Abstract
A characteristic feature of COVID-19 disease is lymphopenia. Lymphopenia occurs early in the clinical course and is a predictor of disease severity and outcomes. The mechanism of lymphopenia in COVID-19 is uncertain. It has been variously attributed to the release of inflammatory cytokines including IL-6 and TNF-α; direct infection of the lymphocytes by the virus; and rapid sequestration of lymphocytes in the tissues. Additionally, we postulate that prostaglandin D2 (PGD2) is a key meditator of lymphopenia in COVID-19. First, SARS-CoV infection is known to stimulate the production of PGD2 in the airways, which inhibits the host dendritic cell response via the DP1 receptor signaling. Second, PGD2 is known to upregulate monocytic myeloid-derived suppressor cells (MDSC) via the DP2 receptor signaling in group 2 innate lymphoid cells (ILC2). We propose targeting PGD2/DP2 signaling using a receptor antagonist such as ramatroban as an immunotherapy for immune dysfunction and lymphopenia in COVID-19 disease.
Keywords: Prostaglandin D2, Lymphopenia, COVID-19, Immunotherapy, Ramatroban, Immunosuppression, SARS-CoV-2
Abbreviations: PGD2, prostaglandin D2; DP1, D-prostanoid receptor 1; DP2, D-prostanoid receptor 2; ILC2, group 2 innate lymphoid cells; MDSC, monocytic myeloid-derived suppressor cells; COX, cyclo-oxygenase; Phospholipase, A2 (PLA2) group IID (PLA2G2D); rDC, respiratory dendritic cell; CCR7, C-C chemokine receptor type 7; Th2, T helper type 2
Lymphopenia is one of the characteristic features of COVID-19 disease in adults, and a predictor of morbidity and mortality [1], [2]. Patients with lymphopenia have more severe disease; correction of lymphopenia correlates with recovery from severe disease, while severe and sustained lymphopenia is associated with fatal outcomes [1], [2]. Consistent with higher mortality in adults with COVID-19, lymphopenia is more common in adults than children. In meta-analyses, 15% of the 1667 children, and over 50% of the 3,062 adults had lymphopenia [3], [4]. Lymphopenia was also observed in 46% of the 80 children, and about 70% of 138 adults in SARS-CoV 2003 infection, and lymphopenia was reported to persist for as long as 1 to 2 years [5], [6], [7].
The mechanisms underlying lymphopenia during SARS-CoV and SARS-CoV-2 infections remain unclear. Lymphocytes have minimal expression of angiotensin converting enzyme 2 (ACE2) [8], [9]. SARS-CoV and SARS-CoV-2 have not been demonstrated to directly infect lymphocytes [9]. Peripheral T lymphocytes, both CD4+ and CD8+, are rapidly reduced in acute SARS-CoV infection possibly due to lymphocytic infiltration and sequestration in specific target organs [10]. Lymphopenia, in the later stages of COVID-19 illness, may have been mediated by thymic involution and atrophy induced by hyperinflammation and cytokine release comprising of IL-6, TNF-α, and IL-1 [11]. However, lymphopenia has been reported to occur concurrently with the onset of clinical symptoms in COVID-19 [1]. We postulate that lymphopenia observed at the onset or during the early stages of COVID-19 illness is caused by increased generation of prostaglandin D2 by the respiratory epithelium.
Prostaglandin D2 (PGD2) is a key eicosanoid generated in respiratory infections. Severe bronchiolitis in infants caused by respiratory syncytial virus (RSV) leads to marked increase in PGD2 in the airways [12]. Mice infected with SARS-CoV also exhibit significant increases in PGD2 concentrations in the bronchoalveolar lavage fluid [13]. SARS-CoV respiratory infection stimulates PGD2 production by increased expression of phospholipase A2 group IID (PLA2G2D), cyclooxygenase-2 (COX-2), and hematopoietic PGD2 synthase (hPGDS) [14]. Furthermore, protein sequences in the spike and nucleocapsid proteins of SARS-CoV activate the expression of the COX-2 gene [15], [16]. Increased expression of PLA2G2D and hPGDS genes also occurs with aging, leading to increased levels of PGD2 in the airways of the elderly [13]. Compared to the 6-week old mice, there is a 300–400% increase in the airways’ PGD2 levels in 12-month old and 22-month old mice [13]. PGD2 action is mediated by binding to two G-protein coupled receptors, D-prostanoid receptor 1 (DP1); and D-prostanoid receptor 2 (DP2), formerly known as chemoattractant receptor-homologous molecule on T helper type 2 cells (CRTH2) [17]. PGD2 has been reported to affect the host’s innate and adaptive immune responses to viruses including SARS-CoV as described below.
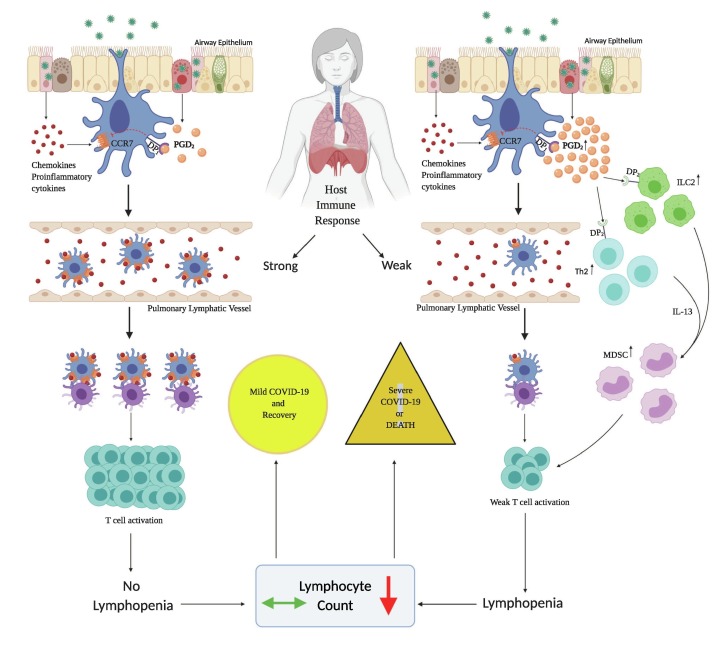
Early in infection, activated respiratory dendritic cells (rDC) undergo a maturation process that includes upregulation of costimulatory ligands, antigen-presenting complexes, and importantly, chemokine receptors such as C–C chemokine receptor type 7 (CCR7) [13]. The elevated levels of chemokine receptors facilitate migration of antigen-bearing rDCs to the local draining lymph nodes (DLNs) in the mediastinum where they participate in initiating adaptive host immune response to the respiratory virus. PGD2/DP1 signaling in the airway epithelial cells leads to the inhibition of CCR7 which suppresses rDC migration to draining lymph nodes. This leads to impairment of T lymphocyte priming and maturation, thereby leading to lymphopenia [13], [18]. Second, PGD2/DP2 signaling stimulates Group 2 innate lymphoid cells (ILC2) and T helper 2 (Th2) cells to secrete interleukin-13 (IL-13). IL-13 upregulates monocyte-macrophage derived suppressor cells (MDSC), which downregulates the T-lymphocyte response, causing lymphopenia [19], [20], [21]. MDSCs mediated impairment of pathogen specific adaptive immune responses has been demonstrated with Hemophilus influenzae respiratory infection [22]. Interestingly, ILC2, despite their scarcity, are the dominant innate lymphoid cell population in the lung, indicating a key role as first responders and amplifiers upon immune challenge at this site [23].
Based on the above findings, we hypothesize that an increase in airway PGD2 levels initiates lymphopenia in COVID-19 (Fig. 1 ). We propose that antagonism of PGD2 synthesis or signaling can prevent lymphopenia or promote recovery of lymphocyte counts in COVID-19 disease. However, suppression of PGD2 synthesis will inhibit PGD2/DP1 signaling which has been demonstrated to attenuate inflammation and reduce vascular permeability [24], [25]. Therefore, selective targeting of PGD2/DP2 signaling, while sparing PGD2/DP1 axis, is necessary to restore immune dysfunction during the symptomatic phase of COVID-19. Ramatroban is a potent, reversible, and selective antagonist of PGD2/DP2 receptors that has been shown to inhibit PGD2 stimulated IL-13 secretion, with an IC-50 of 118 nM [17], [20]. Ramatroban has been used orally as a treatment for allergic rhinitis in Japan for the past 20 years.[26] Given the global disease burden of the COVID-19 pandemic, there is an urgent need to examine the role of eicosanoids including PGD2 in the pathogenesis of the disease, and to investigate the potential immunotherapeutic role of PGD2 antagonists such as ramatroban.
Fig. 1.
Proposed mechanism of lymphopenia in patients with COVID-19. A host specific, exuberant PGD2 response early in infection, initiates DP1 signaling, which inhibits the dendritic cell function by downregulating CCR7, leading to a weak T cell response. PGD2/DP2 signaling stimulates respiratory ILC2 and Th2 cells, which secrete IL-13. IL-13 stimulates proliferation of MDSC cells, thereby downregulating the pathogen specific T cell responses. Excessive PGD2 action via DP1 receptors during the incubation period and DP2 receptors during the symptomatic stage leads to lymphopenia. Lymphopenia is a predictor of morbidity and mortality in COVID-19.
Disclosure of Potential Sources of Conflict of Interest
AG has filed three provisional patent applications for use of PGD2 and thromboxane A2 antagonists, including ramatroban, as a treatment for COVID-19 (Application numbers: 63/003,286 filed on March 31; 2020; 63/005,205 filed on April 3, 2020; and 63/027,751 filed on May 2, 2020). Other authors have not declared conflict of interest. Ramatroban (Baynas®) was approved in Japan for allergic rhinitis in 2000.
Acknowledgments
Acknowledgement
The authors thank Prof. Srinivasa T. Reddy, Ph.D. for his critical review of the manuscript.
Author Contributions
AG conceptualized, created the inventive concept and the framework for the manuscript; KCC and AG wrote the original draft; and both reviewed and edited the final version.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mehy.2020.110122.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Tan L., Wang Q., Zhang D. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Trans Targeted Therapy. 2020;5(1) doi: 10.1038/s41392-020-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Z., Zhou Q., Wang C. Clinical characteristics of children with COVID-19: a rapid review and meta-analysis. Ann Transl Med. 2020;8(10):620. doi: 10.21037/atm-20-3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu J., Ji P., Pang J. Clinical characteristics of 3,062 COVID-19 patients: a meta-analysis. J Med Virol. 2020 doi: 10.1002/jmv.25884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee N., Hui D., Wu A. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348(20):1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- 6.Stockman L.J., Massoudi M.S., Helfand R. Severe acute respiratory syndrome in children. Pediatr Infect Dis J. 2007;26(1):68–74. doi: 10.1097/01.inf.0000247136.28950.41. [DOI] [PubMed] [Google Scholar]
- 7.Li T., Xie J., He Y. Long-term persistence of robust antibody and cytotoxic T cell responses in recovered patients infected with SARS coronavirus. PLoS ONE. 2006;1(1) doi: 10.1371/journal.pone.0000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Catanzaro M., Fagiani F., Racchi M., Corsini E., Govoni S., Lanni C. Immune response in COVID-19: addressing a pharmacological challenge by targeting pathways triggered by SARS-CoV-2. Signal Trans Targeted Therapy. 2020;5(1) doi: 10.1038/s41392-020-0191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li M.-Y., Li L., Zhang Y., Wang X.-S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Diseas Poverty. 2020;9(1) doi: 10.1186/s40249-020-00662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li T., Qiu Z., Zhang L. Significant changes of peripheral T lymphocyte subsets in patients with severe acute respiratory syndrome. J Infect Dis. 2004;189(4):648–651. doi: 10.1086/381535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scarpa R., Costa L., Del Puente A., Caso F. Role of thymopoiesis and inflamm-aging in COVID-19 phenotype. Pediatrics Neonatol. 2020 doi: 10.1016/j.pedneo.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Werder R.B., Lynch J.P., Simpson J.C. PGD2/DP2 receptor activation promotes severe viral bronchiolitis by suppressing IFN-lambda production. Sci Transl Med. 2018;10(440) doi: 10.1126/scitranslmed.aao0052. [DOI] [PubMed] [Google Scholar]
- 13.Zhao J., Zhao J., Legge K., Perlman S. Age-related increases in PGD(2) expression impair respiratory DC migration, resulting in diminished T cell responses upon respiratory virus infection in mice. J Clin Invest. 2011;121(12):4921–4930. doi: 10.1172/JCI59777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vijay R., Hua X., Meyerholz D.K. Critical role of phospholipase A2 group IID in age-related susceptibility to severe acute respiratory syndrome-CoV infection. J Exp Med. 2015;212(11):1851–1868. doi: 10.1084/jem.20150632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan X., Hao Q., Mu Y. Nucleocapsid protein of SARS-CoV activates the expression of cyclooxygenase-2 by binding directly to regulatory elements for nuclear factor-kappa B and CCAAT/enhancer binding protein. Int J Biochem Cell Biol. 2006;38(8):1417–1428. doi: 10.1016/j.biocel.2006.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu M., Gu C., Wu J., Zhu Y. Amino acids 1 to 422 of the spike protein of SARS associated coronavirus are required for induction of cyclooxygenase-2. Virus Genes. 2006;33(3):309–317. doi: 10.1007/s11262-005-0070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kupczyk M., Kuna P. Targeting the PGD2/CRTH2/DP1 signaling pathway in asthma and allergic disease: current status and future perspectives. Drugs. 2017;77(12):1281–1294. doi: 10.1007/s40265-017-0777-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braciale T.J., Kim T.S. Slowing down with age: lung DCs do it too. J Clin Invest. 2011;121(12):4636–4639. doi: 10.1172/JCI61367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arima M., Fukuda T. Prostaglandin D2and TH2 inflammation in the pathogenesis of bronchial asthma. Korean J Internal Med. 2011;26(1):8. doi: 10.3904/kjim.2011.26.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xue L., Gyles S.L., Wettey F.R. Prostaglandin D2 Causes preferential induction of proinflammatory Th2 cytokine production through an action on chemoattractant receptor-like molecule expressed on Th2 cells. J Immunol. 2005;175(10):6531–6536. doi: 10.4049/jimmunol.175.10.6531. [DOI] [PubMed] [Google Scholar]
- 21.Trabanelli S., Chevalier M.F., Martinez-Usatorre A. Tumour-derived PGD2 and NKp30-B7H6 engagement drives an immunosuppressive ILC2-MDSC axis. Nat Commun. 2017;8(1):593. doi: 10.1038/s41467-017-00678-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Santo C., Salio M., Masri S.H. Invariant NKT cells reduce the immunosuppressive activity of influenza A virus–induced myeloid-derived suppressor cells in mice and humans. J Clin Investig. 2008;118(12):4036–4048. doi: 10.1172/JCI36264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mindt B.C., Fritz J.H., Duerr C.U. Group 2 innate lymphoid cells in pulmonary immunity and tissue homeostasis. Front Immunol. 2018;9 doi: 10.3389/fimmu.2018.00840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murata T., Aritake K., Tsubosaka Y. Anti-inflammatory role of PGD2 in acute lung inflammation and therapeutic application of its signal enhancement. Proc Natl Acad Sci USA. 2013;110(13):5205–5210. doi: 10.1073/pnas.1218091110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsubosaka Y., Maehara T., Imai D. Hematopoietic prostaglandin D synthase-derived prostaglandin D2 ameliorates adjuvant-induced joint inflammation in mice. FASEB J. 2019;33(6):6829–6837. doi: 10.1096/fj.201802153R. [DOI] [PubMed] [Google Scholar]
- 26.Ishizuka T., Matsui T., Okamoto Y., Ohta A., Shichijo M. Ramatroban (BAY u 3405): a novel dual antagonist of TXA2 receptor and CRTh2, a newly identified prostaglandin D2 receptor. Cardiovasc Drug Rev. 2004;22(2):71–90. doi: 10.1111/j.1527-3466.2004.tb00132.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.