Abstract
Background:
Cognitive impairment is one of the debilitating consequences of multiple sclerosis (MS) with negative effects on daily life, individual and social activities, quality of life (QOL), and depression. No approved medication is introduced so far for affected individuals. We aimed to evaluate the efficacy of donepezil on cognitive performance, QOL, and depression in MS.
Methods:
This is a double-blinded randomized clinical trial conducted on 100 patients with MS during 2018. Patients were assessed prior to intervention abbreviated mental test (AMT), prospective and retrospective mental questionnaire (PRMQ), everyday memory questionnaire (EMQ), digit span test, Beck depression inventory (BDI), and MSQOL questionnaire. Then patients were randomly divided into two groups of treatment (daily regimen of 10 mg donepezil) and placebo for 3 months. Subjects were reassessed using the same instruments at the end of intervention.
Results:
Fifty patients remained in each group at the end of study. The mean age in donepezil and placebo groups was 31.9 ± 5.89 and 30.65 ± 5.43 years, respectively. EMQ, PRMQ, digit span test, MSQOL, and depression scores improved following donepezil therapy (P < 0.001) while no statistically significant difference was found in the placebo group (P > 0.05). Comparison of two groups also showed more favorable scores in donepezil group with respect to all assessment tools (P < 0.001).
Conclusions:
Donepezil could effectively improve cognitive impairment in MS patients. Also, its positive effect on QOL and depression could result in a smaller number of interventions in this group of patients.
Keywords: Cognitive dysfunction, depression, donepezil, multiple sclerosis, quality of life
Introduction
Cognitive impairment is found in 45--70% of patients with multiple sclerosis (MS) without any gender predominance.[1] MS-related cognitive impairment is shown to affect daily activity, occupational state, and self-confidence notably.[2] The consequences of cognitive impairment (e.g., less participation in social activities) could also exacerbate the condition. For example, less participation in social activities leads to negative psychological disorders (e.g., depression) that worsen the cognitive function.[3] Furthermore, depression and anxiety pose treatment follow-up withdrawals and increase the risk of flare-ups as well as deterioration of cognitive state.[3] Given the debilitating nature of cognitive impairment, effective interventions are required to ease the symptoms in affected individuals.
It has been reported that MS patients with cognitive impairment have some degrees of cholinergic disturbance.[4] Acetylcholine esterase inhibitors (AChEIs) prolong/increase the presence of acetylcholine in the synaptic space and could potentially facilitate memory and learning. These medications are currently being used in patients with Alzheimer's disease.[5] Donepezil, as an AChEI, has been shown to control cognitive impairment and lead to memory reconstruction and reinforcement among MS patients.[6,7] Also, it has an antidepressant function due to the sigma agonism effects.[8]
Despite the importance of cognitive dysfunction in MS, no approved intervention is recognized to benefit these patients.[5] Previous studies evaluating the effect of AChEIs on cognitive function have reported mixed findings.[5] Also, they mostly have failed to address all the possible effects of such interventions on affected individuals. Therefore, we decided to evaluate the efficacy of donepezil on MS patients with cognitive dysfunction, as well as its possible effect on depression rehabilitation and quality of life (QOL).
Methods
Study setting
This is a double-blinded randomized clinical trial conducted on patients with MS referred to Kashani Hospital outpatient Clinic, affiliated to Isfahan University of Medical Sciences, during 2018. MS patients (diagnosed according to the revision of McDonald criteria in 2017[9]), aged ≥18 y/o with extended disability status scale (EDSS)[10] ≤5.5 who could read and write were approached and invited to the screening stage. In this stage, they were asked to complete two screening tests [everyday memory questionnaire (EMQ) 13-item scale[11] and Beck depression inventory (BDI)[12] to assess their cognitive ability and depression state. Patients with mild to moderate cognitive impairment were included and those with severe depression were excluded.
The mild to moderate cognition impairment was defined as the scores of less than 116 in the EMQ. This questionnaire contains 28 questions with the least score of 28 and the most score of 252. The scores of 28--58 are defined as appropriate memory performance, 59--116 as moderate impaired, and 117--252 as the severe impaired memory function.[13]
Also, we excluded patients with other neurological disorders, history of using AChEIs or psychostimulants, abnormal hepatic or kidney function, current use of anticholinergic medications, and known psychiatric illnesses. The study was approved by the ethics committee of Isfahan University of medical sciences (2/13/2016).
Sample size and recruitment
We used the equation for comparing two means with type I error of 1.96 and power of 0.80 to calculate the sample size. Assumptions in two groups (including mean score of cognitive function tests) were pre-specified on the basis of previously published data and our previous experiment on another AChEI.[14,15] We hypothesized that an equivalent outcome will be observed in groups, regarding the memory function and assessed as the mean score of “prospective and retrospective mental questionnaire (PRMQ)” and “EMQ” which are explained below. The greater number was considered as the sample size. Given our assumptions, the required sample size was calculated to be 50 patients in each group.
The study procedure and the intervention were completely explained to the patients and written informed consents were obtained prior to the screening phase. The study protocol was approved by Isfahan University of Medical Sciences Ethics Committee (registration code: 398611). This study is a subset of a larger clinical trial and the main protocol has been registered in the Iranian Registry of Clinical Trials (IRCT2016042227522N1).
The patients were blinded to the type of the intervention as they were unaware about the group that was randomly allocated to. Besides, the psychologist who interviewed with the patients and filled the questionnaires was blinded to the regimen of interviewed participants.
Tools and procedures
As stated before, all patients were asked to fill out “EMQ 28-item scale” before inclusion in the study.[11] After initial screening and recruitment, patients were asked to fill out a set of questionnaires in the pretreatment phase to evaluate their cognitive function (memory and attention components) as well as QOL. Cognitive function was assessed using abbreviated mental test (AMT),[16] PRMQ)[17] and digit span test. Furthermore, the second version of BDI was used to evaluate depression in the screening phase.[12] QOL was also assessed using “multiple sclerosis quality of life questionnaire” (MSQOL).[18]
EMQ measures memory function in everyday life and is consisted of 28 items with 9-point scoring system, with greater scores representing worse memory state.[11] The Persian version of AMT includes 10 questions that evaluate cognitive impairment. The questions ask for age, orientation for time, date, and place, occupation, date of birth, date of revolution in Iran, name of the Iran's leader, invert counting and repeating an address. The reliability of this test determined to be 0.89.[16] PRMQ is consisted of 16 items that target different types of memory failure, as well as the resultant frustration. The items are scored on a five-point scale,[1,2,3,4,5] with higher scores accounting for more frequent memory failures. The reliability of the Persian version is reported to be 0.80.[17] Digit span test evaluates working memory and attention by asking the patients to repeat a list of digits that is being read for them. Higher scores show greater number of numbers and therefore better working memory function.[15] The second version of BDI measures the severity of depressive symptoms. It includes 21 items scoring from 0 (absent or mild) to 3 (severe) and the greater scores show more severe depression. The reliability of the Persian version of the test is 0.84.[12] MSQOL consisted of 54 questions assessing physical health, role limitation due to physical and emotional problems, pain, emotional well-being, energy, health perception, social function, cognitive function, health distress, sexual function, and overall QOL. The higher total scores demonstrate a better QOL in the target subset of questionnaire. The reliability of the Persian version is reported to be 0.96.[18]
Then, the patients were randomly divided into two groups of A (treated with donepezil) and B (treated with placebo). In group A, daily regimen of 10 mg donepezil (Yasnal®; KRKA pharmaceutical company, Slovenia) was started for 3 months. Patients in group B were treated with daily regimen of placebo, similar to donepezil pills in shape, color, and packaging, for 3 months. Medication adherence and adverse events was evaluated through weekly phone call follow-ups. Both patients and the people who were in touch with them for most of the times were asked about the adherence to the medications. Eventually, all patients underwent posttreatment assessment with previously mentioned questionnaires used in the screening and pretreatment assessment phase. The posttreatment assessments were performed within less than 15 days after the discontinuation of the interventions.
Outcome measurement
Patients completed the questionnaires in two time points: before the intervention (including the screening questionnaires) and at most within 15 days following the discontinuation of the interventions. The primary outcome was defined as cognitive function, measured by EMQ, AMT, PRMQ, and “digit span test.” The secondary outcomes were QOL and depression, measured by MSQOL and BDI, respectively. Reported side effects were also recorded and reported as the secondary outcome.
Statistical analysis
Descriptive data were presented in form of means (± standard deviation) and percentages. Chi-squared test was used to compare categorical data between two groups. Independent, t-test or the nonparametric equivalent was used to compare interval data between two groups, as applicable. Analysis of covariance (ANCOVA) was also used to evaluate the effect of intervention in each group. Only patients who completed the study were reported at the end. Statistical analysis was carried out using IBM SPSS version 22 and a P value less than 0.05 was considered as significant.
Results
Study flow chart, and demographic features
We approached 192 patients, 46 patients refused to participate, 26 were excluded, including 12 patients with severe depressive symptoms (based on BDI results) and 14 with moderate to severe cognitive dysfunction in the screening phase. Also, six patients had history of previous use of AChEIs or psychostimulants, two had impaired renal function, five had concomitant neurological disorders, and four had known psychiatric illnesses.
The remaining 103 cases were randomly divided into two groups of placebo (51 cases) and intervention (52 cases); however, a patient in the placebo decided to withdraw in the beginning of the study. In addition, two patients in the treatment group withdrew due to recurrent headaches following donepezil use. The study flow chart is presented in Figure 1.
Figure 1.
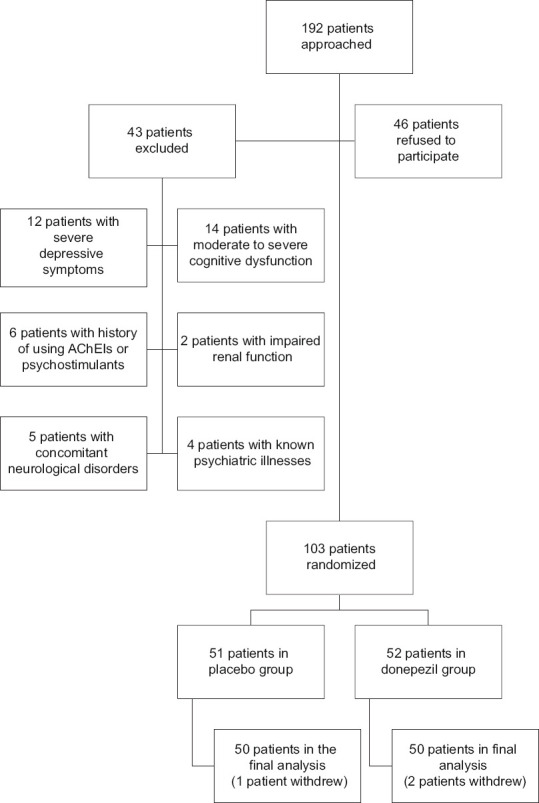
The study flowchart
In donepezil group, 37 patients were females while the control group included 34 females (P = 0.513). The mean age of patients in group A and B were 31.9 ± 5.89 and 30.65 ± 5.43, respectively (P = 0.45). The mean EDSS in group A and B were 2.71 and 2.58, respectively (P = 0.66). Baseline characteristics and demographic features are presented in Table 1.
Table 1.
Demographic features and baseline characteristics of the study groups
| Donepezil group | Placebo group | |
|---|---|---|
| Type of multiple sclerosis disease (n (%)) | ||
| Relapsing remitting | 41 (82%) | 44 (88%) |
| Primary progressive | 3 (6%) | 2 (4%) |
| Secondary progressive | 6 (12%) | 4 (8%) |
| Relapse rate (n (%)) | ||
| Without relapse | 39 (78%) | 38 (76%) |
| Once | 8 (16%) | 10 (20%) |
| Twice | 3 (6%) | 2 (4%) |
| Duration of multiple sclerosis (years) | 6.15 | 5.90 |
| Age | 31.92±5.89 | 30.66±5.43 |
| Female/male | 37 (74%)/13 (26%) | 34 (68%)/16 (32%) |
| Extended disability scale score (mean) | 2.71 | 2.58 |
| Disease modifying therapy | ||
| Fingolimod | 5 (10%) | 7 (14%) |
| Beta interferon | 43 (86%) | 41 (82%) |
| Rituximab | 2 (4%) | 2 (4%) |
| Dominant disease symptom | ||
| Motor | 22 (44%) | 20 (40%) |
| Brain stem | 10 (20%) | 11 (22%) |
| Sensory | 7 (14%) | 8 (16%) |
| Sphincteric | 6 (12%) | 7 (14%) |
| Ocular | 5 (10%) | 4 (8%) |
Assessment questionnaires before and after intervention
Table 2 demonstrates comparison of results from pre-/post-intervention assessments. As it is demonstrated, EMQ, RPMQ, digit span memory, physical and mental health scores of MSQOL, and depression scores significantly improved in donepezil group after intervention (P < 0.05) while no statistically significant change was observed in the placebo (P > 0.05). ANCOVA results are showed in Table 3. It shows independent role of donepezil on improvement of scores from each questionnaire.
Table 2.
Comparison of pre- and post-intervention assessment results between two study groups
| Group questionnaire | Everyday memory questionnaire | Retrospective and prospective memory questionnaire | Digit span test | Multiple sclerosis quality of life questionnaire | Beck depression inventory | ||
|---|---|---|---|---|---|---|---|
| Physical health | Mental health | ||||||
| Placebo | Before intervention | 116.98±36.04 | 53.36±10.68 | 9.80±2.36 | 37.52±10.26 | 34.28±13.70 | 32.74±10.24 |
| After intervention | 106.42±30.55 | 55.32±11.05 | 9.90±2.48 | 34.51±12.84 | 31.96±13.14 | 33.04±9.80 | |
| P | 0.06 | 0.19 | 0.74 | 0.06 | 0.22 | 0.79 | |
| Donepezil | Before intervention | 111.76±39.12 | 43.46±7.56 | 10.44±2.35 | 57.12±10.46 | 50.55±12.77 | 21.48±8.18 |
| After intervention | 74.62±25.24 | 36.70±8.71 | 13.30±2.68 | 66.37±14.97 | 64.75±13.79 | 13.96±7.88 | |
| P | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
Table 3.
Analysis of covariance for assessment of donepezil effect on memory performance, attention, quality of life, and depression in multiple sclerosis patients
| Questionnaire | Donepezil | ||
|---|---|---|---|
| Variable | Factor | F | P |
| Everyday memory | Before intervention | 39.15 | <0.001 |
| Group | 38.85 | <0.001 | |
| Prospective and retrospective memory | Before intervention | 30.16 | <0.001 |
| Group | 45.73 | <0.001 | |
| Digit span memory | Before intervention | 17.41 | <0.001 |
| Group | 41.90 | <0.001 | |
| Physical health | Before intervention | 58.86 | <0.001 |
| Group | 26.19 | <0.001 | |
| Mental health | Before intervention | 20.46 | <0.001 |
| Group | 79.86 | <0.001 | |
| Depression | Before intervention | 78.71 | <0.001 |
| Group | 57.73 | <0.001 | |
Medication adherence and adverse events
Medication adherence and adverse events were evaluated through weekly phone calls. None of the patients reported medication non-adherence in the interviews, however, we did not measure serum levels of the drugs to confirm this.
With respect to the adverse events, three patients in the donepezil group reported nausea and one patient reported diarrhea in one of the follow-ups. Four of the patients started experiencing headaches following donepezil therapy. Two of them reported mild transient headaches one or two times only, while the other two subjects reported recurrent mild to moderate episodes and decided to withdraw the study. For all these patients, we changed the daily regimen to every other day for a week and they were back on the daily regimen after that. In the placebo group, one patient reported gastroesophageal reflux and two reported nausea, but all were mild and resolved without any intervention.
Discussion
Cognitive dysfunction is a major problem in MS patients, leading to other consequences such as decreased QOL, depression, anxiety, etc.[5,19,20] Despite the intensity of the problem, approved interventions are not introduced for these patients. Previous research in the field has resulted in suggesting some interventions, however the mixed results have limited the reliability of these approaches.[21,22] In the current study, we decided to evaluate the efficacy of donepezil treatment on cognitive function, but with a more wholistic approach, by evaluating depression and QOL as secondary outcome measures. This could be beneficial when considering a medication like donepezil in this patient, as the patient may benefit in different aspects from a single intervention.
Given the successful usage of donepezil in Alzheimer disease, it was introduced as a potential treatment in a number of neurologic disorders with cognitive impairment.[23,24] The major mechanism of this medication is through inhibiting acetylcholine esterase, however, its glutaminergic effects are suggested to affect cognition and daily activity in both animals and human.[25] Dysfunction of muscarinic receptors was found as a possible mechanism responsible for memory impairment in multiple sclerosis. Consequently, donepezil was used in these patients to improve memory and cognitive function.[26,27]
In contrary, other major mechanisms of cognitive impairment in MS include myelin destruction (leading to slow transmission of impulses), axonal injuries (leading to main pathways interruptions and development of new elongated slow accessory pathways), and elimination of cognitive modulators.[28] Accordingly, in a systematic review, Cotter et al. argued that acetylcholine esterase impaired function may not be present in all of MS patients with cognitive impairment; thus, all of these patients would not benefit equally from donepezil treatment. They also claimed that this group of medication has no benefit over placebo in improving cognitive impairment.[5]
Based on our findings, donepezil could successfully improve different components of cognitive function, including daily memory, retrospective and prospective memory, and attention. Moreover, it improves depression as well as both physical and mental QOL. Although our findings do not comply with the results from the study by Cottonet al., we believe the observed discrepancy in donepezil treatment outcome is due to the different mechanisms responsible for cognitive impairment in MS patients. Nevertheless, it still could improve cognitive function to some extent, and can also be beneficial in a more wholistic perspective.
QOL is also affected negatively in MS patients. This occurs due to MS progressive nature, the long-term course of disease, development of disabilities, chronic fatigue, and depression.[29] Li et al. showed that donepezil treatment in Alzheimer disease improves QOL.[30] Studies on MS patients have shown improvement of QOL following donepezil therapy and have attributed the observed findings to the possible improved cognitive functionality.[7,31] We also found higher scores of QOL following donepezil therapy among our patients, while the placebo group showed no improvement.
With respect to depression, we found smaller depression scores following donepezil therapy among our patients while the placebo group showed no difference before and after the intervention. Similar to our findings, previous studies have also reported improvement in depression after treatment with donepezil and have attributed the observed change to the improved cognitive function in these patients.[5,32] For instance, long-term use of donepezil for treatment of Alzheimer disease was accompanied with considerable both cognitive and functional benefits.[33,34]
Besides its application in cognition, donepezil could affect other areas as well. A study suggested that donepezil could decrease the brain and spleen interlukin-1β and cyclooxygenase-2 (COX-2), and possibly could reduce systemic inflammation.[24] In contrast, another animal study assessed the efficacy of donepezil on learning and memory of mice following a 15-day period of therapy. At the end of the study, they dissected animals' brains looking for acetylcholine esterase level, as well as oxidative and anti-inflammatory markers. They only reported reduced level of acetylcholine esterase at the end.[35] In the current study, we did not investigate systemic inflammation.
This study was limited by the short duration of treatment, lack of long-term follow-up assessment, lack of assessment for other possible effects of donepezil on systemic inflammation and cytokines, not using other tools to evaluate cognitive function, lack of a reliable method to evaluate medication adherence, and limited number of study population. We recommend further larger studies with longer follow-up duration in future.
Conclusions
Donepezil could effectively improve cognitive impairment in MS patients. Also, it has positive effects on QOL and depression. Therefore, donepezil therapy could cover multiple affected areas in these patients, making them needless of multiple drugs and avoiding consequent side effects and drug interactions. It seems that the earlier initiation of donepezil for patients in the early steps of cognitive dysfunctions can help lessen the severity and decelerate the progression of cognition impairment.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
This study was funded by Vice-Chancellor for Research and Technology of Isfahan University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to thank the managerial team and personnel of multiple sclerosis clinic of Kashani Hospital for their kind cooperation during the study.
References
- 1.Hämäläinen P, Rosti-Otajärvi E. Cognitive impairment in MS: Rehabilitation approaches. Acta Neurol Scand. 2016;134:8–13. doi: 10.1111/ane.12650. [DOI] [PubMed] [Google Scholar]
- 2.Patel VP, Walker LA, Feinstein A. Revisiting cognitive reserve and cognition in multiple sclerosis: A closer look at depression. Mult Scler J. 2018;24:186–95. doi: 10.1177/1352458517692887. [DOI] [PubMed] [Google Scholar]
- 3.de Jong BA, Uitdehaag BM. Anxiety is more important than depression in MS–commentary. Mult Scler J. 2018;24:444–5. doi: 10.1177/1352458518756516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calabrese P, Penner IK. Cognitive dysfunctions in multiple sclerosis–A “multiple disconnection syndrome”? J Neurology. 2007;254:II18–21. doi: 10.1007/s00415-007-2006-5. [DOI] [PubMed] [Google Scholar]
- 5.Cotter J, Muhlert N, Talwar A, Granger K. Examining the effectiveness of acetylcholinesterase inhibitors and stimulant-based medications for cognitive dysfunction in multiple sclerosis: A systematic review and meta-analysis. Neurosci Biobehav Rev. 2018;86:99–107. doi: 10.1016/j.neubiorev.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Christodoulou C, MacAllister WS, McLinskey NA, Krupp LB. Treatment of cognitive impairment in multiple sclerosis. CNS Drugs. 2008;22:87–97. doi: 10.2165/00023210-200822020-00001. [DOI] [PubMed] [Google Scholar]
- 7.Christodoulou C, Melville P, Scherl WF, MacAllister WS, Elkins LE, Krupp LB. Effects of donepezil on memory and cognition in multiple sclerosis. J Neurol Sci. 2006;245:127–36. doi: 10.1016/j.jns.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen L, Lucke-Wold BP, Mookerjee SA, Cavendish JZ, Robson MJ, Scandinaro AL, et al. Role of sigma-1 receptors in neurodegenerative diseases. J Pharmacol Sci. 2015;127:17–29. doi: 10.1016/j.jphs.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17:162–73. doi: 10.1016/S1474-4422(17)30470-2. [DOI] [PubMed] [Google Scholar]
- 10.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS) Neurology. 1983;33:1444–52. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 11.Royle J, Lincoln NB. The everyday memory questionnaire–revised: Development of a 13-item scale. Disabil Rehabil. 2008;30:114–21. doi: 10.1080/09638280701223876. [DOI] [PubMed] [Google Scholar]
- 12.Ghassemzadeh H, Mojtabai R, Karamghadiri N, Ebrahimkhani N. Psychometric properties of a Persian-language version of the beck depression inventory-second edition: BDI-II-PERSIAN. Depress Anxiety. 2005;21:185–92. doi: 10.1002/da.20070. [DOI] [PubMed] [Google Scholar]
- 13.Sunderland A, Harris J, Baddeley A. Everyday Memory, Actions and Absentmindedness. London: Academic Press; 1984. Assessing everyday memory after severe head injury; pp. 191–206. [Google Scholar]
- 14.Huckans M, Pavawalla S, Demadura T, Kolessar M, Seelye A, Roost N, et al. A pilot study examining effects of group-based cognitive strategy training treatment on self-reported cognitive problems, psychiatric symptoms, functioning, and compensatory strategy use in OIF/OEF combat veterans with persistent mild cognitive disorder and history of traumatic brain injury. J Rehabil Res Dev. 2010;47:43–60. doi: 10.1682/jrrd.2009.02.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaygannejad V, Janghorbani M, Ashtari F, Zanjani HA, Zakizade NJ. Effects of rivastigmine on memory and cognition in multiple sclerosis. Can J Neurol Sci. 2008;35:476–81. doi: 10.1017/s0317167100009148. [DOI] [PubMed] [Google Scholar]
- 16.Bakhtiyari F, Foroughan M, Fakhrzadeh H, Nazari N, Najafi B, Alizadeh M, et al. Validation of the persian version of abbreviated mental test (AMT) in elderly residents of Kahrizak charity foundation. Iranian J Diab Metab. 2014;13:487–94. [Google Scholar]
- 17.Zare H, Sahragard M, Khodamoradi S. Investigating of internal consistency and confirmatory factor analysis of prospective and retrospective memory in an iranian sample. Iranian J Cognition Education. 2014;1:33–8. [Google Scholar]
- 18.Ghaem H, Haghighi AB, Jafari P, Nikseresht A. Validity and reliability of the persian version of the multiple sclerosis quality of life questionnaire. Neurolo India. 2007;55:369–75. doi: 10.4103/0028-3886.33316. [DOI] [PubMed] [Google Scholar]
- 19.Babaee S, Shafiei Z, Sadeghi MMM, Nik AY, Valiani M. Effectiveness of massage therapy on the mood of patients after open-heart surgery. Iran J Nursing Midwifery Res. 2012;17(Suppl 1):S120. [PMC free article] [PubMed] [Google Scholar]
- 20.Barzegar M, Badihian S, Mirmosayyeb O, Ashtari F, Jamadi M, Emami S, et al. Comparative study of quality of life, anxiety, depression, and fatigue among patients with neuromyelitis optica spectrum disorder and multiple sclerosis: The first report from Iran. Mult Scler Relat Disord. 2018;22:161–5. doi: 10.1016/j.msard.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 21.Krupp L, Christodoulou C, Melville P, Scherl W, MacAllister W, Elkins L. Donepezil improved memory in multiple sclerosis in a randomized clinical trial. Neurology. 2004;63:1579–85. doi: 10.1212/01.wnl.0000142989.09633.5a. [DOI] [PubMed] [Google Scholar]
- 22.Krupp L, Christodoulou C, Melville P, Scherl W, Pai LY, Muenz L, et al. Multicenter randomized clinical trial of donepezil for memory impairment in multiple sclerosis. Neurology. 2011;76:1500–7. doi: 10.1212/WNL.0b013e318218107a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiong G, Murali DP. Combination drug therapy for Alzheimer's disease. Geriatrics. 2005;60:22–6. [PubMed] [Google Scholar]
- 24.Chen T, Hou R, Xu S, Wu C. Donepezil regulates 1-methyl-4-phenylpyridinium-induced microglial polarization in Parkinson's disease. ACS Chem Neurosci. 2015;6:1708–14. doi: 10.1021/acschemneuro.5b00026. [DOI] [PubMed] [Google Scholar]
- 25.Chan PC, Lee HH, Hong CT, Hu CJ, Wu D. REM sleep behavior disorder (RBD) in dementia with Lewy bodies (DLB) Behav Neurol. 2018;2018:9421098. doi: 10.1155/2018/9421098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ebrahimzade K, Sepas L, Yazdan-Panah R, Abedi GHelich GHeshlaghi M, GHasabi-Alamdari M. Working memory and quality of life in multiple sclerosis patients. J Urmia Univ Med Sci. 2016;27:598–607. [Google Scholar]
- 27.Calabrese P. Neuropsychology of multiple sclerosis. J Neurol. 2006;253:i10–5. doi: 10.1007/s00415-006-1103-1. [DOI] [PubMed] [Google Scholar]
- 28.Hawellek DJ, Hipp JF, Lewis CM, Corbetta M, Engel AK. Increased functional connectivity indicates the severity of cognitive impairment in multiple sclerosis. Proc Natl Acad Sci U S A. 2011;108:19066–71. doi: 10.1073/pnas.1110024108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arnett PA, Barwick FH, Beeney JE. Depression in multiple sclerosis: Review and theoretical proposal. J Int Neuropsychol Soc. 2008;14:691–724. doi: 10.1017/S1355617708081174. [DOI] [PubMed] [Google Scholar]
- 30.Li Q, He S, Chen Y, Feng F, Qu W, Sun H. Donepezil-based multi-functional cholinesterase inhibitors for treatment of Alzheimer's disease. Eur J Med Chem. 2018;158:463–77. doi: 10.1016/j.ejmech.2018.09.031. [DOI] [PubMed] [Google Scholar]
- 31.Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol. 2008;7:1139–51. doi: 10.1016/S1474-4422(08)70259-X. [DOI] [PubMed] [Google Scholar]
- 32.Winkelmann A, Engel C, Apel A, Zettl UK. Cognitive impairment in multiple sclerosis. J Neurol. 2007;254:II35–II42. doi: 10.1007/s00415-007-2010-9. [DOI] [PubMed] [Google Scholar]
- 33.Howard R, McShane R, Lindesay J, Ritchie C, Baldwin A, Barber R, et al. Donepezil and memantine for moderate-to-severe Alzheimer's disease. N Engl J Med. 2012;366:893–903. doi: 10.1056/NEJMoa1106668. [DOI] [PubMed] [Google Scholar]
- 34.Hogan DB. Long-term efficacy and toxicity of cholinesterase inhibitors in the treatment of Alzheimer disease. Can J Psychiatry. 2014;59:618–23. doi: 10.1177/070674371405901202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruhal P, Dhingra D. Inosine improves cognitive function and decreases aging-induced oxidative stress and neuroinflammation in aged female rats. Inflammopharmacology. 2018;26:1317–29. doi: 10.1007/s10787-018-0476-y. [DOI] [PubMed] [Google Scholar]


