Abstract
The mitochondria are a major source of reactive oxygen species (ROS). Superoxide anion (O2•–) is produced by the process of oxidative phosphorylation associated with glucose, amino acid, and fatty acid metabolism, resulting in the production of adenosine triphosphate (ATP) in the mitochondria. Excess production of reactive oxidants in the mitochondria, including O2•–, and its by-product, peroxynitrite (ONOO–), which is generated by a reaction between O2•– with nitric oxide (NO•), alters cellular function via oxidative modification of proteins, lipids, and nucleic acids. Mitochondria maintain an antioxidant enzyme system that eliminates excess ROS; manganese superoxide dismutase (Mn-SOD) is one of the major components of this system, as it catalyzes the first step involved in scavenging ROS. Reduced expression and/or the activity of Mn-SOD results in diminished mitochondrial antioxidant capacity; this can impair the overall health of the cell by altering mitochondrial function and may lead to the development and progression of kidney disease. Targeted therapeutic agents may protect mitochondrial proteins, including Mn-SOD against oxidative stress-induced dysfunction, and this may consequently lead to the protection of renal function. Here, we describe the biological function and regulation of Mn-SOD and review the significance of mitochondrial oxidative stress concerning the pathogenesis of kidney diseases, including chronic kidney disease (CKD) and acute kidney injury (AKI), with a focus on Mn-SOD dysfunction.
Keywords: manganese superoxide dismutase, acute kidney injury, chronic kidney disease, mitochondria, peroxynitrite, posttranslational modification
Introduction
The prevalence of chronic kidney disease (CKD) has been increasing worldwide (Eckardt et al., 2013). CKD is characterized by the gradual loss of renal function over a period of months to years, ultimately leading to end-stage renal disease (ESRD). CKD has also been recognized as an independent risk factor for cardiovascular disease (CVD) (Go et al., 2004; Gansevoort et al., 2013). In contrast, acute kidney injury (AKI), also known as acute renal failure, is defined as the sudden and rapid loss of renal function, often within 48 h (KDIGO, 2012). AKI is closely associated with the increased risk of developing CKD, and progression is directly dependent on the severity of AKI (Fiorentino et al., 2018). Therefore, to suppress these outcomes, it will be necessary to have a better understanding of the underlying fundamental mechanisms related to progression and onset of both CKD and AKI.
Reactive oxygen species (ROS) can damage cellular proteins, lipids, and DNA, ultimately leading to cellular dysfunction (Wang et al., 2018). Mitochondria are the major source of endogenous ROS, which depends directly on the metabolic and redox state of the mitochondria. Metabolic state refers to the efficiency of electron transfer from nutrients to molecular oxygen (O2). Electrons that are leaked from the respiratory chain react with O2 to produce superoxide anions (O2•–). Mitochondria have ROS scavenging systems via which O2•– is converted to H2O2 by the actions of superoxide dismutase (SOD), including Cu/Zn-SOD and manganese superoxide dismutase (Mn-SOD) (Mailloux, 2015; Kim et al., 2017; Wang et al., 2018). Further decomposition of H2O2 to H2O and O2 is catalyzed by other antioxidative enzymes within the mitochondria, including glutathione peroxidase (GPx) and peroxiredoxin (PRx)/thioredoxin (TRx) (Mailloux, 2015; Kim et al., 2017; Wang et al., 2018). At low levels, ROS are second messengers for signal transduction and may serve to regulate cellular adaptation against several stressors. However, oxidative stress results when ROS production exceeds homeostatic levels due to an imbalance between ROS production and decomposition.
The kidney is a highly metabolic organ with high levels of oxidation within cellular mitochondria. Renal tissues have a large energy demand, most notably within the proximal tubular cells; this is related to their role in reabsorbing critical nutrients after glomerular filtration. As such, the kidney is particularly vulnerable to damage caused by oxidative stress. Previous reports have shown that mitochondrial oxidative stress may be directly linked to mechanisms underlying the onset and progression of both CKD and AKI (Che et al., 2014; He et al., 2017). Excess mitochondrial ROS may promote mitochondrial dysfunction, ATP depletion, inflammation, and apoptosis in association with kidney disease (Che et al., 2014; He et al., 2017). Among ROS scavenging enzymes in the mitochondrial matrix, Mn-SOD has a primary responsibility for O2•– scavenging within mitochondria; as such, dysfunction of Mn-SOD results in mitochondrial oxidative stress. Collective experimental evidence has suggested a relationship between Mn-SOD dysfunction and the pathogenesis of kidney disease. In this review, we consider the significance of mitochondrial oxidative stress on the pathogenesis of kidney disease, including CKD and AKI, with a focus on the role of Mn-SOD dysfunction.
Biological Function of Manganese Superoxide Dismutase on Reactions and Transformations of Superoxide Anion
Human Mn-SOD, encoded by the sod2 gene, is located on chromosome 6q25.3 (Creagan et al., 1973) (Figure 1). The sequence of Mn-SOD is highly conserved, with over 40% homology among proteins from human, yeast, and Escherichia coli (Barra et al., 1984). Mn-SOD is a tetrameric enzyme with four identical subunits, each harboring a manganese ion (Mn2+/Mn3+) as a cofactor. Mn-SOD is located primarily within the mitochondrial matrix (Borgstahl et al., 1992; Karnati et al., 2013). By contrast, Cu/Zn-SOD is localized to the mitochondrial inner membrane space.
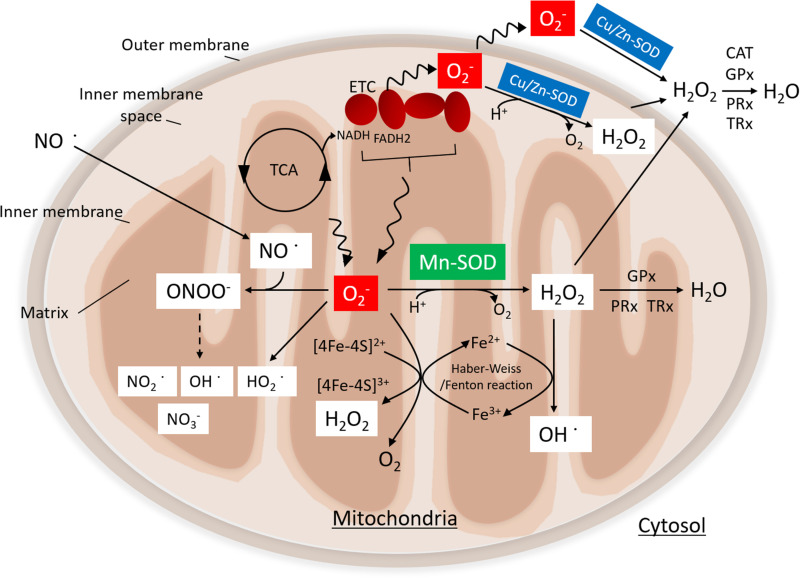
FIGURE 1.
Function of manganese superoxide dismutase (Mn-SOD). Superoxide (O2•–) is produced by the electron transport chain (ETC) during nutrient metabolism by the tricarboxylic acid (TCA) cycle. Mn-SOD is localized in the mitochondrial matrix, where it catalyzes the dismutation of O2•– to H2O2. By contrast, Cu/Zn-SOD, which is located in the inner membrane space of mitochondria, catalyzes the conversion of O2•– to hydrogen peroxide (H2O2). H2O2 is further metabolized by the glutathione peroxidase (GPx) and the peroxiredoxin (PRx)/thioredoxin (TRx) system in mitochondrial matrix or by catalase (CAT) GPx and PRx/TRx in the cytosol. O2•– and nitric oxide (NO•) can react to form peroxynitrite (ONOO–), giving rise to nitrogen dioxide (NO2•) and hydroxyl radical (OH•); eventually, stable nitrite (NO3–) is produced. H2O2 is converted to OH via the Fenton/Haber–Weiss reaction. O2•– can reduce ferric iron (Fe3+) to ferrous iron (Fe2+) in iron–sulfur centers of proteins, ultimately resulting in the production of H2O2. Moreover, the protonation of O2•– generates the hydroperoxyl radical, HO2.
O2•– production in mitochondria is closely linked to mitochondrial production of adenosine triphosphate (ATP), which takes place via electron transfer linked to nutrient (glucose, amino acids, and fatty acids) metabolism. Electrons are by-products of the tricarboxylic acid (TCA) cycle enzymes and respiratory complexes that promote a univalent reduction of oxygen (O2) to O2•–. Superoxide anion (O2•–) is an important signaling molecule but one that can be toxic at high concentrations. SOD enzymes, including Mn-SOD and Cu/Zn-SOD, catalyze the dismutation of O2•– to H2O2 in the mitochondrial matrix and the intermembrane space, respectively (Kawamata and Manfredi, 2010; Karnati et al., 2013; Mailloux, 2015). The GPx and PRx/TRx systems convert H2O2 to H2O within the mitochondrial matrix. Alternatively, H2O2 that has diffused into the cytoplasm is metabolized by catalase, GPx, and PRx/TRx. O2•– can also react with nitric oxide (NO•) to produce peroxynitrite (ONOO–). The decomposition of ONOO– gives rise to highly oxidizing intermediates, including nitrogen dioxide (NO2•), and hydroxyl radical (OH•); eventually, stable nitrite (NO3–) is produced (Tharmalingam et al., 2017; Radi, 2018). As such, elevated levels of O2•– may result in decreased bioavailability of NO and an increased toxic ONOO– production. Additionally, O2•– may reduce ferric iron (Fe3+) to ferrous iron (Fe2+) in iron–sulfur centers in critical proteins, which may lead to enzyme inactivation and concomitant loss of Fe2+ from the enzymes, thereby promoting production of H2O2 (Imlay, 2003). H2O2 may then react with Fe2+ to produce hydroxy radicals (OH•) via the Fenton/Haber–Weiss reaction. Moreover, protonation of O2•– may promote formation of the reactive hydroperoxyl radical HO2.
Oxidative stress induced by the imbalance between ROS production and the scavenging capacity of antioxidant protection mechanisms in the mitochondria leads to the inactivation of endogenous antioxidant systems, impairment of the electron transport, uncoupling of oxidative phosphorylation, and altered membrane permeability. As such, internal oxidative stress-mediated damage may be the main cause of mitochondrial dysfunction. Mn-SOD is the primary antioxidative enzyme that is responsible for scavenging O2•– in the mitochondrial matrix. Therefore, Mn-SOD dysfunction may result in overproduction of highly reactive oxidants, such as ONOO– and OH, which may result in mitochondrial dysfunction and disease development.
Transcriptional Regulation of Manganese Superoxide Dismutase
The sod2 gene has three major regulatory regions, namely, a proximal promoter, a 5′ upstream enhancer region, and an intronic enhancer region, which promotes activation or repression of sod2 gene expression (Xu et al., 2002).
Proximal Promotor of the sod2 Gene
The basic proximal promoter of the sod2 gene has no classical TATA or CAAT box, although it is enriched in CG repeats that contain binding sites for specificity protein 1 (Sp1) and activator protein 2 (AP-2) (Zhu et al., 2001; Xu et al., 2002). Sp1 binding to the promoter activates sod2 gene transcription, whereas AP-2 suppresses the transcriptional activity via competition with Sp1 at its binding site or by limiting its bioavailability via AP-2/Sp1 complex formation. As such, Sp1 and AP-2 regulate the basal level of sod2 gene transcription via antagonistic mechanisms at the proximal promoter region.
5′ Upstream Enhancer Region of the sod2 Gene
The 5′ upstream enhancer region of the sod2 gene includes binding sites for activating protein 1 (AP-1) (Borrello and Demple, 1997), cyclic AMP response element-binding protein (CREB) (Kim et al., 1999), nuclear factor, erythroid 2-like 2 (Nrf2) (Taylor et al., 2008), forkhead box O3 (FoxO3a) (Kops et al., 2002), nuclear factor-kappa B (NF-κB) (Warner et al., 1996), and hypoxia-inducible factor 1α (HIF-1α) (Gao et al., 2013). Binding of transcription factors at these sites within the sod2 5′ enhancer leads to an interaction with the basal transcriptional machinery of the proximal promoter, including Sp1. Nrf2 binds to the antioxidant responsive element (ARE) and acts as a master regulator of ARE-responsive antioxidant genes, including sod2 at homeostasis and in response to induction (Taylor et al., 2008; Li et al., 2015). FoxO-binding elements were identified upstream of the sod2 transcriptional start site; however, only the site at position −1,249 mediates FoxO3a-dependent transcription of sod2 gene in quiescent cells (Kops et al., 2002). In pathological conditions, activation of Akt (protein kinase B) promotes nuclear exclusion of FoxO3a via phosphorylation, thereby downregulating the transcription of sod2 (Kato et al., 2006; Li et al., 2006). FoxO3 is deacetylated by sirtuins, including SIRT1, SIRT2, and SIRT3, resulting in enhanced DNA binding and sod2 gene transcription (Jacobs et al., 2008). HIF-1α represses sod2 gene transcription in association with renal clear cell carcinoma (Gao et al., 2013). High levels of mitochondrial O2•– that result from the repression of sod2 gene expression lead to HIF-1α stabilization. Moreover, peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) also displays antioxidant defense responses via its role in promoting upregulated expression of antioxidative enzymes, including sod2 (Valle et al., 2005; St-Pierre et al., 2006; Lu et al., 2010). PGC-1α also plays an important role in mitochondrial biogenesis and respiration (Wu et al., 1999; Lin et al., 2005). Ding et al. (2017) reported that knockdown of SIRT3 inhibited osteogenic differentiation via its role in decreasing the stability of PGC-1α by increasing its acetylation and via downregulated expression of Mn-SOD; this results in elevated levels of ROS and reduced mitochondrial biogenesis and function. Cherry et al. (2014) reported that PGC-1α promotes mitochondrial biogenesis and induces expression of antioxidative enzyme, most notably Nrf2-mediated Mn-SOD expression in the liver of mice of Staphylococcus aureus-induced peritonitis.
Binding sites for p53 were identified in the 5′ upstream region of the sod2 gene (Pani et al., 2000; Drane et al., 2001). p53 exerts its inhibitory action via the prevention of Sp1 binding to the proximal promoter of sod2 gene (Dhar et al., 2006). Additionally, high levels of p53 expression serve to suppress sod2 expression, whereas low levels of p53 expression promote NF-κB binding to the enhancer region of the sod2 gene and increasing sod2 transcription (Dhar et al., 2010). In contrast, Hussain et al. (2004) reported that the promoter of sod2 contains p53 consensus binding sites, which located at −2,009 to −2,032 bp upstream of the transcription start site and induction of p53 leads to increased promoter activity and gene expression.
Intronic Enhancer Region of the sod2 Gene
The intronic enhancer contains a primary binding site for NF-κB (Jones et al., 1997; Xu et al., 1999). NF-κB positively regulates transcription via binding to the intronic enhancer region, which is located in the second intron of the sod2 gene by promoting interactions with CCAAT/enhancer-binding protein beta (C/EBP-β; Xu et al., 1999; Jones et al., 1997). NF-κB binding at the intronic enhancer region also enhances transcriptional activity of Sp1 in the proximal promoter of sod2 (Ennen et al., 2011).
Posttranslational Regulation of Manganese Superoxide Dismutase Activity
In addition to transcriptional regulation, previous reports indicated that Mn-SOD function is regulated by posttranslational modifications, including nitration, acetylation, phosphorylation, and glutathionylation.
Nitration
NO, a ubiquitous intracellular messenger, plays crucial roles in a variety of biological processes (Beckman and Koppenol, 1996). Overproduction of NO and O2•– leads to the formation of the extremely reactive ONOO–, which can result in lipid peroxidation, DNA damage, and protein nitration. ONOO– can generate covalent modifications of tyrosine (Tyr) residues in proteins, thereby reducing their functionality (Figure 2).
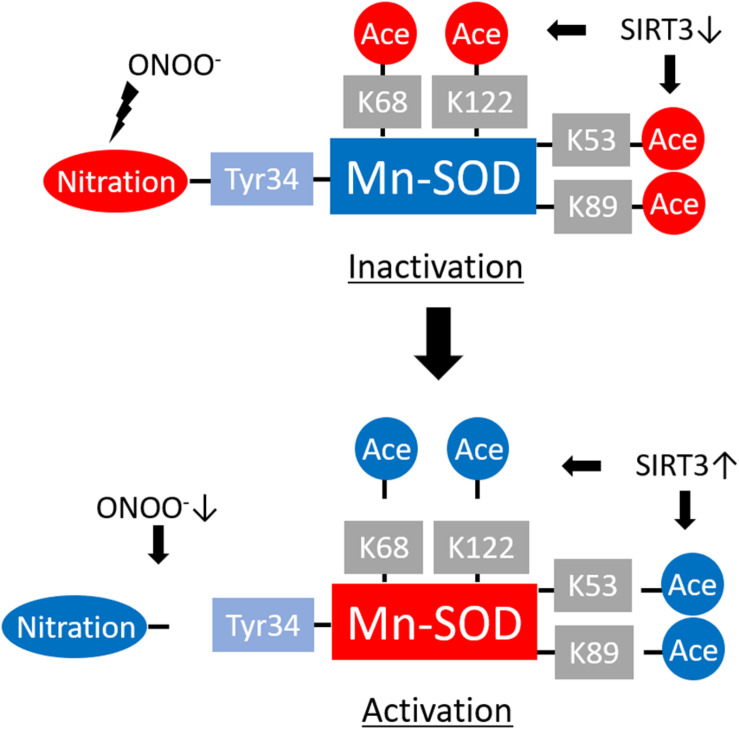
FIGURE 2.
Regulation of manganese superoxide dismutase (Mn-SOD) enzyme activity by translational modifications. ONOO– promotes Mn-SOD inactivation by nitrating the critical Tyr34 at the enzyme active site. Acetylation of Mn-SOD at K68, K122, K53, and K89 residues also induces Mn-SOD inactivation. Reduced levels of ONOO– and activation of NAD-dependent deacetylase sirtuin-3 (SIRT3) lead to Mn-SOD reactivation through denitrification or deacetylation of the target residues.
ONOO– leads to Mn-SOD deactivation via nitration of the critical active-site Tyr34 residue (MacMillan-Crow et al., 1998; Yamakura et al., 1998; Yamakura and Kawasaki, 2010) (Figure 2). Mn-SOD protects cells by O2•– scavenging, thereby preventing the interaction of O2•– with NO. However, Mn-SOD deactivation leads to the amplification of oxidative stress via accumulation of more O2•– that promotes not just only ONOO– formation in the mitochondria but also both the onset and progression of several diseases.
Acetylation
Nε-lysine acetylation is recognized as a critical posttranslational modification that regulates protein function, most notably that of histones (Narita et al., 2019). Non-histone protein acetylation modulates numerous cellular processes, including gene transcription, DNA damage and repair, cell division, signal transduction, protein folding, autophagy, and metabolism.
Sirtuins (SIRTs), which are NAD-dependent protein deacetylases, play significant roles in the regulation of protein acetylation (Guarente, 2011). SIRT3, SIRT4, and SIRT5 have been identified in the mitochondria (Guarente, 2011; Kitada et al., 2019). The deacetylase activity of SIRT3 is substantially higher than that of SIRT4 or SIRT5; as such, SIRT3 plays a critical role in mitochondrial biology and pathophysiological processes, including redox status, via its capacity to regulate acetylation of lysine (K) residues in mitochondrial proteins (Guarente, 2011; van de Ven et al., 2017; Kitada et al., 2019). Mn-SOD is among the target proteins of SIRT3. Chen et al. (2011) found that Mn-SOD K68 is an important site for SIRT3 regulation in human cells, whereas Tao et al. (2010) identified K122 and Qiu et al. (2010) identified K53 and K89 in Mn-SOD as the main sites of Sirt3 deacetylation in mouse cells (Figure 2). Overall, we conclude that SIRT3 deacetylates Mn-SOD at several lysine residues. SIRT3-mediated deacetylation results in enhanced Mn-SOD activity within a given nutrient and redox state and under various pathophysiological conditions. This results in decreased mitochondrial oxidative stress and prevents the progression of cellular aging and aging-related disease.
Other Modifications
Mn-SOD can also be phosphorylated, and this process has a direct impact on enzyme function. Candas et al. (2013) reported that the CyclinB1/CDK1-induced phosphorylation of Mn-SOD at Ser106 led to Mn-SOD activation and increased stability, which was associated with improved mitochondrial function and cellular resistance to apoptosis due to radiation. Additionally, cyclin-dependent kinase 4 (CDK4) located in mitochondria directly phosphorylates Mn-SOD at Ser106, resulting in increased Mn-SOD activity and mitochondrial respiration, notably in skin tissues of mice receiving whole-body low-dose ionizing radiation (Jin et al., 2015). The phosphorylation at Ser106 of Mn-SOD enhances its tetrameric conformation, stability, and enzymatic activity. By contrast, Hopper et al. (2006) reported an inverse correlation between phosphorylation and activity of Mn-SOD in mitochondria isolated from pig heart, and Ca2+-induced dephosphorylation increased Mn-SOD enzymatic activity. Thus, further studies are necessary to clarify how the phosphorylation or dephosphorylation of Mn-SOD is involved in the regulation of Mn-SOD activity.
Additionally, Zhao et al. (2017) identified Mn-SOD oxidation at histidine (His54 and His55), tyrosine (Tyr58), tryptophan (Trp147, Trp149, Trp205, and Trp210), and asparagine (Asn206 and Asn209) residues in human kidney tissues through mass spectrometry. In renal carcinoma cells, Mn-SOD oxidation at histidine (His54 and His55), tyrosine (Tyr58), and tryptophan (Trp147 and Trp149) residues was enhanced and associated with Mn-SOD deactivation (Zhao et al., 2017). John et al. (2009) also found that oxidation at His54 and His55 was associated with Mn-SOD deactivation in human medulloblastoma cells (John et al., 2009).
S-glutathionylation is the term that describes the addition of glutathione to cysteine residues of proteins; this process prevents irreversible oxidation of protein thiols and regulates a diverse array of cellular processes (Mailloux and Willmore, 2014). Mn-SOD can undergo S-glutathionylation within the mitochondria. Hopper et al. (2006) reported S-glutathionylation of recombinant rat Mn-SOD that was generated in E. coli. Mn-SOD cysteine (Cys) 196 was identified as a target of S-glutathionylation in the rat (Castellano et al., 2009). Patil et al. (2013) demonstrated that diamide, a biochemical that oxidizes glutathione and thus promotes protein glutathionylation, promoted reversible reductions in Mn-SOD activity when added to cultured rat renal tubular cells or when used to perfuse rat kidney in vivo.
Physiological Significance of Manganese Superoxide Dismutase on Kidney Disease in Animal Studies
Lessons From Manganese Superoxide Dismutase Gene-Altered Mice
Studies featuring knockout (KO) of Mn-SOD activity via inactivating mutations or due to the complete elimination of Mn-SOD expression have been performed in mice. These manipulations have resulted in massive oxidative stress and neonatal death associated with cardiomyopathy, neurodegeneration, lipid accumulation in the liver and skeletal muscle, and metabolic acidosis (Li et al., 1995; Holley et al., 2012). There were no obvious alterations in mitochondrial structure in Mn-SOD gene-deleted (–/–) mice; however, this gene deletion had a substantial impact on mitochondrial enzyme activity, including significant reductions in the activity of both succinate dehydrogenase and aconitase when compared with wild-type mice. By contrast, Lebovitz et al. (1996) previously generated a line of Mn-SOD knockout mice (SOD2mlBCM/SOD2mlBCM), in which the phenotype of mice is different from Mn-SOD knockout mice produced by other groups. Their mice survived longer than other group’s mice and exhibited extensively swollen and damaged mitochondria within degenerating neurons and cardiac myocytes. These discrepancies on the phenotype of Mn-SOD knockout mice possibly depend on differences in the molecular constructs used to generate the deletions and in the genetic backgrounds on which the mutations were being bred. Williams et al. (1998) reported that Mn-SOD heterozygotes (±) exhibited a 50% decrease in Mn-SOD activity, which was associated with impaired function and increased oxidative stress in the mitochondria in liver cells. Van Remmen et al. (2003) also reported increased and lifelong oxidative damage to DNA in liver, brain, and heart tissues of the Mn-SOD± mice; oxidative damage to DNA was associated with an increased incidence of cancer in these mice.
The physiological and pathological roles of Mn-SOD concerning renal function have been investigated using the heterozygous Mn-SOD± mice and with renal-specific Mn-SOD KO mice. Rodriguez-Iturbe et al. (2007) demonstrated oxidative stress and renal interstitial inflammation in a cohort of Mn-SOD± mice. These alterations were found to be compatible with accelerated renal senescence and the development of salt-sensitive hypertension. Additionally, Parajuli et al. (2011) created kidney-specific Mn-SOD KO mice from two different transgenic mouse lines. Specifically, “floxed” Mn-SOD mice (exon 3 of Mn-SOD allele flanked with LoxP sites in introns 2 and 3) were crossed with transgenic Ksp1.3/Cre (kidney Cre) mice. The Ksp1.3/Cre transgenic mice expressed Cre recombinase specifically in distal tubules, collecting ducts, loops of Henle, ureteric buds, and the developing genitourinary tract of the kidney. As such, the impact of the Mn-SOD KO is limited to these specific parts of the kidney. Kidney-specific Mn-SOD KO mice exhibited normal renal function; however, the localized Mn-SOD KO resulted in modest renal damage, including tubular dilation, epithelial cell enlargement, the formation of casts within the tubular lumen, and the accumulation of nitrotyrosine-modified protein. These data indicate that Mn-SOD plays a crucial role in maintaining redox homeostasis in kidney mitochondria.
Kidney Disease Animal Model Studies
Mitochondrial oxidative stress associated with Mn-SOD dysfunction in the kidney is involved in the pathogenesis of several kidney diseases, including AKI and CKD, as well as in the AKI to CKD transition. Mn-SOD dysfunction has been associated with glomerular and tubulointerstitial fibrosis, inflammation, excess apoptosis of renal cells, and tubular cell damage, as one of the sources of ROS in the mitochondria (Figure 3). We focused on the change of Mn-SOD in the kidney, such as posttranslational modification, activity, and expression levels, in animal models of kidney disease, and showed the summary in Table 1.
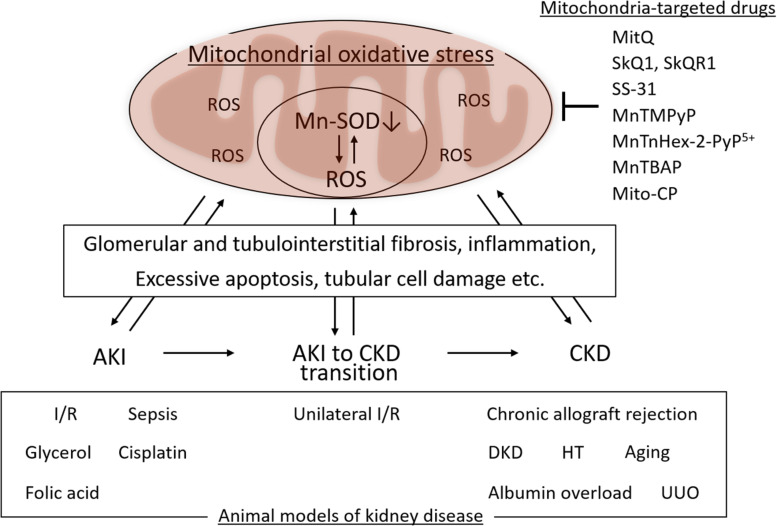
FIGURE 3.
Mitochondrial oxidative, manganese superoxide dismutase (Mn-SOD) dysfunction, and kidney disease. Mitochondrial oxidative stress associated with Mn-SOD dysfunction in the kidney is one of the key factors underlying the pathogenesis of kidney disease, including acute kidney injury (AKI), chronic kidney disease (CKD), as well as the AKI to CKD transition through glomerular and tubulointerstitial fibrosis, inflammation, excessive apoptosis of renal cells, and tubular cell damage. Several mitochondria-targeted drugs may improve the kidney disease through the improvement of mitochondrial oxidative stress. I/R, Ischemia/reperfusion; DKD, diabetic kidney disease; HT, hypertension; UUO, unilateral ureteral obstruction.
TABLE 1.
Change of renal manganese superoxide dismutase (Mn-SOD) in animal models of kidney disease.
| Type of kidney disease | Animal models of kidney disease | Change of Mn-SOD in the kidney | References |
| AKI | I/R-induced AKI models | ||
| I/R mice | Tyrosine nitration of Mn-SOD ↑ | Cruthirds et al., 2003 | |
| Acetylated Mn-SOD ↑ | Ouyang et al., 2019 | ||
| Sepsis-associated AKI models | |||
| CLP mice | Mn-SOD activity ↓ | Patil et al., 2014 | |
| Mn-SOD expression/activity ↓ | Chen G. D. et al., 2018 | ||
| CLP rats | Acetylated Mn-SOD ↑ | Xu, 2016 | |
| Other AKI models | |||
| Glycerol-induced AKI rats | Mn-SOD expression ↓ | Homsi et al., 2011 | |
| Cisplatin-induced AKI mice | Mn-SOD expression ↓ | Rattanavich et al., 2013 | |
| Folic acid-induced AKI mice | Mn-SOD expression ↓ | Zhang et al., 2019 | |
| AKI to CKD transition | Unilateral I/R mice | Mn-SOD expression ↓ | Zhang et al., 2018 |
| CKD | Chronic allograft rejection and associated nephropathy models | ||
| Chronic allograft rejection of rats | Tyrosine nitration of Mn-SOD ↑ | MacMillan-Crow et al., 1996, 2001 | |
| DKD models | |||
| db/db mice | Tyrosine nitration of Mn-SOD ↑ | Kitada et al., 2011 | |
| Mn-SOD expression ↓ | Lu et al., 2013; Hou et al., 2018 | ||
| STZ-induced diabetic Apo E–/– mice | Tyrosine nitration of Mn-SOD ↑ | Xu et al., 2006 | |
| Mn-SOD expression ↓ | Yi et al., 2011 | ||
| Zucker diabetic fatty rats | Acetylated Mn-SOD ↑ | Ogura et al., 2018 | |
| STZ-induced diabetic mice | Mn-SOD expression ↓ | Spencer et al., 2018; Li et al., 2019 | |
| A high-sugar/high-fat or cholesterol diet with STZ-induced diabetic rats | Mn-SOD expression ↓ | Onozato et al., 2004; Chen P. et al., 2018; Zayed et al., 2018 | |
| BTBR ob/ob mice | Mn-SOD expression ↓ | Morigi et al., 2020 | |
| Diabetic SHRs | Mn-SOD expression ↓ | Tang et al., 1997 | |
| KK/Ta-Akita mice | Mn-SOD expression ↑ | Fujita et al., 2009 | |
| Hypertension-related kidney injury models | |||
| Angiotensin II-induced hypertension rats | Tyrosine nitration of Mn-SOD ↑ | Guo et al., 2003 | |
| SHRs treated with the NOS inhibitor | Mn-SOD expression ↑, Mn-SOD activity ↑ | Majzunova et al., 2019 | |
| SHRs fed high-fat diet | Mn-SOD expression ↓ | Chung et al., 2012 | |
| Aging-related kidney injury models | |||
| 24 months old mice (vs. 12 months old mice) | Mn-SOD expression ↓ | Lim et al., 2012 | |
| Klotho–/– mice | Mn-SOD expression ↓ | Kimura et al., 2018 | |
| 16 months old mice (vs. 8 weeks old mice) | Mn-SOD expression ↓ | Hou et al., 2016 | |
| 20 months old mice (vs. 3 months old mice) | Mn-SOD expression ↓ | Teissier et al., 2019 | |
| 52 weeks old rats (vs. 13 weeks old rats) | Mn-SOD expression ↑ | Gomes et al., 2009 | |
| Other CKD models | |||
| Albumin-overload mice | Mn-SOD expression ↓ | Zhuang et al., 2015; Jia et al., 2016 | |
| UUO mice | Mn-SOD expression ↓ | Li et al., 2017; Liu et al., 2017 | |
AKI, Acute kidney disease; CKD, chronic kidney disease; I/R, ischemic/reperfusion; CLP, cecal ligation and puncture; STZ, streptozotocin; SHRs, spontaneously hypertensive rats; UUO, unilateral ureteral obstruction.
Ischemia/Reperfusion-Induced Acute Kidney Injury
Increased oxidative stress is a well-known factor that contributes to renal ischemia/reperfusion (I/R) injury (Noiri et al., 2001). Mitochondria are remarkably sensitive to I/R injury, as I/R rapidly promotes the generation of O2•– and other ROS, including ONOO–, within the injured mitochondria (Walker et al., 2000).
In the kidney of a rodent model of I/R AKI, Cruthirds et al. (2003) reported an acute increase in oxidative stress associated with increased levels of mitochondrial protein nitration, including Mn-SOD and cytochrome c, which resulted in Mn-SOD deactivation and mitochondrial dysfunction. Additionally, renal SIRT3 activity was reduced in response to I/R-induced AKI compared with activity observed among those in the control/sham-operated group. This was also accompanied by increased acetylation of both Mn-SOD and p53 (Ouyang et al., 2019). Taken together, these results suggest that inactivation of Mn-SOD induced by posttranslational modifications, such as nitration and acetylation, results in accelerated renal mitochondrial oxidative stress and contributes to the progression of I/R-induced AKI.
Le Clef et al. (2016) established a unilateral renal I/R model to study the associated mechanisms and therapies directed toward inhibiting the AKI to CKD transition in mice. Zhang et al. (2018) demonstrated that the mitochondrial complex-1 inhibitor, rotenone, slows the progression of AKI to CKD. By the mitochondrial complex-1 inhibitor, the expression of Mn-SOD and ATP synthase subunit β, mitochondrial DNA copy number, were restored, and inflammation and fibrosis were suppressed, in this model. These data suggest that mitochondrial O2•– may play a crucial role in promoting the pathogenesis associated with the transition from AKI to CKD.
Sepsis-Associated Acute Kidney Injury
Oxidative stress plays a crucial role in the development of mitochondrial dysfunction in response to septic AKI (Schrier and Wang, 2004). Patil et al. (2014) reported a decrease in renal Mn-SOD activity in association with the experiment in sepsis mice, which led to mitochondrial oxidative stress. Treatment with Mito-TEMPO, a mitochondrial-targeted mimetic of SOD, resulted in improvements in sepsis-induced AKI, which were directly associated with increased activation of Mn-SOD and decreased mitochondrial oxidative stress. Additionally, Chen G. D. et al., 2018 demonstrated that renal Mn-SOD expression and activity were reduced in sepsis-induced AKI and that insulin therapy reversed mitochondrial dysfunction via the suppression of the oxidative stress associated with upregulation of Mn-SOD. Furthermore, Xu (2016) found that SIRT1/SIRT3 activity was reduced in the renal tubular epithelial cells of rats with sepsis-associated AKI. This finding was accompanied by increasing levels of acetylated Mn-SOD, swollen mitochondria, and elevated levels of cellular apoptosis. Interestingly, activation of SIRT1 via treatment with resveratrol partially restored SIRT3 activity, which led to improved mitochondrial function and reduced levels of apoptosis. Taken together, these data indicate that Mn-SOD dysfunction and associated mitochondrial oxidative stress are both closely linked to mechanisms promoting disease pathogenesis in sepsis-related AKI.
Other Acute Kidney Injury Models
Glycerol-induced AKI results in increased expression of phosphorylated p53 in renal tissues in association with elevated levels of tubular cell apoptosis and oxidative stress due to decreased expression of antioxidative enzymes, including Mn-SOD (Homsi et al., 2011). Treatment with the p53 inhibitor, pifithrin-α, reversed features of glycerol-induced renal injury, including tubular cell apoptosis and oxidative stress via its capacity to restore Mn-SOD expression in the kidney (Homsi et al., 2011).
Oxidative stress and mitochondrial dysfunction related to ATP depletion have been observed in response to cisplatin-induced cellular injury in the kidney (Arany and Safirstein, 2003). Rattanavich et al. (2013) reported that cisplatin treatment results in diminished expression of renal Mn-SOD via the enhanced expression of phospho-p66ShcA and phospho-Foxo3A in studies featuring heterozygous p66ShcA (±) mice. Specifically, increased expression of phospho-p66ShcA and phospho-Foxo3A was detected in the renal tissue of wild-type mice treated with cisplatin in association with reduced expression of both Mn-SOD and catalase. By contrast, renal tissue from cisplatin-treated p66ShcA± mice revealed attenuated phosphorylation of p66ShcA and Foxo3a, together with a restored expression of Mn-SOD and catalase.
In the folic acid (FA)-induced AKI model, mitochondrial DNA copy number and expression of mitochondrial cytochrome c oxidase subunit 1, mitochondrial NADH dehydrogenase subunit 1, and Mn-SOD in the kidney were all reduced. These findings implicate mitochondrial dysfunction and oxidative stress in the pathogenesis of FA-induced AKI (Zhang et al., 2019). However, pretreatment with rotenone aggravated the renal injury, mitochondrial damage, and oxidative stress in this mouse model of AKI.
Chronic Renal Allograft Rejection
Chronic allograft rejection is currently the most important cause of renal transplant failure (Shrestha and Haylor, 2016). At the tissue level, increased oxidative stress observed in response to I/R may contribute to chronic allograft rejection and associated nephropathy. MacMillan-Crow et al. (1996, 2001) previously reported that endogenous tyrosine nitration and inactivation of Mn-SOD were observed in both human recipients and rat models of chronic renal allograft rejection. These data suggest that deactivation of Mn-SOD by ONOO– results in a progressive increase in the production of ONOO–, leading to irreversible oxidative injury to mitochondria in the renal allograft.
Diabetic Kidney Disease
Diabetic kidney disease (DKD) is among the main causes of ESRD and an independent risk factor for CVD (Ninomiya et al., 2009). Mitochondrial dysfunction has been linked to the pathogenesis of DKD, and mitochondrial oxidative stress is closely associated with mitochondrial dysfunction (Sharma, 2017; Xu et al., 2020). Dysfunction or decreased expression of Mn-SOD is one of the major factors associated with mitochondrial oxidative stress in diabetic kidney and, as such, with the pathogenesis of DKD.
The increased detection of nitrotyrosine in proximal tubules in renal biopsy samples from diabetic patients compared with non-diabetic control suggested that oxidant injury within the proximal tubules may play a critical role in the pathogenesis of DKD (Thuraisingham et al., 2000). Reduction of Mn-SOD activity in the kidney due to posttranslational modifications and oxidative stress has also been shown in several diabetic animal models. We previously demonstrated that mitochondrial oxidative stress is enhanced in the kidney of diabetic db/db mice when compared with kidneys from non-diabetic db/m controls. These observations were linked to tyrosine nitration of Mn-SOD and the associated reduction in enzyme activity (Kitada et al., 2011). Administration of resveratrol reversed the level of mitochondrial oxidative stress by limiting the extent of nitration, thereby increasing Mn-SOD activity in the diabetic kidney (Kitada et al., 2011). Similarly, in the kidneys of STZ-induced diabetic apolipoprotein E–/– mice, a decrease in Mn-SOD activity was associated with an increase in Mn-SOD Tyr-34 nitration (Xu et al., 2006). Antagonists of thromboxane A2 receptors limited diabetes-induced renal injury, which was also accompanied by a reduction of tyrosine nitration (Xu et al., 2006). Additionally, we found that mitochondrial oxidative stress was induced by a reduction in Mn-SOD activity via increased acetylation at K68; this finding was associated with a decreased intracellular NAD+/NADH ratio and decreased SIRT3 activity accompanied by increased expression of CD38, a NAD-degrading enzyme, in the kidneys of Zucker diabetic fatty rats (Ogura et al., 2018). Additionally, administration of the CD38 inhibitor, apigenin, resulted in improved mitochondrial oxidative stress through SIRT3 activation and Mn-SOD deacetylation (Ogura et al., 2020).
The expression of Mn-SOD is reduced in several diabetic animal models, including STZ-induced type 1 diabetes (Spencer et al., 2018; Li et al., 2019), a high-sugar/high-fat or cholesterol diet with STZ-induced diabetes (Onozato et al., 2004; Chen P. et al., 2018; Zayed et al., 2018), Apo E–/– mice with STZ-induced diabetes (Yi et al., 2011), db/db mice (Lu et al., 2013; Hou et al., 2018), BTBR ob/ob mice (Morigi et al., 2020), and diabetic spontaneously hypertensive rats (SHR) (Tang et al., 1997). This has been associated with increased oxidative stress in kidney mitochondria. However, the diabetic KK/Ta-Akita mice feature the downregulation of cytosolic Cu/Zn-SOD and extracellular Cu/Zn-SOD, but not mitochondrial Mn-SOD, in the kidney (Fujita et al., 2009). Additionally, Dugan et al. (2013) demonstrated no increased renal disease in STZ-induced diabetes in heterozygous Mn-SOD± mice over that observed in STZ-induced WT mice.
Hypertension-Related Kidney Injury
Oxidative stress in blood vessels and the kidney due to chronic hypertension may result from the dysregulated responses of several vasoconstrictor mechanisms, including those associated with angiotensin II stimulation or with dysfunction of nitric oxide synthase (eNOS). Angiotensin II-induced hypertension promotes an increase in O2•– generation via the activation of NADPH oxidase (Wang et al., 2001; Onozato et al., 2002). The antioxidant response is impaired in hypertensive animals, which results in oxidative stress followed by the development of end-organ damage, including ESRD. Guo et al. (2003) demonstrated that angiotensin II-induced hypertension in rats was accompanied by increased tyrosine nitration and thus deactivation of Mn-SOD that serves to augment oxidative stress in the kidney. Activation of NADPH oxidase also induces the production of O2•–, and ONOO– also may induce eNOS uncoupling, leading to overproduction of O2•– as opposed to NO.
Protein nitration was increased in the renal cortex of SHRs treated with the NOS inhibitor, L-NAME (Majzunova et al., 2019). Renal expression of Mn-SOD was increased in SHR in response to treatment with L-NAME, although total SOD activity remained unchanged (Majzunova et al., 2019). These data suggest that the impaired antioxidant response combined with a deficiency in NO may promote oxidative damage of proteins in the kidney via the production of ONOO– in SHRs. By contrast, SHRs that fed a high-fat diet (HFD) developed renal lipotoxicity, insulin resistance, and hypertension in association with a decrease in PPARα expression. This leads to Akt activation, increased FoxO3a phosphorylation, suppression of the PGC-1α-ERR-1α pathway, reduced expression of Mn-SOD, and increased oxidative stresses (Chung et al., 2012).
Aging-Related Kidney Dysfunction
Aging-related changes lead to the functional decline of several organs, including the kidney. Oxidative stress, particularly mitochondrial oxidative stress, has been recognized as one of the contributors to the aging process (Vlassara et al., 2009; Kume et al., 2010). Reduction or dysfunction of Mn-SOD-mediated mitochondrial oxidative stress may be involved in the pathogenesis associated with the aging kidney.
Lim et al. (2012) reported increased oxidative stress in the kidneys of aged (24 months old) mice compared with that observed among younger mice. This finding was associated with a reduction in Cu/Zn-SOD and Mn-SOD expression in the kidney, which may be mediated by decreases in SIRT1, PGC-1α, estrogen-related receptor (ERR)-1α, PPARα, and Klotho. Klotho has been identified as an antiaging gene that is primarily expressed in the kidney, parathyroid, and choroid plexus (Kuro-o et al., 1997); decreased Klotho expression has been correlated with kidney disease. Klotho exerts resistance against oxidative stress, possibly via the activation of FoxO transcription factors, which lead to increased expression of antioxidative enzymes, including Mn-SOD (Yamamoto et al., 2005). Klotho–/– mice exhibit enhanced nitrotyrosine levels and reduced levels of antioxidative enzymes, including Mn-SOD in the kidney (Kimura et al., 2018). Additionally, Hou et al. (2016) demonstrated that the production of endogenous hydrogen sulfide (H2S) in the aging kidney is insufficient. Exogenous H2S can reduce renal oxidative stress in aging mice via a mechanism that involves enhanced nuclear translocation of Nrf2 and increased expression of antioxidative enzymes, including Mn-SOD (Hou et al., 2016). Moreover, the endogenous advanced glycated end products (AGEs)/receptor for AGEs (RAGE) axis mediates factors associated with the aging process in many tissues and organs, including the kidney. Teissier et al. (2019) showed that older (20 months old) WT mice exhibited renal inflammation and oxidative stress with reduced expression of Mn-SOD compared with 3-month-old counterparts. By contrast, 20-month-old RAGE–/– mice were characterized by lower inflammation and elevated expression levels of Mn-SOD and SIRT1 in the kidney compared with their 20-month-old WT counterparts (Teissier et al., 2019).
By contrast, Gomes et al. (2009) detected increased levels of NADPH oxidase subunits and H2O2 in aged Wistar–Kyoto rats (52 weeks old) compared with those in young rats, although aged rats show no renal functional impairment or marked elevation in blood pressure. The aged rats did exhibit increased expression of antioxidative enzymes, including Mn-SOD, extracellular Cu/Zn-SOD, and catalase (Gomes et al., 2009), and these findings reveal that increases in antioxidant defenses may be useful for counteracting the damaging impact of oxidative stress.
Other Chronic Kidney Disease Animal Models
Albumin overload is a well-known model of renal tubulointerstitial injury caused by inflammation and fibrosis (Eddy, 1989). In the kidney of albumin-overload mice, Mn-SOD expression is reduced, thus promoting mitochondrial oxidative stress and inflammation. This condition can be improved by treatment with an Mn-SOD mimic [Mn (III)tetrakis(4-benzoic acid)porphyrin chloride (MnTBAP)] (Zhuang et al., 2015; Jia et al., 2016).
Unilateral ureteral obstruction (UUO) in a rodent model generates progressive renal fibrosis (Chevalier et al., 2009). Oxidative stress and inflammation are closely associated with the pathogenesis of UUO-induced renal fibrosis (Chevalier et al., 2009). In the kidney of UUO mice, the expression of antioxidative enzymes, including Mn-SOD, is reduced, resulting in mitochondrial oxidative stress, inflammation, and tubular epithelial–mesenchymal transition (EMT; Li et al., 2017; Liu et al., 2017). This condition can be improved by treatment with MnTBAP (Li et al., 2017; Liu et al., 2017).
Physiological Significance of Manganese Superoxide Dismutase on Kidney Disease in Human Studies
Manganese Superoxide Dismutase Gene Polymorphisms on Diabetic Kidney Disease
Ala16Val (rs4880C/T) is a functional Mn-SOD SNP polymorphism in exon 2 of sod2 gene. The substitution of C to T (GCT to GTT), resulting in the conversion from alanine (A) to valine (V), induces a conformational change in the mitochondrial targeting sequence. This results in less efficient transport of Mn-SOD into the mitochondrial matrix and decreases antioxidant activity within the mitochondria (Shimoda-Matsubayashi et al., 1996; Sutton et al., 2003). The relationship of this Mn-SOD gene polymorphism with DKD has been studied widely.
Previous reports revealed an association between the Mn-SOD gene polymorphism and the risk for developing DKD. Möllsten et al. (2007) reported that VV genotype was associated with an increased risk of diabetic nephropathy among Finnish and Swedish patients with type 1 diabetes. Nomiyama et al. (2003) identified the VV genotype with significantly higher frequency in the diabetic patients with nephropathy compared with the AA or VA genotype; however, this was unrelated to the etiology of type 2 diabetes in Japanese patients. By contrast, Houldsworth et al. (2015) demonstrated that the CC (alanine/alanine) genotype in type 1 diabetic patients might protect against diabetic nephropathy. According to another study on Korean type 2 diabetic patients, the alanine-encoding allele was detected at significantly lower frequency among patients with nephropathy who have micro- or macroalbuminuria compared with those patients without nephropathy (Lee et al., 2006). Liu et al. (2009) showed that the AA and VA genotypes were independent factors that protected against the development of nephropathy in Chinese type 2 diabetic patients. Ascencio-Montiel et al. (2013) demonstrated that in type 2 diabetes patients from Mexico, the frequency of the TT (valine/valine) genotype was 6.7% higher in patients with macroalbuminuria than in those in the normo-albuminuria group; the CC (alanine/alanine) genotype was associated with a lower risk of macroalbuminuria than the TT (valine/valine) genotype (Tian et al., 2011). Moreover, in a meta-analysis that included five studies focusing on type 2 diabetic patients, the alanine-encoding allele was associated with a reduced risk of macroalbuminuria (CR 0.65, 95% CI 0.52–0.80) compared with the valine-encoding allele (Tian et al., 2011). Likewise, a meta-analysis that included 17 published articles with both type 1 and 2 diabetic patients revealed a significant association of the alanine-encoding allele with a reduced risk of diabetic microvascular complications and nephropathy in the dominant model (OR 0.788, 95% CI 0.680–0.914 and OR 0.801, 95% CI 0.664–0.967, respectively) (Ascencio-Montiel et al., 2013).
Membranous Nephropathy
Membranous nephropathy (MN) is a leading cause of nephrotic syndrome in adults. Glomerular damage is induced by subepithelial immune deposits that consist mainly of IgG4 and complement components C5b-9. Several podocyte proteins, including aldose reductase (AR) and Mn-SOD, have been identified as renal antigens associated with the pathogenesis of MN (Prunotto et al., 2010). Confocal and immunoelectron microscopy (IEM) displayed co-localization of anti-AR and anti-Mn-SOD IgG4 and C5b-9 in electron-immune deposits in podocytes. Additionally, Murtas et al. (2012) demonstrated that the levels of serum IgG4 directed against AR and Mn-SOD in patients with MN are higher in MN than those in any of the other proteinuria-associated glomerulonephritides, including focal glomerulonephropathy and IgA nephritis.
Mitochondria-Targeted Therapies for Scavenging O2•–
Mitochondria are both sources and targets of ROS. The dysfunction of mitochondria due to oxidative stress may be the causal factor for kidney disease. As such, it will be necessary to develop pharmacological methods aimed at reducing oxidative damage to mitochondria and their components, including Mn-SOD. Several molecules that accumulate within the mitochondria and can scavenge ROS have been developed to address this issue. The beneficial effect of mitochondria-targeted drugs for kidney injury has been shown in several animal models (Table 2). However, those drugs are not delivered to only the kidney; therefore, the risk of adverse effects in systemic administration remains. To address this issue, it is necessary to develop the drugs that specifically deliver to the kidney.
TABLE 2.
Mitochondria-targeted drugs and animal models of kidney disease.
| Mitochondria-targeted drugs | Antioxidative moiety | Animal models of kidney disease showing the benefit of mitochondria-targeted drugs | References |
| MitoQ | Ubiquinone | I/R-induced AKI mice | Dare et al., 2015 |
| Sepsis-associated AKI rats | Lowes et al., 2008 | ||
| Cisplatin-induced AKI mice | Mukhopadhyay et al., 2012 | ||
| Ins2(+/)–(AkitaJ) mice | Chacko et al., 2010 | ||
| db/db mice | Xiao et al., 2017; Han et al., 2018 | ||
| ADPKD model mice (Ksp-Cre PKD1flox/flox) and rats (Han:SPRD Cy/+) | Ishimoto et al., 2017 | ||
| SkQ1 | Plastoquinone | mtDNA mutator mice | Shabalina et al., 2017 |
| SkQR1 | I/R-induced AKI rats | Plotnikov et al., 2011 | |
| Gentamicin-induced AKI rats | Jankauskas et al., 2012 | ||
| SS-31 | Tyr or Dmt (2′,6′-dimethyltyrosine) residues | I/R injury rats | Szeto et al., 2011 |
| PTRA-induced I/R pig | Eirin et al., 2012 | ||
| MnTMPyP | Manganese metalloporphyrin | I/R injury of rats | Liang et al., 2009 |
| MnTnHex-2-PyP5+ | I/R-induced AKI rats | Dorai et al., 2011 | |
| MnTBAP | Albumin-overload model mice | Zhuang et al., 2015; Jia et al., 2016 | |
| UUO model mice | Liu et al., 2017 | ||
| Mito-CP | Nitroxide CP | Cisplatin-induced AKI mice | Mukhopadhyay et al., 2012 |
MnTMPyP, MnP manganese (III) tetrakis (1-methyl-4-pyridyl) porphyrin; MnTnHex-2-PyP5+, Mn (III) meso-tetrakis (N-n-hexylpyridinium-2-yl) porphyrin; MnTBAP, Mn (III) tetrakis (4-benzoic acid) porphyrin chloride; CP, carboxy-proxyl; I/R, ischemic/reperfusion; AKI, acute kidney disease; ADPKD, autosomal dominant polycystic kidney disease; PTRA, percutaneous transluminal renal angioplasty; UUO, unilateral ureteral obstruction.
MitoQ
MitoQ is a derivative of ubiquinone conjugated to triphenylphosphonium (TTP), a lipophilic cation that enables this molecule to enter and accumulate within the mitochondria via an electrochemical gradient (Smith et al., 2003; Smith and Murphy, 2010). In the mitochondrial matrix, MitoQ is reduced to the active antioxidant form, ubiquinol, by the respiratory chain, which serves to protect the mitochondria and their components from oxidative damage (Smith et al., 2003; Smith and Murphy, 2010). Additionally, MitoQ is cleared rapidly from the plasma after intravenous administration and accumulates within the kidney (Porteous et al., 2010; Smith and Murphy, 2010). Previous animal studies have revealed the beneficial effects of MitoQ in association with various kidney disorders, including I/R-induced AKI (Dare et al., 2015; Kezic et al., 2016), sepsis-induced AKI (Lowes et al., 2008), cisplatin-induced kidney injury (Mukhopadhyay et al., 2012), DKD (Chacko et al., 2010; Xiao et al., 2017; Han et al., 2018), and polycystic kidney disease (Ishimoto et al., 2017).
SkQ1 and SkQR1
SkQ1 is a compound of plastoquinonyl-decyl-triphenylphosphonium in which ubiquinone was replaced by plastoquinone (Antonenko et al., 2008). SkQR1 is also a mitochondria-targeted compound and a conjugate of a positively charged rhodamine molecule with plastoquinone (Antonenko et al., 2008). SkQs, including SkQ1 and SkQR1, penetrate planar phospholipid membranes, and as such, they can accumulate in mitochondria (Antonenko et al., 2008). Administration of SkQ1 to mitochondrial DNA mutator mice resulted in diminished renal oxidative stress and mitochondrial dysfunction (Shabalina et al., 2017). In experimental rats with I/R injury, SkQR1 treatment normalized the ROS levels in kidney mitochondria, decreased blood urea nitrogen (BUN) and creatinine levels, and reduced disease-associated mortality, compared with what was observed among the rats without treatment (Plotnikov et al., 2011). Additionally, SkQR1 was also effective for the treatment of gentamicin-induced AKI (Jankauskas et al., 2012).
SS-31
The Szeto–Schiller (SS)-31 peptide, also known as Bendavia, may optimize the mitochondrial phospholipid cardiolipin microdomains. This may lead to reduced electron leak from the inner membrane (Brown et al., 2013). In an experimental model of I/R injury in the kidney, treatment with SS-31 attenuated renal oxidative stress prevented tubular cell apoptosis and necrosis, and reduced inflammation (Szeto et al., 2011). Rapid ATP recovery in mitochondria secondary to administration of SS-31 led to the protection of microvascular endothelial cells, reduced microvascular congestion, and, thus, better reflow to the medulla. In an experimental pig model featuring percutaneous transluminal renal angioplasty, administration of SS-31 resulted in decreased microvascular rarefaction, apoptosis, oxidative stress, tubular injury, and fibrosis, which were all associated with preserved mitochondrial biogenesis in the kidney after reperfusion (Eirin et al., 2012).
Manganese Superoxide Dismutase Mimics
Mn-SOD mimics are stoichiometric scavengers of O2•– and accumulate in mitochondria depending on their positive charge and lipophilicity (Miriyala et al., 2012). Among the cationic metalloporphyrins, the Mn porphyrins (MnP) are the most potent Mn-SOD mimics; these molecules have been designed and optimized for accumulation in the mitochondria where they mimic the action of the Mn-SOD catalytic site (Miriyala et al., 2012). The MnP manganese (III) tetrakis (1-methyl-4-pyridyl) porphyrin (MnTMPyP) functions as a peroxynitrite scavenger. Administration of MnTMPyP resulted in diminished lipid peroxidation and reduced nitrotyrosine content in the proximal tubular area in association with reductions in caspase-3 activation and tubular epithelial cell damage after I/R injury in rats (Liang et al., 2009). Similarly, administration of Mn (III) meso-tetrakis (N-n-hexylpyridinium-2-yl) porphyrin (MnTnHex-2-PyP5+) or Mn (III) tetrakis (4-benzoic acid) porphyrin chloride (MnTBAP) also resulted in protection against the deleterious effects associated with I/R-induced AKI (Dorai et al., 2011) as well as in the albumin-overload kidney injury (Zhuang et al., 2015; Jia et al., 2016) and the UUO model (Liu et al., 2017). Moreover, mitochondria-targeted carboxy-proxyl (Mito-CP) is a five-membered nitroxide CP conjugated to the TTP cation. Administration of Mito-CP resulted in improvements in parameters associated with cisplatin-induced AKI, including kidney dysfunction, renal inflammation, and tubular cell apoptosis, which were equivalent to those of MitoQ (Mukhopadhyay et al., 2012).
Conclusion and Perspectives
Mitochondrial oxidative stress is induced by an imbalance between ROS production and scavenging; the scavenging function may be impaired or destroyed due to dysfunction of antioxidative defense in mitochondria. Mitochondrial oxidative stress contributes to the pathogenesis of kidney disease, including AKI, CKD, and the AKI to CKD transition, and mitochondria-targeted drugs may suppress the onset or progression of kidney disease (Figure 3). Mn-SOD-associated dysfunction is one of the main factors underlying defective antioxidative defense in mitochondria. Mn-SOD dysfunction results in a net increase in ROS and generates a vicious cycle in which mitochondrial oxidative stress is amplified, as Mn-SOD then becomes a target of dysfunctional oxidative modification. An agent with the capacity to scavenge excess ROS in mitochondria would lead to a break in this vicious cycle, serving to preserve Mn-SOD and mitochondrial function and consequently suppress kidney disease. Although Mn-SOD may become dysfunctional in kidney disease, it remains unclear whether mitochondrial oxidative stress due to only Mn-SOD dysfunction is the primary contributor to the pathogenesis of kidney disease. Redox state in mitochondria is regulated by not only Mn-SOD but also other antioxidative enzymes, including Cu/Zn-SOD, GPx, and the PRx/TRx system. However, as Mn-SOD catalyzes the first step in ROS scavenging in mitochondria, treatments targeted at supporting Mn-SOD integrity and function may lead to effective treatments to prevent the onset and progression of kidney disease.
Author Contributions
MK designed, wrote, and edited the manuscript. JX, YO, IM, and DK contributed to the discussion. MK was the guarantor of this work. All authors contributed to the article and approved the submitted version.
Conflict of Interest
Boehringer Ingelheim, Mitsubishi Tanabe Pharma, Kyowa Kirin, Taisho Pharmaceutical Co., and Ono Pharmaceutical Co., contributed to establishing the Division of Anticipatory Molecular Food Science and Technology. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This study was supported in part by the Japan–China Sasakawa Medical Fellowship to JX.
References
- Antonenko Y. N., Avetisyan A. V., Bakeeva L. E., Chernyak B. V., Chertkov V. A., Domnina L. V., et al. (2008). Mitochondria-targeted plastoquinone derivatives as tools to interrupt execution of the aging program. Cationic plastoquinone derivatives: synthesis and in vitro studies. Biochemistry 73 1273–1287. 10.1134/s0006297908120018 [DOI] [PubMed] [Google Scholar]
- Arany I., Safirstein R. L. (2003). Cisplatin nephrotoxicity. Semin. Nephrol. 23 460–464. [DOI] [PubMed] [Google Scholar]
- Ascencio-Montiel I. J., Parra E. J., Valladares-Salgado A., Gómez-Zamudio J. H., Kumate-Rodriguez J., Escobedo-de-la-Peña J., et al. (2013). SOD2 gene Val16Ala polymorphism is associated with macroalbuminuria in Mexican type 2 diabetes patients: a comparative study and meta-analysis. BMC Med. Genet. 14:110. 10.1186/1471-2350-14-110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barra D., Schinina M. E., Simmaco M., Bannister J. V., Bannister W. H., Rotilio G., et al. (1984). The primary structure of human liver manganese superoxide dismutase. J. Biol. Chem. 259 12595–12601. [PubMed] [Google Scholar]
- Beckman J. S., Koppenol W. H. (1996). Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am. J. Physiol. 271 C1424–C1437. [DOI] [PubMed] [Google Scholar]
- Borgstahl G. E., Parge H. E., Hickey M. J., Beyer W. F., Jr., Hallewell R. A., Tainer J. A. (1992). The structure of human mitochondrial manganese superoxide dismutase reveals a novel tetrameric interface of two 4-helix bundles. Cell 71 107–118. 10.1016/0092-8674(92)90270-m [DOI] [PubMed] [Google Scholar]
- Borrello S., Demple B. (1997). NF Kappa B-independent transcriptional induction of the human manganous superoxide dismutase gene. Arch. Biochem. Biophys. 348 289–294. 10.1006/abbi.1997.0355 [DOI] [PubMed] [Google Scholar]
- Brown D. A., Sabbah H. N., Shaikh S. R. (2013). Mitochondrial inner membrane lipids and proteins as targets for decreasing cardiac ischemia/reperfusion injury. Pharmacol. Ther. 140 258–266. 10.1016/j.pharmthera.2013.07.005 [DOI] [PubMed] [Google Scholar]
- Candas D., Fan M., Nantajit D., Vaughan A. T., Murley J. S., Woloschak G. E., et al. (2013). CyclinB1/Cdk1 phosphorylates mitochondrial antioxidant MnSOD in cell adaptive response to radiation stress. J. Mol. Cell Biol. 5 166–175. 10.1093/jmcb/mjs062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano I., Cecere F., De Vendittis A., Cotugno R., Chambery A., Di Maro A., et al. (2009). Rat mitochondrial manganese superoxide dismutase: amino acid positions involved in covalent modifications, activity, and heat stability. Biopolymers 91 1215–1226. 10.1002/bip.21208 [DOI] [PubMed] [Google Scholar]
- Chacko B. K., Reily C., Srivastava A., Johnson M. S., Ye Y., Ulasova E., et al. (2010). Prevention of diabetic nephropathy in Ins2(+/)- (AkitaJ) mice by the mitochondria-targeted therapy MitoQ. Biochem. J. 432 9–19. 10.1042/bj20100308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che R., Yuan Y., Huang S., Zhang A. (2014). Mitochondrial dysfunction in the pathophysiology of renal diseases. Am. J. Physiol. Renal Physiol. 306 F367–F378. [DOI] [PubMed] [Google Scholar]
- Chen G. D., Zhang J. L., Chen Y. T., Zhang J. X., Wang T., Zeng Q. Y. (2018). Insulin alleviates mitochondrial oxidative stress involving upregulation of superoxide dismutase 2 and uncoupling protein 2 in septic acute kidney injury. Exp. Ther. Med. 15 3967–3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P., Shi X., Xu X., Lin Y., Shao Z., Wu R., et al. (2018). Liraglutide ameliorates early renal injury by the activation of renal FoxO1 in a type 2 diabetic kidney disease rat model. Diabetes Res. Clin. Pract. 137 173–182. 10.1016/j.diabres.2017.09.006 [DOI] [PubMed] [Google Scholar]
- Chen Y., Zhang J., Lin Y., Lei Q., Guan K. L., Zhao S., et al. (2011). Tumour suppressor SIRT3 deacetylates and activates manganese superoxide dismutase to scavenge ROS. EMBO Rep. 12 534–541. 10.1038/embor.2011.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry A. D., Suliman H. B., Bartz R. R., Piantadosi C. A. (2014). Peroxisome proliferator-activated receptor gamma co-activator 1-α as a critical co-activator of the murine hepatic oxidative stress response and mitochondrial biogenesis in Staphylococcus aureus sepsis. J. Biol. Chem. 289 41–52. 10.1074/jbc.m113.512483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier R. L., Forbes M. S., Thornhill B. A. (2009). Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney Int. 75 1145–1152. 10.1038/ki.2009.86 [DOI] [PubMed] [Google Scholar]
- Chung H. W., Lim J. H., Kim M. Y., Shin S. J., Chung S., Choi B. S., et al. (2012). High-fat diet-induced renal cell apoptosis and oxidative stress in spontaneously hypertensive rat are ameliorated by fenofibrate through the PPARα-FoxO3a-PGC-1α pathway. Nephrol. Dial. Transplant. 27 2213–2225. 10.1093/ndt/gfr613 [DOI] [PubMed] [Google Scholar]
- Creagan R., Tischfield J., Ricciuti F., Ruddle F. H. (1973). Chromosome assignments of genes in man using mouse-human somatic cell hybrids: mitochondrial superoxide dismutase (indophenol oxidase-B, tetrameric) to chromosome 6 Humangenetik. Redox Biol. 20 203–209. 10.1007/bf00385731 [DOI] [PubMed] [Google Scholar]
- Cruthirds D. L., Novak L., Akhi K. M., Sanders P. W., Thompson J. A., MacMillan-Crow L. A. (2003). Mitochondrial targets of oxidative stress during renal ischemia/reperfusion. Arch. Biochem. Biophys. 412 27–33. 10.1016/s0003-9861(03)00039-0 [DOI] [PubMed] [Google Scholar]
- Dare A. J., Bolton E. A., Pettigrew G. J., Bradley J. A., Saeb-Parsy K., Murphy M. P. (2015). Protection against renal ischemia-reperfusion injury in vivo by the mitochondria targeted antioxidant MitoQ. Redox Biol. 5 163–168. 10.1016/j.redox.2015.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar S. K., Xu Y., Chen Y., St. Clair D. K. (2006). Specificity protein 1-dependent p53-mediated suppression of human manganese superoxide dismutase gene expression. J. Biol. Chem. 281 21698–21709. 10.1074/jbc.m601083200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar S. K., Xu Y., St. Clair D. K. (2010). Nuclear factor kappaB- and specificity protein 1-dependent p53-mediated bi-directional regulation of the human manganese superoxide dismutase gene. J. Biol. Chem. 285 9835–9846. 10.1074/jbc.m109.060715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y., Yang H., Wang Y., Chen J., Ji Z., Sun H. (2017). Sirtuin 3 is required for osteogenic differentiation through maintenance of PGC-1α-SOD2-mediated regulation of mitochondrial function. Int. J. Biol. Sci. 13 254–264. 10.7150/ijbs.17053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorai T., Fishman A. I., Ding C., Batinic-Haberle I., Goldfarb D. S., Grasso M. (2011). Amelioration of renal ischemia-reperfusion injury with a novel protective cocktail. J. Urol. 186 2448–2454. 10.1016/j.juro.2011.08.010 [DOI] [PubMed] [Google Scholar]
- Drane P., Bravard A., Bouvard V., May E. (2001). Reciprocal down-regulation of p53 and SOD2 gene expression-implication in p53 mediated apoptosis. Oncogene 20 430–439. 10.1038/sj.onc.1204101 [DOI] [PubMed] [Google Scholar]
- Dugan L. L., You Y. H., Ali S. S., Diamond-Stanic M., Miyamoto S., DeCleves A. E., et al. (2013). AMPK dysregulation promotes diabetes-related reduction of superoxide and mitochondrial function. J. Clin. Invest. 123 4888–4899. 10.1172/jci66218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckardt K. U., Coresh J., Devuyst O., Johnson R. J., Köttgen A. (2013). Evolving importance of kidney disease: from subspecialty to global health burden. Lancet 382 158–169. 10.1016/s0140-6736(13)60439-0 [DOI] [PubMed] [Google Scholar]
- Eddy A. A. (1989). Interstitial nephritis induced by protein-overload proteinuria. Am. J. Pathol. 135 719–733. [PMC free article] [PubMed] [Google Scholar]
- Eirin A., Li Z., Zhang X., Krier J. D., Woollard J. R., Zhu X. Y., et al. (2012). A mitochondrial permeability transition pore inhibitor improves renal outcomes after revascularization in experimental atherosclerotic renal artery stenosis. Hypertension 60 1242–1249. 10.1161/hypertensionaha.112.199919 [DOI] [PubMed] [Google Scholar]
- Ennen M., Minig V., Grandemange S., Touche N., Merlin J. L., Besancenot V., et al. (2011). Regulation of the high basal expression of the manganese superoxide dismutase gene in aggressive breast cancer cells. Free Radic. Biol. Med. 50 1771–1779. 10.1016/j.freeradbiomed.2011.03.013 [DOI] [PubMed] [Google Scholar]
- Fiorentino M., Grandaliano G., Gesualdo L., Castellano G. (2018). Acute kidney injury to chronic kidney disease transition. Contrib. Nephrol. 193 45–54. 10.1159/000484962 [DOI] [PubMed] [Google Scholar]
- Fujita H., Fujishima H., Chida S., Takahashi K., Qi Z., Kanetsuna Y., et al. (2009). Reduction of renal superoxide dismutase in progressive diabetic nephropathy. J. Am. Soc. Nephrol. 20 1303–1313. 10.1681/asn.2008080844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gansevoort R. T., Correa-Rotter R., Hemmelgarn B. R., Jafar T. H., Heerspink H. J. (2013). Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet 382 339–352. [DOI] [PubMed] [Google Scholar]
- Gao Y. H., Li C. X., Shen S. M., Li H., Chen G. Q., Wei Q., et al. (2013). Hypoxia-inducible factor 1α mediates the down-regulation of superoxide dismutase 2 in von Hippel-Lindau deficient renal clear cell carcinoma. Biochem. Biophys. Res. Commun. 435 46–51. 10.1016/j.bbrc.2013.04.034 [DOI] [PubMed] [Google Scholar]
- Go A. S., Chertow G. M., Fan D., McCulloch C. E., Hsu C. Y. (2004). Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 351 1296–1305. 10.1056/nejmoa041031 [DOI] [PubMed] [Google Scholar]
- Gomes P., Simão S., Silva E., Pinto V., Amaral J. S., Afonso J., et al. (2009). Aging increases oxidative stress and renal expression of oxidant and antioxidant enzymes that are associated with an increased trend in systolic blood pressure. Oxid. Med. Cell Longev. 2 138–145. 10.4161/oxim.2.3.8819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente L. (2011). Franklin H. Epstein lecture: sirtuins, aging, and medicine. N. Engl. J. Med. 364 2235–2244. 10.1056/nejmra1100831 [DOI] [PubMed] [Google Scholar]
- Guo W., Adachi T., Matsui R., Xu S., Jiang B., Zou M. H., et al. (2003). Quantitative assessment of tyrosine nitration of manganese superoxide dismutase in angiotensin II-infused rat kidney. Am. J. Physiol. Heart Circ. Physiol. 285 H1396–H1403. [DOI] [PubMed] [Google Scholar]
- Han Y., Xu X., Tang C., Gao P., Chen X., Xiong X., et al. (2018). Reactive oxygen species promote tubular injury in diabetic nephropathy: the role of the mitochondrial ros-txnip-nlrp3 biological axis. Redox Biol. 2018 32–46. 10.1016/j.redox.2018.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L., Wei Q., Liu J., Yi M., Liu Y., Liu H., et al. (2017). AKI on CKD: heightened injury, suppressed repair, and the underlying mechanisms. Kidney Int. 92 1071–1083. 10.1016/j.kint.2017.06.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley A. K., Dhar S. K., Xu Y., St. Clair D. K. (2012). Manganese superoxide dismutase: beyond life and death. Amino Acids 42 139–158. 10.1007/s00726-010-0600-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homsi E., Mota da Silva S., Jr., Machado de Brito S., Bouçada Inácio Peixoto E., Butori Lopes de Faria J., Janino P. (2011). p53-Mediated oxidative stress and tubular injury in rats with glycerol-induced acute kidney injury. Am. J. Nephrol. 33 49–59. 10.1159/000322836 [DOI] [PubMed] [Google Scholar]
- Hopper R. K., Carroll S., Aponte A. M., Johnson D. T., French S., Shen R. F., et al. (2006). Mitochondrial matrix phosphoproteome: effect of extra mitochondrial calcium. Biochemistry 45 2524–2536. 10.1021/bi052475e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou C. L., Wang M. J., Sun C., Huang Y., Jin S., Mu X. P., et al. (2016). Protective effects of hydrogen sulfide in the ageing kidney. Oxid. Med. Cell Longev. 2016:7570489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y., Shi Y., Han B., Liu X., Qiao X., Qi Y., et al. (2018). The antioxidant peptide SS31 prevents oxidative stress, downregulates CD36 and improves renal function in diabetic nephropathy. Nephrol. Dial. Transplant. 33 1908–1918. 10.1093/ndt/gfy021 [DOI] [PubMed] [Google Scholar]
- Houldsworth A., Hodgkinson A., Shaw S., Millward A., Demaine A. G. (2015). Polymorphic differences in the SOD-2 gene may affect the pathogenesis of nephropathy in patients with diabetes and diabetic complications. Gene 569 41–45. 10.1016/j.gene.2015.04.006 [DOI] [PubMed] [Google Scholar]
- Hussain S. P., Amstad P., He P., Robles A., Lupold S., Kaneko I., et al. (2004). p53-induced up-regulation of MnSOD and GPx but not catalase increases oxidative stress and apoptosis. Cancer Res. 64 2350–2356. 10.1158/0008-5472.can-2287-2 [DOI] [PubMed] [Google Scholar]
- Imlay J. A. (2003). Pathways of oxidative damage. Annu. Rev. Microbiol. 57 395–418. 10.1146/annurev.micro.57.030502.090938 [DOI] [PubMed] [Google Scholar]
- Ishimoto Y., Inagi R., Yoshihara D., Kugita M., Nagao S., Shimizu A., et al. (2017). Mitochondrial abnormality facilitates cyst formation in autosomal dominant polycystic kidney disease. Mol. Cell. Biol. 7:e00337-17. 10.1128/MCB.00337-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs K. M., Pennington J. D., Bisht K. S., Aykin-Burns N., Kim H. S., Mishra M., et al. (2008). SIRT3 interacts with the daf-16 homolog FOXO3a in the mitochondria, as well as increases FOXO3a dependent gene expression. Int. J. Biol. Sci. 4 291–299. 10.7150/ijbs.4.291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankauskas S. S., Plotnikov E. Y., Morosanova M. A., Pevzner I. B., Zorova L. D., Skulachev V. P., et al. (2012). Mitochondria-targeted antioxidant SkQR1 ameliorates gentamycin-induced renal failure and hearing loss. Biochemistry 77 666–670. 10.1134/s0006297912060144 [DOI] [PubMed] [Google Scholar]
- Jia Z., Zhuang Y., Hu C., Zhang X., Ding G., Zhang Y., et al. (2016). Albuminuria enhances NHE3 and NCC via stimulation of mitochondrial oxidative stress/angiotensin II axis. Oncotarget 7 47134–47144. 10.18632/oncotarget.9972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C., Qin L., Shi Y., Candas D., Fan M., Lu C. L., et al. (2015). CDK4-mediated MnSOD activation and mitochondrial homeostasis in radioadaptive protection. Free Radic. Biol. Med. 81 77–87. 10.1016/j.freeradbiomed.2014.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- John J. P., Pollak A., Lubec G. (2009). Complete sequencing and oxidative modification of manganese superoxide dismutase in medulloblastoma cells. Electrophoresis 30 3006–3016. 10.1002/elps.200900168 [DOI] [PubMed] [Google Scholar]
- Jones P. L., Ping D., Boss J. M. (1997). Tumor necrosis factor alpha and interleukin-1beta regulate the murine manganese superoxide dismutase gene through a complex intronic enhancer involving C/EBP-beta and NF-kappaB. Mol. Cell. Biol. 17 6970–6981. 10.1128/mcb.17.12.6970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnati S., Luers G., Pfreimer S., Baumgart-Vogt E. (2013). Mammalian SOD2 is exclusively located in mitochondria and not present in peroxisomes. Histochem. Cell Biol. 140 105–117. 10.1007/s00418-013-1099-4 [DOI] [PubMed] [Google Scholar]
- Kato M., Yuan H., Xu Z. G., Lanting L., Li S. L., Wang M., et al. (2006). Role of the Akt/FoxO3a pathway in TGF-beta1-mediated mesangial cell dysfunction: a novel mechanism related to diabetic kidney disease. J. Am. Soc. Nephrol. 17 3325–3335. 10.1681/asn.2006070754 [DOI] [PubMed] [Google Scholar]
- Kawamata H., Manfredi G. (2010). Import, maturation, and function of SOD1 and its copper chaperone CCS in the mitochondrial intermembrane space. Antioxid. Redox Signal. 13 1375–1384. 10.1089/ars.2010.3212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- KDIGO (2012). KDIGO clinical practice guideline for acute kidney injury. Kidney Int. Suppl. 2 1–138. [Google Scholar]
- Kezic A., Spasojevic I., Lezaic V., Bajcetic M. (2016). Mitochondria-targeted antioxidants: future perspectives in kidney ischemia reperfusion injury. Oxid. Med. Cell Longev. 2016:2950503. 10.1155/2016/2950503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. P., Roe J. H., Chock P. B., Yim M. B. (1999). Transcriptional activation of the human manganese superoxide dismutase gene mediated by tetradecanoylphorbol acetate. J. Biol. Chem. 274 37455–37460. 10.1074/jbc.274.52.37455 [DOI] [PubMed] [Google Scholar]
- Kim Y. S., Gupta Vallur P., Phaëton R., Mythreye K., Hempel N. (2017). Insights into the dichotomous regulation of SOD2 in cancer. Antioxidants 6:E86. 10.3390/antiox6040086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T., Shiizaki K., Akimoto T., Shinzato T., Shimizu T., Kurosawa A., et al. (2018). The impact of preserved Klotho gene expression on antioxidative stress activity in healthy kidney. Am. J. Physiol. Renal Physiol. 315 F345–F352. [DOI] [PubMed] [Google Scholar]
- Kitada M., Kume S., Imaizumi N., Koya D. (2011). Resveratrol improves oxidative stress and protects against diabetic nephropathy through normalization of Mn-SOD dysfunction in AMPK/SIRT1-independent pathway. Diabetes Metab. Res. Rev. 60 634–643. 10.2337/db10-0386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada M., Ogura Y., Monno I., Koya D. (2019). Sirtuins and Type 2 diabetes: role in inflammation, oxidative stress, and mitochondrial function. Front. Endocrinol. 10:187. 10.3389/fendo.2019.00187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kops G. J., Dansen T. B., Polderman P. E., Saarloos I., Wirtz K. W., Coffer P. J., et al. (2002). Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature 419 316–321. 10.1038/nature01036 [DOI] [PubMed] [Google Scholar]
- Kume S., Uzu T., Horiike K., Chin-Kanasaki M., Isshiki K., Araki S., et al. (2010). Calorie restriction enhances cell adaptation to hypoxia through Sirt1-dependent mitochondrial autophagy in mouse aged kidney. J. Clin. Invest. 120 1043–1055. 10.1172/jci41376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuro-o M., Matsumura Y., Aizawa H., Kawaguchi H., Suga T., Utsugi T., et al. (1997). Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390 45–51. 10.1038/36285 [DOI] [PubMed] [Google Scholar]
- Le Clef N., Verhulst A., D’Haese P. C., Vervaet B. A. (2016). Unilateral renal ischemia-reperfusion as a robust model for acute to chronic kidney injury in mice. PLoS One 11:e0152153. 10.1371/journal.pone.0152153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebovitz R. M., Zhang H., Vogel H., Cartwright J., Dionne L., Lu N., et al. (1996). Neurodegeneration, myocardial injury, and perinatal death in mitochondrial superoxide dismutase-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 93 9782–9787. 10.1073/pnas.93.18.9782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. J., Choi M. G., Kim D. S., Kim T. W. (2006). Manganese superoxide dismutase gene polymorphism (V16A) is associated with stages of albuminuria in Korean type 2 diabetic patients. Metabolism 55 1–7. 10.1016/j.metabol.2005.04.030 [DOI] [PubMed] [Google Scholar]
- Li A., Zhang X., Shu M., Wu M., Wang J., Zhang J., et al. (2017). Arctigenin suppresses renal interstitial fibrosis in a rat model of obstructive nephropathy. Phytomedicine 30 28–41. 10.1016/j.phymed.2017.03.003 [DOI] [PubMed] [Google Scholar]
- Li C., Matavelli L. C., Akhtar S., Siragy H. M. (2019). Prorenin receptor contributes to renal mitochondria dysfunction, apoptosis and fibrosis in diabetic mice. Sci. Rep. 9:11667. 10.1038/s41598-019-47055-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Chiu J. F., Mossman B. T., Fukagawa N. K. (2006). Down-regulation of manganese-superoxide dismutase through phosphorylation of FOXO3a by Akt in explanted vascular smooth muscle cells from old rats. J. Biol. Chem. 281 40429–40439. 10.1074/jbc.m606596200 [DOI] [PubMed] [Google Scholar]
- Li T., He S., Liu S., Kong Z., Wang J., Zhang Y. (2015). Effects of different exercise durations on Keap1-Nrf2-ARE pathway activation in mouse skeletal muscle. Free Radic. Res. 49 1269–1274. 10.3109/10715762.2015.1066784 [DOI] [PubMed] [Google Scholar]
- Li Y., Huang T. T., Carlson E. J., Melov S., Ursell P. C., Olson J. L., et al. (1995). Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat. Genet. 11 376–381. 10.1038/ng1295-376 [DOI] [PubMed] [Google Scholar]
- Liang H. L., Hilton G., Mortensen J., Regner K., Johnson C. P., Nilakantan V. (2009). MnTMPyP, a cell-permeant SOD mimetic, reduces oxidative stress and apoptosis following renal ischemia-reperfusion. Am. J. Physiol. Renal Physiol. 296 F266–F276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J. H., Kim E. N., Kim M. Y., Chung S., Shin S. J., Kim H. W., et al. (2012). Age-associated molecular changes in the kidney in aged mice. Oxid. Med. Cell Longev. 2012:171383. 10.1155/2012/171383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J., Handschin C., Spiegelman B. M. (2005). Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 1 361–370. 10.1016/j.cmet.2005.05.004 [DOI] [PubMed] [Google Scholar]
- Liu L., Zheng T., Wang N., Wang F., Li M., Jiang J., et al. (2009). The manganese superoxide dismutase Val16Ala polymorphism is associated with decreased risk of diabetic nephropathy in Chinese patients with type 2 diabetes. Mol. Cell. Biochem. 322 87–91. 10.1007/s11010-008-9943-x [DOI] [PubMed] [Google Scholar]
- Liu M., Zhu Y., Sun Y., Wen Z., Huang S., Ding G., et al. (2017). MnTBAP therapy attenuates the downregulation of sodium transporters in obstructive kidney disease. Oncotarget 9 394–403. 10.18632/oncotarget.23037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowes D. A., Thottakam B. M., Webster N. R., Murphy M. P., Galley H. F. (2008). The mitochondria-targeted antioxidant MitoQ protects against organ damage in a lipopolysaccharide-peptidoglycan model of sepsis. Free Radic. Biol. Med. 45 1559–1565. 10.1016/j.freeradbiomed.2008.09.003 [DOI] [PubMed] [Google Scholar]
- Lu Q., Zhai Y., Cheng Q., Liu Y., Gao X., Zhang T., et al. (2013). The Akt-FoxO3a-manganese superoxide dismutase pathway is involved in the regulation of oxidative stress in diabetic nephropathy. Exp. Physiol. 98 934–945. 10.1113/expphysiol.2012.068361 [DOI] [PubMed] [Google Scholar]
- Lu Z., Xu X., Hu X., Fassett J., Zhu G., Tao Y., et al. (2010). PGC-1 alpha regulates expression of myocardial mitochondrial antioxidants and myocardial oxidative stress after chronic systolic overload. Antioxid. Redox. Signal. 13 1011–1022. 10.1089/ars.2009.2940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMillan-Crow L. A., Crow J. P., Kerby J. D., Beckman J. S., Thompson J. A. (1996). Nitration and inactivation of manganese superoxide dismutase in chronic rejection of human renal allografts. Proc. Natl. Acad. Sci. U.S.A. 93 11853–11858. 10.1073/pnas.93.21.11853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMillan-Crow L. A., Crow J. P., Thompson J. A. (1998). Peroxynitrite-mediated inactivation of manganese superoxide dismutase involves nitration and oxidation of critical tyrosine residues. Biochemistry 37 1613–1622. 10.1021/bi971894b [DOI] [PubMed] [Google Scholar]
- MacMillan-Crow L. A., Cruthirds D. L., Ahki K. M., Sanders P. W., Thompson J. A. (2001). Mitochondrial tyrosine nitration precedes chronic allograft nephropathy. Free Radic Biol. Med. 31 1603–1608. 10.1016/s0891-5849(01)00750-x [DOI] [PubMed] [Google Scholar]
- Mailloux R. J. (2015). Teaching the fundamentals of electron transfer reactions in mitochondria and the production and detection of reactive oxygen species. Redox Biol. 4 381–398. 10.1016/j.redox.2015.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailloux R. J., Willmore W. G. (2014). S-glutathionylation reactions in mitochondrial function and disease. Front. Cell Dev. Biol. 2:68. 10.3389/fcell.2014.00068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majzunova M., Kvandova M., Berenyiova A., Balis P., Dovinova I., Cacanyiova S. (2019). Chronic NOS inhibition affects oxidative state and antioxidant response differently in the kidneys of young normotensive and hypertensive rats. Oxid. Med. Cell Longev. 2019:5349398. 10.1155/2019/5349398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miriyala S., Spasojevic I., Tovmasyan A., Salvemini D., Vujaskovic Z., St. Clair D. (2012). Manganese superoxide dismutase, MnSOD and its mimics. Biochim. Biophys. Acta 1822 794–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möllsten A., Marklund S. L., Wessman M., Svensson M., Forsblom C., Parkkonen M., et al. (2007). A functional polymorphism in the manganese superoxide dismutase gene and diabetic nephropathy. Diabetes Metab. Res. Rev. 56 265–269. 10.2337/db06-0698 [DOI] [PubMed] [Google Scholar]
- Morigi M., Perico L., Corna D., Locatelli M., Cassis P., Carminati C. E., et al. (2020). C3a receptor blockade protects podocytes from injury in diabetic nephropathy. JCI Insight. 5:131849. 10.1172/jci.insight.131849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay P., Horvath B., Zsengeller Z., Zielonka J., Tanchian G., Holovac E., et al. (2012). Mitochondrial-targeted antioxidants represent a promising approach for prevention of cisplatin-induced nephropathy. Free Radic. Biol. Med. 52 497–506. 10.1016/j.freeradbiomed.2011.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtas C., Bruschi M., Candiano G., Moroni G., Magistroni R., Magnano A., et al. (2012). Coexistence of different circulating anti-podocyte antibodies in membranous nephropathy. Clin. J. Am. Soc. Nephrol. 7 1394–1400. 10.2215/cjn.02170312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita T., Weinert B. T., Choudhary C. (2019). Functions and mechanisms of non-histone protein acetylation. Nat. Rev. Mol. Cell Biol. 20 156–174. 10.1038/s41580-018-0081-3 [DOI] [PubMed] [Google Scholar]
- Ninomiya T., Perkovic V., de Galan B. E., Zoungas S., Pillai A., Jardine M., et al. (2009). Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J. Am. Soc. Nephrol. 20 1813–1821. 10.1681/asn.2008121270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noiri E., Nakao A., Uchida K., Tsukahara H., Ohno M., Fujita T., et al. (2001). Oxidative and nitrosative stress in acute renal ischemia. Am. J. Physiol. Renal Physiol. 281 F948–F957. [DOI] [PubMed] [Google Scholar]
- Nomiyama T., Tanaka Y., Piao L., Nagasaka K., Sakai K., Ogihara T., et al. (2003). The polymorphism of manganese superoxide dismutase is associated with diabetic nephropathy in Japanese type 2 diabetic patients. J. Hum. Genet. 48 138–141. [DOI] [PubMed] [Google Scholar]
- Ogura Y., Kitada M., Monno I., Kanasaki K., Watanabe A., Koya D. (2018). Renal mitochondrial oxidative stress is enhanced by the reduction of Sirt3 activity, in Zucker diabetic fatty rats. Redox Rep. 23 153–159. 10.1080/13510002.2018.1487174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura Y., Kitada M., Xu J., Monno I., Koya D. (2020). CD38 inhibition by apigenin ameliorates mitochondrial oxidative stress through restoration of the intracellular NAD+/NADH ratio and Sirt3 activity in renal tubular cells in diabetic rats. Aging (Albany NY) 12, 11325–11336. 10.18632/aging.103410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onozato M. L., Tojo A., Goto A., Fujita T. (2004). Radical scavenging effect of gliclazide in diabetic rats fed with a high cholesterol diet. Kidney Int. 65 951–960. 10.1111/j.1523-1755.2004.00470.x [DOI] [PubMed] [Google Scholar]
- Onozato M. L., Tojo A., Goto A., Fujita T., Wilcox C. S. (2002). Oxidative stress and nitric oxide synthase in rat diabetic nephropathy: effects of ACEI and ARB. Kidney Int. 61 186–194. 10.1046/j.1523-1755.2002.00123.x [DOI] [PubMed] [Google Scholar]
- Ouyang J., Zeng Z., Fang H., Li F., Zhang X., Tan W. (2019). SIRT3 inactivation promotes acute kidney injury through elevated acetylation of SOD2 and p53. J. Surg. Res. 233 221–230. 10.1016/j.jss.2018.07.019 [DOI] [PubMed] [Google Scholar]
- Pani G., Bedogni B., Anzevino R., Colavitti R., Palazzotti B., Borrello S., et al. (2000). Deregulated manganese superoxide dismutase expression and resistance to oxidative injury in p53-deficient cells. Cancer Res. 60 4654–4660. [PubMed] [Google Scholar]
- Parajuli N., Marine A., Simmons S., Saba H., Mitchell T., Shimizu T., et al. (2011). Generation and characterization of a novel kidney-specific manganese superoxide dismutase knockout mouse. Free Radic. Biol. Med. 51 406–416. 10.1016/j.freeradbiomed.2011.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil N. K., Parajuli N., MacMillan-Crow L. A., Mayeux P. R. (2014). Inactivation of renal mitochondrial respiratory complexes and manganese superoxide dismutase during sepsis: mitochondria-targeted antioxidant mitigates injury. Am. J. Physiol. Renal Physiol. 306 F734–F743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil N. K., Saba H., MacMillan-Crow L. A. (2013). Effect of S-nitrosoglutathione on renal mitochondrial function: a new mechanism for reversible regulation of manganese superoxide dismutase activity? Free Radic. Biol. Med. 56 54–63. 10.1016/j.freeradbiomed.2012.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotnikov E. Y., Chupyrkina A. A., Jankauskas S. S., Pevzner I. B., Silachev D. N., Skulachev V. P., et al. (2011). Mechanisms of nephroprotective effect of mitochondria-targeted antioxidants under rhabdomyolysis and ischemia/reperfusion. Biochim. Biophys. Acta 1812 77–86. 10.1016/j.bbadis.2010.09.008 [DOI] [PubMed] [Google Scholar]
- Porteous C. M., Logan A., Evans C., Ledgerwood E. C., Menon D. K., Aigbirhio F., et al. (2010). Rapid uptake of lipophilic triphenylphosphonium cations by mitochondria in vivo following intravenous injection: implications for mitochondria-specific therapies and probes. Biochim. Biophys. Acta 1800 1009–1017. 10.1016/j.bbagen.2010.06.001 [DOI] [PubMed] [Google Scholar]
- Prunotto M., Carnevali M. L., Candiano G., Murtas C., Bruschi M., Corradini E., et al. (2010). Autoimmunity in membranous nephropathy targets aldose reductase and SOD2. J. Am. Soc. Nephrol. 21 507–519. 10.1681/asn.2008121259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X., Brown K., Hirschey M. D., Verdin E., Chen D. (2010). Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 12 662–667. 10.1016/j.cmet.2010.11.015 [DOI] [PubMed] [Google Scholar]
- Radi R. (2018). Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine. Proc. Natl. Acad. Sci. U.S.A. 115 5839–5848. 10.1073/pnas.1804932115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattanavich R., Plagov A., Kumar D., Rai P., Lederman R., Salhan D., et al. (2013). Deficit of p66ShcA restores redox-sensitive stress response program in cisplatin-induced acute kidney injury. Exp. Mol. Pathol. 94 445–452. 10.1016/j.yexmp.2013.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Iturbe B., Sepassi L., Quiroz Y., Ni Z., Wallace D. C., Vaziri N. D. (2007). Association of mitochondrial SOD deficiency with salt-sensitive hypertension and accelerated renal senescence. J. Appl. Physiol. 102 255–260. 10.1152/japplphysiol.00513.2006 [DOI] [PubMed] [Google Scholar]
- Schrier R. W., Wang W. (2004). Acute renal failure and sepsis. N. Engl. J. Med. 351 159–169. [DOI] [PubMed] [Google Scholar]
- Shabalina I. G., Vyssokikh M. Y., Gibanova N., Csikasz R. I., Edgar D., Hallden-Waldemarson A., et al. (2017). Improved health-span and lifespan in mtDNA mutator mice treated with the mitochondrially targeted antioxidant SkQ1. Aging 9 315–339. 10.18632/aging.101174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma K. (2017). Mitochondrial dysfunction in the diabetic kidney. Adv. Exp. Med. Biol. 982 553–562. 10.1007/978-3-319-55330-6_28 [DOI] [PubMed] [Google Scholar]
- Shimoda-Matsubayashi S., Matsumine H., Kobayashi T., Nakagawa-Hattori Y., Shimizu Y., Mizuno Y. (1996). Structural dimorphism in the mitochondrial targeting sequence in the human manganese superoxide dismutase gene. A predictive evidence for conformational change to influence mitochondrial transport and a study of allelic association in Parkinson’s disease. Biochem. Biophys. Res. Commun. 226 561–565. 10.1006/bbrc.1996.1394 [DOI] [PubMed] [Google Scholar]
- Shrestha B., Haylor J. (2016). Evolution of the concept and pathogenesis of chronic renal allograft injury: an updated review. Exp. Clin. Transplant. 14 596–605. [DOI] [PubMed] [Google Scholar]
- Smith R. A., Murphy M. P. (2010). Animal and human studies with the mitochondria-targeted antioxidant MitoQ. Ann. N. Y. Acad. Sci. 1201 96–103. 10.1111/j.1749-6632.2010.05627.x [DOI] [PubMed] [Google Scholar]
- Smith R. A. J., Porteous C. M., Gane A. M., Murphy M. P. (2003). Delivery of bioactive molecules to mitochondria in vivo. Proc. Natl. Acad. Sci. U.S.A. 100 5407–5412. 10.1073/pnas.0931245100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer N. Y., Yang Z., Sullivan J. C., Klein T., Stanton R. C. (2018). Linagliptin unmasks specific antioxidant pathways protective against albuminuria and kidney hypertrophy in a mouse model of diabetes. PLoS One 13:e0200249. 10.1371/journal.pone.0200249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Pierre J., Drori S., Uldry M., Silvaggi J. M., Rhee J., Jager S., et al. (2006). Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 127 397–408. 10.1016/j.cell.2006.09.024 [DOI] [PubMed] [Google Scholar]
- Sutton A., Khoury H., Prip-Buus C., Cepanec C., Pessayre D., Degoul F. (2003). The Ala16Val genetic dimorphism modulates the import of human manganese superoxide dismutase into rat liver mitochondria. Pharmacogenetics 13 145–157. [DOI] [PubMed] [Google Scholar]
- Szeto H. H., Liu S., Soong Y., Wu D., Darrah S. F., Cheng F. Y., et al. (2011). Mitochondria-targeted peptide accelerates ATP recovery and reduces ischemic kidney injury. J. Am. Soc. Nephrol. 22 1041–1052. 10.1681/asn.2010080808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z., Shou I., Wang L. N., Fukui M., Tomino Y. (1997). Effects of antihypertensive drugs or glycemic control on antioxidant enzyme activities in spontaneously hypertensive rats with diabetes. Nephron 76 323–330. 10.1159/000190199 [DOI] [PubMed] [Google Scholar]
- Tao R., Coleman M. C., Pennington J. D., Ozden O., Park S. H., Jiang H., et al. (2010). Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol. Cell. 40 893–904. 10.1016/j.molcel.2010.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. C., Acquaah-Mensah G., Singhal M., Malhotra D., Biswal S. (2008). Network inference algorithms elucidate Nrf2 regulation of mouse lung oxidative stress. PLoS Comput. Biol. 4:e1000166. 10.1371/journal.pcbi.1000166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teissier T., Quersin V., Gnemmi V., Daroux M., Howsam M., Delguste F., et al. (2019). Knockout of receptor for advanced glycation end-products attenuates age-related renal lesions. Aging Cell 18 e12850. 10.1111/acel.12850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tharmalingam S., Alhasawi A., Appanna V. P., Lemire J., Appanna V. D. (2017). Reactive nitrogen species (RNS)-resistant microbes: adaptation and medical implications. Biol. Chem. 398 1193–1208. 10.1515/hsz-2017-0152 [DOI] [PubMed] [Google Scholar]
- Thuraisingham R. C., Nott C. A., Dodd S. M., Yaqoob M. M. (2000). Increased nitrotyrosine staining in kidneys from patients with diabetic nephropathy. Kidney Int. 57 1968–1972. 10.1046/j.1523-1755.2000.00046.x [DOI] [PubMed] [Google Scholar]
- Tian C., Fang S., Du X., Jia C. (2011). Association of the C47T polymorphism in SOD2 with diabetes mellitus and diabetic microvascular complications: a meta-analysis. Diabetologia 54 803–811. 10.1007/s00125-010-2004-5 [DOI] [PubMed] [Google Scholar]
- Valle I., varez-Barrientos A., Arza E., Lamas S., Monsalve M. (2005). PGC-1alpha regulates the mitochondrial antioxidant defense system in vascular endothelial cells. Cardiovasc. Res. 66 562–573. 10.1016/j.cardiores.2005.01.026 [DOI] [PubMed] [Google Scholar]
- van de Ven R. A. H., Santos D., Haigis M. C. (2017). Mitochondrial sirtuins and molecular mechanisms of aging. Trends Mol. Med. 23 320–331. 10.1016/j.molmed.2017.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Remmen H., Ikeno Y., Hamilton M., Pahlavani M., Wolf N., Thorpe S. R., et al. (2003). Life-long reduction in MnSOD activity results in increased DNA damage and higher incidence of cancer but does not accelerate aging. Physiol. Genom. 16 29–37. 10.1152/physiolgenomics.00122.2003 [DOI] [PubMed] [Google Scholar]
- Vlassara H., Torreggiani M., Post J. B., Zheng F., Uribarri J., Striker G. E. (2009). Role of oxidants/inflammation in declining renal function in chronic kidney disease and normal aging. Kidney Int. Suppl. 76 S3–S11. [DOI] [PubMed] [Google Scholar]
- Walker L. M., Walker P. D., Imam S. Z., Ali S. F., Mayeux P. R. (2000). Evidence for peroxynitrite formation in renal ischemia-reperfusion injury: studies with the inducible nitric oxide synthase inhibitor L-N(6)-(1-Iminoethyl)lysine. J. Pharmacol. Exp. Ther. 295 417–422. [PubMed] [Google Scholar]
- Wang H. D., Xu S. Q., Johns D. G., Du Y., Quinn M. T., Cayatte A. J., et al. (2001). Role of NADPH oxidase in the vascular hypertrophic and oxidative stress response to angiotensin II in mice. Circ. Res. 88 947–953. 10.1161/hh0901.089987 [DOI] [PubMed] [Google Scholar]
- Wang Y., Branicky R., Noë A., Hekimi S. (2018). Superoxide dismutases: dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 217 1915–1928. 10.1083/jcb.201708007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner B. B., Stuart L., Gebb S., Wispé J. R. (1996). Redox regulation of manganese superoxide dismutase. Am. J. Physiol. 271 L150–L158. [DOI] [PubMed] [Google Scholar]
- Williams M. D., Van Remmen H., Conrad C. C., Huang T. T., Epstein C. J., Richardson A. (1998). Increased oxidative damage is correlated to altered mitochondrial function in heterozygous manganese superoxide dismutase knockout mice. J. Biol. Chem. 273 28510–28515. 10.1074/jbc.273.43.28510 [DOI] [PubMed] [Google Scholar]
- Wu Z., Puigserver P., Andersson U., Zhang C., Adelmant G., Mootha V., et al. (1999). Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98 115–124. 10.1016/s0092-8674(00)80611-x [DOI] [PubMed] [Google Scholar]
- Xiao L., Xu X., Zhang F., Wang M., Xu Y., Tang D., et al. (2017). The mitochondria-targeted antioxidant MitoQ ameliorated tubular injury mediated by mitophagy in diabetic kidney disease via Nrf2/PINK1. Redox. Biol. 11 297–311. 10.1016/j.redox.2016.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Kitada M., Koya D. (2020). The impact of mitochondrial quality control by Sirtuins on the treatment of type 2 diabetes and diabetic kidney disease. Biochim. Biophys. Acta Mol. Basis Dis. 1866:165756. 10.1016/j.bbadis.2020.165756 [DOI] [PubMed] [Google Scholar]
- Xu S. (2016). SIRT1/3 activation by resveratrol attenuates acute kidney injury in a septic rat model. Oxid. Med. Cell Longev. 2016:7296092. 10.1155/2016/7296092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S., Jiang B., Maitland K. A., Bayat H., Gu J., Nadler J. L., et al. (2006). The thromboxane receptor antagonist S18886 attenuates renal oxidant stress and proteinuria in diabetic apolipoprotein E-deficient mice. Diabetes Metab. Res. Rev. 55 110–119. 10.2337/diabetes.55.01.06.db05-0831 [DOI] [PubMed] [Google Scholar]
- Xu Y., Kiningham K. K., Devalaraja M. N., Yeh C. C., Majima H., Kasarskis E. J., et al. (1999). An intronic NF-kappaB element is essential for induction of the human manganese superoxide dismutase gene by tumor necrosis factor-alpha and interleukin-1beta. DNA Cell Biol. 18 709–722. 10.1089/104454999314999 [DOI] [PubMed] [Google Scholar]
- Xu Y., Porntadavity S., St Clair D. K. (2002). Transcriptional regulation of the human manganese superoxide dismutase gene: the role of specificity protein 1 (Sp1) and activating protein-2 (AP-2). Biochem. J. 362 401–412. 10.1042/bj3620401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakura F., Kawasaki H. (2010). Post-translational modifications of superoxide dismutase. Biochim. Biophys. Acta 1804 318–325. 10.1016/j.bbapap.2009.10.010 [DOI] [PubMed] [Google Scholar]
- Yamakura F., Taka H., Fujimura T., Murayama K. (1998). Inactivation of human manganese-superoxide dismutase by peroxynitrite is caused by exclusive nitration of tyrosine 34 to 3-nitrotyrosine. J. Biol. Chem. 273 14085–14089. 10.1074/jbc.273.23.14085 [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Clark J. D., Pastor J. V., Gurnani P., Nandi A., Kurosu H., et al. (2005). Regulation of oxidative stress by the anti-aging hormone klotho. J. Biol. Chem. 280 38029–38034. 10.1074/jbc.m509039200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi X., Nickeleit V., James L. R., Maeda N. (2011). α-Lipoic acid protects diabetic apolipoprotein E-deficient mice from nephropathy. J. Diabetes Complicat. 25 193–201. 10.1016/j.jdiacomp.2010.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zayed A. E., Saleh A., Gomaa A. M. S., Abd-Elkareem M., Anwar M. M. (2018). Protective effect of ginkgo biloba and magnetized water on nephropathy in induced type 2 diabetes in rat. Oxid. Med. Cell Longev. 2018:1785614. 10.1155/2018/1785614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Sha Y., Wei K., Wu C., Ding D., Yang Y., et al. (2018). Rotenone ameliorates chronic renal injury caused by acute ischemia/reperfusion. Oncotarget 9 24199–24208. 10.18632/oncotarget.24733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Yang Y., Gao H., Zhang Y., Jia Z., Huang S. (2019). Inhibition of mitochondrial complex i aggravates folic acid-induced acute kidney injury. Kidney Blood Press Res. 44 1002–1013. 10.1159/000501934 [DOI] [PubMed] [Google Scholar]
- Zhao Z., Azadzoi K. M., Choi H. P., Jing R., Lu X., Li C., et al. (2017). LC-MS/MS analysis unravels deep oxidation of manganese superoxide dismutase in kidney cancer. Int. J. Mol. Sci. 18:E319. 10.3390/ijms18020319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C. H., Huang Y., Oberley L. W., Domann F. E. (2001). A family of AP-2 proteins down-regulate manganese superoxide dismutase expression. J. Biol. Chem. 276 14407–14413. 10.1074/jbc.m009708200 [DOI] [PubMed] [Google Scholar]
- Zhuang Y., Yasinta M., Hu C., Zhao M., Ding G., Bai M., et al. (2015). Mitochondrial dysfunction confers albumin-induced NLRP3 inflammasome activation and renal tubular injury. Am. J. Physiol. Renal Physiol. 308 F857–F866. [DOI] [PubMed] [Google Scholar]