Abstract
Background
Coronavirus Disease-19 (COVID-19) is associated with a high rate of thrombosis, the pathophysiology of which is not well defined. Viscoelastic testing may identify and characterise hypercoagulable states which are not apparent using conventional coagulation assays.
Objectives
The objective of this study was to undertake viscoelastic evaluation of the coagulation state in critically ill adults with COVID-19–associated respiratory failure
Methods
This was a single-centre observational point prevalence cohort study of adults with COVID-19–associated respiratory failure requiring respiratory support in the intensive care unit. Coagulation status was evaluated using rotational thromboelastometry (ROTEM®) in conjunction with laboratory markers of coagulation.
Results
Six patients fulfilled inclusion criteria. Each patient had one ROTEM® performed. All patients had supranormal clot amplitude at 10 min (A10) and supranormal clot firmness (maximal clot firmness) measured in at least one ROTEM® pathway, and five were supranormal on all pathways. Minimal clot lysis was present on all analyses. Fibrinogen and D-dimer were elevated and routine markers of coagulation within normal ranges in all patients.
Conclusion
Patients with COVID-19–associated respiratory failure admitted to the intensive care unit exhibit a hypercoagulable state which is not appreciable on conventional tests of coagulation. Supranormal clot firmness, minimal fibrinolysis, and hyperfibrinogenaemia are key findings. Further research is required into the pathophysiology of this hypercoagulable state, as well as the harms and benefits of different anticoagulation strategies.
Keywords: Viscoelastic testing, Rotational thromboelastometry, COVID-19, Coronavirus, ARDS
1. Introduction
Our understanding of the mechanisms by which the novel SARS-coronavirus-2, the virus causing coronavirus disease-2019 (COVID-19), causes organ failure and death continues to evolve. Several lines of investigation implicate a procoagulant tendency associated with COVID-19 which leads to microvascular and macrovascular thrombosis. Clinical reports of disproportionate rates of venous and arterial thromboembolism1 are corroborated by histological evidence of thrombotic microangiopathy in postmortem studies of patients dying from COVID-19.2 Intravascular thromboses can lead to local ischaemia, microhemorrhage, and inflammation which, in the lung, is consistent with the observations that a subset of patients exhibit profound hypoxia with relatively preserved lung compliance.3 Furthermore, thrombotic microangiopathy could underlie the acute kidney injury which affects up to 25.2% of patients with COVID-19 admitted to intensive care units (ICUs).4
Rotational thromboelastometry (ROTEM®) and thromboelastography (TEG) are viscoelastic point-of-care tests which provide functional analysis of clot development and fibrinolytic function. In addition to their role in bleeding,5 they are able to identify a variety of patient populations at increased risk of thromboembolic events.6 We undertook an observational cohort study to evaluate the coagulation state, as defined by viscoelastic testing, in patients admitted to the ICU requiring respiratory support for COVID-19–associated lung disease. Our objective was to identify whether these patients exhibited a procoagulant state.
2. Methods
We undertook a single-centre observational point prevelence cohort study in the 43 bed Intensive Care Unit of the Royal Adelaide Hospital, a metropolitan tertiary centre and South Australian designated hospital for COVID-19 in April 2020. Local ethics approval was granted, and waiver of consent obtained (Central Adelaide Local Health Network Human Research Ethics Committee Reg 13102). All patients >18 y old with COVID-19–associated respiratory failure admitted to the ICU were identified using our electronic medical record (Sunrise, Allscripts, USA) from which demographic and clinical data were derived. Diagnosis of SARS-CoV-2 was performed with reverse transcription polymerase chain reaction (RT-PCR) from throat or nasal swab. The Sequential Organ Failure Assessment score was calculated as previously described7, with neurological component excluded owing to limitations in performing this in the sedated patient. Peripheral (arterial) blood was analysed by ROTEM® (ROTEM® Sigma - Tem Innovations GmbH, Germany). EXTEM, INTEM, and FIBTEM pathways, representing contact pathway, tissue factor pathway, and fibrinogen component, respectively,8 were analysed. Contemporaneous laboratory markers of coagulation and relevant blood pathology were recorded. Presence of lupus anticoagulant (LAC) was identified with dilute Russell's viper venom time and sensitive activated partial thromboplastin time tests. Patient records were reviewed 30 days after ICU discharge to determine the incidence of detected thromboembolic disease. Data are described using simple summary statistics using Stata, version 15.1 (StataCorp, College Station, TX, USA).
3. Results
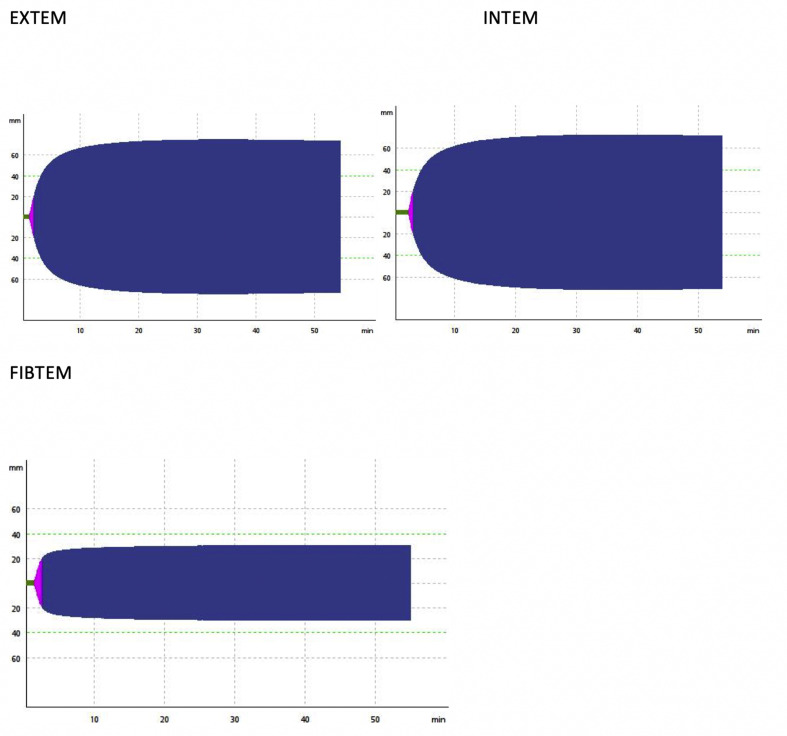
Six patients met the study inclusion criteria during the study period. Demographic and clinical information are summarised in Table 1 . Renal dialysis was provided through prolonged intermittent renal replacement therapy, with sessions complete for >6 h before blood sampling. Three patients were treated with prone positioning for respiratory failure, and no patients received pulmonary vasodilator therapy or extracorporeal membrane oxygenation. All patients were receiving thromboprophylaxis with 40 mg of enoxaparin administered subcutaneously daily. Each patient had one ROTEM® performed. ROTEM® and laboratory results are presented in Table 2 . A representative ROTEM® from our cohort is presented in Fig. 1 .
Table 1.
Demographics (n = 6).
| Demographics (n = 6) | Median [IQR] |
|---|---|
| Age | 69 [64.2 to 73] |
| Male, n (%) | 5 (83) |
| LOS ICU at the time of testing (days) | 14 [10 to 15] |
| APACHE II at admission | 43 [41 to 49.5] |
| APACHE II for 24 h before testing | 75.5 [65.75 to 105.5] |
| SOFA ex GCS | 7.5 [6.25 to 11.75] |
| Ventilated, n (%) | 5 (83) |
| RRT, n (%) | 2 (33) |
| ECMO, n (%) | 0 (0) |
APACHE II = Acute Physiology, Age and Chronic Health Evaluation II; ECMO = extracorporeal membrane oxygenation; GCS = Glasgow Coma Score, ICU = intensive care unit; LOS = length of stay; RRT = renal replacement therapy; SOFA = Sequential Organ Failure Assessment on the day of testing; IQR = interquartile range.
Table 2.
Results.
| Coagulation profile | Outside range, n (%) | Median [IQR] | Normal range | |
|---|---|---|---|---|
| EXTEM | A10 (mm) | 5/6 > NR (83) | 70 [68.25 to 74.75] | 43–63 mm |
| CFT (s) | 2/6 <NR (33) | 48.5 [41 to 60.5] | 46–149 s | |
| MCF (mm) | 5/6 > NR (83) | 74.5 [72.5 to 79.5] | 55–72 mm | |
| ML (%) | 1.5 [1 to 4.25] | 0–15% | ||
| FIBTEM | A10 (mm) | 6/6 > NR (100) | 30.5 [28.25 to 40.25] | 6–21 mm |
| MCF (mm) | 6/6 > NR (100) | 38 [30.5 to 45.5] | 6–21 mm | |
| ML (%) | 0 [0 to 0] | 0–15% | ||
| INTEM | A10 (mm) | 5/6 > NR (83) | 70.5 [66.25 to 71] | 43–62 mm |
| CFT (secs) | 5/6 < NR (83) | 39.5 [34.75 to 51] | 62–130 s | |
| MCF (mm) | 5/6 > NR (83) | 75.5 [72.75 to 77.5] | 51–69 mm | |
| ML (%) | 0.5 [0 to 1.75] | 0–15% | ||
| D-dimer (mg/L) | 6.1 [2.585 to 9.66] | 0–0.59 mg/L | ||
| PT (s) | 14.7 [14.075 to 14.925] | 12–16 s | ||
| INR | 1.1 [1.025 to 1.1] | 0.9–1.2 | ||
| aPTT (s) | 34 [30.25 to 43.75] | 24–38 s | ||
| Fibrinogen (g/L) | 7.5 [7.205 to 8.075] | 1.5–4 g/L | ||
| Platelets x 109/L | 290.5 [213 to 338] | 150–450 × 109/L | ||
| ATIII (%) | 83 [73.25 to 83.75] | 80–125% | ||
| PrC (%) | 113 [100.5 to 122.5] | 65–130% | ||
| PrS (%) | 122 [108 to 143.5] | 65–155% | ||
| Lupus anticoagulant (dRVVT) n = 5 (secs) | 6.1 [2.585 to 9.66] | 31–51 s | ||
A10 = maximum clot amplitude at 10 min; aPTT = activated partial thromboplastin time; ATIII = antithrombin III; CFT = clot formation time; dRVVT = Dilute Russell's viper venom time; INR = international normalised ratio; MCF = maximum clot firmness; ML = lysis index; PrC = protein C; PrS = protein S; PT = prothrombin time; IQR = interquartile range.
Fig. 1.
ROTEM® from a representative patient. ROTEM® = rotational thromboelastometry.
The most salient findings are the supranormal clot amplitude at 10 min and supranormal clot firmness in all analyses. In addition, the INTEM clot formation time was significantly reduced. Clot lysis was minimal (median <2%) in all analyses. In contrast, traditional coagulation parameters (prothrombin time, international normalised ratio, activated partial thromboplastin time, and platelet count) were within normal limits other than elevated fibrinogen and D-dimer. Endogenous anticoagulant proteins (antithrombin III, protein C, and protein S) were within normal range.
No patient had known arterial or venous thrombosis at the time of the study. Two patients later developed venous thromboembolism, one with pulmonary embolism, who underwent no deep vein thrombosis screening, and one with catheter-associated jugular vein thrombus. Both survived. A third patient died in the ICU, with venous thromboembolism not clinically suspected. The other three patients were discharged from our hospital alive without identified thromboembolic disease, including one with a negative dedicated computed tomography pulmonary arteriography scan before hospital discharge.
4. Discussion
Our data indicate that patients with COVID-19 admitted to the ICUs exhibit a prothrombotic tendency as evaluated by viscoelastic testing of clot formation. These data are consistent with both the clinical observation that thromboembolic disease is over-represented in patients with COVID-19 and histological evidence of thrombotic microangiopathy in nonsurvivors of COVID-19. In patients with COVID-19, there is early limited evidence of a procoagulant pattern on ROTEM®9 , 10 and TEG11 , 12 that appears to be persistent beyond admission. This procoagulant pattern is not commonly found in other groups of critically ill patients.[13], [14], [15]
Our cohort had been admitted to the ICU for a median of 14 days at the time of testing. This is the first study to evaluate for a prothrombotic state this late in intensive care admission, and our findings suggest that thromboembolic complications should be considered as a cause of clinical deterioration throughout the ICU admission and potentially beyond.
Virus-associated procoagulant phenotypes have been noted previously in severe acute respiratory syndrome-1 and Middle Eastern respiratory syndrome coronavirus infections16 although mechanisms remain unclear. Viral endotheliitis has been postulated, but the ROTEM® evaluation indicates that the procoagulant state is not dependent on an activated endothelial surface. COVID-19 has been associated with a hyperinflammatory state,17 which may directly contribute to hypercoagulability.
Depression of fibrinolysis, identified by failure of clot lysis at 30 min on TEG, has been described in selected patients with severe COVID-19 infection in a single-centre study, with strong correlation to thromboembolic events and renal injury.18 Our study identified similar results in unselected ICU patients, demonstrating that a persistent reduction in fibrinolysis and elevation in D-dimer was present throughout a small general COVID-19 ICU cohort, adding weight to this mechanism contributing to the increased thrombotic state of COVID-19.
Traditional clotting parameters were normal, consistent with findings from other studies.10 We did not measure factor VIII levels, but it has been previously shown that these are elevated in COVID-19–infected ICU patients.12 Importantly, endogenous antithrombotic proteins such as antithrombin III and proteins C and S were not significantly altered. To our knowledge, this is the first study where these endogenous proteins have been measured in COVID-19 and suggests that deficiencies in these counter-regulatory proteins do not underlie the clotting tendency identified in these patients.
Positive LAC has previously been described19 , 20 in a large proportion of patients. In contrast, antiphospholipid antibodies were absent in the five patients assayed in our cohort. It is unclear whether this is due to the small numbers in our study, differences in assays, or differences in timing of test performance. Of note, our tests were performed relatively late in the hospital presentation, and LAC can be transiently positive in acute viral infection.21
Our study has several limitations. Ours is a small, single-centre study, and findings can only be considered hypothesis generating, requiring validation in a larger multicentre cohort. The absence of a control group limits interpretation of our results; potential confounders include prolonged critical illness, immobility, and comorbidity. Our rate of thromboembolic disease was 33%, lower than reported in some other studies.1 , 19 Routine imaging surveillance for deep vein thrombosis was not undertaken, in keeping with our standard practice before the COVID-19 pandemic and also in light of the potential risks of healthcare worker exposure to patients with COVID-19, potentially explaining why the detected incidence appears low.
Future research priorities include (i) mapping changes in thrombotic tendencies over time, (ii) identifying efficacy and safety of anticoagulation strategies, and (iii) better understanding of the pathogenetic mechanisms of the prothrombotic state induced by SARS-CoV-2 and potentially other coronaviruses.
5. Conclusion
Patients with COVID-19–associated respiratory failure admitted to the ICU have a high prevalence of a hypercoaguable state which is not appreciable on conventional tests of coagulation. This is not explained by alterations in endogenous anticoagulant proteins or LAC. Supranormal clot firmness, minimal fibrinolysis, and hyperfibrinogenaemia are key findings, and further research is required into the drivers of these markers, as well as the influence of anticoagulant therapies on the prothrombotic state and patient outcomes.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Collett - conceptualisation, methodology, formal analysis, investigation, data curation, writing – original draft, writing – final draft.
Gluck - conceptualisation, methodology, investigation, data curation, writing – original draft.
Strickland - conceptualisation, methodology, investigation, data curation, writing – original draft.
Reddi - conceptualisation, methodology, formal analysis, investigation, data curation, writing – original draft, writing – final draft, Supervision.
Conflict of Interest
None of the authors have any interests to declare.
References
- 1.Klok F.A., Kruip M., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gattinoni L., Chiumello D., Caironi P., Busana M., Romitti F., Brazzi L. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46(6):1099–1102. doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centre ICNAaR . 2020. ICNARC report on COVID-19 in critical care. [Google Scholar]
- 5.Wikkelsø A., Wetterslev J., Møller A.M., Afshari A. Thromboelastography (TEG) or rotational thromboelastometry (ROTEM) to monitor haemostatic treatment in bleeding patients: a systematic review with meta-analysis and trial sequential analysis. Anaesthesia. 2017;72:519–531. doi: 10.1111/anae.13765. [DOI] [PubMed] [Google Scholar]
- 6.Harahsheh Y., Ho K.M. Use of viscoelastic tests to predict clinical thromboembolic events: a systematic review and meta-analysis. Eur J Haematol. 2018;100:113–123. doi: 10.1111/ejh.12992. [DOI] [PubMed] [Google Scholar]
- 7.Vincent J.L., Moreno R., Takala J., Willatts S., De Mendonca A., Bruining H. The SOFA (Sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the European society of intensive care medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 8.Akay O.M. The double hazard of bleeding and thrombosis in hemostasis from a clinical point of view: a global assessment by rotational thromboelastometry (ROTEM) Clin Appl Thromb Hemost Offic J Int Acad Clin Appl Thromb Hemost. 2018;24:850–858. doi: 10.1177/1076029618772336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spiezia L., Boscolo A., Poletto F., Cerruti L., Tiberio I., Campello E. COVID-19-Related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb Haemost. 2020;120:998–1000. doi: 10.1055/s-0040-1710018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pavoni V., Gianesello L., Pazzi M., Stera C., Meconi T., Frigieri F.C. Evaluation of coagulation function by rotation thromboelastometry in critically ill patients with severe COVID-19 pneumonia. J Thromb Thrombolysis. 2020;50:281–286. doi: 10.1007/s11239-020-02130-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ranucci M., Ballotta A., Di Dedda U., Bayshnikova E., Dei Poli M., Resta M. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemostasis. 2020;18(7):1747–1751. doi: 10.1111/jth.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Panigada M., Bottino N., Tagliabue P., Grasselli G., Novembrino C., Chantarangkul V. Hypercoagulability of COVID-19 patients in intensive care unit. A report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemostasis. 2020;18(7):1738–1742. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schött U., Larsson A. ROTEM: multiplate monitoring in the ICU and outcome scores. Crit Care. 2014;18:P93. [Google Scholar]
- 14.Crochemore T., Corrêa T.D., Lance M.D., Solomon C., Neto A.S., Guerra J.C.C. Thromboelastometry profile in critically ill patients: a single-center, retrospective, observational study. PloS One. 2018;13 doi: 10.1371/journal.pone.0192965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Müller M.C., Meijers J.C., Vroom M.B., Juffermans N.P. Utility of thromboelastography and/or thromboelastometry in adults with sepsis: a systematic review. Crit Care. 2014;18:R30. doi: 10.1186/cc13721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giannis D., Ziogas I.A., Gianni P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol. 2020;127:104362. doi: 10.1016/j.jcv.2020.104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20(6):355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wright F.L., Vogler T.O., Moore E.E., Moore H.B., Wohlauer M.V., Urban S. Fibrinolysis shutdown correlates to thromboembolic events in severe COVID-19 infection. J Am Coll Surg. 2020;231(2):193–203. doi: 10.1016/j.jamcollsurg.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M., Delabranche X. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harzallah I., Debliquis A., Drenou B. Lupus anticoagulant is frequent in patients with Covid-19. J Thromb Haemost. 2020 doi: 10.1111/jth.14867. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdel-Wahab N., Talathi S., Lopez-Olivo M.A., Suarez-Almazor M.E. Risk of developing antiphospholipid antibodies following viral infection: a systematic review and meta-analysis. Lupus. 2018;27:572–583. doi: 10.1177/0961203317731532. [DOI] [PubMed] [Google Scholar]