INTRODUCTION
Despite controversy regarding patient selection and clinical efficacy, venovenous extracorporeal membrane oxygenation (VV ECMO) use for severe respiratory failure is increasing. 1–3 Potential advantages of VV ECMO over conventional support include the reduction of airway pressures and sedation, early patient mobilization, and reduced time to extubation.4–12 However, early mobilization and extubation can only be achieved if femoral cannula placement is avoided. This can be done by using a dual lumen, single cannula in the internal jugular (IJ) position.
The dual lumen cannula for VV ECMO has multiple drainage orifices in the superior vena cava and inferior vena cava (IVC) and a return limb directing blood across the tricuspid valve.7,12 The cannula traverses the right atrium (RA), and its terminal tip resides 3–5 cm within the IVC (Figure 1).13 Approaches to cannulation and complications with placement have been reported, including perforation of the great vessels or right ventricle, which may prove fatal.7,14–20 Fluoroscopy can be used to visualize the wire traversing the right atrium and entering the IVC before advancement of the cannula.19–20 The need for fluoroscopic guidance in a hybrid operating room or catheterization lab may delay cannulation. Furthermore, it may be dangerous to transport patients who are hemodynamically unstable and dependent on high ventilator settings.
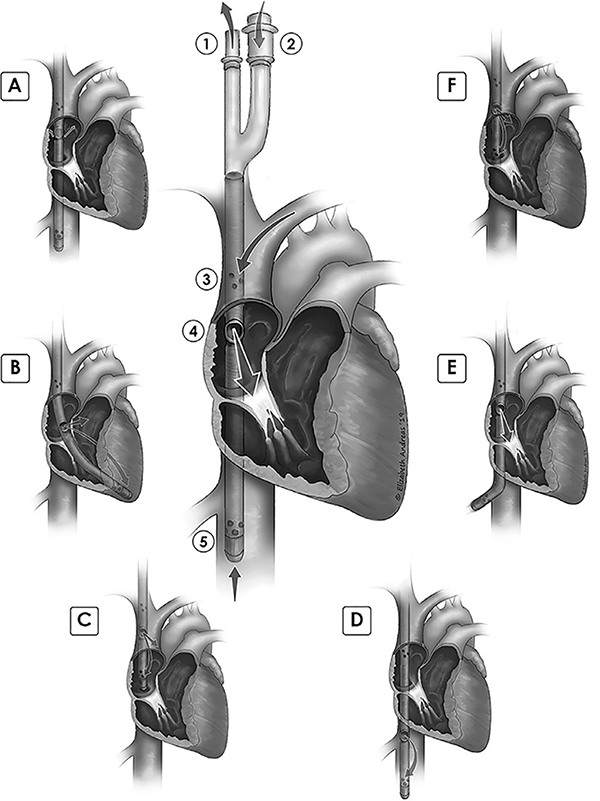
Figure 1: Cannula correct position and malposition illustrations.
Central figure: proper cannula depth and orientation. 1. Bicaval drainage outflow limb. 2. Post-oxygenator return limb. 3. Superior vena cava drainage orifices. 4. Return flow spout, at level of upper fossa ovalis, return jet directed anteriorly, towards center of tricuspid valve. 5. Inferior vena cava drainage orifices, with distal tip located a few centimeters beyond hepatic vein. Malposition illustrations and case numbers: A. Return flow oriented away from tricuspid, jet directed posteriorly, towards interatrial septum- 4 cases. B. Cannula within right ventricle, return flow recirculating by entering distal drainage orifices. Tip is against distal RV lateral wall- 2 cases. C. Distal tip of cannula in right atrium, too shallow. Return flow jet within SVC, likely not visible by TEE. Recirculation of flow from return jet into distal drain orifices- 6 cases. D. Cannula too deep, return flow within IVC, recirculation into distal drainage orifices- 7 cases. E. Tip in hepatic vein. Vein may collapse around distal drainage orifices, leading to chatter and low flow alarms in ECMO circuit- 6 cases. F. Return flow at upper RA/SVC junction, leading to recirculation of flow with some return flow entering distal orifices; distal tip is near RA/IVC junction; note that return flow may be seen with TEE- 3 cases.
Alternative options to fluoroscopy guidance in an interventional suite include portable X-ray and blind placement. Portable x-ray images in intensive care unit (ICU) rooms have quality limitations. Blind placement of a large cannula is problematic because of the need to separate venous drainage (predominantly from the IVC) and return flow (directed to the tricuspid valve) with precise cannula positioning.21,22 Echocardiographic guidance, either alone or accompanied by fluoroscopy, has been described as an alternative to fluoroscopic guidance in an operating room or interventional suite.15,23–25
The authors previously have described their standard institutional practice of placing the dual-lumen single VV ECMO cannula using transesophageal echocardiography (TEE) during cannulation in the ICU.26 In this companion article, the authors summarize analysis of a consecutive case series of VV ECMO cases to determine the success rate of initial cannulation as well as the frequency of subsequent cannula adjustment, and to illustrate common cannula malposition examples diagnosed by echocardiography.
MATERIALS AND METHODS
DATA SHARING
To facilitate research reproducibility, replicability, accuracy and transparency, the datasets generated and/or analyzed during the current study, and the associated analytic code, will be made available indefinitely, following publication, to anyone who wishes to access the data for analysis, on the Open Science Foundation29 (OSF) repository under DOI 10.17605/OSF.IO/KRGF2 at https://osf.io/krgf2/. Data were de-identified in accordance with Section 164.514 of the Health Insurance Portability and Accountability Act.
Study Design
This is a retrospective, observational cohort study of sequential patients managed with VV ECMO at a single tertiary care center that accepts regional referrals for respiratory failure. After Institutional Review Board approval (#104589), two internal institutional databases were queried to identify and cross-check all cases of VV ECMO managed from 2012 to 2018. Data was extracted from the electronic medical record directly into REDCap27 by a trained research coordinator, and directly into an Excel spreadsheet by a trained ECMO coordinator, respectively. Subsequent chart review was performed by the investigators to obtain additional information regarding complications.
Participants
Inclusion criteria included adult or pediatric patients who underwent placement of ECMO in the venovenous configuration, with the dual lumen cannula, for respiratory failure. Exclusion criteria included cannulation at a referring hospital by surgeons who do not use echo guidance in the ICU, insufficient documentation, and cannulation for veno-arterial ECMO.
Covariates and Outcomes
Covariates included: age (years), weight (kilograms), height (centimeters), body mass index (km/m2), sex, cannula size (French, Fr), number and details of each documented echocardiogram (including type of echocardiogram (transesophageal vs transthoracic)), ventricular function at initial placement (right and left ventricular systolic function), cannula position (acceptable vs. problematic location), hemodynamic assessment at the time of study (based on documentation of hypotension or cardiac arrest), clinical interventions necessary to treat instability at the time of study, clinical complications at the time of the echocardiogram, clinical complications from cannulation procedure, cannula adjustment, consequences from subsequent cannula position (after initial placement), duration of ECMO (days), vital status at decannulation, discharge disposition location, and cause of death.
An adequate outflow jet was defined as a turbulent jet visualized by color flow Doppler in the right atrium, originating from the outflow spout, with flow directed towards the tricuspid valve. This was either documented in the medical record at the time of initial imaging, or was determined by review of the video clips. We assessed the following patient and cannulation characteristics for association with the outcome of successful decannulation: age, procedure location (ICU vs other), right and left ventricular function at cannulation, initial cannula position, complications of initial cannulation procedure, consequences of initial cannula malposition, and need for cannula adjustment.
Statistical Analysis
Descriptive statistics, including median (interquartile range; IQR), were used to assess patient characteristics. Categorical characteristics were compared using Fisher exact test and ordinal variables with the use of the Wilcoxon-Mann-Whitney test. Continuous characteristics were compared using independent samples t-test. Coefficients, 95% CI’s and p-values were reported from all models. Statistical analyses were conducted in STATA 15.1. Significance was assessed at the 0.05 level and all tests were two-tailed.
RESULTS
Study Population
From 2012 to 2018, 48 sequential cases of VV ECMO were identified. Three cases were excluded because cannulation had been performed at referring hospitals, and initial cannula position success and any complications of cannulation were unknown. Our final cohort included 45 cases.
Patient Characteristics
Patient characteristics are described in Table 1. Age ranged from 10–75 years (Median 35; Interquartile Range [IQR] 25, 58), 71% were male. The most common causes of respiratory failure were pneumonia, aspiration, or inhalational burn injury. Cannulas ranged from 20–31 Fr; 53% were 27Fr. ECMO duration was 10 (5, 17) days, with the longest run 70 days. Thirty patients (67%) survived to decannulation and 28 (62%) survived to discharge, including 11 patients who were discharged directly to home. (Table 2).
Table 1:
Baseline characteristics of patients
| Variable | n (%) |
|---|---|
| Age* (years) | 35 (25, 58) |
| BMI* (kg/m2) | 32 (24, 38) |
| Height* (cm) | 173 (165, 179) |
| Weight* (kg) | 88 (69, 118) |
| Female Sex | 13 (29%) |
| Cannula Size | |
| 20 | 1 (3%) |
| 23 | 4 (10%) |
| 27 | 21 (53%) |
| 31 | 14 (35%) |
| Duration of ECMO in Days* | 10 (5, 17) |
| Total number of echocardiography studies per patient during ECMO for cannula position* | 2 (1, 2) |
| Clinical Presentation | |
| ARDS | 31 (69%) |
| Burn/Inhalation Injury | 7 (16%) |
| Bacterial Pneumonia | 5 (11%) |
| Viral Pneumonia | 12 (27%) |
| Aspiration | 6 (13%) |
| Not Specified | 1 (2%) |
| Perioperative/Cardiogenic | 7 (16%) |
| Protein Alveolar Proteinosis | 2 (4%) |
| Trauma | 1 (2%) |
| Interstitial Lung Disease | 1 (2%) |
| Post-transplant | 3 (7%) |
| RV function at placement | |
| Normal | 32 (73%) |
| Abnormal | 12 (27%) |
| LV function at placement | |
| Normal | 28 (65%) |
| Hyperdynamic | 2 (5%) |
| Mildly reduced | 8 (19%) |
| Moderate dysfunction | 3 (7%) |
| Severe dysfunction | 2 (5%) |
Median (interquartile range, IQR)
Missing values by group: BMI=1/45, Height=1/45, Weight=1/45, Cannula size=5/45, RV function at placement=1/45, LV function at placement=2/45
Table 2:
Cannula position and complications (n=45 patients)
| Variable | n (%) |
|---|---|
| Complications (any) | 12 (27%) |
| Cardiac arrest | 4 (9%) |
| Pericardial hemorrhage | 1 (2%) |
| Unable to cannulate/aborted | 1 (2%) |
| Hypotension | 1 (2%) |
| Cannula malposition | 5 (11%) |
| Complications (due to procedure)(any) | 6 (13%) |
| Pericardial hemorrhage | 1 (2%) |
| Unable to cannulate/aborted | 1 (2%) |
| Cannula malposition | 5 (11%) |
| Clinical consequences of initial malposition (n=44) | |
| Not Applicable | 39 (89%) |
| Hypoxemia | 4 (9%) |
| Decreased Flow | 1 (2%) |
| Cannulation location | |
| ICU | 40 (89%) |
| Cath Lab | 1 (2%) |
| Operating Room | 4 (9%) |
| Imaging used for cannulation | |
| TEE | 42 (93.3%) |
| TTE + Fluoroscopy | 2 (4.4%) |
| TEE + Fluoroscopy | 1 (2.2%) |
| Clinical consequences of malposition later during ECMO course | |
| None | 27 (61%) |
| Hypoxemia | 13 (30%) |
| Decreased Flow | 3 (7%) |
| Insufficient documentation | 1 (2%) |
| Cannula adjusted during ECMO run | 21 (47%) |
| Survived to successful decannulation | 30 (67%) |
| Disposition among those successfully decannulated from ECMO (n=30) | |
| Home | 11 (37%) |
| Long-Term Acute Care | 7 (23%) |
| Acute Rehabilitation | 9 (30%) |
| Transfer to another hospital | 1 (3%) |
| Died before discharge | 2 (7%) |
| Cause of death on ECMO (n=15) | |
| Multi-system organ failure | 5 (33%) |
| Transition to palliative care | 3 (20%) |
| Neurologic injury | 5 (33%) |
| Other | 2 (13%) |
| Cause of in-hospital death after ECMO (n=2) | |
| Hypoxemia/hypercarbia | 1 (50%) |
| Cardiogenic shock | 1 (50%) |
Missing values by group: Complications (due to procedure)=1/6; Clinical consequences of initial malposition=1/44; Clinical consequences of malposition later during ECMO course=1/44; Disposition among those successfully decannulated from ECLS=1/30
Echocardiography
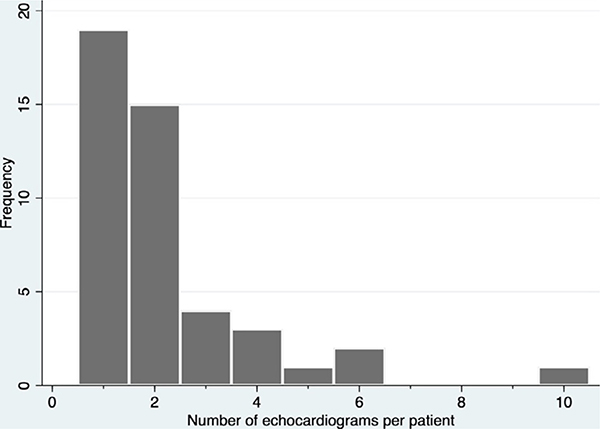
Transesophageal echocardiography (TEE), without fluoroscopy, was performed in 42 (93%) of cases to guide cannulation. Two patients (4%) had contraindications to TEE: one had undergone esophagectomy, and another had had a recent gastric bypass. In these cases, transthoracic echocardiography (TTE) was used in combination with fluoroscopy in the ICU. Forty cases (89%) were performed in the intensive care unit (ICU) and four were in the operating room. In the single case performed in the cath lab, TEE guidance was used along with fluoroscopy. The total number of echo studies reported for both cannulation and subsequent re-evaluation of cannula position was one or two in 34 cases (76%); seven patients (16%) required >3 echo studies (Figure 2). At the time of cannulation, right ventricular systolic function was normal in 32 patients (73%) and abnormal in 12 (27%). Left ventricular systolic function was normal in 28 patients (65%), hyperdynamic in 2 (5%), and abnormal in 13 (30%) (Table 1). RV function could not be interpreted because of inadequate clips in one case, and LV function could not be interpreted because of inadequate clips in two cases.
Figure 2:
Frequency of echocardiography studies per patient
Successful Positioning and Adverse Events at Initial Cannulation
Adverse events during cannulation occurred in 12 cases (27 %) (Table 3). Six adverse events were directly associated with cannulation problems (for an incidence of 13%). The first was a vascular injury (described below). The cannulation in this case was successful when a smaller cannula was used. There were two cases of inadvertent cannula position in the RV, resulting in persistent hypoxemia in both cases, as well as recurrent supraventricular tachycardia in one case. There were two cases of persistent hypoxemia from cannula outflow located in the IVC, and one case of a cannula tip becoming kinked in the right atrium. Finally, for one patient, attempts to cannulate were aborted due to the finding of internal jugular thrombosis. Thus, initial cannulation was successful in 39 of 45 cases (87%), with five cases of initial malposition of the cannula and one aborted attempt. The unacceptable locations were right ventricle (two cases), too deep (two cases) and one case of a hairpin bend of the cannula in the right atrium (Table 3).
Table 3A:
Adverse Events At Time of Cannulation Attributed to Procedure (6/45)
| Complication | Intervention | Outcome |
|---|---|---|
| Pericardial hemorrhage due to SVC Degloving Injury | Smaller cannula used on successful second attempt; four unit blood transfusion | Survived ECMO to lung transplant |
| Cannula displaced into RV, decreased flow, persistent hypoxemia and tachyarrhythmia | Repositioned cannula under echo guidance | Transitioned to palliative care after hemorrhagic stroke |
| Cannula displaced into RV, persistent hypoxemia | Repositioned cannula under echo guidance | Discharged home |
| Cannula too deep, outflow in IVC, persistent hypoxemia | Repositioned cannula under echo guidance | Discharged to LTAC, then to home |
| Cannula too deep, outflow in IVC, persistent hypoxemia | Repositioned cannula under echo guidance | Discharged home |
| Cannula too shallow, kinked in right atrium, persistent hypoxemia | Repositioned cannula under echo guidance | Discharged to acute rehab, then to home |
Of the four cases with a complication of cardiac arrest, in no case was the arrest related directly to the cannulation procedure. Three of the four cases of cardiac arrest peri-cannulation resulted in prompt return of spontaneous circulation after a brief period of chest compressions, and an ultimate outcome of survival to discharge (Table 3B). In the fourth case, the cardiac arrest during cannulation was in the context of multi-system organ failure, and there was persistent shock despite mechanical circulatory support. Further efforts were suspended within hours of cannulation because they were judged to be futile. Finally, there was one case of severe hypotension at the time of cannulation, necessitating vasopressors and fluid challenge (Table 3B).
Table 3B:
Adverse Events At Time of Cannulation Due to Patient Condition
| Complication | Intervention | Outcome |
|---|---|---|
| Internal Jugular Discovered to be thrombosed | Procedure aborted | Managed with mechanical ventilation; survived to discharge |
| Cardiac arrest | Pressors, ACLS, VA ECMO | Support withdrawn due to futility |
| Cardiac arrest | Brief chest compressions | Discharged to LTAC |
| Cardiac arrest | Brief chest compressions | Discharged home |
| Cardiac arrest | Brief chest compressions | Discharged home |
| Hypotension | Vasopressors and fluids | Discharged to LTAC |
Abbreviations
SVC, superior vena cava; RV, right ventricle; IVC, inferior vena cava; LTAC, long-term acute care facility
The most serious adverse event was vascular injury, bleeding, and hemorrhagic shock in a 19-year-old male with a BMI of 19.5kg/m2 and body surface area (BSA) of 1.67m2. The initial attempt to place a 27 Fr cannula failed, as the cannula could not be advanced beyond the superior vena cava/right atrial (SVC/RA) junction. The metal reinforcements became enmeshed in the endothelial lining of the SVC, and removal of the cannula was difficult, necessitating a second operator. A 23 Fr cannula was then placed successfully. On inspection of the 27Fr cannula, the outside wire frame was noted to have bent components, and this area was covered by a sheath of vascular tissue, consistent with an intimal venous degloving injury. During the procedure, the patient became hemodynamically unstable and TEE revealed a new pericardial effusion, without evidence of tamponade (Video Clip 1). The hypotension and bleeding necessitated transfusion of 4 units of packed red blood cells before achieving hemodynamic stability. Pericardial drainage was not necessary. After several days of VV ECMO, the patient required conversion to veno-arterial ECMO because of right ventricular failure and persistent hypoxemia. Ultimately the patient had 65 days of ECMO support and underwent lung transplantation.
One case of malposition of the cannula into the RV was associated with atrial fibrillation and multiple cardioversions, as well as an effusion adjacent to the RV, but without clinical tamponade (Video Clip 2). Low flow was present until the cannula was repositioned. In the other case of malposition of the cannula with tip in the RV, persistent hypoxemia lead to repeat TEE, and the cannula tip was repositioned in the IVC, with immediate improvement in oxygenation.
In three cases, the long, thin, flexible guidewire bent either during dilation in the neck or from repeatedly catching on the Eustachian valve (along the anterior IVC-RA junction). In two of these cases, the patients were obese (BMI 42kg/m2 and 36.6 kg/m2). The situation was addressed in two cases by placing a sheath over the first guidewire, and then advancing a stiffer wire which facilitated Seldinger technique for placement of the distal tip into the IVC. In a third case, a malleable angled wire was used to replace a guidewire with a loop in the RA, so that the guidewire tip could be positioned in the IVC.
There was a single case of aborted cannulation, which was due to a right IJ thrombus. The patient had completed 30 days of ECMO for ARDS and was initially stable after decannulation. However, within a few hours, the patient developed pulmonary edema associated with hypoxemic respiratory failure. Efforts were made to re-access the right IJ for additional ECMO support, but the cannulation attempt was aborted because significant right IJ thrombus was identified. The patient was successfully treated with mechanical ventilation (and without a second run of ECMO) and was discharged to home.
Echocardiographic Evaluation of Cannula Position
During the course of VV ECMO support, echocardiography may be needed to evaluate cannula position when there are clinical signs suggesting malposition, such as hypoxemia, line chatter, low flow, or suck down events. In our cohort, 17 patients (38%) had echocardiographic identification of malposition of the cannula during the ECMO run (Figure 2). The most common cannula position found in hypoxemic patients was too shallow. Other problematic positions were too deep, malrotated, or tip in hepatic vein. The most common intervention was to change the depth or rotate the cannula. Figure 1 illustrates the types of malposition identified by echocardiography and the number of cases of each malposition type. Some patients required multiple echo-guided cannula adjustments (Figure 2).
Association between characteristics and survival
Associations between patient and cannulation characteristics and survival to decannulation are reported in Table 4. Younger age (36 vs 52 years; p<0.01) was the only characteristic associated with survival. There was no significant association between cannula malposition and survival to decannulation. There was no significant association between the need to adjust the cannula during ECMO and survival (Table 4).
Table 4:
Univariate associations with survival
| Variable* | Dead (n=15) | Alive (n=30) | p-value |
|---|---|---|---|
| Age† | 52.3 ± 4.7 | 35.6 ± 3.0 | <0.01 |
| Cannulation Site | 0.45 | ||
| ICU | 14 (93.3%) | 26 (86.7%) | |
| Cath lab | 1 (6.7%) | 0 (0.0%) | |
| OR | 0 (0.0%) | 4 (13.3%) | |
| Right ventricle function | 1.0 | ||
| Normal | 11 (73.3%) | 21 (72.4%) | |
| Abnormal | 4 (26.7%) | 8 (27.6%) | |
| Complications during procedure | 2 (13.3%) | 8 (26.7%) | 0.46 |
| Complications due to cannulation | 1 (6.7%) | 4 (13.3%) | 0.65 |
| Cannula adjustment | 7 (46.7%) | 14 (46.7%) | 1.00 |
| Consequences of initial malposition | 0.05 | ||
| Hypoxemia | 0 (0%) | 4 (14.3%) | |
| Decreased Flow | 1 (6.7%) | 0 (0%) | |
| None | 14 (93.3%) | 24 (85.7%) | |
| Consequences of subsequent malposition | 0.66 | ||
| Hypoxemia | 5 (33.3%) | 8 (27.6%) | |
| Decreased flow | 0 (0%) | 3 (10.3%) | |
| Insufficient document | 1 (6.7%) | 0 (0%) | |
| None | 9 (60%) | 18 (62.1%) | |
Number (percent)
Mean ± Standard Error
Abbreviations: ICU, intensive care unit; Cath lab, catheterization laboratory; OR, operating room
Missing values by group: RV Function=1/45; Consequences of initial malposition=1/44; Consequences of subsequent malposition=1/44
DISCUSSION
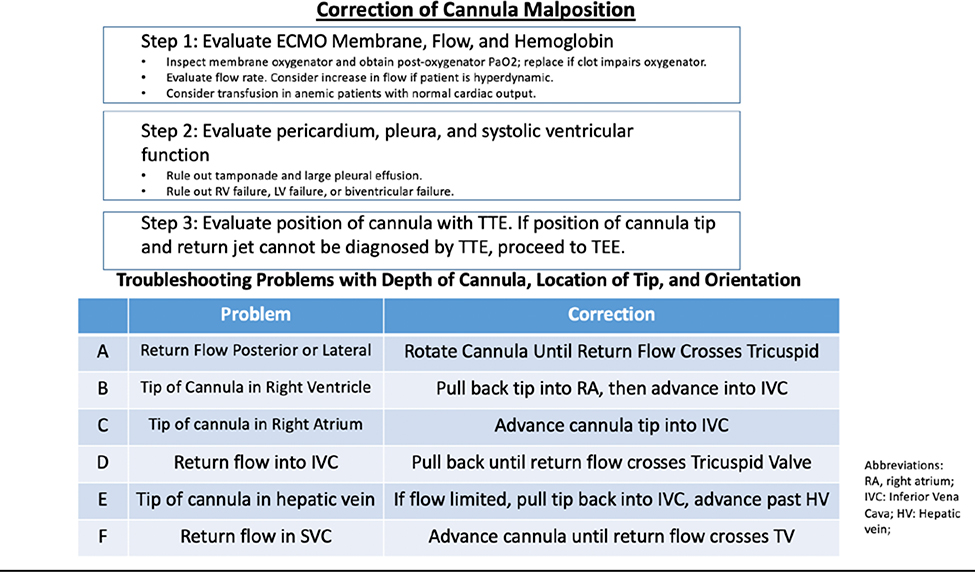
In our series, we observed that 39/45 or 87% of cannulations resulted in an initial proper position of the cannula. Nevertheless, a cardinal finding of the case series is that despite high initial success in cannula positioning, 38% of patients required echo-guided cannula adjustments during the course of ECMO. The precise positioning and orientation of the cannula needed for efficient separation of drainage and return flow leads to clinically significant oxygenation problems for many patients. A step-by-step approach to troubleshooting hypoxemia and cannula malposition during ECMO is presented in Figure 3.
Fig 3.
Stepwise approach to troubleshooting persistent hypoxemia during VV ECMO.
Even with echocardiographic guidance, the cannula tip was inadvertently positioned (or migrated soon after cannulation) into the right ventricle in 2 cases (4%). This potentially catastrophic consequence is important to recognize, as blind advancement in the right ventricle can cause RV perforation. ECMO oxygenation was inadequate because of recirculation. Fortunately, no injuries to the right ventricle were evident in our series.
The guidewire may bend—or even loop—at the level of the IVC/RA junction when the Eustachian valve is prominent, or when there is an angle between the inferior RA and IVC. This bend in the guidewire then directs the cannula tip anteriorly as it is advanced through the right atrium, which can subsequently catch on the Eustachian valve (Video Clip 3, Video Clip 4). Efforts to advance the cannula when the tip has engaged the Eustachian valve reinforce the anterior curve of the wire and cannula tip.
It may be prudent to take extra steps to control the tip of the guidewire and cannula when there is a large Eustachian valve and/or prominent angulation between the IVC and RA. For example, a small caliber dilator can be placed over the initial wire to allow safe advancement of a stiffer wire so that the guidewire position crossing the RA and ending deep in the IVC follows a straight course. An alternative strategy is to place a catheter with an angled tip over-the-wire.
While our series primarily utilized TEE, we have noted that TTE is complementary and sometimes superior to TEE for cannula assessment. The lower esophageal sphincter can cause shadowing of the inferior RA/IVC junction, and a subcostal TTE view of this area may be more informative than the TEE bicaval or transgastric views focused on the right atrium. In one case, it was difficult to demonstrate that the cannula tip was in the RV by TEE, but obvious using TTE (Video Clips 5–7). A second TEE was carried out with more attention directed to looking at the transgastric RV inflow from multiplane angles to interrogate the posterior aspect of the tricuspid valve, which revealed the cannula clearly crossing from the RA into the RV (Video Clip 2). Additional structures which may be imaged more easily by subcostal TTE than by TEE include the hepatic vein and the IVC distal to the hepatic vein (particularly if transgastric TEE views are limited). When the distal cannula is not visible in the IVC, the parasternal RV inflow view may prove to be useful to evaluate whether the tip is in the RV (Video Clip 8).
Other investigators have reported complications encountered during TEE guidance for placement of the dual lumen single cannula. In a large survey study from France, TEE guidance was used in 35 of 52 total cases.15 In this study, complications included 2 patients with myocardial perforation (4%), one of whom died (2%). This is comparable to our observed rate of great vessel trauma (2%), though the imaging modality for the two patients in that study was not reported. In the Conrad study of 190 percutaneous cannulations, only 15 were the dual lumen cannula, and nearly all VV ECMO cases involved fluoroscopic guidance.28 They reported a low complication rate, which included one great vessel perforation during adult VV ECMO performed under fluoroscopic (but not ultrasound) guidance (1/63 patients; 2%). A study of 720 patients who underwent percutaneous cannulation for ECMO included 76 patients who received the dual-lumen cannula, but complications and placement technique for this subset were not reported separately.17
Fluoroscopic guidance has been recommended to diagnose malposition during placement and to prevent complications.28 In our series, acceptable positioning was achieved in 87% of patients, with 93% of procedures guided by TEE without fluoroscopy. Serious consequences of vessel or myocardial perforation were comparable to those reported from two large studies reporting outcomes, one of which utilized fluoroscopic guidance. Some considerations in favor of echo guidance include the ability to evaluate RV and LV systolic function, tricuspid regurgitation, and pericardial effusions, the avoidance of radiation, and the portability of echo machines. Considerations in favor of fluoroscopy include identification of wire position without respect to the angulation or plane of the wire, and a wider variety of angled and stiff wires and introducers are available in the cath lab or hybrid operating room interventional suite.
Limitations and Future Directions
Limitations of this study include absence of a comparable cohort of patients undergoing fluoroscopy for a direct comparison of the safety and effectiveness of TEE-guided cannulation in the ICU. Other limitations include small case volume and a single site. For serious but rare complications, a much larger population of patients undergoing VV ECMO would be needed to provide robust estimates of the risks. Because there was no prospective surveillance of all patients for cannula malposition, it is possible that the documented cases of problematic cannula position underrepresent the actual number. However, it seems likely that clinically relevant cannula malposition was detected in the majority of cases, because the dual lumen cannula only works for patients with severe lung injury when return flow and venous drainage have a very specific location and orientation in the RA and IVC.
We elected to include two patients who had contraindications to TEE, four patients cannulated in the OR, and one patient cannulated in the cath lab, because our interest was to define not only the initial success of cannula placement (87%), but also to describe how often the cannula was adjusted during the course of ECMO (38%).
CONCLUSIONS
Cannulation for VV ECMO using the dual-lumen bicaval cannula is feasible using TEE guidance in the ICU. Persistent hypoxemia and low flow may indicate cannula malposition. The need to reposition the cannula during the ECMO run is common. TEE can be helpful in diagnosis of cannula malposition and to guide repositioning. More research is needed to determine the relative risks, costs, and outcomes of different cannulation strategies and different image guidance techniques for VV ECMO.
Supplementary Material
Video Clip 1: Transgastric short axis, with new pericardial effusion associated with SVC injury.
Video Clip 2: Transgastric biplane view showing tip of cannula in right ventricle.
Video Clip 3: Modified bicaval view showing tip of cannula abutting Eustachian valve.
Video Clip 4: Modified bicaval view showing color flow Doppler, with evidence of intermittent obstruction of distal venous inflow orifice by Eustachian valve.
Video Clip 5: Transesophageal echocardiogram in mid esophagus with a four chamber view, showing cannula in right atrium, but out-of-plane in right ventricle, leading to a false assurance that the cannula was not misplaced in the right ventricle.
Video Clip 6: Subcostal view of the same patient as in clip 5, focused on the right ventricle, with cannula crossing the tricuspid valve and ending in the right ventricle apex.
Video Clip 7: Subcostal TTE, with Color flow Doppler demonstrating interaction between right ventricular apical myocardium and drainage orifices of tip of misplaced cannula, clearly illustrating that the tip is in the right ventricle. This position is unacceptable, because return flow of oxygenated blood enters distal cannula inflow orifices and because there is a risk of arrhythmia and damage to the right heart valves and myocardium.
Video Clip 8: Transthoracic echocardiogram right ventricular inflow view, showing cannula traversing tricuspid valve and ending well within right ventricle. This is a view of the right ventricle which may reveal the true course of the cannula when four-chamber views are unrevealing.
ACKNOWLEDGEMENTS
We would like to thank Chloe Skidmore, MS for her assistance in formatting and preparation
Conflicts of interest and source of funding:
Dr. Tonna was supported by a career development award (K23HL141596) from the National Heart, Lung, And Blood Institute (NHLBI) of the National Institutes of Health (NIH). This study was also supported, in part, by the University of Utah Study Design and Biostatistics Center, with funding in part from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant 5UL1TR001067-02 (formerly 8UL1TR000105 and UL1RR025764). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. None of the funding sources were involved in the design or conduct of the study, collection, management, analysis or interpretation of the data, or preparation, review or approval of the manuscript. None of the authors report any conflicts of interest related to this manuscript.
Contributor Information
Matthew J. Griffee, Department of Anesthesiology.
Joshua M. Zimmerman, Department of Anesthesiology.
Stephen H. McKellar, Division of Cardiothoracic Surgery.
Joseph E Tonna, Division of Emergency Medicine; Division of Cardiothoracic Surgery; Department of Surgery.
REFERENCES
- 1.Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. The Lancet 2009;374:1351–63. [DOI] [PubMed] [Google Scholar]
- 2.Combes A, Hajage D, Capellier G, et al. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. N Engl J Med 2018;378:1965–75. [DOI] [PubMed] [Google Scholar]
- 3.Bratton SL, Chan T, Barrett CS, et al. Metrics to Assess Extracorporeal Membrane Oxygenation Utilization in Pediatric Cardiac Surgery Programs. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies 2017;18:779–86. [DOI] [PubMed] [Google Scholar]
- 4.Serpa Neto A, Schmidt M, Azevedo LC, et al. Associations between ventilator settings during extracorporeal membrane oxygenation for refractory hypoxemia and outcome in patients with acute respiratory distress syndrome: a pooled individual patient data analysis : Mechanical ventilation during ECMO. Intensive Care Med 2016;42:1672–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nosotti M, Rosso L, Tosi D, et al. Extracorporeal membrane oxygenation with spontaneous breathing as a bridge to lung transplantation. Interact Cardiovasc Thorac Surg 2013;16:55–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anton-Martin P, Thompson MT, Sheeran PD, et al. Extubation during pediatric extracorporeal membrane oxygenation: a single-center experience. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies 2014;15:861–9. [DOI] [PubMed] [Google Scholar]
- 7.Kuhl T, Michels G, Pfister R, et al. Comparison of the Avalon Dual-Lumen Cannula with Conventional Cannulation Technique for Venovenous Extracorporeal Membrane Oxygenation. Thorac Cardiovasc Surg 2015;63:653–62. [DOI] [PubMed] [Google Scholar]
- 8.Abrams D, Combes A, Brodie D. Extracorporeal membrane oxygenation in cardiopulmonary disease in adults. J Am Coll Cardiol 2014;63:2769–78. [DOI] [PubMed] [Google Scholar]
- 9.Chavez J, Bortolotto SJ, Paulson M, et al. Promotion of progressive mobility activities with ventricular assist and extracorporeal membrane oxygenation devices in a cardiothoracic intensive care unit. Dimens Crit Care Nurs 2015;34:348–55. [DOI] [PubMed] [Google Scholar]
- 10.Boling B, Dennis DR, Tribble TA, et al. Safety of Nurse-Led Ambulation for Patients on Venovenous Extracorporeal Membrane Oxygenation. Prog Transplant 2016;26:112–6. [DOI] [PubMed] [Google Scholar]
- 11.Bain JC, Turner DA, Rehder KJ, et al. Economic Outcomes of Extracorporeal Membrane Oxygenation With and Without Ambulation as a Bridge to Lung Transplantation. Respir Care 2016;61:1–7. [DOI] [PubMed] [Google Scholar]
- 12.Camboni D, Philipp A, Lubnow M, et al. Extracorporeal membrane oxygenation by single-vessel access in adults: advantages and limitations. ASAIO J 2012;58:616–21. [DOI] [PubMed] [Google Scholar]
- 13.Brodie D, Bacchetta M. Extracorporeal membrane oxygenation for ARDS in adults. The New England Journal of Medicine 2011;365:1905–14. [DOI] [PubMed] [Google Scholar]
- 14.Hirose H, Yamane K, Marhefka G, et al. Right ventricular rupture and tamponade caused by malposition of the Avalon cannula for venovenous extracorporeal membrane oxygenation. J Cardiothorac Surg 2012;7:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chimot L, Marque S, Gros A, et al. Avalon(c) bicaval dual-lumen cannula for venovenous extracorporeal membrane oxygenation: survey of cannula use in France. ASAIO J 2013;59:157–61. [DOI] [PubMed] [Google Scholar]
- 16.Anekwe DE, Koo KK, de Marchie M, et al. Interprofessional Survey of Perceived Barriers and Facilitators to Early Mobilization of Critically Ill Patients in Montreal, Canada. J Intensive Care Med 2017:885066617696846. [DOI] [PubMed] [Google Scholar]
- 17.Rupprecht L, Lunz D, Philipp A, et al. Pitfalls in percutaneous ECMO cannulation. Heart, lung and vessels 2015;7:320–6. [PMC free article] [PubMed] [Google Scholar]
- 18.Ngai CW, Ng PY, Sin WC. Bicaval dual lumen cannula in adult veno-venous extracorporeal membrane oxygenation-clinical pearls for safe cannulation. J Thorac Dis 2018;10:S624–S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burns J, Cooper E, Salt G, et al. Retrospective Observational Review of Percutaneous Cannulation for Extracorporeal Membrane Oxygenation. ASAIO J 2016;62:325–8. [DOI] [PubMed] [Google Scholar]
- 20.Conrad SA, Grier LR, Scott LK, et al. Percutaneous Cannulation for Extracorporeal Membrane Oxygenation by Intensivists. Critical Care Medicine 2015;43:1010–5. [DOI] [PubMed] [Google Scholar]
- 21.Serraino GF, Jiritano F, Rossi M, et al. Bedside Emergency Percutaneous Extracorporeal Membrane Oxygenator with Bicaval Dual-Lumen Cannula. Heart Surg Forum 2018;21:E290–E3. [DOI] [PubMed] [Google Scholar]
- 22.Hayes D Jr., Yates AR, Duffy VL, et al. Rapid placement of bicaval dual-lumen catheter in a swine model of venovenous ECMO. J Invest Surg 2014;27:27–31. [DOI] [PubMed] [Google Scholar]
- 23.Chacon MM, Shillcutt SK. Intraoperative Transesophageal Echocardiography-Guided Placement of Bicaval Dual-Lumen Extracorporeal Membrane Oxygenation Cannula. CASE (Phila) 2017;1:116–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayes D Jr., Preston TJ, Davis IC, et al. Contrast transthoracic echocardiography and the placement of a bicaval dual-lumen catheter in a Swine model of venovenous extracorporeal membrane oxygenation. Artificial organs 2013;37:574–6. [DOI] [PubMed] [Google Scholar]
- 25.Dolch ME, Frey L, Buerkle MA, et al. Transesophageal echocardiography-guided technique for extracorporeal membrane oxygenation dual-lumen catheter placement. ASAIO J 2011;57:341–3. [DOI] [PubMed] [Google Scholar]
- 26.Griffee MJ, Tonna JE, McKellar SH, et al. Echocardiographic Guidance and Troubleshooting for Venovenous Extracorporeal Membrane Oxygenation Using the Dual-Lumen Bicaval Cannula. J Cardiothorac Vasc Anesth 2018;32:370–8. [DOI] [PubMed] [Google Scholar]
- 27.Fisher MR, Forfia PR, Chamera E, et al. Accuracy of Doppler Echocardiography in the Hemodynamic Assessment of Pulmonary Hypertension. American Journal of Respiratory and Critical Care Medicine 2009;179:615–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conrad SA, Grier LR, Scott LK, et al. Percutaneous cannulation for extracorporeal membrane oxygenation by intensivists: a retrospective single-institution case series. Crit Care Med 2015;43:1010–5. [DOI] [PubMed] [Google Scholar]
- 29.Foster ED, Deardorff A. Open Science Framework (OSF). Journal of the Medical Library Association : JMLA 2017;105:203–6. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video Clip 1: Transgastric short axis, with new pericardial effusion associated with SVC injury.
Video Clip 2: Transgastric biplane view showing tip of cannula in right ventricle.
Video Clip 3: Modified bicaval view showing tip of cannula abutting Eustachian valve.
Video Clip 4: Modified bicaval view showing color flow Doppler, with evidence of intermittent obstruction of distal venous inflow orifice by Eustachian valve.
Video Clip 5: Transesophageal echocardiogram in mid esophagus with a four chamber view, showing cannula in right atrium, but out-of-plane in right ventricle, leading to a false assurance that the cannula was not misplaced in the right ventricle.
Video Clip 6: Subcostal view of the same patient as in clip 5, focused on the right ventricle, with cannula crossing the tricuspid valve and ending in the right ventricle apex.
Video Clip 7: Subcostal TTE, with Color flow Doppler demonstrating interaction between right ventricular apical myocardium and drainage orifices of tip of misplaced cannula, clearly illustrating that the tip is in the right ventricle. This position is unacceptable, because return flow of oxygenated blood enters distal cannula inflow orifices and because there is a risk of arrhythmia and damage to the right heart valves and myocardium.
Video Clip 8: Transthoracic echocardiogram right ventricular inflow view, showing cannula traversing tricuspid valve and ending well within right ventricle. This is a view of the right ventricle which may reveal the true course of the cannula when four-chamber views are unrevealing.