine is contraindicated in immunocompromised individuals. Hepatitis A and B antibody status should be checked, and if the patient tested negative for anti-HBs or anti-HAV total, vaccines should be administered. HPV vaccination is also recommended to female patients aged between 9 and 26 years for the prevention of cervical cancer (47).
Anti-integrin therapy
VDZ is a humanized, monoclonal IgG1 antibody that blocks the heterodimer α4β7 integrin and inhibits migration and leukocytes adhesion. In contrast to non-selective α4β1 integrin antibody natalizumab, anti-α4β7 integrin antibody VDZ is gut specific. Probably this is the main reason for the good safety profile of VDZ established from clinical studies and real-life data (59). The mechanism of action of VDZ is not expected to increase the risk for malignancies, although longer term data are needed for a comprehensive assessment. Colombel et al. have evaluated the safety data from six trials (2830 patients had 4811 PYs of VDZ exposure) VDZ was not associated with an increased risk for infections/serious infections. Serious clostridial infections, sepsis, and tuberculosis were reported infrequently (≤0.6% of patients). Most importantly, no cases of progressive multifocal leukoencephalopathy were observed (60). VDZ is a foreign protein, and allergic reactions should be expected. Fortunately, infusion reactions have been reported in <5% of cases (61). The current Turkish Health Ministry regulation requires screening for tuberculosis and neurological consultation prior to VDZ treatment although neither the risk for TB infections nor progressive multifocal leukoencephalopathy prevalence was higher than placebo.
Details to consider before starting treatment with biological agents
If the clinical condition of the patient that the treatment planned allows, it is wise to avoid starting anti-TNF and immunomodulatory treatments concomitantly. Because if serious adverse events occur, it will be impossible to determine which one of these two agents causes the events. Before starting treatment with biological agents, albumin levels of the patients must be evaluated as the clearance of these agents increases in case of hypoalbuminemia and leads to decrease at the efficiency of drugs (62).
SECTION 4
When to start biologics in UC? And how to use them effectively?
Currently, anti-TNF and anti-integrin agents are included in established treatment protocols for the management of patients with UC. The success of biologics is assessed on the basis of clinical response, clinical remission, mucosal healing, and histological recovery and colectomy rates. Studies on biologics that are used in the management of UC are summarized in Table 4.
Table 4.
The efficacy of biologics versus placebo in patients with ulcerative colitis (adapted from reference (63)).
| Study | Anti-TNF | Inflammatory Activity Treatment experience | Treatment phase | Doses | Clinical response* (%) | Clinical remission** (%) | Steroid-free remission (%) | Mucosal healing*** (%) | Colectomy (%) |
|---|---|---|---|---|---|---|---|---|---|
| ACT1 (63) | IFX | Mayo score ≥6, endoscopic subscore ≥2 KS+AZA/6-MP | Induction/maintenance | 5 mg/kg Weeks 0, 2, and 6 Every 8 weeks, iv |
5 mg/kg, 10 mg/kg, placebo Week 8 (69.4 vs. 61 vs. 37.2) Week 54 (45.5 vs. 44 vs. 19.8) |
Week 8 (38.3 vs. 14.9) Week 54 (34.7 vs. 16.5) |
Week 30 (21.5 vs. 7.2) | Week 8 (62 vs. 33.9) Week 54 (45.5 vs. 18.2) |
Week 54 (10 vs. 17) |
| ACT2 (64) | IFX | Mayo score ≥6, endoscopic subscore ≥2 KS+AZA/6-MP+5-ASA | Induction/maintenance | 5 mg/kg Weeks 0, 2, and 6 Every 8 weeks, iv |
5 mg/kg, 10 mg/kg, placebo Week 8 (64.5 vs. 29.3) Week 54 (47.1 vs. 26) |
Week 8 (33.9 vs. 5.7) Week 54 (25.6 vs. 10.6) |
Week 8 ( 60.3 vs. 30.9) Week 30 (47.1 vs. 46.31) |
||
| ULTRA-1 (65) | ADA | Mayo score ≥6, endoscopic subscore ≥2 KS+AZA/6-MP | Induction | 160/80/40 Every 2 weeks, SC |
Week 8 (54.6 vs. 44.6) | Week 8 (18.5 vs. 10) | Week 16 (31 vs. 16) | Week 8 (46.9 vs. 41.5) | |
| ULTRA-2 (66) | ADA | Mayo score ≥6, endoscopic subscore ≥2 KS+AZA/6-MP | Maintenance | 160/80/40 Every 2 weeks, SC |
Week 8 (50.4 vs. 34.6) Week 52 (30.2 vs. 18.3) |
Week 8 (16.5 vs. 9.3) Week 52 (17.3 vs. 8.5) |
Week 52 (13.3 vs. 5.7) | Week 8 (50.4 vs. 34.6) Week 52 (30.2 vs. 18.3) |
Week 16 (31 vs. 16) Week 52 (13.3 vs. 5.7) |
| PURSUIT- SC (67) | GLM | Mayo score 6–12 Endoscopic subscore >2 |
Induction | Weeks 0 and 2 200 and 100 mg SC Every 2 weeks, 50–100 mg SC |
Week 6 (51 vs. 30.3) | Week 6 (17.8 vs. 6.4) | Week 54 (34.4 vs. 20.7) | Week 6 (17.8 vs. 6.4) | |
| PURSUIT-M (68) | GLM | Patients responsive to GLM induction treatment | Induction/maintenance | Week 54 (49.7 vs. 31.2) | Week 54 (27.8 vs. 15.6) | Week 54 (42.4 vs26.6) | Week 54 (34.4 vs. 20.7) * | ||
| GEMINI-1 (69) | VDZ | Mayo score 6–12 endoscopic subscore ≥2 |
Induction/maintenance | Loading dose of 300 mg iv Weeks 0 and 2 Every 4 and 8 weeks |
Week 6 (47.1 vs. 25.5) | Week 52 (44.8 vs. 15.9) | Week 52 (45.2 vs. 13.9) | Week 6 (40.9 vs. 16.1) |
Cilinical response; ≥30% or ≥ points reduction from the baseline in Mayo scores, rectal bleeding subscore 0 or 1.
Clinical remission.
Mucosal healing; Mayo edoscopic subscore: 0.
The role of anti-TNF agents in the management of patients with UC
The mechanism of the pathogenesis in UC is a reduction in Th1 responses and an increase in Th2 responses. However, Th2 response is atypical in nature, IL-13 expression has been observed in CD4+ T lymphocytes (70), while increased IL-1β, IL-4, IL-5, IL-8, IFN-γ and TNF-α levels have been detected. Increased TNF-α levels have been also observed in mucosal and fecal samples. The efficacy of anti-TNF agents in patients with UC is based on this fact (71).
Infliximab (IFX), adalimumab (ADA), and golimumab (GM) have been approved for the treatment of UC. In well-designed, multicenter, randomized, controlled studies, anti-TNF agents have been proven to be superior over placebo in a number of parameters including clinical response, clinical remission, steroid-free remission, and mucosal healing in patients with moderate to severe UC unresponsive to conventional agents (64–69).
Induction of remission
Anti-TNF agents are indicated as the second-line treatment for inducing remission in UC, in patients with moderate to severe UC refractory or intolerant to oral CSs and/or immunomodulatory agents or if steroids are contraindicated, and in steroid-dependent patients if steroid-free remission cannot be achieved with immunomodulatory (IM) agents (72–76).
As there are no studies comparing anti-TNF agents against one another, it has been suggested that these agents exhibit approximately equal efficacy based on placebo-controlled studies and meta-analyses.
Anti-TNF agents may be used in combination with AZA/6-MP. The UC-SUCCSESS study demonstrated that AZA given in addition to IFX prevented the development of ADAs, increased IFX “trough” levels and consequently, increased the efficacy of IFX (77).
Anti-TNF agents may be used as a rescue treatment following 3 days of treatment with iv CSs in patients with acute severe UC (ASUC) and as an alternative to second-line cyclosporine therapy. They should be used in AZA-experienced patients who are on multidrug regimens, and when cyclosporine is contraindicated or not feasible (e.g., if serum levels cannot be measured) (72–74). Only IFX should be used as anti-TNF agent in patients with ASUC. The efficacy and colectomy rates associated with anti-TNF agents were higher or equal to those of cyclosporine (78,79).
In the assessment of therapeutic efficacy, treatment should be reviewed in the absence of symptomatic response 8–10 weeks after anti-TNF loading (75). VDZ is an alternative biologic therapy to be used in cases of primary unresponsiveness.
Maintenance of remission
Remission is aimed to maintain steroid-free clinical and endoscopic improvement achieved in UC.
Anti-TNF agents should be used in the maintenance of remission in patients who have responded to induction treatment with anti-TNF agents. Anti-TNF agents may be used alone or in combination with AZA/6-MP to maintain remission (72,74).
“Anti-drug antibodies” (ADAs) and drug levels should be measured in case of loss of efficacy during the maintenance therapy and alternative options, such as the use of anti-TNF agents at higher doses or the use of immunomodulatory agents should be considered.
Anti-integrin agents in the treatment of UC
Currently, VDZ is the sole anti-integrin agent to be used in the treatment of UC. VDZ is a biologic agent that acts against α4β7 integrin and blocks the interactions of α4β7 integrin with “mucosal addressing cell adhesion molecule-1” on lymphocytes and endothelial surface, specifically in the gastrointestinal tract (80). In this way, VDZ inhibits inflammatory cell trafficking to the tissue.
Induction of remission
As with anti-TNF agents, VDZ is indicated in the second-line treatment for inducing remission in UC, in patients with moderate to severe UC, refractory or intolerant to oral CSs and/or IM therapy or in steroid-dependent patients or if steroids are contraindicated, and steroid-free remission cannot be achieved with IM agents or as an alternative biologic agent in case of primary or secondary loss of response to anti-TNF agents (73,75).
VDZ is particularly more effective in anti-TNF-naive patients than in those who are anti-TNF experienced (69).
The efficacy of combination therapy with VDZ and immunomodulatory agents has not been established (81).
A definite period has not been specified for the assessment of the efficacy of the treatment. It is recommended to wait until week 16 of the treatment.
VDZ is not indicated in patients with ASUC.
Maintenance of remission
Real-world data suggest that the efficacy of VDZ may increase cumulatively. VDZ may be used effectively in the maintenance of remission based on this observation (81).
VDZ therapy should be continued until steroid-free remission is achieved in patients if induction therapy is successful.
Anti-TNF agents or anti-integrin agents?
Recently, it has been found in a randomized trial which was the first head to head trial comparing two biologic treatment (VDZ vs. adalimumab) in patients with active moderate to severe UC that VDZ showed superior efficacy over adalimumab in achieving clinical remission and mucosal healing at week 52. However, there was no significant difference in steroid-free remission between these two drug regimens (82).
In patients with moderate to severe UC refractory to conventional treatment, anti-TNF agents are recommended in pregnant patients, in patients with ASUC, and in patients with extraintestinal manifestations, whereas anti-integrin agents are recommended in older patients, in patients with a history of serious, opportunistic infections, or in patients with extraintestinal cancers (83).
VDZ is positioned as first-line biologic treatment, such as anti-TNF agents, in patients with moderate to severe UC according to the recent guidelines published by the British Society of Gastroenterology and American College of Gastroenterology (84,85).
SECTION 5
When to start biologics in CD? And how to use them effectively?
Although the introduction of biologics, notably anti-TNF agents in the treatment of IBD, has strengthened clinicians’ hand over the last 20 years, cure is not possible yet. Therefore, by switching to biologics before giving enough time to conventional treatment, a potentially appropriate treatment option would be prematurely excluded. Though slowly, increasing number of alternative treatments to biologics leads to confusion when deciding about which treatment is the treatment of choice under such conditions. Some of the important clinical studies on biological treatments are summarized in Table 5.
Table 5.
Clinical studies of biologics.
| Study | Biologic | Treatment phase | Study information reference | No. Of patients | Dose | Clinical response | Clinical remission |
|---|---|---|---|---|---|---|---|
| ACCENT-I | Infliximab | Induction and maintenance | R, DB, III (114) | 573 | (Induction: all patients, 5 mg/kg week 0) Group 1: placebo Group 2: 5 mg/kg Group 3: 10 mg/kg |
58% at week 2 | At week 30 Group 1: 21% Group 2: 39% Group 3: 45% |
| SANDS BE et al. (2004) | Infliximab | Fistula closure in fistulizing Crohn’s disease | R, DB, III (104) | 306 | (All patients 5 mg/kg weeks 0, 2, and 6) Group 1: placebo Group 2: 5 mg/kg every 2 months |
69% at Week 14 | Fistula closure at week 54 Group 1: 19% Group 2: 36% |
| CLASSIC-I | Adalimumab | Induction, comparisons of dozing schedules | R, DB, III (115) | 299 | Group 1: placebo Group 2: 40 mg/20 mg (weeks 2–4) Group 3: 80 mg/40 mg (weeks 2–4) Group 4: 160 mg/80 mg (weeks 2–4) |
- | At week 4* Group 1: 12% Group 2: 18% Group 3: 24% Group 4: 36% |
| CHARM | Adalimumab | Induction and maintenance comparisons of dozing Schedules | R, DB, III (116) | 854** | (All patients week 0: 80 mg, week 2: 40 mg) Group 1: placebo Group 2: 40 mg (every 2 weeks) Group 3: 40 mg (every week) |
91% at week 4 | At week 56 Group 1: 12% Group 2: 36% Group 3: 41% |
| PRECISE-II | Certolizumab | Induction and maintenance | R, DB, III (117) | 668 | (All patients 400 mg weeks 0, 2, and 4) Group 1: placebo Group 2: 400 mg/month |
64% at week 6 At week 26 Group 1: 36% Group 2: 63% |
At week 26 Group 1: 29% Group 2: 48% |
| GEMINI-II | Vedolizumab | Induction and maintenance comparisons of dozing Schedules | R, DB, III (118) | 368,747*** | 368 patients (induction) Group 1: placebo Group 2: vedolizumab |
Induction: week 6: Group 1: 6.8% Group 2: 14.5% |
Maintenance: Every 8 weeks Group: 39% Every 4 weeks Group: 36.6% Placebo: 21%,6 |
| UNITI I-II-IM# | Ustekinumab | Induction and maintenance comparisons of dozing schedules | R, DB, III (119) | UNITI-I: 741 UNITI-II: 628 UNITI-IM: 397 | UNITI-I, II: 130 mg, 6 mg/kg, placebo UNITI-IM: 90 mg 8 weeks or 12 weeks | Induction: week 6 34.3%, 33.7%, 21.5%, 51.7%, 55.5%,28.7%, respectively (UNITI-I, II) |
Maintenance: (UNITI-IM) week 44 Placebo: 35.9% Every 8 weeks: 53.1% Every 12 weeks: 48.8% |
CDAI <220.
Clinical response criteria: CDAI >70 regression. Patients with clinical response (778/854; 91%) were divided into groups.
In the Gemini study, 368 patients received placebo or VDZ in the induction phase, 747 patients received open label 300 mg VDZ at 0 and 2 weeks, and then patients with clinical response (n=461) received 300 mg as maintenance at week 4 or week 8.
741 anti-TNF refractory or intolerant patients were included in the UNITI-I study, 628 patients refractory or intolerant to conventional treatment were included in the UNITI-II study, and 397 patients who had response were included in the UNITI-IM study.
R: randomized; DB: double blind.
Role of anti-TNF agents in the treatment of CD
In general, anti-TNFs are recommended in the treatment of moderate to severe CD after the failure of conventional treatments (31,86). However, the decision on conventional treatment failure still depends on personal perception, as the treatment duration and issues, such as the assessment of comorbidities and side effects, have not been clearly defined yet.
The basement of current biologic use has been extended by patients who are not having any alternative treatment option and suffer from chronic active disease in mild to moderate intensity in both endoscopic and clinic activities.
Anti-TNF agents can be used to induce remission in case of prior failure of remission induction therapy or if CSs are contraindicated. Anti-TNF treatment should be considered in patients who have become steroid-dependent or steroid refractory while receiving steroids or IM agents or if disease exacerbations cannot be kept under control. Switching from an IM therapy to another IM therapy or anti-TNF agents may be considered in patients intolerant to previous IM or who have developed side effects (31,87).
Management planning should be based on the assessment of disease activity and risk factors. There is no gold standard test or scoring scales to assess disease activity. Clinician’s assessment based on clinical and endoscopic findings and data from medical history and examination of the patient is essential. Tests or scoring scales may provide the physician with only a partial objectivity.
Age <40 years at the time of diagnosis, ileal/ileocolic involvement, long-segment disease, and perianal involvement have been considered as risk factors for progressive disease (88). It is well known that the combination of anti-TNF and IM has an additive effect in a patient who has not received these treatments before (89). Therefore, it appears to be rational to start with combination therapy including anti-TNF and IM agents to induce and maintain remission to prevent long-term complications in patients with multiple prognostic risk factors. Even though, which patients are more likely to benefit from long-term intensive treatment, it is a matter of debate (90,91).
We believe that anti-TNF agents and IM agents should be started with an interval of a few weeks, and CBCs and blood chemistry should be assessed before adding anti-TNF agents to IM therapy in patients considered for combination therapy, to avoid the dilemma of choosing which drug is the cause and subsequent discontinuation of both drugs.
Anti-TNF agents are indicated in the treatment of moderate to severe CD after the failure of conventional treatments.
Biologics may play a role in the treatment of chronic active, mild to moderate CD in patients who are non-responder to/unable to use IM agents.
Combination treatment with TNF blockers and IM agents may be started in patients with risk factors for progressive disease.
The components of combination therapy should be started separately with an interval of a few weeks.
Whether combination therapy (IM+anti-TNF) or monotherapy (anti-TNF) should be used when treatment with immunomodulatory agents fails?
There are no studies demonstrating the extra benefits of adding anti-TNF agents to full-dose immunomodulatory agents in patients who failed to achieve clinical response/remission under IM therapy. Nevertheless, this approach does not provide any additional clinical benefits; several studies have suggested that this therapeutic approach might further increase the risk for opportunistic infections (92). On the other hand, it is well established that keeping low-dose IM in combination with anti-TNF agents reduces the immunogenicity of these biologics. Although anti-TNF agents are used as monotherapy by certain groups, it may be more rational to administer immunomodulatory therapy at lower doses to prevent anti-TNF antibodies from developing.
There is no evidence of additional benefits of continuing IM therapy at full doses in patients who have switched to anti-TNF therapy after a treatment failure with IM agents.
Combination with low-dose IM therapy with anti-TNF to prevent the development of antibodies may be more reasonable.
Whether the dose should be increased when anti-TNF therapy fails? Whether to use another anti-TNF agent or switch to another therapy with a different mechanism of action?
Primary non-response should be considered in case of non-response after the initiation of the therapy or a partial response which disappears within the first 3 months, whereas a secondary non-response should be considered if non-response occurs after 3 months of treatment. Primary non-response occurs in approximately one-third of patients who receive anti-TNF therapy, and secondary non-response occurs over time in approximately half of patients initially responsive to anti-TNF therapy (72). A second anti-TNF response is more common in patients intolerant to the first anti-TNF agent and least common among patients who exhibit primary non-response (93). In case of primary non-response, it is more rationale to switch to another group of biologics which are more likely to induce a response rather than trying another anti-TNF agent (94).
Dose increases are useless if the blood anti-TNF level is within therapeutic range (mechanistic loss of effect) for secondary non-responders to anti-TNF agents. There are studies investigating the success of a second anti-TNF agent in such cases (95,96). The recommendation is to switch to out of class biologics.
Patients who switched to adalimumab after developing antibodies against infliximab were found to be more likely to develop antibodies against this therapy too (97). In a study investigating secondary loss of response, the highest rate of patients who benefited from switching to a second anti-TNF agent after treatment failure with an anti-TNF agent was in the group of patients who had low drug levels. One can argue that this group of patients might also benefit from higher doses of the first anti-TNF agent (96). Low-dose MTX or AZA may be added to the regimen if a second anti-TNF agent will be given to a secondary non-responder due to neutralizing antibodies against the first anti-TNF agent. These patients may switch from anti-TNF therapy to another class of biologics.
Medical history and clinical assessment are of paramount importance in case of secondary loss of response, particularly when blood drug levels cannot be measured. If the patients are clinically doing well after receiving treatment but deteriorate just before the next dose, this means that this patient may benefit from dose intensification or escalation.
In case of loss of response in patients non-compliant with treatment, requiring frequent treatment interruptions or developing infusion reactions, the presence of antibodies is more likely.
If a sudden clinical deterioration occurs following an infection or acute abscess formation, this may suggest the consumption of anti-TNF by infection-related TNF-alpha and consequent reduction in drug levels. In such patients, taking infectious process under control may provide clinical benefit by increasing the drug level. In a similar way, conditions associated with increased inflammatory load, such as infections, abscesses, or active sacroiliitis, drug level measurements may be misleading as these measurements are usually preplanned.
Regarding therapeutic drug level measurements, we have adopted a dynamic, reactive approach integrating medical history and clinical data of the patient and drug level measurements. Considering the conditions in our country, we believe that it is possible to manage the disease on the basis of clinical manifestations, medical history, and drug level measurements, when required, but putting the secondary importance to measuring antibody levels to lower healthcare costs.
Switching to another biologic with different mechanism of action may be more appropriate in primary non-responders.
Drug levels and clinical signs and symptoms may guide disease management in patients with loss of response.
Presence of antibodies or mechanistic loss of effectiveness underlying as the cause of secondary non-response possibly suggests poor success rate in the same biologic group. The class of biologic agent may be changed in this group of patients.
VDZ in the management of CD
VDZ is a monoclonal antibody that acts against α4β7 integrin and is a gastrointestinal system (GIS)-selective integrin inhibitor. In contrast to anti-TNFs having the potential of activity at any inflammatory foci, VDZ targets intestinal inflammation alone, and this feature limits its use in clinical practice. However, high GIS selectivity is the reason why cases of PML associated with natalizumab have not been seen with VDZ. Currently, no cases of PML have been reported with VDZ either globally or in Turkey. Therefore, regarding the current practice in Turkey, the abolishment of prescription requirement of being signed by a neurologist would be more convenient for patients and would reduce labor loss. There are no such requirements in many countries where VDZ is available.
The current place of VDZ in the reimbursement system of our country is after anti-TNF failure or intolerance in CD. Although VDZ has been mainly used after anti-TNF failure based on real-world data, favorable sustained remission rates have raised the topic of use of VDZ after conventional treatment (31,98,99). Prior anti-TNF agent use, perianal involvement, current smoking has a negative impact on treatment response (98). Actually, this real-world experience is applicable to all biologic agents. Real-world data suggest that VDZ is as effective as anti-TNF agents following the failure of conventional treatment (99). In a study presented in ECCO 2018, better 12-month remission rates were reported with VDZ, particularly in colonic involvement, in 538 patients matched for demographics and risk groups (100). Therefore, VDZ may be used after conventional treatment in patients with CD with colonic involvement but without extraintestinal manifestations. Optimum response is achieved at approximately 12–16 weeks after the onset of the treatment, and any decision of non-response should not be made before week 24, particularly in patients with limited treatment options (98). Although it may appear logical to add low-dose immunomodulatory therapy to VDZ to prevent the development of antibodies, there is no sufficient evidence about this subject.
In a meta-analysis assessing the side effects associated with VDZ, no increases were observed with respect to important side effects, such as mortality, cancer, progressive multifocal leukoencephalopathy (PML), and serious infections (60,101). Owing to the low potential of infection side effects, VDZ and ustekinumab may be preferred in patients who are considered to be susceptible to infectious side effects (60,101,102).
Although VDZ is generally used in CD after anti-TNF failure, it has been increasingly used as first-line biologic treatment after conventional treatment failure.
The effect of VDZ was found to be superior in anti-TNF naive patients than in anti-TNF experienced patients.
As the effects of VDZ appear late, one should wait 24 weeks at least to consider treatment failure particularly in patients with limited treatment options.
Hypothetically, GIS selectivity of VDZ may prevent it from being effective in extraintestinal manifestations of IBD.
VDZ and ustekinumab appear to be safer than anti-TNF agents with respect to infectious side effects.
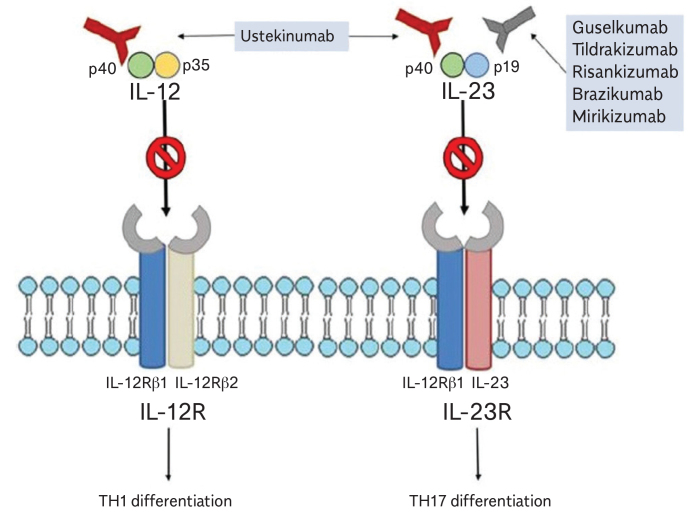
Ustekinumab in the management of CD
Ustekinumab is a humanized IgG1 antibody targeting the p40 subunit of interleukins 12 and 23. It has been initially approved by the Food and Drug Administration (FDA) for the treatment of psoriasis. In a study in either primary or secondary non-responders to anti-TNF therapies, ustekinumab was found to be superior than placebo in both inducing and maintaining remission (103).
Ustekinumab is not reimbursed for the treatment of CD in our country. Therefore, it is used off-label to treat patients who have not responded to other biologic therapies. There are no studies comparing ustekinumab and VDZ after anti-TNF failure. However, ustekinumab may be preferred when a rapid onset of action is required and in patients with CD associated with extraintestinal manifestations or coincidental psoriasis (104).
Phase studies and real-world data indicate that ustekinumab is safe and ustekinumab is associated with fewer infection side effects than anti-TNF agents (102).
Long-term, head-to-head comparative studies are needed to provide an appropriate response to “Whether VDZ or ustekinumab should be used in CD following conventional treatment failure?”.
A group of biologic agents that will have less or no impact on the effects of other biologics should be primarily preferred following conventional treatment. Although anti-TNF part of this issue is partially known, there is no answer to this question yet.
Ustekinumab may be preferred when a rapid onset of action is required and in patients with CD associated with extraintestinal manifestations or coincidental psoriasis.
There are no head-to-head comparative studies investigating ustekinumab versus VDZ following anti-TNF agents.
Use of biologics in patients with CD who present with complex perianal fistula
Perianal involvement is one of the major risk factors for poor prognosis. Considering all treatment options, clinical and endoscopic response rates are lower in these groups of patients than in other groups. Therefore, particularly in the presence of other risk factors, it appears to be rationale to initiate anti-TNF therapy in combination with conventional treatment in patients with perianal involvement, if not contraindicated.
Biologic agents have been proven to be superior over placebo in perianal fistula healing based on subgroup analyses of phase studies. Furthermore, infliximab has been investigated in randomized, controlled, prospective studies (105,106). However, because of the inadequacy of parameters for fistula closure, whether these findings indicate a favorable long-term outcome and their sustainability have not been investigated.
The inclusion of antimicrobial therapy into the management plan may occasionally increase treatment success, although it can be transient. Complex nature of the fistula, age of the patient, and concurrent abscesses may have a negative impact on treatment success. In case of the presence of an abscess in fistulizing CD, anti-TNF therapy should be initiated under antimicrobial therapy after the abscess is drained and a seton should be placed to prevent the development of new abscesses.
Perianal involvement is one of the major risk factors for poor prognosis.
Biologic agents and particularly infliximab have been proven to be more effective than placebo in the closure of perianal complex fistula in short term and intermediate term.
Combined use of biologics and antimicrobial therapy increases the chance of treatment success.
In the presence of an abscess, anti-TNF therapy should be given under antimicrobial therapy after the abscess is drained and a seton is placed.
The use of biologics in CD after surgical resection
The requirement for surgical treatment is diminished after the introduction of biologic agents in the treatment of CD (107,108). The 5-year clinical recurrence rate is approximately 50%, and the endoscopic recurrence rate is approximately 90% following surgery (109). However, it remains unknown what is the real meaning of post-resection endoscopic score and how much it really figures out a progressive process. Furthermore, observational comments on anastomosis ulcers rather highlight the local ischemic process and may not show any progression further. Risk factors for developing postoperative recurrences include penetrating disease behavior, current smoking, and history of second surgical resection (110).
During the postoperative period in CD, the main treatment strategy recommended by the guidelines has been to prevent disease recurrences (31,110). Although the efficacy of 5-ASA was low in studies, it has been widely used during the postoperative period owing to its high safety profile (111). Guidelines recommend the use of AZA and/or anti-TNFs in patients at high risk of recurrence based on a low evidence level. Although metronidazole and ornidazole have a partial effect on the prevention of recurrence, this effect is not long lasting, and toxicity is a major concern (112).
The reason of starting prophylactic treatment during the postoperative period is to prevent bowel damage and, subsequently, to protect patients from additional surgery, function loss, and comorbidity. On the other hand, although meta-analyses demonstrated a limited benefit of AZA and anti-TNF therapy, there are conflicting data on their effect on the prevention of postoperative clinical recurrences (113,114). Another important consideration is that the medication will not provide any further benefit in patients who develop recurrence under prophylaxis and this means further restriction in treatment options which are already restricted in these patients.
Therefore, a close monitoring is recommended using combined parameters, such as clinical signs and symptoms, intestinal ultrasound, calprotectin, acute phase, and colonoscopy, when required. Treatment should be given in an accelerated manner from local treatment to biologics. Intensive treatment appears to be more appropriate in patients with residual active disease segment after surgery and those who have undergone re-surgery. Although IM and anti-TNF therapies may not prevent surgery, they may be beneficial during the postoperative period (115).
Risk factors for postoperative recurrences include penetrating disease behavior, current smoking, and a history of prior surgical resection.
Guidelines recommend the use of AZA and/or anti-TNFs in patients at high risk of recurrence based on a low evidence level.
Close monitoring using multiple parameters and accelerated treatment is recommended regarding disease recurrences during the postoperative period.
Even though a TNF agent fails preoperatively, it should be used for prophylaxis and in disease activation.
SECTION 6
How to monitor patients using biologic therapies
Patients with IBD should be carefully monitored during the treatment. Phenotype of disease, activity (active (mild-moderate-severe) or remission), extraintestinal involvement, and complications must be evaluated at each outpatient visit. In routine daily practice, it might be difficult to calculate clinical activity index, such as CD Activity Index (CDAI) and UC activity index (Mayo score or Truelove and Witz severity index or clinical activity index). We can estimate the disease activity according to symptoms and laboratory results (Table 6) (122).
Table 6.
Clinical activity estimates for Crohn’s disease according to symptoms and laboratory workup.
| Mild (CDAI 150–220) | Moderate (CDAI 220–450) |
|---|---|
| √ No obstruction, fever, dehydration, abdominal mass, and tenderness | √ Intermittent vomiting |
| √ Elevated CRP | √ Weight loss >10% |
| √ Weight loss <10% | √ Abdominal tenderness without obvious ileus and abdominal mass |
| Severe (CDAI >450) | |
| √ Refractory symptoms in spite of the effective therapy | |
| √ Cachexia (BMI 18 kg/m2) or presence of obstruction, abscess | |
The simple clinical colitis activity index (SCCAI) can be helpful in UC. The SCCAI is composed of six items: bowel frequency during the day, bowel frequency during the night, urgency for defecation, blood in stool, general well-being, and extraintestinal manifestations (arthritis, erythema nodosum, pyoderma gangrenosum, and uveitis). Clinicians are able to categorize two types of patients based on the SCCAI: patients with inactive disease (SCCAI score <5) and patients with active disease (SCCAI score ≥5). Bowel movements >3 times during the night, bloody stool, and presence of any extracolonic manifestations represent active disease (123,124).
Evaluation of biologic therapy response in every visit is mandatory. Follow-up visits should be done every 2 or 3 months depending on which biologic is being used.
Clinical response should be evaluated according to the CDAI or Harvey-Bradshaw index in CD. Treatment response is defined as a decrease of 70–100 points from the baseline CDAI. Remission is accepted as CDAI scores <150 points. Relapse is defined as a flare of symptoms (CDAI >150 points) in clinical remission during the follow-up period. Early relapse is accepted as any relapses within 3 months. Relapse can be rare (<1/year), frequent (>1/year), or continuous (31). In every flare, complications must be evaluated. If there is a complication, such as bowel perforation, persistent or recurrent obstruction, abdominal abscess not amenable to percutaneous drainage, intractable hemorrhage, dysplasia/cancer, or medically refractory disease, surgery is indicated. Surgery can be an alternative in localized disease (<30 cm) instead of medical treatment change (42).
In every visit, evaluation should be based on clinical, laboratory (CRP, whole blood test, and fecal calprotectin), and/or imaging, when clinically needed (computerized tomography/magnetic resonance imaging/ultrasound, endoscopy), if applicable (31). Clinical response can be evaluated by the SCCAI in UC. We have to consider the impact of disease on the patient (symptoms, life quality, fatigue, and disability), complicated disease course (anatomic damage, resection, perianal disease, frequency of flare, and extraintestinal involvement), and presence of inflammation (CRP, mucosal lesions, upper GIS involvement, and disease extent) (125). Biomarkers, such as fecal calprotectin, are important for the management of disease. The cut-off level should be accepted as <150 μg/g for fecal calprotectin. Presence of complications related to the disease or drugs must be carefully evaluated. If any complication is detected, we have to discontinue the biologic treatment.
In routine practice, endoscopic evaluation should be classified as follows: mild, edema/erythema; moderate, erosion and superficial ulcers (involved surface 10%–30%); and severe, deep large ulcers >2 cm. Evaluation of the ileum for postoperative endoscopic recurrence by colonoscopy within a year (generally after 6 months) after ileocolonic resection may help guide further therapy. Postoperative endoscopic evaluation must be rated by the Rutgeert’s scoring system (126).
The rate of loss of response to anti-TNF is approximately 30%–40% annually. Primary non-responder rate is 20%–30%. Therefore, evaluation of the response to biologic treatment is important to prevent the unnecessary use of biologic agents (127). Clinical response is generally observed during 2–4 weeks. The maximum waiting period for clinical response must be 12–16 weeks. If induction of remission is successful, maintenance therapy will continue. Clostridium difficile, cytomegalovirus, and enteric pathogens must be analyzed in refractory patients during the induction therapy (31). Biologic drug failure can occur via three mechanisms:
Mechanistic failure: absence of antidrug antibody, enough trough level (change the class)
Immune-mediated drug failure: antidrug antibody positive, low or undetectable trough concentrations (add immunomodulator or switch anti-TNF)
Non-immune-mediated drug failure: subtherapeutic drug trough concentrations and absent antidrug antibodies (increase the dose).
Therapeutic target trough levels for anti-TNF were accepted as follows: infliximab >7.5 μg/mL, adalimumab >5 μg/mL, and certolizumab pegol >20 μg/mL (31).
Non-inflammatory causes (abscess, infections, stricture, and cancer) must be kept in mind in primary or secondary non-responder patients (128). Proactive anti-TNF drug monitoring does not increase clinical remission or prevent the loss of response (129). Trough levels were negatively correlated with CRP levels (130). Serum drug levels should be monitored in primary non-responder patients or in patients with loss of response to anti-TNFs, if applicable. A recently published study showed that any increase in the infliximab dose must be based on symptoms. Drug levels and antibody monitoring have no additional benefits for the CS-free clinical remission in a larger proportion of patients (131). On the other hand, fecal calprotectin is informative in predicting relapse and primary non-response to anti-TNF therapy in IBD (132). The rate of the discontinuation of treatment because of adverse events is higher in the combination therapy (at least 3 drugs) (odds ratio=3.225, p<0.001). The rate of anti-TNF discontinuation because of adverse events is approximately 10%–20% (133). Exposure to different types of biological treatments is associated with specific changes in immune profiles (134). We have to evaluate all possible clinical conditions (e.g., hypoalbuminemia, smoking, primary non-responder, and non-inflammatory reasons) for non-responder patients. In addition to the routine biochemical analyses and whole blood test, LDH and protein electrophoresis must be included in the laboratory workup in every follow-up visit. Lymphoma risk is high, especially in combination therapies in very young (<18 years) or elderly patients (>65 years) (42). A careful physical examination is mandatory during the biologic therapy.
Endoscopic evaluation is helpful for the management of disease. There is no clear suggestion for timing. After 6–12 months of biologic therapy, endoscopic evaluation can be performed. If the patient is symptomatic and there is a mucosal lesion, therapy should be revised. Although the patient is asymptomatic, mucosal lesion can be seen. In this clinical condition, we can discuss for therapy change with the patient. However, we must not forget that the clinical and endoscopic remission rate is 40% for anti-TNFs and a deep remission rate is 30% (135). We have to answer many questions, if we perform routine follow-up endoscopy in asymptomatic patients during treatment with biologic treatment (cost-effectivity, informed consent of patients, and interval of endoscopy). On the other hand, we have to perform colonoscopy for colon cancer surveillance according to the guidelines (every 1 or 2 years) in UC and CD.
As a summary,
Evaluate clinical response to therapy
Clinical follow-up visit should be performed every 2–3 months (effectivity/adverse events)
In asymptomatic patients, wait and observe clinical/laboratory findings
Surgery must be advised when needed.
SECTION 7
Should we stop biologics? If yes, when and how?
Since late 1990s, evolving therapies, such as anti-TNFs, for CD and UC have been increasingly used. Whereas there is almost a consensus in which patients to start anti-TNF, when to start biologics or how to start biologics, it is not clear yet when to stop such kind of treatments or even in which patients with inflammatory diseases (IBD) we can stop these medications. Once these medications have gained popularity, concerns have been raised about their possible side effects. Most bothersome side effects include serious infections and opportunistic infections (45,136), melanoma/non-melanoma skin cancers (137), and lymphoproliferative disorders (138). In patients under combination treatments consisting of TPs and anti-TNFs, these risks are even higher (45,136).
Other reasons which give rise to thought of quitting anti-TNFs are increased healthcare costs or national regulations and some specific situations, such as pregnancy. Despite the arguments in favor of stopping anti-TNFs, there is no consensus yet on in which patients we can stop or de-escalate anti-TNFs because of loss of efficacy concerns. In an observational study from England, the authors showed relapse rates of 36% in CD and 42% in UC 1 year after the discontinuation of anti-TNF therapy (139).
In another prospective, multicenter, observational study from Czech Republic in 78 patients with IBD in off-steroid clinical and endoscopic remission, anti-TNF therapy was stopped, and patients were followed up. 53% of patients with CD and 53% of patients with UC relapsed by the end of the follow-up period with a median time to relapse of 8 months in CD and 14 months in UC, respectively (140).
Knowing that there is an increased risk of relapse after the discontinuation of anti-TNFs, there is an absolute need to clarify the determinants of the risk of relapse. A recent review evaluating the factors associated with the risk of relapse after stopping anti-TNFs in patients with IBD revealed that younger age, smoking, longer disease duration, perianal fistulizing disease, low hemoglobin levels, high leucocyte counts, high fecal calprotectin levels, and high serum anti-TNF levels were found to be the factors associated with higher relapse rates, whereas mucosal healing appeared to be protective against relapse (141). Another review regarding factors predicting relapse after the discontinuation of anti-TNFs showed that elevated fecal calprotectin, elevated CRP and leucocyte count, absence of mucosal healing, smoking, perianal disease, young age at diagnosis, disease location (ileocolonic disease), male sex, elevated trough levels, prior anti-TNF use, and prior immunomodulatory failure might be associated with higher relapse rates (142). Another study showed that ileocolonic disease, prior anti-TNF use, and prior bowel surgery might increase the risk for relapse after the discontinuation of anti-TNF therapy (140).
The French inflammatory bowel diseases group (GETAID) performed a prospective study to investigate potential associations between demographic, clinical, and biologic factors and the time to relapse using a Cox model. Parameters included in the analysis were as follows: male sex, CS use, previous surgical resection, hemoglobin level, leucocyte count, CDEIS scores, hs-CRP levels, infliximab levels, and fecal calprotectin levels. The authors produced a model assessing the risk for relapse. According to these models, patients with <4 factors appeared to have a relapse risk of <20%, whereas patients who had >6 factors had a 100% likelihood of relapse (143).
Overall, mucosal healing appears to be one of the most important factors preventing disease flare after stopping anti-TNFs. In CD, the discontinuation of anti-TNF therapy based on clinical remission alone is associated with a relapse rate of 42% after 1 year of follow-up, whereas this rate is reduced to 26% when such decision is based on both clinical remission and endoscopic remission (144). Similar differences were also observed among patients with UC. Relapse rates were 50% and 33%, respectively, after discontinuation based on clinical remission or endoscopic remission alone in patients with UC (144).
A European expert panel discussing when to stop biologic treatment in CD safely came to a conclusion as withdrawal of anti-TNF monotherapy should be considered after 2 years in case of both clinical remission and mucosal remission are achieved or after 4 years of clinical remission. The same panel reached to a conclusion for patients treated with combination treatment including anti-TNFs and immunomodulatory drugs that the withdrawal of anti-TNF therapy should be considered after 2 years of clinical remission (145).
In a recent review, the authors proposed a risk-based de-escalation algorithm (146). According to this algorithm, if the patient is receiving steroids, immunomodulatory, and anti-TNF agents, we should stop CSs. If a low-risk patient is receiving immunomodulatory and anti-TNF agents, we should stop immunomodulatory agents, whereas in high-risk patients, we should continue the combination treatment. If a patient is receiving monotherapy, we might stop anti-TNFs in case of deep remission especially in an elderly patient. In another study (147), the author described patient profiles favoring treatment continuation and profiles favoring treatment withdrawal. According to this study, factors favoring continuation include young age with extensive or complicated disease, patients with complex perianal CD, patients with extensive UC, patients with previous surgeries, persisting endoscopic lesions, or elevated CRP and/or fecal calprotectin in patients with CD. Factors favoring treatment withdrawal are older age without previous surgeries, old age with limited extent of UC, side effects related to anti-TNFs, absence of residual trough level, and poor adherence to medication.
A brief summary of risk factors are summarized in Table 7.
Table 7.
Risk factors for relapsing disease after stopping medical treatment.
| Favoring high risk for relapse | Favoring low risk for relapse | |
|---|---|---|
| Age | Young | Elder |
| Previous surgery | Yes | No |
| Prior anti-TNF use | Yes | No |
| Complex perianal disease | Yes | No |
| Extensive ulcerative colitis | Yes | No |
| Anti-TNF naïve | No | Yes |
| Elevated fecal calprotectin | Yes | No |
| High anti-TNF trough levels | Yes | No |
| Endoscopic remission | No | Yes |
| Male sex | Yes | No |
In summary,
Following the discontinuation of anti-TNF agents in patients in clinical remission, approximately half of the patients will relapse.
Before deciding when to stop anti-TNF, it is recommended to classify patients with IBD into low risk for relapse and high risk for relapse groups.
Clinical factors regarding high risk for relapse are young age, previous surgeries, ileocolonic involvement in CD and extensive colitis in UC, and complex perianal fistulas in CD.
Combining endoscopic assessment, fecal calprotectin and anti-TNF trough levels with clinical factors listed above will help to determine low- or high-risk patients more precisely.
SECTION 8
New upcoming treatments in IBD
Anti-TNF agents are the most widely used biologic agents in the treatment of IBD. These agents have been shown to be steroid-sparing, to reduce IBD-related hospitalizations and surgeries, induce mucosal healing, and improve patients’ quality of life. However, these treatments are not effective in all patients, and some patients may not respond to the treatment (40%) or some patients who respond to this treatment can lose their response over time (10%–45%) (104). These agents are well-tolerated, but their use can be associated with some adverse effects, such as a risk for infections especially tuberculosis and a risk for malignancy, such as non-Hodgkin lymphoma and non-melanoma skin cancers (148).
Several novel agents targeting different inflammatory pathways in IBD are available or under investigation. Two new classes of biologic drugs, integrin-antagonists for both UC and CD, and an interleukin (IL)-12/23-antagonist ustekinumab for CD are now available. The first Janus kinase (JAK) inhibitor, tofacitinib, for UC will enter the market in the near future. In the present study, ustekinumab, anti-IL23 agents, JAK inhibitors, and sphingosine-1-phosphate receptor modulators will be discussed in detail.
Anti-IL12/IL23 agents
Ustekinumab
Ustekinumab (Stelara©, Janssen) is a monoclonal IgG1 antibody targeting the p40 subunit of IL-12 and IL-23 which leads to a downregulation of cytokine expression in both Th1 and Th17 pathways (149–151) (Figure 2). It was recently approved for the treatment of moderately to severely active CD on the basis of the demonstrated efficacy in induction (UNITI-1 and UNITI-2) and maintenance (IM-UNITI) studies in both TNF antagonist naive patients and patients who fail to respond to a TNF antagonist (152,153).
Figure 2.
Molecules targeting IL-12/IL-23 axis in IBD (148).
Patients in the 8-week UNITI-1 and -2 induction trials were randomized at week 0 to receive a single iv infusion of ustekinumab at a dose of 130 mg, a single iv infusion of ustekinumab at a dose of ~6 mg/kg body weight or placebo (154).
In the UNITI-1 study (n=741), ustekinumab induced clinical remission in 18.5% of patients who failed to respond or who were intolerant to anti-TNF at week 6 (vs. 8.9% of patients on placebo, p=0.002) and in 20.9% of these patients at week 8 (vs. 7.3% of patients on placebo, p<0.001). In the UNITI-2 study (n=638), ustekinumab led to a higher rate of clinical remission at week 6 (34.9% vs. 17.7% in the placebo arm, p<0.001) and week 8 (40.2% vs. 19.6% in the placebo arm, p<0.001) in anti-TNF naive patients. In the pooled UNITI-1 and UNITI-2 population (n=155), ustekinumab induced a greater reduction in SES-CD than placebo (n=97) from baseline to week 8 (−2.8 vs. −0.7, p=0.012), thus showing the effect of ustekinumab on mucosal healing (154).
Patients who completed the UNITI-1 or -2 studies could progress to the IM-UNITI maintenance trial at week 8. The IM-UNITI study showed that two-thirds of patients on ustekinumab had sustained clinical remission at week 44 (vs. 45.6% of those on placebo, p=0.007) (155). Ustekinumab was generally well-tolerated as either induction or maintenance therapy; serious infections and malignancies were rare (154,155).
Anti-IL23 agents
Increased knowledge on the role of IL-23 has allowed for the development of effective therapeutic progresses by blocking the IL-23 mediated pathways (156). These more selective anti-IL23 p19 agents are:
Brazikumab (MEDI2070, formerly AMG139, Allergan)
Risankizumab (BI655066, ABBV066, AbbVie)
Guselkumab (CNTO1959, Janssen)
Tildrakizumab (MK3222, Merck&Co.)
Two multicenter, randomized, placebo-controlled phase II clinical trials have evaluated risankizumab and brazikumab (157). The humanized IgG1 monoclonal antibody risankizumab was tested in a randomized, double-blind, placebo-controlled phase II study (n=121) in moderately-to-severely active CD. Patients were randomized equally to receive iv 200 mg of risankizumab, 600 mg of risankizumab, or placebo at weeks 0, 4, and 8. The 600 mg risankizumab dose achieved significantly higher clinical (37% vs. 15.0%, p=0.0252) and endoscopic remission (20% vs. 3.0%, p=0.0107) rates at week 12 (158).
Another humanized monoclonal antibody brazikumab was evaluated in a double-blind, placebo-controlled phase II trial (n=119) in patients with moderate to severe CD who had failed to respond to a TNF antagonist. Patients were randomly assigned to receive either brazikumab (700 mg) or placebo iv at weeks 0 and 4. A clinical response was achieved in 49.2% of patients who received brazikumab at week 8 compared with 26.7% of those assigned to placebo (p=0.010) (159). Confirmatory phase 3 studies are underway.
Mirikizumab is currently being studied in two phase II trials including patients with CD. Two other IL-23 p19 antibodies, tildrakizumab and guselkumab, are also likely to be studied for IBD in the future (153).
JAK inhibitors
JAKs are a family of intracellular protein tyrosine kinases: JAK1, JAK2, JAK3, and tyrosine kinase 2. These are crucial to the downstream regulation of inflammatory mediators. Transcription factor STATs (signal transducer and activation of transcription) are activated by binding to transmembrane receptors. JAK inhibition results in suppression of B and T cells but retains regulatory T cell function, therefore, is an important target in IBD (160).
Tofacitinib
Tofacitinib (Xeljanz, Pfizer) is an orally administered small molecule that predominantly inhibits JAK1 and JAK3 (161) (Figure 3). Recent phase III data showed a significant treatment effect in three clinical trials in UC (162).
Figure 3.
The JAK-STAT signaling pathway. JAK inhibitors are new therapeutic agents which are currently being investigated in clinical trials in IBD. First-generation JAK inhibitors (e.g., tofacitinib) target multiple JAKs, whereas second-generation JAK inhibitors (e.g., filgotinib) selectively target one JAK (161).
In the induction trials (OCTAVE 1 and OCTAVE 2), 598 and 541 patients, respectively, were randomly assigned to receive induction therapy with tofacitinib (10 mg twice daily) or placebo for 8 weeks. Clinical remission occurred in 18.5% of the patients in the 10 mg tofacitinib at week 8 group versus 8.2% in the placebo group (p=0.007) in the OCTAVE 1 trial and in 16.6% versus 3.6% (p<0.001) in the OCTAVE 2 trial. Patients who completed induction trials with a clinical response were participated in the OCTAVE SUSTAIN trial. In this trial, remission at week 52 occurred in 34.3% (5 mg) and 40.6% (10 mg) versus 11.1% in the placebo group (p<0.001) (153,160,162,163).
Filgotinib
Filgotinib (Galapagos; GLPG0634, GS-6034) which is a JAK1-selective inhibitor was investigated in a phase II study in CD showing positive results at week 10. 47% of patients treated with 200 mg filgotinib daily achieved clinical remission (vs. 23% treated with placebo, p=0.0077) (163–165). Further trials in fistulizing CD and small bowel disease, as well as phase III trials in UC and CD, are underway (153–163).
Upadacitinib
Preliminary results of a phase II study in CD with JAK1-selective inhibitor upadacitinib (ABT-494, AbbVie) showed higher rates of endoscopic remission (with 24 mg at week 16, 22 vs. 0% in the placebo group, p=0.01) and clinical response (with 6 mg and 24 mg twice daily, 57% and 61% respectively, vs. 32% with placebo, p=0.05) than placebo (166). The drug is being evaluated for UC and in an upcoming phase III trial for CD (153,163).
Sphingosine-1-phosphate receptor modulators
Naive T cells routinely circulate between the blood and lymphatic tissue. They enter the lymph nodes through high endothelial venules and egress through the efferent lymphatic vessel into the bloodstream. The sphingosine-1-phosphate (S1P) receptor family consists of five receptors (S1P1–S1P5). The egress process is governed by interactions between S1P1 receptors on lymphocytes and their ligand S1P which is expressed on the cell surface of lymphatic endothelial cells. Upon stimulation, the cell surface-associated S1P receptors become degraded, leading to sequestration of B and T lymphocytes in the peripheral lymphoid organs and reduced trafficking of these cells to inflamed tissue (149).
Ozanimod
Ozanimod (RPC1063, Celgene), oral S1P receptor 1 and 5 agonists, demonstrated efficacy in a phase II clinical trial for the treatment of UC (167). 197 patients were randomly assigned to receive either placebo or 0.5 or 1 mg of oral ozanimod daily. The 1 mg dose group showed higher rate of clinical remission than the placebo group (16 vs. 6%, p=0.048 at week 8 and 21 vs. 6%, p=0.01 at week 32). Ozanimod is currently being tested in a phase III trial in UC and a phase II trial in CD.
Another selective S1P modulator, etrasimod (APD334), is being evaluated in a placebo-controlled phase II trial in UC (153,168).
SECTION 9
Use of conventional and biological agents in the management of IBD in special conditions including pregnancy, lactation, and malignancy
Pregnancy, breastfeeding, and a history of malignancy are usually among the exclusion criteria in phase studies of drugs and important clinical studies. Pregnant patients usually receive additional drugs for the treatment and control of their disease, other than those which are under investigation for fetal toxicity. Occasionally, IBD or rheumatic diseases themselves may harm the unborn child.
In a similar way, the incidence of certain cancer types is increased during the course of IBD and rheumatic diseases. As a result, investigating a drug in pregnant or lactating patients or in patients with a history of malignancy means additional investigation of many complex confounding factors and, consequently, a slow accumulation of information.
Use of conventional therapy during pregnancy and lactation
Methotrexate
Fetal malformations have been observed in patients who got pregnant during treatment with MTX (167). MTX should be discontinued immediately in patients who got pregnant during treatment with MTX, and folic acid supplementation should be started. Pregnant patients exposed to MTX should be followed up by experienced physicians at an obstetrics clinic. Washout period for MTX should be 3 months in women who are planning to conceive. MTX should be switched to pregnancy-safe alternatives 3 months before conception, and folic acid supplementation should be started (170).
MTX is excreted into human milk in small amounts; however, available information about its use during lactation is limited (171). Lactating mothers should not use MTX.
Paternal low-dose MTX appears to be safe (172). However, potential adverse effects associated with weekly doses >20 mg on sperm quality should be kept in mind.
AZA, 6-MP
A number of studies have investigated the use of TPs in pregnancy, and this group of drugs has been found to be safe during pregnancy and lactation, in general (173–175). The use of AZA during pregnancy has been linked to low birth weight in certain studies; however, a meta-analysis suggested that low birth weight might be associated with disease activity rather than exposure to AZA during pregnancy (176). It is not known whether the incidence of any diseases is increased in the adulthood or in the old age in children born to women exposed to AZA during pregnancy. In conclusion, considering risk/benefit balance, low-dose AZA (<2 mg/kg) may be used during pregnancy. Measurements of enzyme activity are rather important in these patients.
AZA and its metabolites are excreted into human milk in small amounts, and AZA metabolites were not found in serum samples of infants breastfed by mothers who were taking AZA (177,178). Therefore, the use of AZA in lactating mothers is considered safe; however, long-term outcomes are not known. In general, cessation of lactation is not recommended in lactating mothers who are on AZA (171,179). Paternal exposure to low-dose AZA is safe (180).
Corticosteroids
Steroids can cross the placenta; however, fetal exposure is very low as maternal steroids are metabolized in the placenta (180). Although steroids do not cause major congenital defects, certain studies have demonstrated a potential increase in the prevalence of cleft lip and palate, in line with animal studies (181). On the other hand, some studies failed to demonstrate any associations between CSs and these abnormalities (182,183). In conclusion, prednisolone, methylprednisolone, and oral budesonide may be used in the management of IBD at any stages of pregnancy, and any increase in the risk for major fetal malformations is not expected (171,180,184). Study results on whether there is an increased risk for cleft lip and palate are conflicting, but mostly favor no association. Steroids used in the management of IBD are safe for paternal use (185).
5-ASA and SASP
Although an association was found between the use of 5-ASA and premature births, in certain studies, disease activity is a major confounder in these studies (186). A meta-analysis failed to show any association between the use of 5-ASA and the risk for fetal malformation, premature birth, or abortions (187). Concerns have been raised about the potential negative impact on fetal neurological development caused by dibutyl phthalate (DBP), a substance which provides 5-ASA tablets with delayed release characteristic (188). 5-ASA formulations which contain this substance should be avoided during pregnancy. 5-ASA formulations available in our country do not contain DBP. 5-ASA can be used at any stages of pregnancy.
SASP can also be used during pregnancy (189). Sulfapyridine, the moiety of the product responsible for folic acid deficiency, is a sulfonamide antibiotic. Therefore, folic acid supplementation is required during pregnancy, particularly in the first trimester (189).
SASP and 5-ASA are safe in lactating mothers (190–192). They should be kept in mind as a rare cause of diarrhea in newborn.
Paternal use of SASP has been linked to oligospermia (193). Folic acid supplementation should be started, and SASP should be discontinued for 3 months in male patients who fail to conceive (180).
Thalidomide
Thalidomide is contraindicated during pregnancy and lactation. The use of condoms is recommended as the excretion of thalidomide into the semen has been established during paternal use (194).
Cyclosporine
Although cyclosporine is mainly used in rheumatic diseases and transplantation clinics, cyclosporine therapy may also be required in patients with UC. The consensus is that it may be used at the lowest effective dose, at any stages of pregnancy (195). Pregnant patients on cyclosporine should be closely monitored with respect to well-known side effects including diabetes, nephrotoxicity, hypertension, or electrolyte imbalances. Although paternal cyclosporine use has been considered safe, studies on paternal use are insufficient in number and sample size (179).
Use of biologics during pregnancy
TNF-α plays an important role in the implantation and development of the placenta (196). Therefore, hypothetically, TNF blockage may be associated with an increased risk for in-utero developmental abnormalities and abortions. However, an increased prevalence of developmental abnormalities or abortions has not been observed in pregnant patients who had been accidentally exposed to anti-TNF agents (173,180,197).
TNF inhibitors with immunoglobulin structure begin to cross the placenta increasingly by the end of the second trimester. Detectable levels of these medications in the blood of infants until 6 or 7 months after the delivery have raised questions about the administration of live vaccines and developmental disorders of the immune system (198,199). Among anti-TNF agents, only certolizumab pegol may be used throughout the entire pregnancy owing to its low placental transfer rate due to its structural characteristics (198). However, it is recommended to discontinue other anti-TNFs approximately 24–26 weeks of gestation (199). Some authors recommend discontinuing infliximab in week 16 of pregnancy due to hypothetically increased risk for infections in the newborn (180).
On the other hand, no consensus has been established regarding the way to follow in case of active disease or high risk of disease activation. Any IBD activation which may occur following the discontinuation of anti-TNF therapy is a major risk factor for both mother and baby (200). Furthermore, no association has been demonstrated between the use of anti-TNF agents throughout the entire pregnancy and increased prevalence of malformations, and postpartum long-term safety of these agents has been established (201,202). Therefore, we believe that anti-TNF agents should not be discontinued in the last trimester of the pregnancy in patients at high risk of disease activation and in those who exhibit a partial response to anti-TNF therapy, while necessary measures should be taken after the delivery. Clinicians should also be careful about infectious side effects under anti-TNF treatment in pregnancy (202).
If anti-TNF agents are considered in the management of CD, patients who are planning to conceive may be started on certolizumab due to its better safety profile. In the event that another anti-TNF agent (infliximab or adalimumab) is used and cannot be discontinued in the third trimester due to active disease or high activation risk, then live vaccines should be deferred >7 months of age in infants born to these mothers. Other vaccines can be timely administered, and normal immune responses have been obtained to these vaccines (203,204).
There are limited data on the use of either ustekinumab or VDZ during pregnancy. Fetal toxicity has not been observed in animal studies. Both medications are allocated to category B by the FDA. If necessary, these medications can be used after the assessment of the benefit/risk balance (205,206). Long-term prediction for these medications is that they will be used in a similar way to anti-TNFs.
The use of biologics during lactation
Anti-TNFs may be used during lactation. All biologics are excreted into human milk in very small amounts (207,208). In addition, biologics may be partially degraded in the stomach due to their protein structure. An increased risk for infections has not been observed in infants breastfed by mothers exposed to anti-TNF (197,201,202). Cessation of lactation is not recommended in patients who are on/are started on an anti-TNF agent; however, current information and recommendations should be followed. There are reports indicating that VDZ is excreted into human milk in small amounts; however, detailed data on the effects of the amount excreted into milk are not available yet (208). Further data are needed on the safety of ustekinumab and VDZ during lactation.
Anti-TNF agents should be discontinued by the end of the second trimester in patients who are in remission and at low risk for disease activation.
Certolizumab pegol crosses the placenta in negligible amounts due to its distinct structure and may be used at any stages of pregnancy.
Certolizumab may be preferred in patients who are planning to conceive and who will be treated with an anti-TNF agent.
Live vaccines should be deferred >1 year of age in infants born to mothers exposed to an anti-TNF agent other than certolizumab throughout the entire pregnancy due to disease activation or a high risk for activation.
Biologics are excreted into human milk in very small amounts.
Cessation of lactation is not recommended in mothers who are on/are started on an anti-TNF agent; however, current information and recommendations on such use should be followed.
Summary of recommendations regarding the use of medications during pregnancy and lactation is given in Table 8.
Table 8.
Medication use in the management of IBD during pregnancy and lactation.
| Medication | Use during the first trimester | Use during the 2nd and 3rd trimesters | Pregnancy category | Use during lactation | Recommendations |
|---|---|---|---|---|---|
| Thiopurines | Allowed | Allowed | D | Allowed | They can be used in low-risk patients with or without dose reduction according to the benefit/risk balance |
| MTX | Contraindicated | Contraindicated | X | Not recommended | MTX should be discontinued, and folic acid supplementation should be started 3 months before conception. MTX should be immediately discontinued if pregnancy occurs during treatment with MTX, close monitoring and folic acid supplementation are required during pregnancy |
| Cyclosporine | Allowed | Allowed | C | Allowed | The lowest effective dose should be given |
| Glucocorticoids | Allowed | Allowed | C | Allowed | It can be used at any stages of pregnancy. There are controversial data on an increased prevalence of clef lift an palate |
| 5-ASA, sulfasalazine | Allowed | Allowed | B | Allowed | Formulations containing dibutyl phthalate are available abroad. These formulations are not recommended during pregnancy |
| Thalidomide | Contraindicated | Contraindicated | X | Contraindicated | Major teratogenicity, use of condoms is recommended due to paternal teratogenicity |
| Anti-TNF | Allowed | Allowed* | B | Allowed | *TNFs other than certolizumab should be discontinued approximately 24 weeks of gestation. Treatment may be continued in patients at high risk of disease activation. In this case, live vaccines should be deferred beyond 7 months of age in infants born to these mothers |
| Vedolizumab | Limited data | Limited data | B | Limited Data | Vedolizumab can be used with caution if necessary |
| Ustekinumab | Limited data | Limited data | B | Limited Data | Ustekinumab can be used with caution if necessary |
| Metronidazole | Allowed** | Allowed | B | Not recommended | **The use of metronidazole >1 week is not recommended throughout the pregnancy and particularly during the first trimester. The excretion of metronidazole into human milk is proportional to plasma concentrations. Temporary cessation of breastfeeding is recommended during treatment with metronidazole. |
| Ciprofloxacin | Contraindicated | Not recommended | C | Not recommended | Minor musculoskeletal malformations observed in animal studies have not been observed in studies in women exposed to ciprofloxacin during pregnancy. Ciprofloxacin is contraindicated during pregnancy as safer alternatives are available. |
Use of conventional therapy and biologics in patients with malignancies
Long-term follow-up of transplant patients has demonstrated that immunosuppressants might contribute to the development of malignancies and the risk for malignancies might increase with the duration and combination of immunosuppression (209). The associations between TPs and non-melanoma skin cancers and lymphoma have been established (210,211). However, a number of clinical studies with anti-TNFs have failed to demonstrate an increased risk for cancers. Although MTX have not been investigated as extensively as these medications, the increase in the risk for cancers is not as high as with TPs, and this is the reason why MTX has been the treatment of choice in the management of IBD during the last 4–5 years for patients under the age of 30 years and over the age of 65 years (211).
After the introduction of anti-TNF agents in daily practice, the important role of TNF in inflammation and its ability to kill a number of cancer cells in in vitro and animal studies have raised questions about the potential ability of anti-TNF agents to trigger the development of malignancies (212,213). Rare malignancies in patients exposed to these treatments and even tumor regression following the discontinuation of anti-TNFs have been reported in the literature (214–216).
On the other hand, real-world data have not suggested the expected response to treatment with TNF in a variety of cancer types (217). On the contrary, certain studies suggested that TNF might promote tumor growth in patients with breast, lung, pancreas, or kidney cancer (217–220). In certain clinical and laboratory studies, even conventional or targeted chemotherapy was used in combination with an anti-TNF agent to optimize anti-tumor activity (221–222). Therefore, it should be known that TNF may exhibit both pro-neoplastic and anti-neoplastic effects.
The assessment of real-world data for anti-TNFs revealed that a slight increase occurred in the risk for the development of melanoma (210). Data on the development of lymphoma are conflicting (210–211). A recently published study with a large sample size have demonstrated that monotherapy with anti-TNFs might slightly increase the risk for the development of lymphoma. The risk for the development of lymphoma has been found to be higher with combination therapy with an anti-TNF agent and TP than with treatment with TP or an anti-TNF alone (223). Results for non-melanoma skin cancers are conflicting.
No evidence of an increased risk for malignancy has been detected in phase studies and real-world data of VDZ and ustekinumab. Data from a large population of patients with psoriasis did not reveal any increased risk for malignancy (102). Hypothetically, VDZ may be safe regarding extraintestinal malignancies due to GIS selectivity; however, real-world data on the use of VDZ are limited.
Studies on cancer development with anti-TNFs cover a period of 15 years at most. Longer term outcomes of the exposure to biologics are not known yet. Although larger studies predict that either anti-TNF agents (224,225) or immunomodulatory agents (225) do not increase the risk for recurrence or a secondary malignancy in patients with a known malignancy, immunosuppressives should not be given, unless there is a clear-cut indication. In general, the management plan should start with the modality and drug class with the least malignancy potential (surgery, 5-ASA, SASP, and oral budesonide). The consensus on the initiation of immunosuppressive therapy in patients with cancer is to wait 2 years after treatment, whereas this period should be at least 5 years in cancers which are known to recur at high rates under immunosuppressive treatment including skin, bladder, and kidney cancers; sarcoma; leukemia; or myeloma (209,210). We believe that the length of this period may be adjusted according to the risk/benefit balance based on the shared decision and approval of the patient and corresponding specialists (oncologists, hematologists, and dermatologists) in patients with active disease experiencing a significant decrease in the quality of life. Anti-TNFs, steroids, and MTX may be used without waiting, if indicated, in patients who are at risk for developing life-threatening short-term and long-term complications (toxic megacolon, short bowel syndrome, and subileus).
Furthermore, one should keep in mind that targeted therapies which have been recently introduced in oncology practice may also induce the activation of IBD or IBD-like colitis (226). This issue is likely to become a growing challenge for gastroenterologists in the future.
Rules for the use of conventional therapy and biologics in patients with malignancies are as follows:
It still remains unknown how anti-TNFs will act in a given malignancy. Anti-TNFs and other immunosuppressive agents should be discontinued in patients with newly diagnosed malignancy.
Anti-TNF agents should be avoided in patients with known melanoma/history of melanoma.
Case-based assessment is required in the management of active IBD.
Hypothetically, VDZ may be safe in patients with extraintestinal malignancy; however, real-world data are limited.
Malignancy potential of ustekinumab has not been observed in limited series; however, there are limited data from patients with a history of malignancy.
Surgery may be the treatment of choice in patients with short segment involvement.
When colitis is active, biologics may be used in patients with terminal-stage malignancy, as the primary objective is to improve the quality of life and pain relief.
Footnotes
Peer-review: Externally peer-reviewed.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study was supported by Takeda Turkey with an unconditional educational grant.
REFERENCES
- 1.Marshall JK, Irvine EJ. Rectal corticosteroids versus alternative treatments in ulcerative colitis: A meta-analysis. Gut. 1997;40:775–81. doi: 10.1136/gut.40.6.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mulder CJ, Fockens P, Meijer JW, von der Heide H, Wiltink EH, Tytgat GN. Beclomethasone dipropionate (3mg) versus 5-aminosalicylic acid (2g) versus the combination of both (3mg/2g) as retention enemas in active ulcerative proctitis. Eur J Gastroenterol Hepatol. 1996;8:549–53. doi: 10.1097/00042737-199606000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Gionchetti P, Rizello F, Venturi A, et al. Comparison of mesalazine suppositories in proctitis and distal proctosigmoiditis. Aliment Pharmacol Ther. 1997;11:1053–7. doi: 10.1046/j.1365-2036.1997.00259.x. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe M, Nishino H, Sameshima Y, Ota A, Nakamura S, Hibi T. Randomised clinical trial: evaluation of the eficacy of mesalasine (mesalamine) suppositories in patients with ulcerative colitis and active rectal inflammation- a placebo-controlled study. Aliment Pharmacol Ther. 2013;38:264–73. doi: 10.1111/apt.12362. [DOI] [PubMed] [Google Scholar]
- 5.Gionchetti P, Rizello F, Venturi A, et al. Comparison of oral versus rectal mesalazine in the treatment of ulcerative proctitis. Dis Colon Rectum. 1998;41:93–7. doi: 10.1007/BF02236902. [DOI] [PubMed] [Google Scholar]
- 6.Sandborn WJ, Kamm MA, Lichtenstein GR, Lyne A, Butler T, Joseph RE. MMX Multi Matrix System mesalazine for the induction remission in the patients withmild-to-moderate ulcerative colitis: A combine analysis of two randomised, double-blind, placebo-controlled trials. Aliment Pharmacol Ther. 2007;26:205–15. doi: 10.1111/j.1365-2036.2007.03361.x. [DOI] [PubMed] [Google Scholar]
- 7.Ford AC, Khan KJ, Achkar JP, Moayyedi P. Efficacy of oral versus topical or combined oral and topical 5-aminosalicylates, in ulcerative colitis: Systemic review and meta-analysis. Am J Gastroenterol. 2012;107:167–76. doi: 10.1038/ajg.2011.410. [DOI] [PubMed] [Google Scholar]
- 8.Propert CS, Dignass Au, Lindgren S, Oudkerk Pool M, Marteau P. Combined oral and rectal mesalazine for the treatment of mild to moderately active ulcerative colitis: Rapid symptom resolution and improvements in quality of life. J Crohns Colitis. 2014;8:200–7. doi: 10.1016/j.crohns.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 9.Khan N, Abbas AM, Bazzano LA, Koleva YN, Krousel-Wood M. Long-term oral mesalazine adherence and the risk of disease flare in ulcerative colitis: Nationwide 10 year retrospective cohort from the veterans affairs healthcare system. Aliment Pharmacol Ther. 2012;36:755–64. doi: 10.1111/apt.12013. [DOI] [PubMed] [Google Scholar]
- 10.Travis SP, Danese S, Kupcinskas L, et al. Once-daily budesonide MMX in active, mild to moderate ulcerative colitis: results from the randomised CORE II study. Gut. 2014;63:433–41. doi: 10.1136/gutjnl-2012-304258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danese S, Siegel CA, Peyrin-Biroulet L. Review article: Integrrating budesonide-MMX into treatment algorithms for mild to moderate ulcerative colitis. Aliment Pharmacol Ther. 2014;39:1095–103. doi: 10.1111/apt.12712. [DOI] [PubMed] [Google Scholar]
- 12.Ford Ac, Berstein CN, Khan KJ, et al. Glucocorticosteroid therapy in ınflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. 2011;106:590–9. doi: 10.1038/ajg.2011.70. [DOI] [PubMed] [Google Scholar]
- 13.Turner D, Walsh CM, Steinhart Ah, et al. Respons to corticosteroids in sever ulcerative colitis: a systematic review of the literatüre and the meta-regression. Clin Gastroenterol Hepatol. 2007;5:103–10. doi: 10.1016/j.cgh.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 14.Burger D, Travis S. Conventional medical management of ınflammatory bowel disease. Gastroenterology. 2011;140:1827–37. doi: 10.1053/j.gastro.2011.02.045. [DOI] [PubMed] [Google Scholar]
- 15.Dignass A, Lindsay JO, Sturm A, et al. Second evidence-based consensus on the diagnosis and management of ulcerative colitis part 2: Current management. J Crohns Colitis. 2012;6:991–1030. doi: 10.1016/j.crohns.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Feagen BG, Macdonald JK. Oral 5-aminosalicylic acid for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2012;10:Cd000544. doi: 10.1002/14651858.CD000544.pub3. [DOI] [PubMed] [Google Scholar]
- 17.Ford AC, Achkar JP, Khan KJ, et al. Efficacy of 5-aminosalisylates in ulcerative colitis: Systemic review and meta-analysis. Am J Gastroenterol. 2011;106:601–16. doi: 10.1038/ajg.2011.67. [DOI] [PubMed] [Google Scholar]
- 18.Marshall JK, Thabane M, Steinhart AH, Newman JR, Anand A, Irvine EJ. Rectal 5-aminosalicylic acid maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2012;11:CD004118. doi: 10.1002/14651858.CD004118.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moody GA, Eaden JA, Helyes J, Mayberry JF. Oral or rectal administration of drugs in IBD? Aliment Pharmacol Ther. 1997;11:999–1000. [PubMed] [Google Scholar]
- 20.Harbord M, Eliakim R, Bettenworth D, et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 2: Current Management. J Crohns Colitis. 2017;11:769–84. doi: 10.1093/ecco-jcc/jjx009. [DOI] [PubMed] [Google Scholar]
- 21.Gisbert JP, Linares PM, McNicholl Ag, Mate J, Gomollon F. Meta-analysis: The efficacy of azathioprine and mercaptopurine in ulcerative colitis. Aliment Pharmacol Ther. 2009;30:126–37. doi: 10.1111/j.1365-2036.2009.04023.x. [DOI] [PubMed] [Google Scholar]
- 22.Ardizzone S, Maconi G, Russo A, et al. Randomised controlled trial of azathioprine and 5-ASA for treatment of steroid dependent ulcerative colitis. Gut. 2006;55:47–53. doi: 10.1136/gut.2005.068809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chebli CA, Chaves LDdM, Primental FF, et al. Azathioprine maintains long-term steroid-free remission through 3 years in patients with steroid-dependent ulcerative colitis. Inflamm Bowel Dis. 2010;16:613–9. doi: 10.1002/ibd.21083. [DOI] [PubMed] [Google Scholar]
- 24.Fraser AG, Orchard TR, Jewell DP. The efficacy of azatioprine for the treatment of ınflammatory bowel disease: A 30 year review. Gut. 2002;50:485–9. doi: 10.1136/gut.50.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morena-Rncon E, Benitz JM, Servano-Ruiz FJ, et al. Prognosis of patients with ulcerative colitis in sustained remission after thiopurines withdrawal. Inflamm Bowel Dis. 2015;21:1564–71. doi: 10.1097/MIB.0000000000000400. [DOI] [PubMed] [Google Scholar]
- 26.Ford A, Kane S, Khan K, et al. Efficacy of 5-aminosalicylates in Crohn’s disease: systematic review and meta-analysis. Am J Gastroenterol. 2011;106:617–29. doi: 10.1038/ajg.2011.71. [DOI] [PubMed] [Google Scholar]
- 27.Hanauer SB, Stromberg U. Oral pentasa in the treatment of active Crohn’s disease: A meta- analysis of double-blind, placebo-controlled trials. Clin Gastroenterol Hepatol. 2004;2:379–88. doi: 10.1016/S1542-3565(04)00122-3. [DOI] [PubMed] [Google Scholar]
- 28.Dignass A, van Assche G, Lindsay JO, et al. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: current management. J Crohns Colitis. 2010;4:28–62. doi: 10.1016/j.crohns.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Tremaine WJ, Schroeder KW, Harrison JM, et al. A randomized, double-blind, placebo-controlled trial of the oral mesalamine (5-ASA) preparation, Asacol, in the treatment of symptomatic Crohn’s colitis and ileocolitis. J Clin Gastroenterol. 1994;19:278–82. doi: 10.1097/00004836-199412000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Singleton JW, Hanauer SB, Gitnick GL, et al. Mesalamine capsules for the treatment of active Crohn’s disease: results of a 16-week trial. Pentasa Crohn’s Disease Study Group. Gastroenterology. 1993;104:1293–301. doi: 10.1016/0016-5085(93)90337-C. [DOI] [PubMed] [Google Scholar]
- 31.Lichtenstein GR, Loftus EV, Isaacs KL, et al. ACG Clinical Guideline: Management of Crohn’s Disease in Adults. Am J Gastroenterol. 2018;113:481–517. doi: 10.1038/ajg.2018.27. [DOI] [PubMed] [Google Scholar]
- 32.Ransford RA, Langman MJ. Sulphasalazine and mesalazine: Serious adverse reactions re-evaluated on the basis of suspected adverse reaction reports to the Committee on Safety of Medicines. Gut. 2002;51:536–9. doi: 10.1136/gut.51.4.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loftus EV, Jr, Kane SV, Bjorkman D. Systematic review: Short-term adverse effects of 5- aminosalicylic acid agents in the treatment of ulcerative colitis. Aliment Pharmacol Ther. 2004;19:179–89. doi: 10.1111/j.0269-2813.2004.01827.x. [DOI] [PubMed] [Google Scholar]
- 34.Van der Woude CJ, Kolacek S, Dotan I, et al. European Crohn’s Colitis Organization (ECCO). European evidence-based consensus on reproduction in inflammatory bowel disease. J Crohns Colitis. 2010;4:493–510. doi: 10.1016/j.crohns.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 35.Kalkan İH, Dağli U. Pregnancy and ulcerative colitis. Turk J Gastroenterol. 2012;23(Suppl 2):41–7. doi: 10.4318/tjg.2012.0623. [DOI] [PubMed] [Google Scholar]
- 36.Greenberg GR, Feagan BG, Martin F, et al. Oral budesonide for active Crohn’s disease. Canadian Inflammatory Bowel Disease Study Group. N Engl J Med. 1994;331:836–41. doi: 10.1056/NEJM199409293311303. [DOI] [PubMed] [Google Scholar]
- 37.Tremaine WJ, Hanauer SB, Katz S, et al. Budesonide CIR capsules (once or twice daily divided-dose) in active Crohn’s disease: A randomized placebocontrolled study in the United States. Am J Gastroenterol. 2002;97:1748–54. doi: 10.1111/j.1572-0241.2002.05835.x. [DOI] [PubMed] [Google Scholar]
- 38.Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infections and mortality in association with therapies for Crohn’s disease: TREAT registry. Clin Gastroenterol Hepatol. 2006;4:621–30. doi: 10.1016/j.cgh.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 39.Thomas CW, Myhre GM, Tschumper R, et al. Selective inhibition of inflammatory gene expression in activated T lymphocytes: a mechanism of immune suppression by thiopurines. J Pharmacol Exp Ther. 2005;312:537–45. doi: 10.1124/jpet.104.074815. [DOI] [PubMed] [Google Scholar]
- 40.Mantzaris GJ. Thiopurines and Methotrexate Use in IBD Patients in a Biologic Era. Curr Treat Options Gastro. 2017;15:84–104. doi: 10.1007/s11938-017-0128-0. [DOI] [PubMed] [Google Scholar]
- 41.Doherty G, Bennett G, Patil S, et al. Interventions for prevention of postoperative recurrence of Crohn’s disease. Cochrane Database Syst Rev. 2009;4:Cd006873. doi: 10.1002/14651858.CD006873.pub2. [DOI] [PubMed] [Google Scholar]
- 42.Gomollón F, Dignass A, Annese V, et al. 3rd EUROPEAN Evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: Part 1: Diagnosis and medical management. J Crohns Colitis. 2017;11:3–25. doi: 10.1093/ecco-jcc/jjw168. [DOI] [PubMed] [Google Scholar]
- 43.Feagan BG, Rochon J, Fedorak RN, et al. Methotrexate for the treatment of Crohn’s disease. The North American Crohn’s Study Group Investigators. N Engl J Med. 1995;332:292–7. doi: 10.1056/NEJM199502023320503. [DOI] [PubMed] [Google Scholar]
- 44.McDonald JW, Wang Y, Tsoulis DJ, et al. Methotrexate for induction of remission in refractory Crohn’s disease. Cochrane Database Syst Rev. 2014;8:CD003459. doi: 10.1002/14651858.CD003459.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Toruner M, Loftus EV, Harmsen WS, et al. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology. 2008;134:929–36. doi: 10.1053/j.gastro.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 46.Keane J, Gershon S, Wise RP, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001;345:1098–104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 47.Rahier JF, Ben-Horin S, Chowers Y, et al. European Crohn’s and Colitis Organisation (ECCO). European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2009;3:47–91. doi: 10.1016/j.crohns.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 48.Altunöz ME, Senates E, Yesil A, Calhan T, Ovünç AO. Patients with inflammatory bowel disease have a lower response rate to HBV vaccination compared to controls. Dig Dis Sci. 2012;57:1039–44. doi: 10.1007/s10620-011-1980-8. [DOI] [PubMed] [Google Scholar]
- 49.Gisbert JP, Villagrasa JR, Rodríguez-Nogueiras A, Chaparro M. 2-Efficacy of hepatitis B vaccination and revaccination and factors impacting on response in patients with inflammatory bowel disease. Am J Gastroenterol. 2012;107:1460–6. doi: 10.1038/ajg.2012.79. [DOI] [PubMed] [Google Scholar]
- 50.Carrera E, Manzano R, Garrido E. Efficacy of the vaccination in inflammatory bowel disease. World J Gastroenterol. 2013;19:1349–13. doi: 10.3748/wjg.v19.i9.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gisbert JP, Menchén L, García-Sánchez V, Marín I, Villagrasa JR, Chaparro M. Comparison of the effectiveness of two protocols for vaccination (standard and double dosage) against hepatitis B virus in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2012;35:1379–85. doi: 10.1111/j.1365-2036.2012.05110.x. [DOI] [PubMed] [Google Scholar]
- 52.Gisbert JP, Chapparo M, Esteve M. Review article: prevention and management of hepatitis B and C infection in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2011;33:619–33. doi: 10.1111/j.1365-2036.2010.04570.x. [DOI] [PubMed] [Google Scholar]
- 53.Viganò M, Degasperi E, Aghemo A, Lampertico P, Colombo M. Anti-TNF drugs in patients with hepatitis B or C virus infection: safety and clinical management. Expert Opin Biol Ther. 2011;12:193–207. doi: 10.1517/14712598.2012.646986. [DOI] [PubMed] [Google Scholar]
- 54.Walsh AJ, Weltman M, Burger D, et al. Implementing guidelines on the prevention of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2013;7:449–56. doi: 10.1016/j.crohns.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 55.Singh S, Nagpal SJ, Murad MH, et al. Inflammatory Bowel Disease Is Associated with an Increased Risk of Melanoma A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2014;12:210–8. doi: 10.1016/j.cgh.2013.04.033. [DOI] [PubMed] [Google Scholar]
- 56.Annese V, Beaugerie L, Egan L, et al. European Evidence-based Consensus: Inflammatory Bowel Disease and Malignancies. J Crohns and Colitis. 2015;1:945–65. doi: 10.1093/ecco-jcc/jjv141. [DOI] [PubMed] [Google Scholar]
- 57.Lakatos PL, Miheller P. Is There an Increased Risk of Lymphoma and Malignancies Under Anti-TNF Therapy in IBD? Current Drug Targets. 2010;11:179–86. doi: 10.2174/138945010790309867. [DOI] [PubMed] [Google Scholar]
- 58.Bernheim O, Colombel JF, Ullman TA, Laharie D, Beaugerie L, Itzkowitz SH. The management of immunosuppression in patients with inflammatory bowel disease and cancer. Gut. 2013;62:1523–8. doi: 10.1136/gutjnl-2013-305300. [DOI] [PubMed] [Google Scholar]
- 59.Tilg H, Kaser A. Vedolizumab, a humanized mab against the alpha4beta7 integrin for the potential treatment of ulcerative colitis and Crohn’s disease. Curr Opin Investig Drugs. 2010;11:1295–304. [PubMed] [Google Scholar]
- 60.Colombel JF, Sands BE, Rutgeerts P, et al. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut. 2017;66:839–51. doi: 10.1136/gutjnl-2015-311079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rosario M, Fox I, Milch C, et al. Pharmacokinetic/pharmacodynamic relationship and immunogenicity of vedolizumab in adults with inflammatory bowel disease: additional results from GEMINI 1 and 2. Inflamm Bowel Dis. 2013;19(Suppl 1) doi: 10.1097/01.MIB.0000438818.81129.5b. Abstract p-140. [DOI] [Google Scholar]
- 62.Mould DR, Green B. Pharmacokinetics and pharmacodynamics of monoclonal antibodies: concepts and lessons for drug development. Bio Drugs. 2010;24:23–39. doi: 10.2165/11530560-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 63.Himatsu T. The Ideal Anti-TNF Patients: Indications for Anti-TNF Therapy in Ulcerative Colitis. Front Gastrointest Res. 2015;34:178–84. doi: 10.1159/000381601. [DOI] [Google Scholar]
- 64.Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–76. doi: 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- 65.Reinisch W, Sandborn WJ, Hommes DW, et al. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: Results of a randomised controlled trial. Gut. 2011;60:780–7. doi: 10.1136/gut.2010.221127. [DOI] [PubMed] [Google Scholar]
- 66.Sandborn WJ, van Assche G, Reinisch W, et al. Adalimumab induces and maintains clinical remisshion in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2012;142:257–65.e1–3. doi: 10.1053/j.gastro.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 67.Sandborn WJ, Feagan BG, Marano C, et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014;146:85–95. doi: 10.1053/j.gastro.2013.05.048. [DOI] [PubMed] [Google Scholar]
- 68.Sandborn WJ, Feagan BG, Marano C, et al. Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014;146:96–109.e1. doi: 10.1053/j.gastro.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 69.Feagan BG, Rutgeerts P, Sands B, et al. Vedolizumab as Induction and Maintenance Therapy for Ulcerative Colitis. N Eng J Med. 2013;369:699–709. doi: 10.1056/NEJMoa1215734. [DOI] [PubMed] [Google Scholar]
- 70.Yamamoto Furusho JK. Inflammatory bowel disease therapy: blockade of cytokines and cytokine signaling pathways. Curr Opin Gastroenterol. 2018;34:187–93. doi: 10.1097/MOG.0000000000000444. [DOI] [PubMed] [Google Scholar]
- 71.Lam M, Bressler B. Biologic Therapy in Moderate-to-Severe Ulcerative Colitis: Infliximab. In: Baumgart DC, editor. Crohn’s Disease and Ulcerative Colitis. Second Edition. Ch 42. 2017. pp. 429–32. [DOI] [Google Scholar]
- 72.Ford AC, Sandborn WJ, Khan KJ, et al. Efficacy of biological therapies in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. 2011;106:644–59. doi: 10.1038/ajg.2011.73. [DOI] [PubMed] [Google Scholar]
- 73.Harbord M, Eliakim R, Bettenworth D, et al. Third European Evidence- based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 2: Current Management. J Crohns Colitis. 2017;12:769–84. doi: 10.1093/ecco-jcc/jjx009. [DOI] [PubMed] [Google Scholar]
- 74.Gisbert JP, Chaparro M. Acute severe ulcerative colitis: State of the art treatment. Best Prac Res Clin Gastroenterol. 2018;33:59–69. doi: 10.1016/j.bpg.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 75.Bessler B, Marshall JK, Bernstein CH, et al. Clinical Practice Guidelines for the Medical Management of Nonhospitalized Ulcerative Colitis: The Toronto Consensus. Gastroenterology. 2015;148:1035–58. doi: 10.1053/j.gastro.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 76.Dignass A, Eliakim R, Magro F, et al. Second European evidence-based consensus on the diagnosis and managment of ulcerative colitis Part 1: Difinitions and diagnosis. J Cronhs Colitis. 2012;6:965–90. doi: 10.1016/j.crohns.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 77.Panaccione R, Gosch S, Middleton S, et al. Combination Therapy with Infliximab and Azathioprin is Superior to Monotherapy with eiter Agent in Ulcerative Colitis. Gastroentrology. 2014;146:392–400. doi: 10.1053/j.gastro.2013.10.052. [DOI] [PubMed] [Google Scholar]
- 78.Laharie D, Bourreille A, Branche J, et al. Cyclosporin versus infliximab in patients with severe ulcerative colitis refractory to intravenous steroids: A parallel, open-label randomised controlled trial. Lancet. 2012;380:1909–15. doi: 10.1016/S0140-6736(12)61084-8. [DOI] [PubMed] [Google Scholar]
- 79.Narula N, Marshall JK, Colombel JF, et al. Systemic Review and Meta-Anaysis: Infliximab or cyclosporine as Rescue Therapy in Patients with Severe Ulcerative Colitis Refractory to Steroids. Am J Gastroenterol. 2016;111:477–91. doi: 10.1038/ajg.2016.7. [DOI] [PubMed] [Google Scholar]
- 80.Wyant T, Fedyk E, Abhyankar B. An overview of the mechanism of action of the monoclonal antibody vedolizumab. J Crohns Colitis. 2016;10:1437–44. doi: 10.1093/ecco-jcc/jjw092. [DOI] [PubMed] [Google Scholar]
- 81.Engel T, Ungar B, Yungg DE, Shomron BH, et al. Vedolizumab in IBD - Lesson From Real-world Expriwncw: A Systematic Review and Pooled Analysis. J Crohns Colitis. 2017;2:245–57. doi: 10.1093/ecco-jcc/jjx143. [DOI] [PubMed] [Google Scholar]
- 82.Sands BE, Peyrin-Biroulet L, Loftus EV, Jr, et al. Vedolizumab versus Adalimumab for Moderate to Severe Ulcerative Colitis. N Eng J Med. 2019;381:1215–26. doi: 10.1056/NEJMoa1905725. [DOI] [PubMed] [Google Scholar]
- 83.Pouillon L, Van Stappen J, Bossuyt P, et al. Should we use anti-tumor necrosis factor agents or vedolizumab as first-line biological therapy in ulcerative colitis? Best Prac Res Clin Gastroetentrol. 2018;33:17–25. doi: 10.1016/j.bpg.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 84.Lamb CA, Kennedy NA, RAine T, et al. British Society of Gastroenterology consensus guidelines on the management of Inflammatory bowel disease in adults. GUT. 2019;68(Suppl 3):1–106. doi: 10.1136/gutjnl-2019-318484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD. ACG Clinical Guideline. Ulcerative Colitis in Adults. Am J Gas-troenterol. 2019;114:384–413. doi: 10.14309/ajg.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 86.Van Dullemen H, van Deventer S, Hommes D, et al. Treatment of Crohn’s disease with anti-tumor necrosis factor chimeric monoclonal antibody (cA2) Gastroenterology. 1995;109:129–35. doi: 10.1016/0016-5085(95)90277-5. [DOI] [PubMed] [Google Scholar]
- 87.Gomollón F, Dignass A, Annese V, et al. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn’s Disease: Part 1: Diagnosis and Medical Management. J Crohns Colitis. 2016;11:3–25. doi: 10.1093/ecco-jcc/jjw168. [DOI] [PubMed] [Google Scholar]
- 88.Torres J, Caprioli F, Katsanos KH, et al. Predicting outcomes to optimize disease management in inflammatory bowel diseases. J Crohns Colitis. 2016;10:1385–94. doi: 10.1093/ecco-jcc/jjw116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Colombel J, Sandborn W, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Eng J Med. 2010;362:1383–95. doi: 10.1056/NEJMoa0904492. [DOI] [PubMed] [Google Scholar]
- 90.Colombel JF, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn’s disease [CALM]: a multicentre, randomised, controlled phase 3 trial. Lancet. 2018;390:2779–89. doi: 10.1016/S0140-6736(17)32641-7. [DOI] [PubMed] [Google Scholar]
- 91.Boyapati RK, Ho GT, Satsangi J. Top-down in the Long Term in Crohn’s Disease. J Crohns Colitis. 2018;12:513–4. doi: 10.1093/ecco-jcc/jjy027. [DOI] [PubMed] [Google Scholar]
- 92.Osterman MT, Haynes K, Delzell E, et al. Effectiveness and Safety of Immunomodulators with Anti-TNF Therapy in Crohn’s Disease. Clin Gastroenterol Hepatol. 2015;13:1293–1301.e5. doi: 10.1016/j.cgh.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gisbert JP, Marin AC, McNicholl AG, et al. Systematic review with meta-analysis: the efficacy of a second anti-TNF in patients with inflammatory bowel disease whose previous anti-TNF treatment has failed. Aliment Pharmacol Ther. 2015;41:613–23. doi: 10.1111/apt.13083. [DOI] [PubMed] [Google Scholar]
- 94.Armuzzi A, Gionchetti P, Daperno M, et al. Expert consensus paper on the use of vedolizumab for the management of patients with moderate-to-severe inflammatory bowel disease. Dig Liver Dis. 2016;48:360–70. doi: 10.1016/j.dld.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 95.Roblin X, Marotte H, Rinaudo M, et al. Association between pharmacokinetics of adalimumab and mucosal healing in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2014;12:80–4.e2. doi: 10.1016/j.cgh.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 96.Roblin Xavier, Vérot Céline, Paul Stéphane, et al. Is the Pharmacokinetic Profile of a First Anti-TNF Predictive of the Clinical Outcome and Pharmacokinetics of a Second Anti-TNF? Inflamm Bowel Dis. 2018;24:2078–85. doi: 10.1093/ibd/izy111. [DOI] [PubMed] [Google Scholar]
- 97.Frederiksen MT, Ainsworth MA, Brynskov J, et al. Antibodies against infliximab are associated with de novo development of antibodies to adalimumab and therapeutic failure in infliximab-to-adalimumab switchers with IBD. Inflamm Bowel Dis. 2014;20:1714–21. doi: 10.1097/MIB.0000000000000138. [DOI] [PubMed] [Google Scholar]
- 98.Dulai PS, Singh S, Jiang X, et al. The Real-World Effectiveness and Safety of Vedolizumab for Moderate-Severe Crohn’s Disease: Results From the US VICTORY Consortium. Am J Gastroenterol. 2016;111:1147–55. doi: 10.1038/ajg.2016.236. [DOI] [PubMed] [Google Scholar]
- 99.Kopylov Uri, Verstockt Bram, Biedermann Luc, et al. Effectiveness and Safety of Vedolizumab in Anti-TNF-Naïve Patients with Inflammatory Bowel Disease-A Multicenter Retrospective European Study. Inflamm Bowel Dis. 2018;24:2442–51. doi: 10.1093/ibd/izy155. [DOI] [PubMed] [Google Scholar]
- 100.Bohm M, Sagi SV, Fischer M, et al. Comparative effectiveness of vedolizumab and tumour necrosis factor-antagonist therapy in Crohn’s disease: a multicentre consortium propensity score-matched analysis. 2018 ECCO Congress OP25. [Google Scholar]
- 101.Wang MC, Zhang LY, Han W, et al. PRISMA- Efficacy and safety of vedolizumab for inflammatory bowel diseases: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 2014 Dec;93:e326. doi: 10.1097/MD.0000000000000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Papp K, Gottlieb AB, Naldi L, Pariser D, Ho V, Goyal K. Safety Surveillance for Ustekinumab and Other Psoriasis Treatments From the Psoriasis Longitudinal Assessment and Registry (PSOLAR) J Drugs Dermatol. 2015;14:706–14. [PubMed] [Google Scholar]
- 103.Sandborn WJ, Gasink C, Gao LL, et al. Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N Engl J Med. 2012;367:1519–28. doi: 10.1056/NEJMoa1203572. [DOI] [PubMed] [Google Scholar]
- 104.Armuzzia A, Ardizzoneb S, Bianconec L, et al. Ustekinumab in the management of Crohn’s disease: Expert opinion. Digestive and Liver Dis. 2018;50:653–60. doi: 10.1016/j.dld.2018.02.017. [DOI] [PubMed] [Google Scholar]
- 105.Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn’s disease. N Engl J Med. 1999;340:1398–405. doi: 10.1056/NEJM199905063401804. [DOI] [PubMed] [Google Scholar]
- 106.Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med. 2004;350:876–85. doi: 10.1056/NEJMoa030815. [DOI] [PubMed] [Google Scholar]
- 107.Frolkis AD, Dykeman J, Negron ME, et al. Risk of surgery for inflammatory bowel diseases has decreased over time: A systematic review and meta-analysis of population-based studies. Gastroenterology. 2013;145:996–1006. doi: 10.1053/j.gastro.2013.07.041. [DOI] [PubMed] [Google Scholar]
- 108.Rungoe C, Langholz E, Andersson M, et al. Changes in medical treatment and surgery rates in inflammatory bowel disease: a nationwide cohort study 1979–2011. Gut. 2014;63:1607–16. doi: 10.1136/gutjnl-2013-305607. [DOI] [PubMed] [Google Scholar]
- 109.De Cruz P, Kamm MA, Prideaux L, et al. Postoperative recurrent luminal Crohn’s disease: a systematic review. Inflamm Bowel Dis. 2012;18:758–77. doi: 10.1002/ibd.21825. [DOI] [PubMed] [Google Scholar]
- 110.Paolo G, Axel D, Silvio D, et al. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn’s Disease 2016: Part 2: Surgical Management and Special Situations. J Crohns Colitis. 2017;11:135–49. doi: 10.1093/ecco-jcc/jjw169. [DOI] [PubMed] [Google Scholar]
- 111.Cottone M, Camma C. Mesalamine and relapse prevention in Crohn’s disease. Gastroenterology. 2000;119:597. doi: 10.1053/gast.2000.16153. [DOI] [PubMed] [Google Scholar]
- 112.Rutgeerts P, Hiele M, Geboes K, et al. Controlled trial of metronidazole treatment for prevention of Crohn’s recurrence after ileal resection. Gastroenterology. 1995;108:1617–21. doi: 10.1016/0016-5085(95)90121-3. [DOI] [PubMed] [Google Scholar]
- 113.Regueiro M, Feagan BG, Zou B, et al. Infliximab Reduces Endoscopic, but Not Clinical, Recurrence of Crohn’s Disease After Ileocolonic Resection. Gastroenterology. 2016;150:1568–78. doi: 10.1053/j.gastro.2016.02.072. [DOI] [PubMed] [Google Scholar]
- 114.D’Haens GR, Vermeire S, Van Assche G, et al. Therapy of metronidazole with azathioprine to prevent postoperative recurrence of Crohn’s Disease: A controlled randomized trial. Gastroenterology. 2008;135:1123–9. doi: 10.1053/j.gastro.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 115.De Cruz P, Kamm MA, Hamilton AL, et al. Crohn’s disease management after intestinal resection: a randomised trial. Lancet. 2015;385:1406–17. doi: 10.1016/S0140-6736(14)61908-5. [DOI] [PubMed] [Google Scholar]
- 116.Hanauer S, Feagan B, Lichtenstein G, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–9. doi: 10.1016/S0140-6736(02)08512-4. [DOI] [PubMed] [Google Scholar]
- 117.Hanauer S, Sandborn W, Rutgeerts P, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: the CLASSIC trial. Gastroenterology. 2006;130:323–33. doi: 10.1053/j.gastro.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 118.Colombel J, Sandborn W, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: The CHARM trial. Gastroenterology. 2007;132:52–65. doi: 10.1053/j.gastro.2006.11.041. [DOI] [PubMed] [Google Scholar]
- 119.Schreiber S, Khaliq-Kareemi M, Lawrance I, et al. Maintenance therapy with certolizumab pegol for Crohn’s disease. N Eng J Med. 2007;357:239–50. doi: 10.1056/NEJMoa062897. [DOI] [PubMed] [Google Scholar]
- 120.Sandborn W, Feagan B, Rutgeerts P, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Eng J Med. 2013;369:711–21. doi: 10.1056/NEJMoa1215739. [DOI] [PubMed] [Google Scholar]
- 121.Feagan BG, Sandborn WJ, Gasink C, et al. Ustekinumab as Induction and Maintenance Therapy for Crohn’s Disease. N Engl J Med. 2016;375:1946–60. doi: 10.1056/NEJMoa1602773. [DOI] [PubMed] [Google Scholar]
- 122.Gajendran M, Loganathan P, Catinella AP, Hashash JG. A comprehensive review and update on Crohn’s disease. Dis Mon. 2018;64:20–57. doi: 10.1016/j.disamonth.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 123.Bennebroek Evertsz’ F, Nieuwkerk PT, Stokkers PC, et al. The patient simple clinical colitis activity index (P-SCCAI) can detect ulcerative colitis (UC) disease activity in remission: A comparison of the P-SCCAI with clinician-based SCCAI and biological markers. J Crohns Colitis. 2013;7:890–900. doi: 10.1016/j.crohns.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 124.Marín-Jiménez I, Nos P, Domènech E, et al. Diagnostic Performance of the Simple Clinical Colitis Activity Index Self-Administered Online at Home by Patients With Ulcerative Colitis: CRONICA-UC Study. Am J Gastroenterol. 2016;111:261–8. doi: 10.1038/ajg.2015.403. [DOI] [PubMed] [Google Scholar]
- 125.Peyrin-Biroulet L, Panés J, Sandborn WJ, et al. Defining Disease Severity in Inflammatory Bowel Diseases: Current and Future Directions. Clin Gastroenterol Hepatol. 2016;14:348–54. doi: 10.1016/j.cgh.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 126.Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M. Predictability of the postoperative course of Crohn’s disease. Gastroenterology. 1990;99:956–63. doi: 10.1016/0016-5085(90)90613-6. [DOI] [PubMed] [Google Scholar]
- 127.Papamichael K, Gils A, Rutgeerts P, et al. Role for therapeutic drug monitoring during induction therapy with TNF antagonists in IBD: evolution in the definition and management of primary nonresponse. Inflamm Bowel Dis. 2015;21:182–97. doi: 10.1097/MIB.0000000000000202. [DOI] [PubMed] [Google Scholar]
- 128.Ding NS, Hart A, De Cruz P. Systematic review: predicting and optimizing response to anti-TNF therapy in Crohn’s disease - algorithm for practical management. Aliment Pharmacol Ther. 2016;43:30–51. doi: 10.1111/apt.13445. [DOI] [PubMed] [Google Scholar]
- 129.González AE, Lara DO, Segura PS, et al. Proactive measurement of infliximab trough levels vs. Clinical management in inflammatory bowel disease: Multi-centre study. J Crohns Colitis. 2018;12(S1):S319–20. doi: 10.1093/ecco-jcc/jjx180.552. [DOI] [Google Scholar]
- 130.Orfanoudaki E, Gazouli M, Foteinogiannopoulou K, Theodouraki E, Legaki E, Koutroubakis I. Infliximab trough levels are decreasing over time in patients with inflammatory bowel disease on maintenance treatment with infliximab. J Crohn Colitis. 2018;12(S1):S330–1. doi: 10.1093/ecco-jcc/jjx180.574. [DOI] [PubMed] [Google Scholar]
- 131.D’Haens G, Vermeire S, Lambrecht G, et al. Increasing Infliximab Dose Based on Symptoms, Biomarkers, and Serum Drug Concentrations Does Not Increase Clinical, Endoscopic, and Corticosteroid-Free Remission in Patients With Active Luminal Crohn’s Disease. Gastroenterology. 2018;154:1343–51. doi: 10.1053/j.gastro.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 132.Pavlidis P, Gulati S, Dubois P, et al. Early change in faecal calprotectin predicts primary non- response to anti-TNFα therapy in Crohn’s disease. Scand J Gastroenterol. 2016;51:1447–52. doi: 10.1080/00365521.2016.1205128. [DOI] [PubMed] [Google Scholar]
- 133.Godat S, Fournier N, Safroneeva E, et al. Swiss IBD Cohort Study Group. Frequency and type of drug-related side effects necessitating treatment discontinuation in the Swiss Inflammatory Bowel Disease Cohort. Eur J Gastroenterol Hepatol. 2018;30:612–20. doi: 10.1097/MEG.0000000000001078. [DOI] [PubMed] [Google Scholar]
- 134.de Bruyn M, Ballet V, Verstockt S, et al. Temporal changes in immune pathways with consecutive biological therapies as measuredbyserum proteomics. J Crohns Colitis. 2018;12(S1):S3. doi: 10.1093/ecco-jcc/jjx180.002. [DOI] [Google Scholar]
- 135.Pai RK, Geboes K. Disease activity and mucosal healing in inflammatory bowel disease: A new role for histopathology? Virchows Arch. 2018;472:99–110. doi: 10.1007/s00428-017-2156-5. [DOI] [PubMed] [Google Scholar]
- 136.Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infection and mortality in patients with Crohn’s disease: more than 5 years of follow-up in the TREAT registry. Am J Gastroenterol. 2012;107:1409–22. doi: 10.1038/ajg.2012.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Long MD, Martin CF, Pipkin CA, et al. Risk of melanoma and nonmelanoma skin cancer among patients with inflammatory bowel disease. Gastroenterology. 2012;143:390–9. doi: 10.1053/j.gastro.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Siegel CA, Marden SM, PErsing SM, Larson RJ, Sands BE. Risk of lymphoma associated with combination anti-tumor necrosis factor and immunomodulator therapy for the treatment of Crohn’s disease: a meta-analysis. Clin Gastroenterol Hepatol. 2009;7:874–81. doi: 10.1016/j.cgh.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kennedy NA, Warner B, Johnston EL, et al. Relapse after withdrawal from anti-TNF therapy for inflammatory bowel disease: an observational study, plus systematic review and meta-analysis. Aliment Pharmacol Ther. 2016;43:910–23. doi: 10.1111/apt.13547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bortlik M, Duricova D, Machkova N, et al. Discontinuation of anti-tumor necrosis factor therapy in inflammatory bowel disease patients: a prospective observation. Scand J Gastroenterol. 2016;51:196–202. doi: 10.3109/00365521.2015.1079924. [DOI] [PubMed] [Google Scholar]
- 141.Gisbert JP, Marin AC, Chaparro M. Systematic review: factors associated with relapse of inflammatory bowel disease after discontinuation of anti-TNF therapy. Aliment Pharmacol Ther. 2015;42:391–405. doi: 10.1111/apt.13276. [DOI] [PubMed] [Google Scholar]
- 142.Torres J, Boyapati RK, Kennedy NA, Louis E, Colombell JF, Satsangi J. Systematic Review of Effects of Withdrawal of Immunomodulators or Biologic Agents From Patients With Inflammatory Bowel Disease. Gastroenterology. 2015;149:1716–30. doi: 10.1053/j.gastro.2015.08.055. [DOI] [PubMed] [Google Scholar]
- 143.Louis E, Mary JY, Vernier-Massouille G, et al. Maintenance of remission among patients with Crohn’s disease on antimetabolite therapy after infliximab therapy is stopped. Gastroenterology. 2012;142:63–70. doi: 10.1053/j.gastro.2011.09.034. [DOI] [PubMed] [Google Scholar]
- 144.Doherty G, Katsanos KH, Burish J, et al. European Crohn’s and Colitis Organisation Topical Review on Treatment Withdrawal [‘Exit Strategies’] in Inflammatory Bowel Disease. J Crohns Colitis. 2018;12:17–31. doi: 10.1093/ecco-jcc/jjx101. [DOI] [PubMed] [Google Scholar]
- 145.Pittet V, Froehlich F, Maillard MH, et al. When do we dare to stop biological or immunomodulatory therapy for Crohn’s disease? Results of a multidisciplinary European expert panel. J Crohns Colitis. 2013;7:820–6. doi: 10.1016/j.crohns.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 146.Pariente B, Laharie D. Review article: why, when and how to de-escalate therapy in inflammatory bowel diseases. Aliment Pharmacol Ther. 2014;40:338–53. doi: 10.1111/apt.12838. [DOI] [PubMed] [Google Scholar]
- 147.Louis E. Stopping Biologics in IBD-What Is the Evidence? Inflamm Bowel Dis. 2018;24:725–31. doi: 10.1093/ibd/izx098. [DOI] [PubMed] [Google Scholar]
- 148.Chan HC, Ng SC. Emerging biologics in inflammatory bowel disease. J Gastroenterol. 2017;52:141–50. doi: 10.1007/s00535-016-1283-0. [DOI] [PubMed] [Google Scholar]
- 149.Hindryckx P, Casteele NV, Novak G, et al. The Expanding Therapeutic Armamentarium for Inflammatory Bowel Disease: How to Choose the Right Drug[s] for Our Patients? J Crohns Colitis. 2018;12:105–19. doi: 10.1093/ecco-jcc/jjx117. [DOI] [PubMed] [Google Scholar]
- 150.Shi HY, Ng SC. The state of the art on treatment of Crohn’s disease. J Gastroenterol. 2018;53:989–98. doi: 10.1007/s00535-018-1479-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Frieder J, Kivelevitch D, Haugh I, Watson I, Menter A. Anti-IL-23 and Anti-IL-17 Biologic Agents for the Treatment of Immune-Mediated Inflammatory Conditions. Clin Pharmacol Ther. 2018;103:88–101. doi: 10.1002/cpt.893. [DOI] [PubMed] [Google Scholar]
- 152.Verstockt B, Ferrante M, Vermeire S, Van Assche G. New treatment options for inflammatory bowel diseases. J Gastroenterol. 2018;53:585–90. doi: 10.1007/s00535-018-1449-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Duijvestein M, Battat R, Casteele NV, D’Haens GR, Sandborn WJ, Khanna R, Jairath V, Feagan B. Novel Therapies and Treatment Strategies for Patients with Inflammatory Bowel Disease. Curr Treat Options Gastro. 2018;16:129–46. doi: 10.1007/s11938-018-0175-1. [DOI] [PubMed] [Google Scholar]
- 154.Lamb YN, Duggan ST. Ustekinumab: A Review in Moderate to Severe Crohn’s Disease. Drugs. 2017;77:1105–14. doi: 10.1007/s40265-017-0765-6. [DOI] [PubMed] [Google Scholar]
- 155.Feagan BG, Sandborn WJ, Gasink C, et al. Ustekinumab as Induction and Maintenance Therapy for Crohn’s Disease. N Engl J Med. 2016;375:1946–60. doi: 10.1056/NEJMoa1602773. [DOI] [PubMed] [Google Scholar]
- 156.Furfaro F, Gilardi D, Allocca M, et al. IL-23 Blockade for Crohns disease: next generation of anti-cytokine therapy. Expert Rev Clin Immunol. 2017;13:457–67. doi: 10.1080/1744666X.2017.1279055. [DOI] [PubMed] [Google Scholar]
- 157.Sedda S, Bevivino G, Monteleone G. Targeting IL-23 in Crohn’s disease. Expert Rev Clin Immunol. 2018;18:1–7. doi: 10.1080/1744666X.2018.1524754. [DOI] [PubMed] [Google Scholar]
- 158.Feagan BG, Sandborn WJ, D’Haens G, et al. Induction therapy with the selective interleukin-23 inhibitor risankizumab in patients with moderate-to-severe Crohn’s disease: a randomised, double-blind, placebo-controlled phase 2 study. Lancet. 2017;389:1699–709. doi: 10.1016/S0140-6736(17)30570-6. [DOI] [PubMed] [Google Scholar]
- 159.Sands BE, Chen J, Feagan BG, et al. Efficacy and safety of MEDI2070, an antibody against interleukin 23, in patients with moderate to severe Crohn’s disease: a phase 2a study. Gastroenterology. 2017;153:77–86. doi: 10.1053/j.gastro.2017.03.049. [DOI] [PubMed] [Google Scholar]
- 160.White JR, Phillips F, Monaghan T, et al. Review article: novel oral-targeted therapies in inflammatory bowel disease. Aliment Pharmacol Ther. 2018;47:1610–22. doi: 10.1111/apt.14669. [DOI] [PubMed] [Google Scholar]
- 161.De Vries LCS, Wildenberg ME, De Jonge WJ, D’Haens GR. The Future of Janus Kinase Inhibitors in Inflammatory Bowel Disease. J Crohns and Colitis. 2017;2:885–93. doi: 10.1093/ecco-jcc/jjx003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sandborn WJ, Su C, Sands BE, et al. Tofacitinib as Induction and Maintenance Therapy for Ulcerative Colitis. N Engl J Med. 2017;376:1723–36. doi: 10.1056/NEJMoa1606910. [DOI] [PubMed] [Google Scholar]
- 163.Fiorino G, D’Amico F, Italia A, Gilardi D, Furfaro F, Danese S. JAK inhibitors: Novel developments in management of ulcerative colitis. Best Pract Res Clin Gastroenterol. 2018;32–33:89–93. doi: 10.1016/j.bpg.2018.05.015. [DOI] [PubMed] [Google Scholar]
- 164.Vermeire S, Schreiber S, Petryka R, et al. Clinical remission in patients with moderate-to-severe Crohn’s disease treated with filgotinib (the FITZROY study): results from a phase 2, double-blind, randomised, placebo controlled trial. Lancet. 2017;389:266–75. doi: 10.1016/S0140-6736(16)32537-5. [DOI] [PubMed] [Google Scholar]
- 165.Labetoulle R, Paul S, Roblin X. Filgotinib for the treatment of Crohn’s disease. Expert Opin Investig Drugs. 2018;27:295–300. doi: 10.1080/13543784.2018.1442433. [DOI] [PubMed] [Google Scholar]
- 166.Sandborn WJ, Feagan BG, Panes J, et al. Safety and efficacy of ABT- 494 (upadacitinib), an oral Jak1 inhibitor, as induction therapy in patients with Crohn’s disease: results from Celest. Gastroenterology. 2017;152:S1308–S9. doi: 10.1016/S0016-5085(17)34357-3. [DOI] [Google Scholar]
- 167.Sandborn WJ, Feagan BG, Wolf DC, et al. Ozanimod induction and maintenance treatment for ulcerative colitis. N Engl J Med. 2016;374:1754–62. doi: 10.1056/NEJMoa1513248. [DOI] [PubMed] [Google Scholar]
- 168.Peyrin-Biroulet L, Christopher R, Behan D, Lassen C. Modulation of sphingosine-1-phosphate in inflammatory bowel disease. Autoimmun Rev. 2017;16:495–503. doi: 10.1016/j.autrev.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 169.Hernandez-Diaz S, Werler MM, Walker AM, et al. Folic acid antagonists during pregnancy and the risk of birth defects. N Engl J Med. 2000;343:1608–14. doi: 10.1056/NEJM200011303432204. [DOI] [PubMed] [Google Scholar]
- 170.Weber-Schoendorfer C, Chambers C, Wacker E, et al. Pregnancy outcome after methotrexate treatment for rheumatic disease prior to or during early pregnancy: a prospective multicenter cohort study. Arthritis Rheumatol. 2014;66:1101–10. doi: 10.1002/art.38368. [DOI] [PubMed] [Google Scholar]
- 171.Nguyen GC, Seow CH, Maxwell C, et al. The Toronto Consensus Statements for the Management of Inflammatory Bowel Disease in Pregnancy. Gastroenterol. 2016;150:734–757. doi: 10.1053/j.gastro.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 172.Weber-Schoendorfer C, Hoeltzenbein M, Wacker E, Meister R, Schaefer C. No evidence for an increased risk of adverse pregnancy outcome after paternal low-dose methotrexate: an observational cohort study. Rheumatology. 2014;53:757–63. doi: 10.1093/rheumatology/ket390. [DOI] [PubMed] [Google Scholar]
- 173.Casanova MJ, Chaparro M, Domènech E, et al. Safety of thiopurines and anti-TNF-a drugs during pregnancy in patients with inflammatory bowel disease. Am J Gastroenterol. 2013;108:433–40. doi: 10.1038/ajg.2012.430. [DOI] [PubMed] [Google Scholar]
- 174.Goldstein LH, Dolinsky G, Greenberg R, et al. Pregnancy outcome of women exposed to azathioprine during pregnancy. Birth Defects Res A Clin Mol Teratol. 2007;79:696–701. doi: 10.1002/bdra.20399. [DOI] [PubMed] [Google Scholar]
- 175.Shim L, Eslick GD, Simring AA, Murray H, Weltman MD. The effects of azathioprine on birth outcomes in women with inflammatory bowel disease (IBD) J Crohns Colitis. 2011;5:234–8. doi: 10.1016/j.crohns.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 176.Gonzalez-Suarez B, Sengupta S, Moss AC. Impact of inflammatory bowel disease activity and thiopurine therapy on birth weight: A meta-analysis. World J Gastroenterol. 2017;23:8082–9. doi: 10.3748/wjg.v23.i45.8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sau A, Clarke S, Bass J, et al. Azathioprine and breastfeeding: Is it safe? BJOG. 2007;114:498–501. doi: 10.1111/j.1471-0528.2006.01232.x. [DOI] [PubMed] [Google Scholar]
- 178.Singh M, Qualie J, Currie A, Howarth ES, Khare MM. Is breastfeeding safe with azathioprine? Obstet Med. 2011;4:104–7. doi: 10.1258/om.2011.110013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Xu L, Han S, Liu Y, et al. The influence of immunosuppressants on the fertility of males who undergo renal transplantation and on the immune function of their offspring. Transpl Immunol. 2009;22:28–31. doi: 10.1016/j.trim.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 180.Flint J, Panchal S, Hurrell A, et al. BSR and BHPR guideline on prescribing drugs in pregnancy and breastfeeding-Part I: standard and biologic disease modifying anti-rheumatic drugs and corticosteroids. Rheumatology. 2016;55:1693–7. doi: 10.1093/rheumatology/kev404. [DOI] [PubMed] [Google Scholar]
- 181.Park-Wyllie L, Mazzotta P, Pastuszak A, et al. Birth defects after maternal exposure to corticosteroids: prospective cohort study and meta-analysis of epidemiological studies. Teratology. 2000;62:385–92. doi: 10.1002/1096-9926(200012)62:6<385::AID-TERA5>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 182.Bay Bjorn AM, Ehrenstein V, Hundborg HH, et al. Use of corticosteroids in early pregnancy is not associated with risk of oral clefts and other congenital malformations in offspring. Am J Ther. 2014;21:73–80. doi: 10.1097/MJT.0b013e3182491e02. [DOI] [PubMed] [Google Scholar]
- 183.Hviid A, Molgaard-Nielsen D. Corticosteroid use during pregnancy and risk of orofacial clefts. CMAJ. 2011;183:796–804. doi: 10.1503/cmaj.101063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Beaulieu DB, Ananthakrishnan AN, Issa M, et al. Budesonide induction and maintenance therapy for Crohn’s disease during pregnancy. Inflamm Bowel Dis. 2009;15:25–8. doi: 10.1002/ibd.20640. [DOI] [PubMed] [Google Scholar]
- 185.Engeland A, Bjørge T, Daltveit AK, et al. Effects of preconceptional paternal drug exposure on birth outcomes: cohort study of 340 000 pregnancies using Norwegian population-based databases. Br J Clin Pharmacol. 2012;75:1134–41. doi: 10.1111/j.1365-2125.2012.04426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Marteau P, Tennenbaum R, Elefant E, et al. Foetal outcome in women with inflammatory bowel disease treated during pregnancy with oral mesalazine microgranules. Aliment Pharmacol Ther. 1998;12:1101–8. doi: 10.1046/j.1365-2036.1998.00417.x. [DOI] [PubMed] [Google Scholar]
- 187.Rahimi R, Nikfar S, Rezaie A, et al. Pregnancy outcome in women with inflammatory bowel disease following exposure to 5-aminosalicylic acid drugs: a meta-analysis. Reprod Toxicol. 2008;25:271–5. doi: 10.1016/j.reprotox.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 188.Gallinger ZR, Nguyen GC. Presence of phthalates in gastrointestinal medications: is there a hidden danger? World J Gastroenterol. 2013;19:7042–7. doi: 10.3748/wjg.v19.i41.7042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Vermeire S, Carbonnel F, Coulie PG, et al. Management of inflammatory bowel disease in pregnancy. J Crohns Colitis. 2012;6:81123. doi: 10.1016/j.crohns.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 190.Silverman DA, Ford J, Shaw I, et al. Is mesalazine really safe for use in breastfeeding mothers? Gut. 2005;54:170–1. doi: 10.1136/gut.2004.048058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Esbjorner E, Jarnerot G, Wranne L. Sulphasalazine and sulphapyridine serum levels in children to others treated with sulphasalazine during pregnancy and lactation. Acta Paediatr Scand. 1987;76:137–42. doi: 10.1111/j.1651-2227.1987.tb10430.x. [DOI] [PubMed] [Google Scholar]
- 192.Nielsen OH, Maxwell C, Hendel J. IBD medications during pregnancy and lactation. Nat Rev Gastroenterol Hepatol. 2014;11:116–27. doi: 10.1038/nrgastro.2013.135. [DOI] [PubMed] [Google Scholar]
- 193.Birnie GG, McLeod TI, Watkinson G. Incidence of sulphasalazine-induced male infertility. Gut. 1981;22:452–5. doi: 10.1136/gut.22.6.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Teo SK, Chandula RS, Harden JL, Stirling DI, Thomas SD. Sensitive and rapid method for the determination of thalidomide in human plasma and semen using solid-phase extraction and liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;767:145–51. doi: 10.1016/S1570-0232(01)00563-3. [DOI] [PubMed] [Google Scholar]
- 195.Bar Oz B, Hackman R, Einarson T, et al. Pregnancy outcome after cyclosporine therapy during pregnancy: a meta-analysis. Transplantation. 2001;71:1051–5. doi: 10.1097/00007890-200104270-00006. [DOI] [PubMed] [Google Scholar]
- 196.Alijotas-Reig J, Esteve-Valverde E, Ferrer-Oliveras R, Llurba E, Gris JM. Tumor Necrosis Factor-Alpha and Pregnancy: Focus on Biologics. An Updated and Comprehensive Review. Clinic Rev Allerg Immunol. 2017;53:40–53. doi: 10.1007/s12016-016-8596-x. [DOI] [PubMed] [Google Scholar]
- 197.Mahadevan U, Martin CF, Sandler RS, et al. PIANO: A 1000 patient prospective registry of pregnancy outcomes in women with ibd exposed to immunomodulators and biologic therapy. Gastroenterology. 2012;142:S149. doi: 10.1016/S0016-5085(12)60561-7. [DOI] [Google Scholar]
- 198.Mahadevan U, Wolf DC, Dubinsky M, et al. Placental Transfer of Anti-Tumor Necrosis Factor Agents in Pregnant Patients with Inflammatory Bowel Disease. Clin Gastroenterol Hepatol. 2013;11:286–e24. doi: 10.1016/j.cgh.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.van der Woude CJ, Ardizzone S, Bengtson MB, et al. The Second European Evidenced-Based Consensus on Reproduction and Pregnancy in Inflammatory Bowel Disease. J Crohns Colitis. 2015;9:107–24. doi: 10.1093/ecco-jcc/jju006. [DOI] [PubMed] [Google Scholar]
- 200.Cornish J, Tan E, Teare JP, et al. A meta-analysis on the influence of inflammatory bowel disease on pregnancy. Gut. 2006;56:830–7. doi: 10.1136/gut.2006.108324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Chaparro M, Verreth A, Lobaton T, et al. Long-Term Safety of In Utero Exposure to Anti-TNFα Drugs for the Treatment of Inflammatory Bowel Disease: Results from the Multicenter European TEDDY Study. Am J Gastroenterol. 2018;113:396–403. doi: 10.1038/ajg.2017.501. [DOI] [PubMed] [Google Scholar]
- 202.Luu M, Benzenine E, Michiels C, et al. Continuous Anti-TNFα Use Throughout Pregnancy: Possible Complications For the Mother But Not for the Fetus. A Retrospective Cohort on the French National Health Insurance Database (EVASION) Am J Gastroenterol. 2018;113:1669–77. doi: 10.1038/s41395-018-0176-7. [DOI] [PubMed] [Google Scholar]
- 203.Mahadevan U, Kane SV, Church JA, Vasiliauskas EA, Sandborn WJ, Dubinsky MC. The effect of maternal peripartum infliximab use on neonatal immune response. Gastroenterol. 2008;134:A69-A. doi: 10.1016/S0016-5085(08)60323-6. [DOI] [Google Scholar]
- 204.Kaine JL, Kivitz AJ, Birbara C, Luo AY. Immune responses following administration of influenza and pneumococcal vaccines to patients with rheumatoid arthritis receiving adalimumab. J Rheumatol. 2007;34:272–9. [PubMed] [Google Scholar]
- 205.Mahadevan U, Vermeire S, Lasch K, et al. Vedolizumab exposure in pregnancy: outcomes from clinical studies in inflammatory bowel disease. Aliment Pharmacol Ther. 2017;45:941–50. doi: 10.1111/apt.13960. [DOI] [PubMed] [Google Scholar]
- 206.Watson N, Wu K, Farr P, Reynolds NJ, Hampton PJ. Ustekinumab exposure during conception and pregnancy in patients with chronic plaque psoriasis: a case series of 10 pregnancies. Br J Dermatol. 2019;180:195–6. doi: 10.1111/bjd.17086. [DOI] [PubMed] [Google Scholar]
- 207.Matro R, Martin CF, Wolf D, Shah SA, Mahadevan U. Exposure Concentrations of Infants Breastfed by Women Receiving Biologic Therapies for Inflammatory Bowel Diseases and Effects of Breastfeeding on Infections and Development. Gastroenterology. 2018;155:696–704. doi: 10.1053/j.gastro.2018.05.040. [DOI] [PubMed] [Google Scholar]
- 208.Julsgaard M, Kjeldsen J, Bibby BM, Brock B, Baumgart DC. Vedolizumab Concentrations in the Breast Milk of Nursing Mothers With Inflammatory Bowel Disease. Gastroenterology. 2018;154:752–4. doi: 10.1053/j.gastro.2017.08.067. [DOI] [PubMed] [Google Scholar]
- 209.Gutierrez-Dalmau A, Campistol JM. Immunosuppressive therapy and malignancy in organ transplant recipients: a systematic review. Drugs. 2007;67:1167–98. doi: 10.2165/00003495-200767080-00006. [DOI] [PubMed] [Google Scholar]
- 210.Annese V, Beaugerie L, Egan L, et al. European Evidence-based Consensus: Inflammatory Bowel Disease and Malignancies. J Crohns Colitis. 2015;9:945–65. doi: 10.1093/ecco-jcc/jjv141. [DOI] [PubMed] [Google Scholar]
- 211.Wong PKK, Bagga H, Barrett C, et al. A Practical Approach to the Use of Conventional Synthetic, Biologic and Targeted Synthetic Disease Modifying Anti-Rheumatic Drugs for the Treatment of Inflammatory Arthritis in Patients with a History of Malignancy. Curr Rheumatol Rep. 2018;20:64. doi: 10.1007/s11926-018-0774-9. [DOI] [PubMed] [Google Scholar]
- 212.Carswell EA, Old LJ, Kassel RL, et al. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci USA. 1975;72:3666. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Imperato AK, Bingham CO, Abramson SB. Overview of benefit/risk of biological agents. Clin Exp Rheumatol. 2004;22(Suppl 35):S108–14. [PubMed] [Google Scholar]
- 214.Martinez-Escala ME, Posligua AL, Wickless H, Rutherford A, Sable KA, Rubio-Gonzalez B. Progression of undiagnosed cutaneous lymphoma after anti-tumor necrosis factor-alpha therapy. J Am Acad Dermatol. 2018;78:1068–76. doi: 10.1016/j.jaad.2017.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Bradley W, Howard J, David F, James KK, Pauline TT. Resolution of Metastatic Colon Cancer upon Withdrawal of Anti-TNF Therapy for Crohn’s Disease. J Gastrointest Cancer. 2019;50:665–7. doi: 10.1007/s12029-018-0107-2. [DOI] [PubMed] [Google Scholar]
- 216.John CR, Ilaria B, Andrea P, et al. Merkel Cell Carcinomas Arising in Autoimmune Disease Affected Patients Treated with Biologic Drugs, Including Anti-TNF. Clin Cancer Res. 2017;23:3929–34. doi: 10.1158/1078-0432.CCR-16-2899. [DOI] [PubMed] [Google Scholar]
- 217.Mocellin S, Rossi CR, Pilati P, Nitti D. Tumor necrosis factor, cancer and anticancer therapy. Cytokine Growth Factor Rev. 2005;16:35–53. doi: 10.1016/j.cytogfr.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 218.Ohri CM, Shikotra A, Green RH, Waller DA, Bradding P. Tumour necrosis factor-alpha expression in tumour islets confers a survival advantage in non-small cell lung cancer. BMC Cancer. 2010;10:323. doi: 10.1186/1471-2407-10-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Sedger LM, McDermott MF. TNF and TNF-receptors: From mediators of cell death and inflammation to therapeutic giants-past, present and future. Cytokine Growth Factor Rev. 2014;25:453–72. doi: 10.1016/j.cytogfr.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 220.Orosz P, Echtenacher B, Falk W, Rüschoff J, Weber D, Männel DN. Enhancement of experimental metastasis by tumor necrosis factor. J Exp Med. 1993;177:1391–8. doi: 10.1084/jem.177.5.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Bertrand F, Montfort A, Marcheteau E, et al. TNFα blockade overcomes resistance to anti-PD-1 in experimental melanoma. Nat Commun. 2017;8:2256. doi: 10.1038/s41467-017-02358-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Huang D, Xue J, Li S, Yang D. Oxaliplatin and infliximab synergize to induce regression of colon cancer. Oncol Lett. 2018;15:1517–22. doi: 10.3892/ol.2017.7468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Lemaitre M, Kirchgesner J, Rudnichi A, et al. Association between Use of Thiopurines or Tumor Necrosis Factor Antagonists Alone or in Combination and Risk of Lymphoma in Patients With Inflammatory Bowel Disease. JAMA. 2017;318:1679–86. doi: 10.1001/jama.2017.16071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Raaschou P, Söderling J, Turesson C, et al. Tumor necrosis factor inhibitors and cancer recurrence in Swedish patients with rheumatoid arthritis: A nationwide population-based cohort study. Ann Intern Med. 2018;169:291–9. doi: 10.7326/M17-2812. [DOI] [PubMed] [Google Scholar]
- 225.Shelton E, Laharie D, Scott FI, et al. Cancer recurrence following immune-suppressive therapies in patients with immune-mediated diseases: a systematic review and meta-analysis. Gastroenterology. 2016;151:97–109.e4. doi: 10.1053/j.gastro.2016.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Yamauchi R, Araki T, Mitsuyama K, et al. The characteristics of nivolumab-induced colitis: an evaluation of three cases and a literature review. BMC Gastroenterol. 2018;18:135. doi: 10.1186/s12876-018-0864-1. [DOI] [PMC free article] [PubMed] [Google Scholar]