Abstract
Background and study aims
Frontlines healthcare workers (HCWs) during the coronavirus disease 2019 (COVID-19) pandemic are at increased risk of infection by SARS-CoV-2, but there are limited data on the prevalence of COVID-19 among HCWs in Egypt. This study aimed to assess SARS-CoV-2 infection among HCWs providing gastroenterological services.
Subjects and methods
Seventy-four HCWs at the gastroenterological service of Al-Manial University Hospital, the main hospital of the largest tertiary university hospitals complex in Egypt (Kasr Al-Ainy Faculty of Medicine, Cairo University) were tested using real-time reverse transcription–polymerase chain reaction (RT-PCR) on nasopharyngeal samples, and rapid serological IgM/IgG tests (RST). A questionnaire was used to collect demographic, occupational and clinical data.
Results
Of the 74 HCWs, 10 tested positive by RT-PCR (13.5%). In 9/74 (12.2%) HCWs, antibodies could be detected by RST: three with both IgM and IgG lines; six with IgM line only and none with IgG line only. Frequency of positive tests was more among subjects with minor symptoms compared to completely asymptomatic HCWs (50% vs 16.1%, respectively). Neither age, gender or occupation was a risk factor for SARS-CoV-2 infection.
Conclusions
Point prevalence of COVID-19 in gastroenterology HCWs is 13.5% by RT-PCR. Continued measures are warranted to assure HCWs safety and reduce transmission from healthcare settings to the community during COVID-19 pandemic. Presence of positive test results among asymptomatic HCWs illustrates the importance of screening all HCWs irrespective of symptoms.
Keywords: COVID-19, SARS-CoV-2, HCWs, GI endoscopy
Introduction
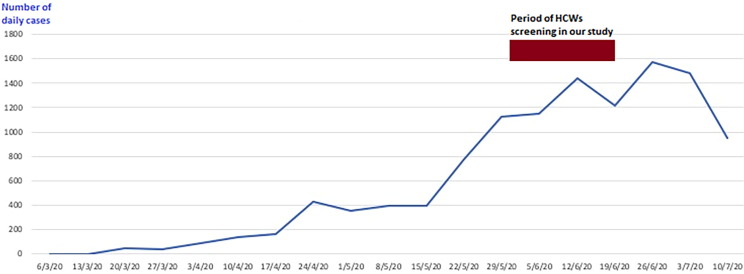
Since its emergence, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) causing the coronavirus disease 2019 (COVID-19) has become a global threat [1]. Egypt has been hit by this pandemic with the first confirmed case officially announced on 14 February 2020 [2]. The number of cases increased substantially to reach 26,384 cases by June 1st and exceed 80 000 cases by July 10th 2020 [3], [4]. It is assumed that there is a significant number of unreported cases due to several reasons [5]. Fig. 1 demonstrates the evolution of officially reported numbers of confirmed cases per day at weekly intervals till preparation of the manuscript.
Fig. 1.
Officially reported COVID-19 cases by day at weekly intervals according to Ministry of Health and Population, Egypt.
Healthcare workers (HCWs) have been significantly affected by the pandemic worldwide, as well as in Egypt [6]. Understanding the dynamics of SARS-CoV-2 infection in this population is essential to guide formulation of appropriate infection control measures [7].
Kasr Al-Aini Faculty of Medicine, Cairo University and its affiliated hospitals, is the largest university hospitals complex in Egypt with about 5600 beds, an outpatient clinic and emergency departments. It is a tertiary care referral centre delivering health services in all specialties. The gastroenterology services provided at Al-Manial University Hospital; the major hospital of this complex, include an inpatient ward with 40 beds, an outpatient clinic, an intensive care unit with 5 beds, a liver transplantation unit and a gastrointestinal endoscopy unit serving inpatients, outpatients as well as emergency cases.
Although gastroenterology departments are not primarily involved in management of COVID-19 patients during the current pandemic, re-arrangements in workflow and staff were undertaken to ensure safety of personnel as well as patients. Most services apart from those related to emergencies and non-deferrable indications were ceased. Strict regulations on the use of personal protective equipment (PPE) have been issued by the hospital administration.
In spite of numerous publications describing measures of infection control and protection of HCWs in gastrointestinal units [8], only few published studies describe the real-world outcomes of their implementation. This work has been conducted to determine the extent of infection by real-time reverse transcription polymerase chain reaction (RT-PCR) and rapid serological test (RST) for SARS-CoV-2 among frontline HCWs providing gastrointestinal services.
Subjects and methods
Ethical committee approval has been issued for the study. Between June 1st and 14th, 2020, all 138 healthcare workers employed in the gastroenterology service of Al-Manial University Hospital, Cairo University were invited to participate in the study according to the eligibility criteria: active clinical work in the department, no involvement in COVID-19 wards. Patients with a combination of major symptoms (fever, new persistent cough) were considered suspicious of COVID-19 and were temporarily excluded from work and were not entitled to enter the study [9]. Presence of isolated other minor symptoms, however, was not an exclusion criterion in order not to underestimate the extent of infection in the studied population [10]. Written informed consent was obtained from all participants.
At enrolment, participants completed a questionnaire comprising demographic data, occupation, past medical history, exposure to suspected or confirmed COVID-19 cases, application of recommended hand hygiene and personal protective equipment (PPE), in addition to symptoms compatible with COVID-19. Subjects were defined as symptomatic if presented with any of the following in the 14 days preceding the test: fatigue, myalgia, sore throat, rhinorrhoea, headache, ageusia or dysgeusia, anosmia, diarrhoea, nausea, or vomiting.
Molecular detection of SARS-CoV-2 (RT-PCR)
Nasopharyngeal swabs were collected for SARS-CoV-2 real-time reverse transcription polymerase chain reaction (RT-PCR) testing using TaqPath™ COVID‑19 CE‑IVD RT‑PCR Kit, 1000 reactions (Cat. No. A48067) from Thermofisher SCIENTIFIC. Viral RNA was extracted by QUIAGEN extraction Kit. The purified nucleic acid was reverse transcribed into cDNA and amplified using the TaqPath™ COVID‑19 RT‑PCR Kit in one step using Fast Dx Applied Biosystems 7500 real-time PCR instruments. In the process, probes annealed to three target sequences specific to SARS-CoV-2: ORF1ab, nucleocapsid (N) and spike (S) primers/probes for bacteriophage MS2. Two of the three genes and the MS2 (positive control) must be positive or the result was considered invalid.
SARS-CoV-2 rapid IgG-IgM test (RST)
Simultaneously, immunoglobulins were detected by COVID-19 IgM/IgG antibody rapid diagnostic test (Artron Laboratories, Burnaby, Canada). It is a qualitative lateral flow immunochromatographic assay for the rapid determination of presence or absence of both anti-SARS-CoV-2-IgM and anti-SARS-CoV-2-IgG in human specimens with a sensitivity of 93.4% and specificity of 97.7% as reported by the manufacturer. The result was read visually after 15–20 min.
Subjects who tested negative on RT-PCR but positive on RST were re-invited for taking a second nasopharyngeal swab for repeat RT-PCR within one week of the initial swab.
Statistical analysis
Continuous variables were described with medians and ranges. Categorical variables were described as frequency and percentages. The Pearson Chi-Square test was used as appropriate. A 2-sided p < 0.05 was considered statistically significant.
Results
In the fourteen-days period of the study, 74 HCWs (30 males and 44 females, with a median age of 32 years) accepted to participate in the study, representing 58.7% of the eligible working personnel. 40.5% of the enrolled HCWs were physicians (including residents and house officers), 37.8% were nurses, 12.2% involved in cleaning and patient transportation and 9.5% were administrative employees (Table 1 ). None of them reported known household contact with an infected person.
Table 1.
Occupational characteristics and results of RT-PCR and rapid serological test (RST) of HCWs screened for SARS-CoV-2.
| Occupation | Total working personnel (n = 138)* | Screened participants (n = 74)** | RT-PCR |
Rapid serological test (RST) |
|||
|---|---|---|---|---|---|---|---|
| Baseline PCR + ve** | 2nd PCR + ve** | IgM only + ve** | IgG only + ve** | IgM & IgG + ve** | |||
| Physicians | 59 | 30 (40.5%) | 3 (4.1%) | 1 (1.3%) | 1 (1.3%) | 0 (0%) | 1 (1.3%) |
| Nurses | 48 | 28 (37.8%) | 3 (4.1%) | 0 (0%) | 2 (2.7%) | 0 (0%) | 1 (1.3%) |
| Patient transporters/cleaners | 18 | 9 (12.2%) | 1 (1.3%) | 0 (0%) | 2 (2.7%) | 0 (0%) | 1 (1.3%) |
| Administrative employees | 13 | 7 (9.5%) | 2 (2.7%) | 0 (0%) | 1 (1.3%) | 0 (0%) | 0 (0%) |
| Total HCWs | 138 | 74 | 9 (12.2%) | 1 (1.3%) | 6 (8.1%) | 0 (0%) | 3 (4.1%) |
Including 12 HCWs already tested positive for SARS-CoV-2 via PCR and home-isolated.
Percentages expressed in relation to total number of screened participants (74).
It is worth mentioning that, in parallel, twelve HCWs (8.7% of the total personnel: 9 nurses and 3 physicians) diagnosed with SARS-CoV-2 infection after a vacation at the end of May 2020, were self-isolating during the time of the study, and excluded from initial analysis.
At baseline, the point prevalence of COVID-19, determined by detection of SARS-CoV-2 RNA in nasopharnygeal swabs was 12.2% (n = 9/74). Similarly, in 9 of 74 (12.2%) HCWs, antibodies could be detected by the rapid serological test (RST): Three participants showed evident both IgM and IgG lines (two of them with positive nasopharyngeal swab and one with negative swab); whereas, six showed well demarcated line of IgM at the RST with negative swab. None of the participants showed isolated IgG at baseline. After one week, all subjects with any positive RST result and negative nasopharyngeal swabs were re-tested for RT-PCR and only one of them tested positive (originally positive IgM at baseline).
Taken altogether, HCWs with at least one positive test (RT-PCR and/or RST) were 16 (21.6% of screened HCWs). The characteristics of these individuals are summarised in Table 2 . The median age of positive and negative HCWs was 32 and 31.5 years, respectively. The frequency of positive tests ranged from 13.3% among screened physicians to 21.4% among nurses, to 33.3% among patient transporters/cleaners and 42.9% among administrative employees. Three of the participants with positive tests had diabetes (n = 1) and hypertension (n = 2) in their medical history.
Table 2.
Characteristics of the 74 HCWs screened for SARS-CoV-2 and individuals with positive RT-PCR and/or rapid serological test (RST).
| Overall Screened HCWs (n = 74) | SARS-CoV-2 negative RT-PCR & RST (n = 58) | SARS-CoV-2 positive RT-PCR and/or RST (n = 16) | p-Value* | |
|---|---|---|---|---|
| Age in years: median (range) | 32 (23–48) | 31.5 (24–48) | 32 (23–43) | |
| Gender: n (%) - Female - Male |
44 (59.5%) 30 (40.5%) |
36 (62%) 22 (38%) |
8 (50%) 8 (50%) |
p = 0.68 |
| Occupation: n (%) - Physician - Nurse - Administrative employees - Patient transporters and cleaners |
30 (40.5%) 28 (37.8%) 7 (9.5%) 9 (12.2%) |
26 (44.8%) 22 (37.9%) 4 (6.9%) 6 (10.3%) |
4 (25%) 6 (37.4%) 3 (18.8%) 3 (18.8%) |
p = 0.28 |
| Symptoms at sampling: n (%) | 12 (16.2%) | 6 (10.3%) | 6 (37.5%) | p ≤ 0.01** |
| Exposure to suspected or confirmed COVID-19 | 56 (75.7%) | 44 (75.9%) | 12 (75%) | p = 0.83 |
| Proper hand hygiene practise | 72 (97.3%) | 57 (98.3%) | 15 (93.8%) | p = 0.13 |
| PPE when indicated: - Always, as recommended - Occasionally |
71 (95.9%) 3 (4.1%) |
56 (96.6%) 2 (3.4%) |
15 (93.8%) 1 (6.2%) |
p = 0.20 |
| Comorbidities: n (%) | 9 (12.2%) | 6 (10.3%) | 3 (18.8%) | p = 0.64 |
From chi-squared test.
Statistically significant.
Among HCWs with symptoms at time of swab, the frequency of positive tests was 50%, while among asymptomatic HCWs the frequency was significantly lower (16.1%). Among the 10 RT-PCR positive subjects, 6 reported concomitant mild symptoms consisting of sore throat (n = 2), headache (n = 3), diarrhoea (n = 1), myalgia (n = 1), rhinorrhea (n = 1), loss of smell and/or taste (n = 1) and fatigue (n = 1) but none had fever, cough or dyspnoea. None of the IgM positive/RT-PCR negative subjects had any symptoms at baseline.
Approximately, 75% of the total screened HCWs and of the positive RT-PCR &/or RST individuals reported exposure to suspected or confirmed COVID-19 individuals during work in the last two weeks. Most participants (greater than90%) confirmed that PPE was available at the workplace and they adhered to the recommendations of their use, as well as proper hand hygiene practise.
Inclusively, we added the 12 HCWs who had been simultaneously diagnosed to have positive SARS-CoV-2 via PCR to the ten patients diagnosed in our study and their characteristics are summarised in Table 3 . Among these RT-PCR positive subjects, 16 (72.7%) had concomitant symptoms consisting of fever (n = 4), cough (n = 3), dyspnoea (n = 1), sore throat (n = 2), headache (n = 5), diarrhoea (n = 2), myalgia (n = 5), rhinorrhea (n = 1), loss of smell and/or taste (n = 2) and fatigue (n = 6). On assessment of infection control measures, 95.5% of them reported proper hand hygiene practise, whereas, 31.8% of them reported inadequate PPE use (p ≤ 0.01). Furthermore, five of them had co-morbidities: Diabetes (n = 2), hypertension (n = 2) and bronchial asthma (n = 1).
Table 3.
Characteristics of total HCWs with laboratory-diagnosed COVID-19 by RT-PCR in the gastrointestinal department.
| SARS-CoV-2 Positive PCR (n = 22)* | |
|---|---|
| Age in years: median (range) | 32 (23–56) |
| Gender: n (%) - Female - Male |
15 (68.2%) 7 (31.8%) |
| Occupation: n (%) - Physician - Nurse - Administrative employees - Patient transporters and cleaners |
7 (31.8%) 12 (54.5%) 2 (9.1%) 1 (4.5%) |
| Symptoms at sample: n (%) | 16 (72.7%) |
| Contact with suspected or confirmed COVID-19 | 16 (72.7%) |
| Proper hand hygiene practise | 21 (95.5%) |
| PPE when indicated: - Always, as recommended - Occasionally |
15 (68.2%) 7 (31.8%) |
| Comorbidities: n (%) | 5 (22.7%) |
12 subjects diagnosed for SARS-CoV-2 via RT-PCR in addition to the 10 patients diagnosed in our study.
Discussion
During the 2003 SARS epidemic, HCWs accounted for more than 20% of all cases [11]. Similarly, reports of significant morbidity and mortality due to SARS-CoV-2 infection among HCWs have been emerging, raising concerns about possible collapse of healthcare systems, in addition to transmission from healthcare settings to the community [12]. To the best of our knowledge, this is the first study aiming at describing the impact of COVID‐19 on HCWs providing gastrointestinal services in a university hospital in Egypt.
In our study, the total SARS-CoV-2 cases confirmed by RT-PCR was 10/74 (13.5% of screened HCWs). Previous studies in developed countries reported variable infection rates in HCWs. In a study on 957 employees in a German university hospital, 52 of them (5.4%) tested positive for SARS-CoV-2 by PCR [13]. A similar rate was observed in a Dutch study on 1353 HCWs of whom 86 (6%) tested positive for SARS-CoV-2 via nasal swab [14]. In an Italian study, 138/1573 HCWs (8.8%) tested positive for SARS-CoV-2 infection via PCR [15]. Higher rates of infection of HCWs were observed in studies from the United Kingdom and Spain in March 2020, where 282/1533 symptomatic HCWs (18%) and 791/2085 (38%) were confirmed to be infected by SARS-CoV-2 infection via RT-PCR, respectively [16], [17]. On the contrary, data from developing countries are scarce. For instance, South African Health Minister reported on 6 May that 511 HCWs had tested positive for SARS-CoV-2 (7% of total national HCWs) [18].
Although gastrointestinal health services have been significantly reduced during the current pandemic, endoscopic procedures represent a source of aerosolization, putting the endoscopy staff at potential risk of SARS-CoV-2 infection from droplet inhalation [19]. Regarding infection rates in endoscopy units, one study in a referral endoscopy centre in Milan in April 2020, showed 2/38 HCWs positive by nasopharyngeal swabs (5.3%) [20]. A retrospective case series recorded on 968 HCWs from 41 endoscopy units in Northern Italy, showed that 42/968 HCWs in these units (4.3%) tested positive for COVID-19. In addition, 29 units (70.7%) did not report any case of infection in their endoscopy teams, whereas, 6 units reported high rates of infection (more than 10%) [21].
Rates of SARS-CoV-2 infection in HCWs during a pandemic might provide a snapshot of its prevalence in the community [17]. Despite denial by all screened HCWs in our study, we cannot exclude infection caused by non-recognised household contacts in the context of widespread community transmission of SARS-CoV-2 at the time of our study, coinciding with rapidly escalating case numbers in Egypt in May and June 2020, towards the epidemiological peak (Fig. 1).
One of the strengths of our study, is adding serological testing to molecular testing, the current standard method for diagnosing COVID-19, allowing more reliable capture of SARS-CoV-2 cases [6]. In 9/74 HCWs (12.2%), antibodies could be detected by RST, raising number of HCWs with at least one positive test (RT-PCR and/or RST) to 16 (21.6% of screened HCWs). In a similar study in a referral hospital in Belgium, 41/326 HCWs were confirmed by RT-PCR and/or serology representing an overall infection rate of 12.6% [6].
Results of RST should be interpreted cautiously and need further validation to determine their accuracy and reliability [22]. In our study, for HCWs with IgM detected by RST while negative at nasopharyngeal swabs, we had to consider the possibility of falsely positive RST or falsely negative nasopharyngeal swab, considering its estimated sensitivity for SARS-CoV-2 detection to be around 70% [23]. We tried to exclude the second possibility by repeating the swab within one week but we found the same results except in one physician who turned out positive. Similar scenarios might represent an epidemiological concern, as it could further spread the infection unnoticed, hampering the efficacy of the screening strategy.
In our study, neither age nor gender were risk factors for SARS-CoV-2 infection. When stratified according to occupation, the frequency of positive tests was higher among screened nurses compared to physicians. Surprisingly, positive test frequencies were lower among subsets with direct patient-near contact (physicians and nurses) than those without (cleaners and administrative employees) suggesting better compliance of the former groups. Consequently, screening of the latter groups and increasing their awareness about infection control measures should not be overlooked, even in non-COVID-19 wards.
A critical measure to reduce nosocomial transmission by respiratory droplets is the strict adherence to hand hygiene and PPE guidelines by all HCWs with direct contact with confirmed or suspected SARS-CoV-2 infected patients [24]. However, barriers against adoption of these strategies exist, including shortages of medical resources and cultural factors [25]. In our study, most HCWs (greater than 90%) confirmed proper adherence to proper hand hygiene. However, on assessment of individuals with positive swabs, 7/22 (31.8%) reported inadequate PPE use, highlighting the importance of enforcement of stricter standards of appropriate usage, including correct donning and doffing [26].
Importantly, another strength of our study is offering the opportunity to test asymptomatic volunteers. In most settings, testing of HCWs for COVID-19 has so far been restricted to individuals who are symptomatic themselves or have symptomatic household contacts. It should be underlined that in our department, 6/22 subjects (27.3%) were infected but displayed no symptoms even none of minor symptoms, meaning that at least one quarter of those infected could be missed with a symptom-based screening strategy. Therefore, it seems reasonable to tailor screening of HCWs based on resources available, taking in consideration that screening all HCWs irrespective of symptoms in high-resource settings, is the best approach to limit intra-hospital spread [10]. Additionally, through this study, we were able to alleviate the HCW’s anxiety for themselves and their relatives.
In conclusion, the point prevalence of COVID-19 in HCWs in the studied gastroenterology service is 13.5%. Continued measures are needed to ensure healthcare worker safety and reduce transmission from healthcare settings to the community during the current pandemic. Our data illustrates the importance of screening HCWs irrespective of symptoms.
Funding
This work was funded by a grant from the Ideation Fund (grant 7177) of the Academy of Scientific Research and Technology, Egypt.
Trial registration
ClinicalTrials.gov, NCT04424017
Declaration of competing interests
The authors declare that they have no known competing interests that may influence the work reported in this paper.
Acknowledgment
We would like to acknowledge the valuable logistic support provided by Dr. Tarek El-Mahdy.
References
- 1.Del Rio C., Malani P.N. 2019 novel coronavirus-important information for clinicians. JAMA. 2020;323(11):1039–1040. doi: 10.1001/jama.2020.1490. [DOI] [PubMed] [Google Scholar]
- 2.WHO, Coronavirus disease 2019 (COVID-19) Situation Report – 26. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200215-sitrep-26-covid-19.pdf?sfvrsn=a4cc6787_2 accessed on 15 February 2020.
- 3.Daily report on COVID-19 by Egyptian Ministry of Health and Population https://www.facebook.com/egypt.mohp/photos/a.123442675873020/177239867159967 accessed on 1 June 2020.
- 4.Daily report on COVID-19 by Egyptian Ministry of Health and Population. https://www.facebook.com/egypt.mohp/photos/a.123442675873020/196941851856435 accessed on 10 July 2020.
- 5.Hassany M., Abdel-Razek W., Asem N. Estimation of COVID-19 Burden in Egypt. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30319-4. S1473-3099(20)30319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin C., Montesinos I., Dauby N. Dynamic of SARS-CoV-2 RT-PCR positivity and seroprevalence among high-risk health care workers and hospital staff. J Hosp Infect. 2020 doi: 10.1016/j.jhin.2020.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y., Tong X., Wang J. High SARS-CoV-2 antibody prevalence among healthcare workers exposed to COVID-19 patients. J Infect. 2020 doi: 10.1016/j.jinf.2020.05.067. S0163-4453(20)30344-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Musa S. Hepatic and gastrointestinal involvement in Coronavirus Disease 2019 (COVID-19): what do we know till now? Arab J Gastroenterol. 2020;21(1):3–8. doi: 10.1016/j.ajg.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rivett L., Sridhar S., Sparkes D. Screening of healthcare workers for SARS-CoV-2 highlights the role of asymptomatic carriage in COVID-19 transmission. Elife. 2020;11(9) doi: 10.7554/eLife.58728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO. Population-based age-stratified seroepidemiological investigation protocol for COVID-19 virus infection. Version: 1.1. Date: 17 March 2020. Accessed 15 May 2020.
- 11.Koh D., Lim M.-K., Ong C.-N. Occupational health response to SARS. Emerg Infect Dis. 2005;11:167–168. doi: 10.3201/eid1101.040637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kursumovic E., Lennane S., Cook T.M. Deaths in healthcare workers due to COVID-19: the need for robust data and analysis. Anaesthesia. 2020 doi: 10.1111/anae.15116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwierzeck V., Correa-Martinez C.L., Schneider SARS-CoV-2 in the employees of a large university hospital. Dtsch Arztebl Int. 2020;117:344–345. doi: 10.3238/arztebl.2020.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kluytmans-van den Bergh M., Buiting A., Pas S. Prevalence and clinical presentation of health care workers with symptoms of coronavirus disease 2019 in 2 Dutch hospitals during an early phase of the pandemic. JAMA Network Open. 2020;3(5) doi: 10.1001/jamanetworkopen.2020.9673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lombardi A., Consonni D., Carugno M. Characteristics of 1,573 healthcare workers who underwent nasopharyngeal swab for SARS-CoV-2 in Milano, Lombardy Italy. Clin Microbiol Infect. 2020 doi: 10.1016/j.cmi.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keeley A.J., Evans C., Colton H. Roll-out of SARS-CoV-2 testing for healthcare workers at a large NHS Foundation Trust in the United Kingdom, March 2020. Euro Surveill. 2020;25:1–4. doi: 10.2807/1560-7917.ES.2020.25.14.2000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Folgueira MD, Muñoz-Ruipérez C, Alonso-López MA, et al. SARS-CoV-2 infection in Health Care Workers in a large public hospital in Madrid, Spain, during March 2020. April 27, 2020 preprint. doi: https://doi.org/10.1101/2020.04.07.20055723.
- 18.Grobler R. Coronavirus: 511 healthworkers positive, 26 hospitalised and 2 have died – Zweli Mkhize. News24, 6 May 2020. https://www.news24.com/SouthAfrica/News/coronavirus-511-health-workers-positive-26-hospitalised-and-2-have-died-zweli-mkhize-20200506 (accessed 10 July 2020).
- 19.Wu C., Chen X., Cai Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan. China JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dioscoridi L, Carrisi C. COVID-19 Exposure risk of healthcare personnel in digestive endoscopy: a prospective study. May 2020. Preprint DOI: 10.21203/rs.3.rs-31812/v1.
- 21.Repici A., Aragona G., Cengia G., On behalf of the ITALIAN GI-COVID19 Working Group Low risk of covid-19 transmission in GI endoscopy. Gut. 2020 doi: 10.1136/gutjnl-2020-321341. [DOI] [PubMed] [Google Scholar]
- 22.Tang Y.W., Schmitz J.E., Persing D.H. The laboratory diagnosis of COVID-19 infection: current issues and challenges. J Clin Microbiol. 2020 doi: 10.1128/JCM.00512-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang Y., Zhang H., Xie J., Lin M., Ying L. Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verbeek J.H., Rajamaki B., Ijaz S. Personal protective equipment for preventing highly infectious diseases due to exposure to contaminated body fluids in healthcare staff. Cochrane Database Syst Rev. 2020;4:CD011621. doi: 10.1002/14651858.CD011621.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.ASGE Quality Assurance in Endoscopy Committee, et al. Gastrointest Endosc 2018; 87:1167–1179. [DOI] [PubMed]
- 26.Black J., Bailey C., Przewrocka J. COVID-19: the case for health-care worker screening to prevent hospital transmission. Lancet (London, England) 2020;395(10234):1418–1420. doi: 10.1016/S0140-6736(20)30917-X. [DOI] [PMC free article] [PubMed] [Google Scholar]