Abstract
African Americans have a 2–4-fold greater incidence of endstage kidney disease (ESKD) than whites, which has long raised the possibility of a genetic cause for this disparity. Recent advances in genetic studies have shown a causal association of polymorphisms at the Apolipoprotein L1 (APOL1) gene with the markedly increased risk for the non-diabetic component of the overall disparity in ESKD in African Americans. Although APOL1-associated kidney disease is thought to account for a substantial proportion of ESKD in African Americans, not all the increased risk of ESKD is accounted for, and a complete cataloging of disparities in genetic causes of ESKD eludes our current understanding of genetic associated kidney disease. Genetic testing aids the screening, diagnosis, prognosis and treatment of diseases with a genetic basis. Widespread use of genetic testing in clinical practice is limited by the small number of actionable genetic variants, limited health literacy of providers and patients, and underlying complex ethical, legal and social issues. This perspective will review racial and ethnic differences associated with genetic diseases and development of ESKD in African Americans, and will discuss potential uncertainties associated with our current understanding of penetrance of genetically-linked kidney disease and population attributable risk percent.
Chronic Kidney Disease (CKD) in African Americans (AAs)
Chronic kidney disease (CKD) affects an estimated 26 million Americans,1 of whom 600,000 have endstage kidney disease (ESKD).1 AAs have a 2–4-fold greater incidence of ESKD than whites, and although rates of ESKD appear to be plateauing,1 racial and ethnic disparities in ESKD incidence and prevalence remain. Despite trials showing improvement in CKD outcomes associated with better control of CKD risk factors such as diabetes and hypertension, and the decreasing incidence of ESKD for other minority groups such as Native Americans,2–4 AAs continue to have the highest incidence of ESKD nationwide, and currently comprise 12.2% of the US population, but account for over 31.5% of the patients with ESKD on dialysis.5 Determinants of health such as public policies,6 access to health care, social and cultural factors, individual behavior, and biologic and/or genetic factors contribute to these disparities in ESKD.
A higher proportion of genetic African ancestry in admixed AAs and Hispanic/Latino Americans was found to associate with higher serum creatinine levels and lower eGFR, especially within the normal range values.7 Further, hypertensive families of African descent in the Seychelles demonstrated significant heritability estimates of glomerular filtration rate (GFR) suggesting the familial aggregation of this trait and need for continued efforts to understand the genetic determinants of kidney disease disparities.8
Recent advances in genetic studies have linked polymorphisms of the Apolipoprotein L1 (APOL1) gene with an increased incidence of non-diabetic ESKD in AAs, which is thought to account for roughly 70% of the disparity in ESKD incidence between AAs and whites.9–11 Other genetic diseases such as sickle cell trait are also associated with development of chronic kidney disease and contribute to this disparity.12 Genome-wide association studies (GWASs) typically detect common variants of small to modest effects, and have revealed a few loci for renal function, which associate with certain renal traits in AAs. These loci include DOK6 and FNDC1 for urine albumin creatinine ratio (UACR) and KCNQ1 for estimated glomerular filtration rate (eGFR). The strength of the statistical significance of these loci is described as ‘suggestive’, in this largest but modestly-sized GWAS dataset in AAs.13 GWAS conducted in a prospective CKD cohort, has also revealed one single nucleotide protein (SNP), rs653747, located in RNA coding gene LINCOO923, which associates with eGFR decline among non-diabetic blacks.14
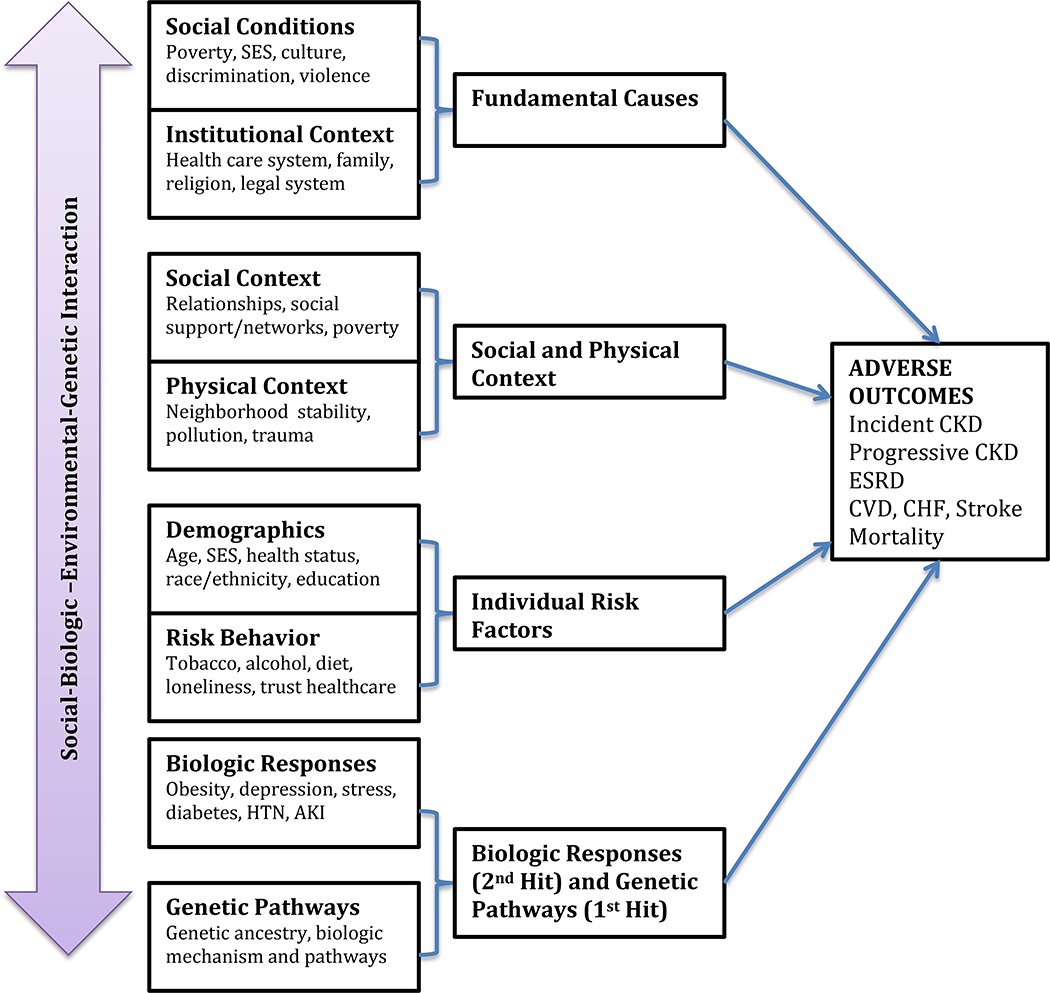
This perspective will review the current literature on what is known and unknown regarding disparities associated with genetic diseases and development of ESKD specifically in AAs and those with recent African ancestry (Figure 1).
Figure 1.
The association of determinants of health with adverse outcomes in kidney disease
APOL1 and Risk of ESKD
For over the past quarter century, AAs consistently have had a 2–4-fold greater incidence of ESKD than whites, which has raised the concern and search for genetic causes for these differences in rates. Over the years, many investigators have searched for potentially inherited genetic causes for kidney disease, which led to the discovery of a variant of the myosin heavy chain 9 (MYH9) gene on chromosome 22 and linkage to ESKD.15,16 Further evaluation by two sets of independent investigators, led to the discovery that two APOL1 polymorphic variants (named G1 and G2) located on chromosome 22 in tight linkage with MYH9, were most likely to be associated with this increased risk of kidney disease in AAs.9,17 Initial case control studies and later population-based studies confirmed the association of APOL1 with development of ESKD and chronic kidney disease (CKD).
Case Control Studies in APOL1
Case control studies among AAs with non-diabetic kidney disease showed that AAs with two APOL1 high risk variants (G1/G1, G2/G2), or compound heterozygotes (G1/G2), were at 10.5-fold (95% Confidence Intervals (CI) 6.0–18.4) greater risk of biopsy-proven focal segmental glomerulosclerosis (FSGS)-associated ESKD, 7.3-fold greater risk of hypertensive ESKD (95% CI 5.6–9.5), and 29-fold greater risk of HIV associated nephropathy (HIVAN) ESKD when compared to AAs with one or no copies of the APOL1 alleles.9,18 Initial population studies estimate that 30%−35% of AAs carry at least one risk allele,19 while 12%−13% carry two of the high-risk alleles.20 While these studies reflect etiology-dependent differences in relative risk, it should be recalled that case control studies, which are classically conducted with small population samples of known cases, in the above examples with biopsy proven disease, and carefully chosen controls, can be associated with high case association when compared to rates obtained from population-based studies, and therefore invite larger cohort studies.
Population-Based Studies in APOL1
Subsequent population-based studies showed that APOL1 high-risk variant alleles were associated with an increased risk of CKD,21 a 5.7-fold (95% CI 3.6–8.9) greater odds of incident albuminuria, 22 lower age of dialysis initiation,23,24 greater risk of incident hypertension, diabetes, but not acute kidney injury (AKI), hospitalizations, and mortality compared to whites.25,26 Diabetes27 and socioeconomic factors were not found to attenuate the risk of CKD progression.28,29 Although AAs were found to have a greater risk of development of CKD, CKD progression, and ESKD, as well as other adverse outcomes, the relative risk was much lower than that previously attributed to development of biopsy-proven FSGS-, hypertensive-, and HIVAN-associated ESKD based on the case-control studies.26 Data from the Dallas Heart Study,21 Coronary Artery Risk Development in Young Adults (CARDIA) study,22 Chronic Renal Insufficiency Cohort (CRIC), and African American Study of Kidney Disease and Hypertension (AASK) studies27 confirmed that APOL1 high-risk variants were associated with greater risk of albuminuria, CKD progression, and ESKD. In addition, among transplant recipients who received kidneys from cadaveric donors positive for APOL1, APOL1 was associated with increased risk of transplant failure. However, APOL1 high-risk status in transplant recipients was not associated with any adverse outcomes.30 Incident cardiovascular events were also shown to be associated with APOL1 high-risk variants among AAs from the Jackson Heart Study (JHS),31 but not between all subjects with and without high-risk variants in the larger ARIC study.26 Larger population-based cohort studies are needed to address discrepancies in these findings.
Mechanisms of action of APOL1
APOL1 protein derives from a family of six apolipoproteins that are relatively conserved in both primates and humans.32 APOL1 is thought to have developed relatively recently and is found to cluster on chromosome 22.33 The APOL1 gene arose approximately 30 million years ago, and is present in humans as well as some non-human primates.34 APOL1 genetics are thought to follow a recessive inheritance pattern, in which affected individuals receive alleles from both parents. G1 is associated with 2 amino acids substitutions (S324G and I384M), while G2 is associated with 6 base-pair deletion, which accounts for 2 amino acids (NY388–389).9 Possessing two of the high-risk APOL1 alleles, G1 or G2, is postulated to be associated with the inheritance of “risk for disease” through a “toxic gain of function” or “loss of protection” mechanism, instead of the classic “loss of function” mechanism, usually associated with recessive genetic polymorphisms.34 APOL1 is not essential for development of kidneys, as a person has been found who is null/null for APOL1 alleles, who was found to have normal kidney function and no albuminuria, although kidney structure was not documented.35 Animal studies show that APOL1 is made primarily by the liver, but is expressed in many organs including the kidney, and is upregulated in inflammatory states, which may contribute to its pathophysiology in disease.34,36 Recent basic research also suggests that APOL1 may be responsible for increased cellular uptake of HIV in HIVAN, leading to accentuated immune responses and worse kidney disease in patients with active HIV infections, who are not sufficiently suppressed by antiretroviral therapy.37 In fact, it has been shown that patients with suppressed HIV viral loads below 400 copies/ml are less susceptible to developing HIVAN in the setting of positive APOL1 variants.38
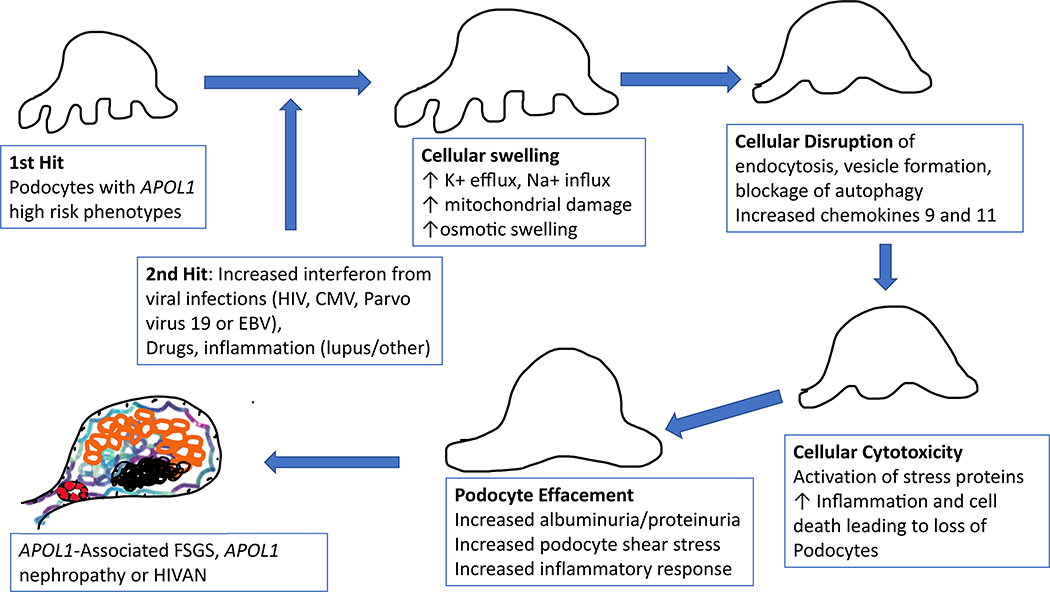
APOL1 polymorphisms are known to be a trypanolytic factor in humans, which protected carriers from African trypanosomes, the causative parasite in African sleeping sickness. Carriers of APOL1 high-risk alleles are protected from African sleeping sickness; G2 confers resistance to Trypanosoma brucei rhodesiense, and G1 decreases severity of infection from T.b. Gambiense.39 The mechanism of resistance to T.b. rhodesiense is still somewhat unclear. It may be due to either insertion into the lysosomal membrane resulting in lysosomal swelling, or due to interaction with the plasma membrane or mitochondria.40 APOL1 confers protection by insertion of an ion channel into trypanosomes, which increases the risk of cellular and mitochondrial lysing, leading to death. Mechanisms of APOL1 toxicity and development of kidney disease in humans may be due to the ability of APOL1 to form ion channels in podocytes and other renal cells such as endothelium. Abnormalities in endosomal trafficking are also a potential underlying mechanism of APOL1 disease.33,41 In vitro studies have shown that APOL1 may be implicated in autophagy or apoptosis of certain cells and may interact with proteins that are critical for glomerular function (slit diaphragm).42 APOL1 may also compromise the ability of certain cell types, such as podocytes, to migrate, eventually leading to depletion in podocyte cell number, thereby, increasing the risk of certain glomerular diseases.42 Terminally differentiated podocytes may be the target forAPOL1-associated disease, but more research is needed to clarify specific pathways (Figure 2).43,44 Certainty regarding the mechanisms of APOL1 and development of FSGS, hypertensive-associated nephropathy or HIVAN are not currently known, but are under intense investigation.40
Figure 2.
The first and second hit hypothesis of APOL1 pathology in kidney disease
Combined Effects of APOL1 High Risk Alleles and GSTM1 Null Allele
Compared with the active allele, the null variant of the antioxidant gene glutathione-S-transferease-u1 (GSTM1), GSTM1(0) has been associated with a more rapid rate of progression of hypertensive CKD in AAs. It has been shown that the joint effect of GSTM1 null and APOL1 high-risk alleles leads to an increased rate of CKD progression compared to those with GSTM1 active/APOL1 low-risk alleles, and confers the highest risk of adverse renal outcomes among hypertensive AAs45. Using the GSTM1 active/APOL1 low-risk as the reference group, the hazard ratios for the composite outcome of incident ESRD and change in estimated glomerular filtration rate (eGFR) were as follows: [Group, hazard ratio; 95% confidence interval (p-value)] [GSTM1 active/APOL1 high-risk group, 2.13; 0.76 to 5.90 (p=0.15]; [GSTM1 null/APOL1 low-risk group, 2.05; 1.08 to 3.88 (p=0.03]; [GSTM1 null/APOL1 high-risk group, 3.0; 1.51 to 5.96 (p=0.002].45
Type 2 Diabetes Mellitus (T2DM)
Disparities in incidence of Type 2 Diabetes Mellitus (T2DM)
An estimated 25.8 million people in the US have T2DM and diabetes-associated ESKD is the primary cause of kidney failure in the US. AAs are over-represented in this patient population,46 and AAs over the age of 40 have approximately two-fold higher greater prevalence of type 2 diabetes compared to European Americans (27.1% vs. 15.5%).47 Genetic factors have been implicated.48 Data from a large curated database of geographically-annotated, disease-associated single nucleotide polymorphisms (SNPs) with human variation measurements in 51 populations across 8 continents from the Human Genome Diversity Panel (HGDP) were analyzed and showed that genetic risk for type 2 diabetes (T2DM) was highest in African populations, intermediate in Middle Eastern and European populations, and lowest in Asian populations. It has been suggested that variation in genetic risk not caused by genetic drift is likely a result of environmental adaptation, which may explain why certain factors affect disease susceptibility. As populations have migrated toward the East, individuals have acquired decreased genetic risk.49 This is likely due to the impact of migration in addition to dynamic local environments, which encourage genetic adaptations that favor beneficial traits.50 There is evidence that climate, diet, and living conditions have led to genetic risk differences in T2DM.51 Single nucleotide polymorphisms (SNPs), including those influencing glucose levels and susceptibility to type 2 diabetes, show variation in allele frequencies across populations with strong signals associating with polar ecoregions, and with foraging, which is typically associated with a diet rich in roots and tubers.51
Ethnic differences in course of diabetes and diabetic complications
It has been suggested that different ethnic groups have variable risk associated with a specific gene linked to diabetes52 and ethnic differences exist in the course of diabetic nephropathy, which is likely due to the presence of causative genetic factors.52 Specifically, ethnic differences in susceptibility to diabetic nephropathy and ESKD have been demonstrated. 53
Ethnic disparities have been demonstrated in the incidence of diabetic complications in a large diabetic cohort after adjustment for demographics, socioeconomics, behavior, and clinical profile. Compared to whites, Asians and Latinos had lower rates of myocardial infarction (MI), congestive heart failure (CHF) and stroke while blacks had lower incidence of MI, but identical rates of CHF and stroke. Asians had one-third the incidence of lower extremity amputations compared to whites, while blacks and Latinos had identical rates to whites. Asians, Latinos and blacks had higher incidence of ESKD than whites. AAs, Hispanics and Native Americas have a higher incidence of diabetic ESKD and longer survival after ESKD onset, and will likely constitute the bulk of the population of diabetic ESKD patients in the future. These ethnic disparities in diabetic complications could not be explained by socioeconomic (SES) disparities, modifiable behavioral outcomes or clinical characteristics. Ethnic differences in genetic susceptibility have been implicated.53
Conversely, a recent post hoc analysis of a randomized clinical trial, showed no differences in incident CKD, eGFR decline or ESKD by population, suggesting that improved comprehensive and standardized management might eradicate some of the population differences seen with kidney disease outcomes in type 2 diabetes. However, it is important to note that the population in this study had a low prevalence of CKD.54
Genes and AAs’ susceptibility to developing Type 2 diabetes mellitus (T2DM) (with or without ESKD)
Emerging research involving fine mapping of reported T2DM loci in AAs (with or without ESKD) has identified index single nucleotide polymorphism (SNP) at the TCF7L2, KLF14, KCNQ1, ADCY5, CDKAL1, JAZF1, GCKR and HMGA2 loci with significant association with T2DM (p < 0.05) in these patients.55 Two additional novel susceptibility loci, which explain the phenotypic variance of Type 2 diabetes in AAs, have also been identified.56 An additional genome wide association study (GWAS) identified diabetic nephropathy susceptibility genes. These novel single nucleotide polymorphisms in AAs, distinct from other ethnicities, contribute to susceptibility to T2DM with ESKD.57 SNP rs7560163 reached genome-wide significance for association with T2DM, while four other loci had less impressive evidence of association.57
Rare Genetic Variants and Risk of Diabetic ESKD in AAs
Although APOL1 accounts for a significant proportion of ESKD in AAs, not all cases of ESKD can be ascribed to APOL1. Investigators continue to search for additional genetic variants that might be implicated in development of kidney disease in AAs, a population at the highest risk for development of ESKD. Rare coding variants in Nephrin (NPHS1),58 Cubilin (CUBN) and Megalin (LRPs) genes, involved in slit diaphragm transport and transportation of albumin across renal tubular epithelial cells respectively, are associated with albuminuria, and may be associated with ESKD in AAs.
Nephrin is an 85 KD transmembrane protein component of the normal glomerular filtration barrier, which is expressed in podocytes. Polymorphisms of the gene were first described with Congenital nephrotic syndrome of the Finnish type (NPHS1), an autosomal recessive disease associated with massive proteinuria in utero first described in several Finnish families 1961.59,60 Using exome sequencing in an African American population with type 2 diabetes (T2DM) associated ESKD, Bonomo and colleagues evaluated coding variants in NPHS1, and associations with T2DM-ESKD in discovery and replication cohorts.58 NPHS1 variant rs35238405 (T233A) was associated with T2DM-ESKD and non-diabetic ESKD in AAs after adjustment for APOL1. It has been hypothesized that this mutation compromises the critical structural region bridging the Ig-C2 and Ig-C3 domains by altering the secondary structure of a B-pleated sheet.58 The authors also found two additional variants (H800R and Y1174H) were associated with protection from ESKD. The authors conclude that variants in the nephrin gene are associated with both increased risk of, and protection from ESKD. While population-attributable risk is low (Table 2), interaction with APOL1 may lead to increased risk of ESKD. Although this study supports NPHS1 as a potential nephropathy susceptibility gene, more definitive confirmatory studies in larger replication population-based cohorts are necessary.
Table 2:
Approximate Population-Attributable Risk Estimates for Genetic Disorders and Incidence in African Americans
| Genetic Variant | Population Prevalence | ESKD Population Attributable Risk % |
|---|---|---|
| APOL1 High Risk ESKD in general population26 | 13% | 19% |
| Diabetic Kidney Disease27 | 15% | 13% |
| High Risk APOL1 and Biopsy-proven FSGS-ESKD18 | 13% | 67% |
| High Risk APOL1 and Biopsy-proven Hypertensive ESKD9 | 13% | 45% |
| High Risk APOL1 and HIVAN-ESKD | 13% | 78.4% |
| Sickle Cell Trait12 | 8% | 9.9% |
| NPHS1 T233A58 | - | 1.1% |
| NPHS1 Y1174H (protection)58 | - | 2.43% |
| NPHS1 H800R (protection)58 | - | 4.56% |
Similarly, polymorphism in the cubilin (CUBN) and megalin (LRP2) genes have been associated with diabetes-associated ESKD in AAs. Cubilin and megalin proteins complex together to form a functional receptor complex in the proximal tubule that reabsorbs filtered urinary albumin. Polymorphisms in CUBN gene have been shown to be associated with albuminuria in individuals of European and recent African ancestry.61 Ma and colleagues found CUBN SNP rs1801239 was associated with T2DM-ESKD in blacks (OR 1.31, 95% CI 1.03–1.67), while a novel LRP2 missense variant was associated with protection from T2DM-associated ESKD in blacks (OR 0.47, 95% CI 0.29–0.75).62 Additional confirmatory studies are needed to support these novel findings.
Hypertension and other genetic findings
Cytochrome P4A11 arachidonic acid monooxygenase (CYP4A11) oxidizes endogenous arachidonic acid to 20-hydroxyeicosatetraenoic acid, which is natriuretic and a renal vasoconstrictor. The CYP4A11 T8590C polymorphism has been associated with hypertension in African-American men. Data from the African American Study of Kidney Disease (AASK) shows higher systolic blood pressure (CC 156.5 +/− 22.6 versus 148.4 +/− 24.3 mmHg in CT and TT combined; p = 0.04). and pulse pressure (p = 0.04) in men with the 8590CC genotype. African-American men with the CYP4A11 T8590C polymorphism who were randomized to the lower blood pressure arm of the AASK trial also had higher systolic and diastolic BP at 36 month follow-up. This genotype has also been associated with an increased cumulative incidence of outcome measures of ESRD or death in black men with proteinuria (p = 0.003), after adjusting for randomization and clinical characteristics.63
Increased Cytochrome P450 3A5 (CYP3A5) of the CYP3A family activity disrupts the metabolism of cortisol in the kidneys and results in sodium and water retention. Therefore, CYP3A5 was implicated in blood pressure regulation and it was suggested that it could be a potential risk factor for the development of hypertension.64 However, the data from different populations in different countries are conflicting. Furthermore, a meta-analysis evaluating the association between the CYP3A5 polymorphism and blood pressure showed no relationship between the CYP3A5 polymorphism and hypertension in neither white nor black populations.65
People with recent African ancestry in the US have been shown to have low plasma renin activity and are considered to be “salt-sensitive”.66,67 Therefore, in investigating reasons for the burden of hypertension in this population, the central role of the epithelial sodium channel (ENAC), which handles sodium transport across membranes and blood pressure regulation in the kidney, has been of interest. In addition to rare monogenetic disorders associated with ENAC such as Liddle’s syndrome, the role of gene polymorphisms of ENAC in hypertension has been investigated. The T594M polymorphism of ENAC was found exclusively in blacks and associated with hypertension in a population of hemodialysis subjects in South London.68 However, further research needs to be done to investigate the potential role of Amiloride, which blocks ENAC, as a new pathway for treatment of high blood pressure in a select population of black hypertensive patients.68
In a bid to explain the higher incidence and prevalence of hypertension, and its associated target organ damage in AAs compared to Caucasians, it has also been shown that TGF-β1 protein as well as TGF-β1 mRNA levels are higher in hypertensives compared with normotensives. More importantly, TGF-β1 protein levels have been shown to be highest in blacks with hypertension. 69 Prior work had shown that African American ESRD patients hyper- express TGF-β1 compared with Caucasian ESRD patients; this has been postulated as a mechanism for the increased prevalence of kidney failure in AAs.70
Renin Angiotensin and Aldosterone System (RAAS) polymorphisms and ESKD
Well-known factors that influence progression of CKD to ESRD such as diabetes,71 hypertension72, proteinuria,73 and race74 have been attributed in part to a genetic component; however, genomic factors explaining this complex phenotype are not well understood. RAAS has been a focus of interest in this area because of its vasoconstrictive properties and pro-inflammatory effect of constituents, which may contribute to inflammation and renal fibrosis.75,76 The search for a genetic basis for kidney disease led to significant interest in RAAS polymorphisms, because these were thought to contribute to the development and course of non-diabetic kidney disease; however, available data are conflicting in this regard.77 The genes encoding angiotensinogen (AGT), angiotensin converting enzyme (ACE) and angiotensin II type 1 receptor (AT1) have been investigated as potential candidates contributing to kidney disease, because of the importance of the proteins encoded by these genes in the regulation of intrarenal hemodynamics and hypertension.
Angiotensinogen
Some evidence links polymorphisms in AGT gene with the development of hypertension78, and possibly kidney disease, in populations from France78, Utah,78 and Japan;79 however, further studies have failed to support these findings in African American80, European81 or Japanese82 populations.83 The TT polymorphism of the AGT gene, which replaces threonine for methionine at codon 235, attracted significant interest due to the higher frequency of homozygosity in AAs84 compared to whites. The TT polymorphism was linked to higher levels of plasma AGT78 and altered renal control mechanisms (in whites), perhaps due to more intrarenal formation of angiotensin II. One study that compared renal hemodynamic responses of AAs and whites to the ACE inhibitor, Captopril, and an infusion of angiotensin II, found differences suggesting that AAs have increased levels of angiotensin II in their kidneys. It is thought that the higher levels of angiotensin II in AAs may correlate with an increased frequency of the TT polymorphism in AAs. Interestingly, plasma AGT levels correlate with body mass index (BMI), and adipocytes express AGT mRNA in abundance, therefore supporting the hypothesis that environmental factors such as obesity could contribute to the effects of TT polymorphism in AAs, since it has been shown that AAs have increased body weight compared to other populations.83,85
Angiotensin-Converting Enzyme
The insertion/deletion of a 287 base pair alu repeat sequence is the best-studied polymorphism of the ACE gene, which has been investigated in relation to diabetic nephropathy, kidney disease, and cardiovascular disease in non-diabetic patients. The presence of the double deletion (DD) polymorphism may signal a higher rate of progression of kidney disease in non-diabetic kidney disease,86 and has also been linked to a faster decline of renal function and reduced responses of blood pressure and proteinuria to ACE inhibitors in type 1 diabetics.87 The insertion (II) genotype in hypertensive albuminuric insulin-dependent type 1 diabetics (IDDM), has been associated with the greatest reduction in albuminuria [mean (95% CI)] [61% (34 to 77)%] vs. [22% (3 to 37)%] in the insertion deletion (ID) and [31% (13 to 46)%] in the DD genotypes (p<0.01). Patients with the II genotype also have a more pronounced reduction in mean arterial blood pressure (MABP) compared to patients with ID and DD genotype (p=0.02).87
A comprehensive genomic study of CKD progression examined the joint contribution of RAAS variants to CKD progression.88 A single-marker, gene and pathway-based analyses of the associations of common variants found 12 RAAS candidate genes associated with CKD progression phenotypes of white and black participants from the Chronic Renal Insufficiency Cohort (CRIC) study.88 Candidate genes AGT and RENBP were consistently linked to risk of renal events in independent sub-populations of white and black subjects from the CRIC study. Two individual AGT variants, rs5051 and rs699, were associated with renal events among whites (p = 0.06 and 0.03 respectively) and blacks (p=0.05 and 0.17 respectively) after adjustment for systolic blood pressure. Additional RAAS pathway candidate genes were also associated with renal events in white and black CRIC participants. Interestingly, ACE and CYPIIB2 were associated with CKD progression phenotypes in whites while ACE2 and AGTR2 were associated with CKD progression in blacks.88
Other Potential Genetic Variants of Interest
UMOD Gene
Uromodulin is a Tamm-Horsfall protein, which is a glycoprotein synthesized by the thick ascending limb of the loop of Henle (TAL). It is the most abundant protein in normal human urine.89 Uromodulin is involved in the regulation of salt transport, protection against urinary tract infections (UTIs), decreased propensity for forming calcium crystals, and provision of innate immunity by binding immunoglobulins and cytokines and/or activating monocytes and dendritic cells.89 It is encoded by the UMOD gene, one of the most impressive loci associated with CKD in the general population, with a consistently large effect on eGFR and CKD risk, across different ethnic groups (OR [95% confidence interval]) (1.24 [1.19 to 2.19]) for CKD, increasing up to (1.35 [1.18 to 1.54]) if eGFRcrea <45ml/min/1.73m2.89 UMOD remains the only locus that demonstrates genome-wide level of significance for its association with incident CKD and incident ESRD. Dominant mutations in UMOD lead to the discovery of ADTKD, a rare disorder associated with hyperuricemia, gout and tubulointerstitial disease, found to be associated with CKD and renal failure.89
In at-risk individuals, higher levels of uromodulin are protective of kidney damage. However, the lead UMOD risk variant (allele T at rs4293393) has been associated with a dose-dependent increase in uromodulin expression, which is thought to be associated with salt-sensitive hypertension and CKD. This ancestral allele has 70% to 80% prevalence in Africans and Europeans and >90% prevalence in East Asians.89
Sickle Cell Disease (SCD) and Sickle Cell Trait (SCT) and development of CKD/ESKD
Sickle Cell Disease (SCD) is defined as homozygosity in hemoglobin S gene (SS), which results in an amino acid substitution that leads to conformational changes in the rheology of red blood cells (RBCs). Although the absolute number of people affect with SCD in the US is unknown, current estimates suggest 100,000 people are affected, and that approximately 1 in 365 AAs and 1 in 16,300 Hispanic births are associated with SCD. SCD follows recessive inheritance, and is associated with protection from development of malaria in endemic areas of the world, such as sub-Saharan Africa and the Mediterranean coast. SCD is associated with sickling of RBCs that leads to hypoxia in major organs, bones and muscle, resulting in painful crisis, vascular thrombosis, and early renal disease. Kidney disease is one of the most common complications of SCD, and manifests itself as a spectrum of disease termed sickle-hemoglobin kidney disease, ranging from early alterations in urinary concentrating ability (hyposthenuria), to medullary ischemia and microinfarctions due to RBC sickling.90 These pathologic changes result in release of cytokines, leading to hyperfiltration, inflammation, and endothelial dysfunction. Clinically, patients can present with hematuria (microscopic and gross), albuminuria, and papillary necrosis, which is the precursor to CKD and ESKD.90
Sickle Cell Trait (SCT) affects over 300 million people worldwide, and is found in 1 in 12 AAs, with a prevalence of 8–9%. SCT was thought to be a benign genetic finding until SCT was shown to be associated with prevalent and incident CKD, rapid kidney function decline, and albuminuria in population-based cohort studies,12 Using five large, prospective, population-based studies: ARIC (1987–2013), Jackson Heart Study (JHS) (2000–2012), CARDIA (1985–2006), Multi-Ethnic Study of Atherosclerosis (MESA) (2000–2012), and the Women’s Health Initiative (WHI) (1993–2012), 15,975 self-identified AAs with and without SCT were evaluated for CKD, incident CKD, albuminuria, and decline in eGFR. Individuals with SCT had an increased risk of CKD (odds ratio (OR) 1.57 [95% CI 1.34–1.84], incident CKD (OR 1.79 [95% CI 1.45–2.20), and decline in eGFR (OR 1.32 [95% CI 1.07–1.61], compared to non-carriers. SCT was also associated with albuminuria (OR 1.86 [95% CI 1.49–2.31].12 There was no interaction with APOL1 high-risk alleles and those with both APOL1 and SCT did not have an increased risk of CKD. Initial population-based and case control studies did not show associations of SCT with development of ESKD, but a larger observational study with the REasons for Geographic and Racial Differences in Stroke (REGARDS) population showed a 2-fold (95% CI 1.44–2.04) greater risk of incident ESKD in those with SCT compared to those without.91 Risk of ESKD was independent of APOL1, diabetes, and hypertension. Mechanisms of development of CKD are similar to those in SCD, but attenuated by the reduced amount of hemoglobin S physiologic effects on RBCs.
CYP3A5*1 Polymorphisms and Genetic Differences in Metabolism Of Immunosuppressants In Transplant Recipients
It has been shown that African American transplant recipients require higher doses of Tacrolimus in order to achieve the same mean blood levels an immunosuppression as whites.92 This has been linked in part to the presence of the wild type gene, CYP3A5*1 (one of the genes in the cytochrome P450 system) in more than half of AAs, while being absent in 60 to 90% of whites, and leads to a rapid rate of metabolism of Tacrolimus in AAs.93
Conclusions
New findings of genetic variants explain some, but not all of the racial/ethnic differences of increased risk of ESKD in AAs and those of recent African ancestry in the US. Modern genetic methods may lead to novel findings of additional variants and associations with ESKD risk. Penetrance of genetic variants in complex diseases such as kidney disease and hypertension remain unexplained, which may support gene and environment interactions on disease presentation. Mechanisms of disease pathology continue to remain elusive and are under intense investigation, results of which are necessary for the future development of potential interventions. Although APOL1 is a strong candidate for the association of development of kidney disease in AAs, mechanistic studies have not confirmed a definitive mechanism for ESKD development. Independent replication of smaller GWAS findings in larger African American cohort populations are needed to confirm findings and facilitate the discovery of causal variants. Genetic penetrance of complex diseases depends not only on the genetic abnormality, but also interactions with genetic and environmental influences. Epigenetic differences along with sociocultural differences may also in part explain some racial and ethnic differences in ESKD development and disease penetrance. Population-attributable risk (PAR) estimates allows one to use prevalence and incidence data to try to determine the effects of certain population characteristics and development of certain diseases. Given the prevalence of APOL1 and other genetic diseases in the African American community, an excess risk of kidney disease remains unaccounted for among AAs (Table 2). Continued assessment of social and biologic determinants of health that can be attenuated, need further evaluation to determine if the incidence and progression of kidney disease in those with known genetic variants found to be associated with ESKD, can be decreased.
Table 1:
Risk of Adverse Outcome Associated with APOL1
| Type of Study | Study, Author, and Year | Disease | High Risk AA vs. Low Risk AA OR/HR/IRR/RRR Multivariable Analysis* | 95% Confidence Interval | High Risk AA vs White OR/HR/IRR/RRR Multivariable Analysis* | 95% Confidence Interval |
|---|---|---|---|---|---|---|
| Case Control | Genovese9 2010 | Hypertensive ESKD | OR= 7.3 | 5.6–9.5 | - | - |
| Case Control | Kopp18 2011 | FSGS HIVAN |
OR=16.9 OR=29.2 | 11.0–26.5 13.1–68.5 | - | - |
| Cohort | Dallas Heart Study Friedman94 2011 | HTN ACR Egfr<60 |
- | - | OR=1.7 OR=2.8 OR=3.9 |
P=.003 1.8–4.4 1.9–2.9 |
| Cohort | CRIC Parsa27 2013 | ESKD | - | - | HR=2.16 | 1.62–2.89 |
| Cohort | CARDIA Peralta22 2016 | Incident Albuminuria | OR=2.93 | 1.86–4.62 | OR=3.89 | 2.43–6.22 |
| Cohort | ARIC Grams26 2016 | Incident HTN Diabetes ESKD |
- | - | IRR=1.21 IRR=1.41 IRR=2.84 |
1.00–1.45 1.20–1.67 1.88–4.30 |
| Cohort | CARDIA Chen95 2017 | Incident HTN | RRR=1.04 | 0.56–1.94 | - | - |
| RCT Secondary analysis | AASK Parsa27 2013 | ESKD/Doubling Creatinine | HR=1.88 | 1.46–2.44 | - | - |
| RCT secondary analysis | AASK Chen96 2017 | Proteinuria in HTN-Attributed CKD | HR=1.72 | 1.27–2.32 | - | - |
Multivariable analysis: OR=Odds Ratio, HR=hazard Ratio, IRR=Incident Rate Ratio, RRR=Relative Risk Ratio. High risk APOL1 African Americans include (G1/G1, G2/G2/ G1/G2), low risk APOL1 African Americans <2 alleles (G1/G0, G2/G0, G0/G0).
Definitions: ESKD=endstage kidney disease, Coronary Artery Risk Development in Young Adults (CARDIA) study, Chronic Renal Insufficiency Cohort (CRIC), and African American Study of Kidney Disease and Hypertension (AASK), and Atherosclerosis Risk in Communities (ARIC). RCT=randomized controlled trial, hypertension (HTN, albumin to creatinine ratio (ACR), focal segmental glomerulosclerosis (FSGS), HIV associated nephropathy (HIVAN), chronic kidney disease (CKD).
Acknowledgements of Support
This manuscript was supported by Dr. Young’s National Institutes of Health (NIH) National Institutes of Health (NIH) National Human Genomes Research Institute grant 1R01HG007879–01A1. Dr. Umeukeje was previously supported by an NIH K12HD043483–17 grant and currently supported by NIH 1K23DK114566–01A1 award. Dr. Young is also supported in part by funding from the Veterans Affairs Puget Sound Health Care System. The Veterans Affairs does not endorse any of the statements or opinions advocated by this manuscript. We would also like to acknowledge Ms. Rachel Lane Walden, MLIS, Librarian at the Library Liaison for the School of Nursing and Eskind Biomedical Library at Vanderbilt University.
Footnotes
None of the authors has any conflicts of interest to disclose.
References
- 1.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA : the journal of the American Medical Association. 2007;298(17):2038–2047. [DOI] [PubMed] [Google Scholar]
- 2.Appel LJ, Wright JT Jr., Greene T, et al. Intensive blood-pressure control in hypertensive chronic kidney disease. The New England journal of medicine. 2010;363(10):918–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Group DER, de Boer IH, Sun W, et al. Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. The New England journal of medicine. 2011;365(25):2366–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wright JT Jr., Bakris G, Greene T, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. Jama. 2002;288(19):2421–2431. [DOI] [PubMed] [Google Scholar]
- 5.USRDS. Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. 2012. [DOI] [PubMed]
- 6.Brancati FL, Whittle JC, Whelton PK, Seidler AJ, Klag MJ. The excess incidence of diabetic end-stage renal disease among blacks. A population-based study of potential explanatory factors [see comments]. JAMA. 1992;268(21):3079–3084. [PubMed] [Google Scholar]
- 7.Udler MS, Nadkarni GN, Belbin G, et al. Effect of Genetic African Ancestry on eGFR and Kidney Disease. Journal of the American Society of Nephrology : JASN. 2015;26(7):1682–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bochud M, Elston RC, Maillard M, et al. Heritability of renal function in hypertensive families of African descent in the Seychelles (Indian Ocean). Kidney international. 2005;67(1):61–69. [DOI] [PubMed] [Google Scholar]
- 9.Genovese G, Friedman DJ, Ross MD, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science (New York, NY). 2010;329(5993):841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tzur S, Rosset S, Shemer R, et al. Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Human genetics. 2010;128(3):345–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dummer PD, Limou S, Rosenberg AZ, et al. APOL1 Kidney Disease Risk Variants: An Evolving Landscape. Seminars in nephrology. 2015;35(3):222–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naik RP, Derebail VK, Grams ME, et al. Association of sickle cell trait with chronic kidney disease and albuminuria in African Americans. JAMA : the journal of the American Medical Association. 2014;312(20):2115–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu CT, Garnaas MK, Tin A, et al. Genetic association for renal traits among participants of African ancestry reveals new loci for renal function. PLoS genetics. 2011;7(9):e1002264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parsa A, Kanetsky PA, Xiao R, et al. Genome-Wide Association of CKD Progression: The Chronic Renal Insufficiency Cohort Study. Journal of the American Society of Nephrology. 2017;28(3):923–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kao WH, Klag MJ, Meoni LA, et al. MYH9 is associated with nondiabetic end-stage renal disease in African Americans. Nature genetics. 2008;40(10):1185–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kopp JB, Smith MW, Nelson GW, et al. MYH9 is a major-effect risk gene for focal segmental glomerulosclerosis. Nature genetics. 2008;40(10):1175–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tzur S, Rosset S, Shemer R, et al. Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Human genetics. 2010;128(3):345–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kopp JB, Nelson GW, Sampath K, et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. Journal of the American Society of Nephrology : JASN. 2011;22(11):2129–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedman DJ, Kozlitina J, Genovese G, Jog P, Pollak MR. Population-based risk assessment of APOL1 on renal disease. Journal of the American Society of Nephrology : JASN. 2011;22(11):2098–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wasser WG, Tzur S, Wolday D, et al. Population genetics of chronic kidney disease: the evolving story of APOL1. Journal of nephrology. 2012;25(5):603–618. [DOI] [PubMed] [Google Scholar]
- 21.Foster MC, Coresh J, Fornage M, et al. APOL1 variants associate with increased risk of CKD among African Americans. Journal of the American Society of Nephrology : JASN. 2013;24(9):1484–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peralta CA, Bibbins-Domingo K, Vittinghoff E, et al. APOL1 Genotype and Race Differences in Incident Albuminuria and Renal Function Decline. Journal of the American Society of Nephrology : JASN. 2016;27(3):887–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanji Z, Powe CE, Wenger JB, et al. Genetic variation in APOL1 associates with younger age at hemodialysis initiation. Journal of the American Society of Nephrology : JASN. 2011;22(11):2091–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tzur S, Rosset S, Skorecki K, Wasser WG. APOL1 allelic variants are associated with lower age of dialysis initiation and thereby increased dialysis vintage in African and Hispanic Americans with non-diabetic end-stage kidney disease. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2012;27(4):1498–1505. [DOI] [PubMed] [Google Scholar]
- 25.Reeves-Daniel AM, DePalma JA, Bleyer AJ, et al. The APOL1 gene and allograft survival after kidney transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11(5):1025–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grams ME, Rebholz CM, Chen Y, et al. Race, APOL1 Risk, and eGFR Decline in the General Population. Journal of the American Society of Nephrology : JASN. 2016;27(9):2842–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parsa A, Kao WH, Xie D, et al. APOL1 risk variants, race, and progression of chronic kidney disease. The New England journal of medicine. 2013;369(23):2183–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen TK, Choi MJ, Kao WH, et al. Examination of Potential Modifiers of the Association of APOL1 Alleles with CKD Progression. Clinical journal of the American Society of Nephrology : CJASN. 2015;10(12):2128–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tamrat R, Peralta CA, Tajuddin SM, Evans MK, Zonderman AB, Crews DC. Apolipoprotein L1, income and early kidney damage. BMC nephrology. 2015;16:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freedman BI, Pastan SO, Israni AK, et al. APOL1 Genotype and Kidney Transplantation Outcomes From Deceased African American Donors. Transplantation. 2016;100(1):194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ito K, Bick AG, Flannick J, et al. Increased burden of cardiovascular disease in carriers of APOL1 genetic variants. Circulation research. 2014;114(5):845–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Page NM, Butlin DJ, Lomthaisong K, Lowry PJ. The human apolipoprotein L gene cluster: identification, classification, and sites of distribution. Genomics. 2001;74(1):71–78. [DOI] [PubMed] [Google Scholar]
- 33.Kruzel-Davila E, Wasser WG, Skorecki K. APOL1 Nephropathy: A Population Genetics and Evolutionary Medicine Detective Story. Seminars in nephrology. 2017;37(6):490–507. [DOI] [PubMed] [Google Scholar]
- 34.Friedman DJ. A Brief History of APOL1: A Gene Evolving. Seminars in nephrology. 2017;37(6):508–513. [DOI] [PubMed] [Google Scholar]
- 35.Johnstone DB, Shegokar V, Nihalani D, et al. APOL1 null alleles from a rural village in India do not correlate with glomerulosclerosis. PloS one. 2012;7(12):e51546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Madhavan SM, O’Toole JF, Konieczkowski M, Ganesan S, Bruggeman LA, Sedor JR. APOL1 localization in normal kidney and nondiabetic kidney disease. Journal of the American Society of Nephrology : JASN. 2011;22(11):2119–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taylor HE, Khatua AK, Popik W. The innate immune factor apolipoprotein L1 restricts HIV-1 infection. Journal of virology. 2014;88(1):592–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Estrella MM, Li M, Tin A, et al. The association between APOL1 risk alleles and longitudinal kidney function differs by HIV viral suppression status. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2015;60(4):646–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cooper A, Ilboudo H, Alibu VP, et al. APOL1 renal risk variants have contrasting resistance and susceptibility associations with African trypanosomiasis. eLife. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Toole JF, Bruggeman LA, Madhavan S, Sedor JR. The Cell Biology of APOL1. Seminars in nephrology. 2017;37(6):538–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beckerman P, Bi-Karchin J, Park AS, et al. Transgenic expression of human APOL1 risk variants in podocytes induces kidney disease in mice. Nature medicine. 2017;23(4):429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lan X, Jhaveri A, Cheng K, et al. APOL1 risk variants enhance podocyte necrosis through compromising lysosomal membrane permeability. American journal of physiology Renal physiology. 2014;307(3):F326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kruzel-Davila E, Wasser WG, Aviram S, Skorecki K. APOL1 nephropathy: from gene to mechanisms of kidney injury. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2016;31(3):349–358. [DOI] [PubMed] [Google Scholar]
- 44.Beckerman P, Susztak K. APOL1: The Balance Imposed by Infection, Selection, and Kidney Disease. Trends Mol Med. 2018;24(8):682–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bodonyi-Kovacs G, Ma JZ, Chang J, et al. Combined Effects of GSTM1 Null Allele and APOL1 Renal Risk Alleles in CKD Progression in the African American Study of Kidney Disease and Hypertension Trial. Journal of the American Society of Nephrology : JASN. 2016;27(10):3140–3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.US Department of Health and Human Services C, Centers for Disease Control and Prevention. Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States. 2011.
- 47.Cowie CC, Rust KF, Ford ES, et al. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988–1994 and 2005–2006. Diabetes care. 2009;32(2):287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rotimi C, Cooper R, Cao G, Sundarum C, McGee D. Familial aggregation of cardiovascular diseases in African-American pedigrees. Genetic Epidemiology. 1994;11(5):397–407. [DOI] [PubMed] [Google Scholar]
- 49.Corona E, Chen R, Sikora M, et al. Analysis of the genetic basis of disease in the context of worldwide human relationships and migration. PLoS genetics. 2013;9(5):e1003447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ziaeian B, Araujo KL, Van Ness PH, Horwitz LI. Medication reconciliation accuracy and patient understanding of intended medication changes on hospital discharge. Journal of general internal medicine. 2012;27(11):1513–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hancock AM, Witonsky DB, Ehler E, et al. Colloquium paper: human adaptations to diet, subsistence, and ecoregion are due to subtle shifts in allele frequency. Proceedings of the National Academy of Sciences of the United States of America. 2010;107 Suppl 2:8924–8930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Freedman BI, Bostrom M, Daeihagh P, Bowden DW. Genetic factors in diabetic nephropathy. Clinical journal of the American Society of Nephrology : CJASN. 2007;2(6):1306–1316. [DOI] [PubMed] [Google Scholar]
- 53.Karter AJ, Ferrara A, Liu JY, Moffet HH, Ackerson LM, Selby JV. Ethnic disparities in diabetic complications in an insured population. JAMA : the journal of the American Medical Association. 2002;287(19):2519–2527. [DOI] [PubMed] [Google Scholar]
- 54.Gerber C, Cai X, Lee J, et al. Incidence and Progression of Chronic Kidney Disease in Black and White Individuals with Type 2 Diabetes. Clinical journal of the American Society of Nephrology : CJASN. 2018;13(6):884–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ng MC, Saxena R, Li J, et al. Transferability and fine mapping of type 2 diabetes loci in African Americans: the Candidate Gene Association Resource Plus Study. Diabetes. 2013;62(3):965–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ng MC, Shriner D, Chen BH, et al. Meta-analysis of genome-wide association studies in African Americans provides insights into the genetic architecture of type 2 diabetes. PLoS genetics. 2014;10(8):e1004517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Palmer ND, McDonough CW, Hicks PJ, et al. A genome-wide association search for type 2 diabetes genes in African Americans. PloS one. 2012;7(1):e29202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bonomo JA, Ng MC, Palmer ND, et al. Coding variants in nephrin (NPHS1) and susceptibility to nephropathy in African Americans. Clinical journal of the American Society of Nephrology : CJASN. 2014;9(8):1434–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kestila M, Lenkkeri U, Mannikko M, et al. Positionally cloned gene for a novel glomerular protein--nephrin--is mutated in congenital nephrotic syndrome. Molecular cell. 1998;1(4):575–582. [DOI] [PubMed] [Google Scholar]
- 60.Norio R Heredity in the congenital nephrotic syndrome. A genetic study of 57 finnish FAMILIES WITH A REVIEW OF REPORTED CASES. Annales paediatriae Fenniae. 1966;12:Suppl 27:21–94. [PubMed] [Google Scholar]
- 61.Boger CA, Chen MH, Tin A, et al. CUBN is a gene locus for albuminuria. Journal of the American Society of Nephrology : JASN. 2011;22(3):555–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ma J, Guan M, Bowden DW, et al. Association Analysis of the Cubilin (CUBN) and Megalin (LRP2) Genes with ESRD in African Americans. Clinical journal of the American Society of Nephrology : CJASN. 2016;11(6):1034–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gainer JV, Lipkowitz MS, Yu C, et al. Association of a CYP4A11 variant and blood pressure in black men. Journal of the American Society of Nephrology : JASN. 2008;19(8):1606–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Givens RC, Lin YS, Dowling AL, et al. CYP3A5 genotype predicts renal CYP3A activity and blood pressure in healthy adults. Journal of applied physiology (Bethesda, Md : 1985). 2003;95(3):1297–1300. [DOI] [PubMed] [Google Scholar]
- 65.Zhang YP, Zuo XC, Huang ZJ, et al. CYP3A5 polymorphism, amlodipine and hypertension. Journal of human hypertension. 2014;28(3):145–149. [DOI] [PubMed] [Google Scholar]
- 66.Weinberger MH, Miller JZ, Luft FC, Grim CE, Fineberg NS. Definitions and characteristics of sodium sensitivity and blood pressure resistance. Hypertension (Dallas, Tex : 1979). 1986;8(6 Pt 2):Ii127–134. [DOI] [PubMed] [Google Scholar]
- 67.Rossier BC, Bochud M, Devuyst O. The Hypertension Pandemic: An Evolutionary Perspective. Physiology. 2017;32(2):112–125. [DOI] [PubMed] [Google Scholar]
- 68.Swift PA, Macgregor GA. Genetic variation in the epithelial sodium channel: a risk factor for hypertension in people of African origin. Advances in renal replacement therapy. 2004;11(1):76–86. [DOI] [PubMed] [Google Scholar]
- 69.Suthanthiran M, Li B, Song JO, et al. Transforming growth factor-beta 1 hyperexpression in African-American hypertensives: A novel mediator of hypertension and/or target organ damage. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(7):3479–3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Suthanthiran M, Khanna A, Cukran D, et al. Transforming growth factor-beta 1 hyperexpression in African American end-stage renal disease patients. Kidney international. 1998;53(3):639–644. [DOI] [PubMed] [Google Scholar]
- 71.Brancati FL, Whelton PK, Randall BL, Neaton JD, Stamler J, Klag MJ. Risk of end-stage renal disease in diabetes mellitus: a prospective cohort study of men screened for MRFIT. Multiple Risk Factor Intervention Trial. JAMA : the journal of the American Medical Association. 1997;278(23):2069–2074. [PubMed] [Google Scholar]
- 72.Klag MJ, Whelton PK, Randall BL, et al. Blood pressure and end-stage renal disease in men. The New England journal of medicine. 1996;334(1):13–18. [DOI] [PubMed] [Google Scholar]
- 73.Peterson JC, Adler S, Burkart JM, et al. Blood pressure control, proteinuria, and the progression of renal disease. The Modification of Diet in Renal Disease Study. Annals of internal medicine. 1995;123(10):754–762. [DOI] [PubMed] [Google Scholar]
- 74.Cowie CC, Port FK, Wolfe RA, Savage PJ, Moll PP, Hawthorne VM. Disparities in incidence of diabetic end-stage renal disease according to race and type of diabetes. The New England journal of medicine. 1989;321(16):1074–1079. [DOI] [PubMed] [Google Scholar]
- 75.Mezzano SA, Ruiz-Ortega M, Egido J. Angiotensin II and renal fibrosis. Hypertension (Dallas, Tex : 1979). 2001;38(3 Pt 2):635–638. [DOI] [PubMed] [Google Scholar]
- 76.Remuzzi G, Perico N, Macia M, Ruggenenti P. The role of renin-angiotensin-aldosterone system in the progression of chronic kidney disease. Kidney international Supplement. 2005(99):S57–65. [DOI] [PubMed] [Google Scholar]
- 77.Schmidt S, Ritz E. Genetics of the renin-angiotensin system and renal disease: a progress report . Current opinion in nephrology and hypertension. 1997;6(2):146–151. [DOI] [PubMed] [Google Scholar]
- 78.Jeunemaitre X, Soubrier F, Kotelevtsev YV, et al. Molecular basis of human hypertension: role of angiotensinogen. Cell. 1992;71(1):169–180. [DOI] [PubMed] [Google Scholar]
- 79.Hata A, Namikawa C, Sasaki M, et al. Angiotensinogen as a risk factor for essential hypertension in Japan. The Journal of clinical investigation. 1994;93(3):1285–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rotimi C, Morrison L, Cooper R, et al. Angiotensinogen gene in human hypertension. Lack of an association of the 235T allele among African Americans. Hypertension (Dallas, Tex : 1979). 1994;24(5):591–594. [DOI] [PubMed] [Google Scholar]
- 81.Brand E, Chatelain N, Keavney B, et al. Evaluation of the Angiotensinogen Locus in Human Essential Hypertension. Hyertension. 1998;31:725–729. [DOI] [PubMed] [Google Scholar]
- 82.Kato N, Sugiyama T, Morita H, Kurihara H, Yamori Y, Yazaki Y. Angiotensinogen gene and essential hypertension in the Japanese: extensive association study and meta-analysis on six reported studies. Journal of hypertension. 1999;17(6):757–763. [DOI] [PubMed] [Google Scholar]
- 83.Price DA, Crook ED. Kidney disease in African Americans: genetic considerations. Journal of the National Medical Association. 2002;94(8 Suppl):16s–27s. [PMC free article] [PubMed] [Google Scholar]
- 84.Rotimi C, Puras A, Cooper R, et al. Polymorphisms of Renin-Angiotensin Genes Among Nigerians, Jamaicans, and African Americans. Hypertension (Dallas, Tex : 1979) 1996;27(3):558–563. [DOI] [PubMed] [Google Scholar]
- 85.Jones DW. What is the role of obesity in hypertension and target organ injury in African Americans? The American journal of the medical sciences. 1999;317(3):147–151. [DOI] [PubMed] [Google Scholar]
- 86.Samuelsson O, Attman P-O, Larsson R, et al. Angiotensin I-converting enzyme gene polymorphism in non-diabetic renal disease. Nephrology Dialysis Transplantation. 2000;15(4):481–486. [DOI] [PubMed] [Google Scholar]
- 87.Jacobsen P, Rossing K, Rossing P, et al. Angiotensin converting enzyme gene polymorphism and ACE inhibition in diabetic nephropathy. Kidney international. 1998;53(4):1002–1006. [DOI] [PubMed] [Google Scholar]
- 88.Kelly TN, Raj D, Rahman M, et al. The role of renin-angiotensin-aldosterone system genes in the progression of chronic kidney disease: findings from the Chronic Renal Insufficiency Cohort (CRIC) study. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2015;30(10):1711–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Devuyst O, Pattaro C. The UMOD Locus: Insights into the Pathogenesis and Prognosis of Kidney Disease. Journal of the American Society of Nephrology : JASN. 2018;29(3):713–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Naik RP, Derebail VK. The spectrum of sickle hemoglobin-related nephropathy: from sickle cell disease to sickle trait. Expert review of hematology. 2017;10(12):1087–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Naik RP, Irvin MR, Judd S, et al. Sickle Cell Trait and the Risk of ESRD in Blacks. Journal of the American Society of Nephrology : JASN. 2017;28(7):2180–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nadkarni GN, Horowitz CR. Genomics in CKD: Is This the Path Forward? Advances in chronic kidney disease. 2016;23(2):120–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lamba JK, Lin YS, Schuetz EG, Thummel KE. Genetic contribution to variable human CYP3A-mediated metabolism. Advanced drug delivery reviews. 2002;54(10):1271–1294. [DOI] [PubMed] [Google Scholar]
- 94.Friedman DJ, Kozlitina J, Genovese G, Jog P, Pollak MR. Population-based risk assessment of APOL1 on renal disease. Journal of the American Society of Nephrology : JASN. 2011;22(11):2098–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen TK, Estrella MM, Vittinghoff E, et al. APOL1 genetic variants are not associated with longitudinal blood pressure in young black adults. Kidney international. 2017;92(4):964–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen TK, Tin A, Peralta CA, et al. APOL1 Risk Variants, Incident Proteinuria, and Subsequent eGFR Decline in Blacks with Hypertension-Attributed CKD. Clinical journal of the American Society of Nephrology : CJASN. 2017;12(11):1771–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.TD K, NS W. Epidemiologic Methods: studying the occurrences of illness. New York, NY: Oxford University Press; 2003. [Google Scholar]
- 98.Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions. American journal of public health. 1998;88(1):15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]