ABSTRACT
Background:
Tribulus terrestris has antioxidant and free-radical-scavenging properties. Malathion is the most common organophosphate, which is capable to produce free radicals and induce disturbance on some of male reproductive parameters. This study was designed to evaluate the effects of T. terrestris extract against damage induced by Malathion to the reproductive parameter of male rats.
Materials and Methods:
In this experimental study, 48 male Wistar rats were randomly assigned to eight groups: first group, sham group (normal saline); second group, Malathion (250 mg/kg) group; third to fifth groups, T. terrestris groups (2.5, 5, and 10 mg/kg body weight, respectively); and sixth to eight groups, Malathion + T. terrestris groups (2.5, 5, and 10 mg/kg). Tribulus terrestris extract (2.5, 5, and 10 mg/kg body weight, respectively) administrated orally, and daily for 8 weeks. The sperm parameters, testis malondialdehyde (MDA), serum total antioxidant capacity, serum testosterone level, and the height of germinal layer were evaluated and analyzed statistically.
Results:
All the values of male reproductive parameters reduced significantly in the Malathion group as compared to the sham group (P < 0.01) except MDA level, which increased significantly. The T. terrestris and T. terrestris + Malathion treatments in all doses increased the whole parameters significantly as compared to the Malathion group (P < 0.01) except MDA level, which decreased significantly. No significant changes were observed in all T. terrestris groups as compared to the sham group (P > 0.05).
Conclusion:
Tribulus terrestris extract administration attenuates the toxic effects of Malathion on some of the male reproductive parameters.
KEYWORDS: Malathion, reproductive parameters, Tribulus terrestris
INTRODUCTION
Human infertility is a highly critical process influenced by many factors such as the age of parents, maternal conditions, smoking, socioeconomic status, genetics, and pesticides.[1] Swan et al.[2] showed that pesticides might elevate the male infertility status. Occupational exposure to pesticides along with its detrimental effects on male fertility cause the delayed pregnancy without contraceptive use, miscarriage, stillbirth, and reduced birth weight.[3] Malathion is an unsystematic organic phosphorous compound belonging to the family of organophosphates.[4] This toxin is extensively used in agricultural fields. The reduced weight of sex organs, increased abnormality, and sperms deaths have been reported due to the administration of organophosphates.[5] Fortunato et al. showed that Malathion could induce the production of free radicals and oxidative stress.[6,7,8] In normal conditions, there is an imbalance status between elimination and production of free radicals in the body of living organisms.[9] Antioxidant enzymes are responsible for detoxification of free radicals.[10] Organophosphates are able to change the antioxidant system of cells, cause membrane lipid peroxidation, and induce cell membrane damage via the production of free radicals.[11] Increased lipid peroxidation and production of free radicals from the metabolism of organophosphates have been proposed as the main mechanisms involved in the impairment of cells.[12] Organophosphates can affect the structure of sperm chromatin by changing in phosphorylation of protamines.[13] Organophosphates are alkylating agents altering the sperm chromatin structure by bonding to protamine and deoxyribonucleic acid (DNA), which lead to sperm degeneration.[14] Many medicinal plants have great antioxidant potential.[15] For example, herbal species such as Tribulus terrestris have been confirmed for the progress of natural antioxidant preparations in the parts of medication and nourishment.[16]Tribulus terrestris increases the fertilization capacity and reproductive functions in rats.[17]Tribulus terrestris belongs to the family Zygophyllaceae. It is used in Asia and Europe to treat sexual dysfunctions.[18]Tribulus terrestris contains naturally dynamic materials such as flavonoids, steroids, alkaloids, saponins, unsaturated fats, tannins, and vitamins.[19,20] Adaay and Mattar[21] stated that T. terrestris extract improves the mice sperm parameters. Tribulus terrestris diminishes the levels of free radicals and malondialdehyde (MDA), which represents its potential to scavenge free radicals.[22,23] Zheleva-Dimitrova et al.[24] showed that regarding the high antioxidant activity of T. terrestris, this plant could be used in infertility treatment. According to the antioxidant effects of T. terrestris, it seems that this material can protect the male reproductive parameters against Malathion-induced oxidative damage. A review of the literature shows that no study has evaluated the effects of T. terrestris against Malathion-induced oxidative stress in reproductive parameters of male rats. Therefore, this study aimed to determine the effects of T. terrestris against Malathion-induced oxidative stress in the reproductive parameters of male rats.
MATERIALS AND METHODS
Extract preparation
The plant of Tribulus terrestris was collected from Kermanshah, Iran, in May 2018. The plant was verified by a botanist, then the extract was prepared as follows: the plant was cleaned and the leaves and stems were desiccated in shadow for 5 days and then grounded using a grinder. Next, 100g of the powder was added to ethanol 70%. The acquired solution was reserved in a warm water bath (36℃) under dark condition for 12h. Thereafter, the solution was progressively poured on Buchner funnel filter paper and cleaned by a vacuum pump. Then the use of a rotary device took place to get the extra solvent. The isolation process continued until the concentrated extract was obtained. The obtained extract was dissolved in distilled water and administered orally.[23]
Animals
This experimental study was performed on 48 male Wistar rats (weighing 220–250g) at Kermanshah University of Medical Sciences. All animals were treated in accordance with guidelines of National Institute of Health for the Care and Use of Laboratory Animals approved by Research Deputy at Kermanshah University of Medical Sciences based on World Medical Association Declaration Ethic of Helsinki (IR.KUMS.REC.1397.499). The rats were maintained based on a regular diet and water and libitrum with a 12:12h light and dark cycle at 23°C ± 2°C in an animal room of Medical School of Kermanshah University of Medical Sciences by considering 1-week adaptation prior to the experiment.[15]
Study groups and treatment of animals
The rats were randomly divided into eight groups (n = 6): First group, the sham group, received normal saline equivalent to the same amount in other experimental groups. Second group, the group of Malathion, in which, the rats were given Malathion at a dose of 250 mg/kg (1/50, lethal dose 50 [LD50]) body weight per day (single dose) through gavage for 8 weeks at 10 AM before T. terrestris extract administration. Third to fifth groups, the T. terrestris extract administration groups, each animal in these groups, respectively, received 2.5, 5, and 10 mg/kg of T. terrestris extract orally for 8 weeks at 10 AM. Sixth to eighth groups, T. terrestris + Malathion administration groups, in this group, each animal received single dose (250 mg/kg) of Malathion via gavage in order to induce the damage of reproductive parameters, then they, respectively, received 2.5, 5, and 10 mg/kg of T. terrestris extract orally for 8 weeks at 10 am.[25,26]
Animals’ dissection and sampling
At the end of the treatment period, all rats were deeply anesthetized by intraperitoneal injection of Ketamine (100 mg/kg) and Xylazine (10 mg/kg). The blood sample was collected from the heart without thoracotomy. The samples were kept in an incubator at 37°C for 20min and then centrifuged at 255× g for 15min. The blood serum was isolated and part of which was kept at –70°C for assessment of total antioxidant capacity (TAC) and testosterone levels. Then, the chest and abdomen of the animals were cut, respectively. The epididymis tail was isolated from the testes and placed in Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 (DMEM/F-12)/fetal bovine serum 5% culture medium. The left testis was removed from the abdominal cavity and was fixed in a 10% formalin solution for histological and morphometric examinations and the right one for the MDA level estimations, in the respect of the groups.[27]
Sperm cell collection
Both cauda epididymides from each animal were crushed and conserved in a warmed petri dish containing 10- mL Hank’s balanced salt solution at 37°C. The spermatozoa were immersed in the buffer. After 15min, the cauda was removed and the suspension was slightly shaken to spread the sperms better than observed by a light microscope at ×400 magnification.[28]
Progressive motility
In this method, four degrees of sperm motility were studied based on World Health Organization protocol, class A: progressive motility. Progressive motility of the sperm cells of each sample was examined by an optical microscope under ×40 magnification in 10 fields of view. For this purpose, at first, about 50 μL of semen liquid culture medium was taken and placed on a slide culture, which was previously cleaned and dried by alcohol. Then the slide culture was placed on this and examined by the microscope. Sperm cell counting was performed through a cell count device, and about 100 sperm cells were counted in each sample. In all experimental and control groups, the counting was repeated.[15]
Survival rate
In this method, eosin staining was used to identify the living sperm cells from dead ones. This staining is based on the infiltration of stain through the membrane of dead cells and its disposal by the membrane of living cells. At the end of the given time, approximately 20 μL of the medium containing semen fluid was collected from each petri dish, and then mixed with an equal volume (about 20 μL) of eosin staining solution. After approximatley2–5min, a part of the mixture was poured onto a neobar slide culture. The prepared slide culture was examined with ×40 magnification. At least 100 sperm cells were calculated from each random sample from the 10 fields of view and eventually the percentage of live sperm cells was documented.[27]
Sperm cell morphology
The normal sperm cell morphology was assessed through the examination of sperm smears from the right cauda epididymis. An aliquot of the sample was used to form the smears for assessment of malformations in spermatozoa. Eosin or nigrosine staining was used to guesstimate the normal spermatozoa morphology. One drop of eosin stain was added to the suspension and mixed slightly. The slides were then observed by a light microscope at ×400 magnification. A total of 400 spermatozoa were studied on respective slides (4000 cells in each group) for irregularities of the head and tail.[15]
Sperm calculation
To analyze the quantity of sperm cells, 400 μL of the sperm suspension was diluted through the formaldehyde fixative (Sigma, St. Louis, USA). Approximately, 15 μL was moved from the diluted solution into a hemocytometer by a Pasteur pipette. The hemocytometer was located into a Petri dish with dampened filter paper and waited to stand for 10min. The stable sperms were counted and assessed per 250 small squares of the hemocytometer using an objective lens at ×40 magnification. The number of sperm per mm3 equated the number of sperm counted × the dilution/number counted in mm2 × the depth of the chamber.[27]
The tissue preparation and staining for germinal layer seminiferous tubule evaluation
The left testis was dissected and Paraffin embedded blocks prepared according to the following steps including fixation, washing, dehydrating by increasing concentration of ethanol, clearing by xylene, and embedding in soft paraffin. In this stage, 5-µm coronal histological thin sections were cut from paraffin-embedded blocks, and five sections per animal were chosen. For the unification of the section selection, the first section was the 4th and the last was the 24th (five sections interval) and finally, the routine protocol for hematoxylin and eosin staining was implemented. More than 20 sections were prepared from each block. The germinal layer of the seminiferous tubules was measured using a Motic camera and software (Moticam 2000, Madrid, Spain). The average diameter (μm) of the germinal layer of the seminiferous tubules was determined for each testis.[28]
Testosterone level measurement
The collected blood samples were centrifuged to achieve the serum with the following characteristics: 23°C, 15min, and 5000× g. The serum samples were then kept in a deep freezer (–18oC) to analyze the serum testosterone level through the enzyme-linked immunosorbent assay (Abcam 108666, Santa Cruz, USA) technique.[15]
Detection of testis malondialdehyde levels
MDA levels in right testis tissues were evaluated as an index of lipid peroxidation. In this regard, homogenizing of the samples was carried out by homogenization buffer containing 1.15% potassium chloride solution and centrifuged at 1500× g for 10min. Then, the homogenated samples were added to a reaction mixture containing, acetic acid (pH 3.5), thiobarbituric acid, and distilled water. After boiling the mixture at 95°C for 1h and centrifuging at 3000× g for 10min, the absorbency of the supernatant was measured by spectrophotometry at 550-nm light length.[29]
Estimation of total antioxidant capacity in serum
To measure the TAC that is the basis of the oxidation colorimetry resuscitation, an acquisition kit (Cat No. TAC-96A; ZellBio GmbH, Munich, Germany) was purchased. The kit contains one reagent ready to use, buffer X-100, dye powder, reaction suspension solution, standard, and a microplate of 96 wells. In this assay, the TAC was equivalent to some antioxidant in the sample that was compared with ascorbic acid as standard. The kit’s sensitivity was equal to 0.1mM, the diagnostic range was mM 2–125 / 0, and the final absorbance was read at 490nm and unit conversion was performed.[29]
Statistical analysis
The Kruskal–Wallis test was used to examine data normality and the homogeneity of variance at a according to the significance level of 0.05. The data were analyzed by Statistical Package for the Social Sciences software program, (IBM, SPSS version 16.0, New York, USA) using one-way analysis of variance postulation followed by Tukey’s post hoc test, and P < 0.05 was considered significant. The variables were represented as mean ± standard error of mean.
RESULTS
Progressive sperm motility and sperm cell viability
Malathion caused a significant reduction in sperm cell viability and progressive motility as compared to the sham group (P < 0.01). No significant differences were observed in T. terrestris groups in comparison with sham group (P > 0.05). Also, sperm cell viability and progressive motility in all treated T. terrestris and Malathion + T. terrestris groups increased significantly compared to the Malathion group (P < 0.01) [Table 1].
Table 1.
Effect of Malathion, Tribulus terrestris, and T. terrestris + Malathion on sperm parameters in male rats (n = 6 for each group)
| Groups | Mean of sperm count (million/per milliliter) | Sperm progressive motility (%) | Sperm viability (%) |
|---|---|---|---|
| Sham | 84.25 ± 5.36 | 18.22 ± 2.52 | 74.85 ± 3.55 |
| Mal | 32.36 ± 2.55* | 2.04 ± 0.98* | 38.47 ± 2.84* |
| TT 2.5 mg/kg | 86.04 ± 4.74† | 20.32 ± 2.32† | 75.67 ± 4.42† |
| TT 5 mg/kg | 86.77 ± 6.22† | 21.44 ± 1.71† | 77.12 ± 3.63† |
| TT 10 mg/kg | 87.39 ± 5.89† | 21.10 ± 1.94† | 77.32 ± 2.34† |
| TT + Mal 2.5 mg/kg | 51.74 ± 3.47¶ | 8.66 ± 1.11¶ | 50.08 ± 3.71¶ |
| TT + Mal 5 mg/kg | 54.94 ± 2.36¶ | 9.78 ± 0.83¶ | 54.99 ± 1.25¶ |
| TT + Mal 10 mg/kg | 55.52 ± 3.33¶ | 9.13 ± 1.47¶ | 56.08 ± 2.09¶ |
TT = Tribulus terrestris, Mal = Malathion
Data were presented as mean ± standard error of mean
* P < 0.01 compared to the normal control group
† P < 0.01 compared to Malathion group
¶ P < 0.01 compared to Malathion l group
Sperm cell count and normal morphology
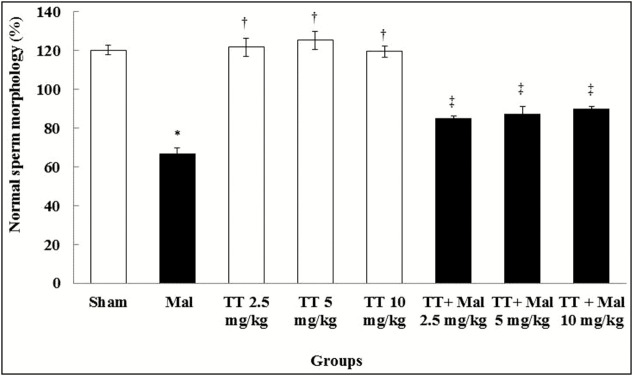
The sperm cell count and morphological normality reduced significantly in the Malathion group compared to the sham group (P < 0.01). No significant differences were found to be experiential in the T. terrestris groups as compared with the sham group (P > 0.05). However, the sperm cell count and normal morphology were enhanced significantly in all treated T. terrestris and Malathion + T. terrestris groups as compared with the Malathion group (P < 0.01) [Table 1] and [Graph 1].
Graph 1.
Comparison of normal sperm cell morphology in treatment groups. *Significant difference compared to the sham group (P < 0.01). †Significant difference compared to the Malathion group (P < 0.01). ‡Significant difference compared to the Malathion group (P < 0.01). TT = Tribulus terrestris, Mal = Malathion
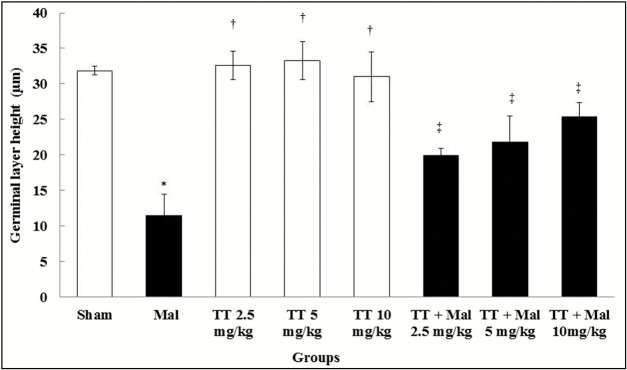
Height of germinal layer in seminiferous tubules
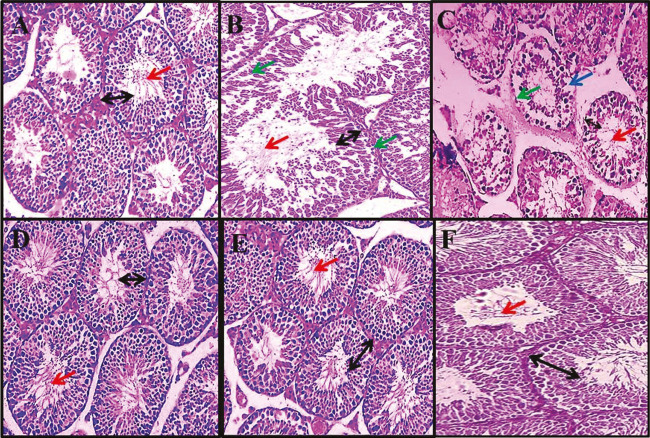
Malathion caused a significant reduction in the height of the germinal layer of seminiferous tubules in comparison with the sham group (P < 0.01). No significant changes were observed in T. terrestris groups in comparison with the sham group (P > 0.05). The germinal layer of seminiferous tubule height in entirely treated T. terrestris and Malathion + T. terrestris groups increased significantly as compared to the Malathion group (P < 0.01) [Graph 2] and [Figure 1].
Graph 2.
Comparison of germinal layer seminiferous tubule height in treatment groups. *Significant difference compared to the sham group (P < 0.01). †Significant difference compared to the Malathion group (P < 0.01). ‡Significant difference compared to the Malathion group (P < 0.01). TT = Tribulus terrestris, Mal = Malathion
Figure 1.
Effect of Malathion, Tribulus terrestris, and T. terrestris + Malathion on seminiferous tubules (magnification ×400). Normal seminiferous tubule structure was observed in the sham group (A), T. terrestris group (10 mg/kg) (F), T. terrestris + Malathion group (5 mg/kg) (D), and T. terrestris + Malathion group (10 mg/kg) (E). A decrease in height of germinal layer in seminiferous tubules, destruction of the cells sequence, vacuolization, and reduce sperm cells density were observed in the Malathion group (B and C). The black arrow identifies the height of the germinal layer, the red arrow identifies sperm cells density, the green arrow identifies irregularities in the structure of the margin of tubules (destruction of the membrane seminiferous tubules structure), and the blue arrow identifies vacuolization
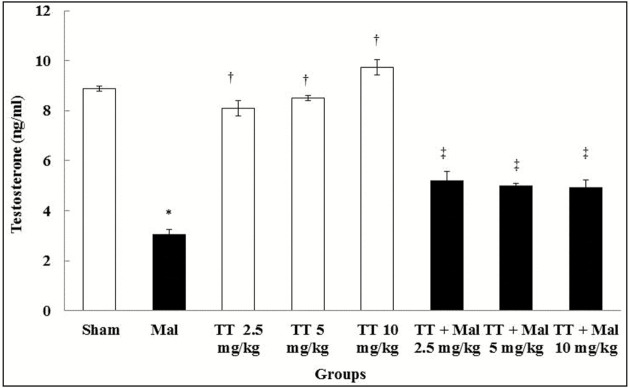
Testosterone hormone levels
Malathion caused a significant decrease in the testosterone hormone levels compared to the sham group (P < 0.01). No significant changes were observed in T. terrestris groups in comparison with the sham group (P > 0.05). Furthermore, testosterone hormone levels in all treated T. terrestris and Malathion + T. terrestris groups improved significantly compared to the Malathion group (P < 0.01) [Graph 3].
Graph 3.
Comparison of testosterone hormone level in treatment groups. *Significant difference compared to the sham group (P < 0.01). †Significant difference compared to the Malathion group (P < 0.01). ‡Significant difference compared to the Malathion group (P < 0.01). TT = Tribulus terrestris, Mal = Malathion
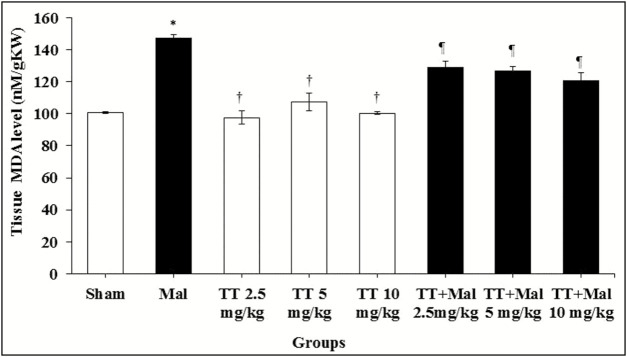
Malondialdehyde levels
Serum levels of MDA showed a significant increase in the Malathion group compared to the sham group (P < 0.01). Also, a significant decrease in MDA levels was observed in all T. terrestris and T. terrestris + Malathion groups compared to the Malathion group (P < 0.01), whereas no significant effect was observed on the levels of MDA in all T. terrestris groups compared to the sham group (P > 0.05) [Graph 4].
Graph 4.
Comparison of testis MDA level between groups. *P < 0.01 compared to the sham group. †P < 0.01 compared to the Malathion group. ¶P < 0.01 compared to the Malathion group. MDA = malondialdehyde, TT = Tribulus terrestris, Mal = Malathion
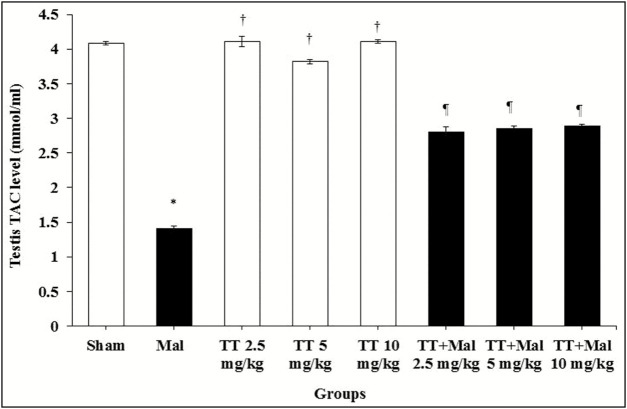
Total antioxidant capacity levels
The results of measured TAC levels in the study groups showed a significant decrease in the Malathion group compared to the sham group (P < 0.01). Also, a significant increase in TAC levels was observed in all T. terrestris and T. terrestris + Malathion groups compared to the Malathion group (P < 0.01), whereas no significant effect was found on the levels of TAC in all T. terrestris groups compared to the sham group (P > 0.05) [Graph 5].
Graph 5.
TAC level change in the male rats. *P < 0.01 compared to the sham group. †P < 0.01 compared to Malathion group. ¶P < 0.01 compared to the Malathion group. TAC = total antioxidant capacity, TT = Tribulus terrestris, Mal = Malathion
DISCUSSION
Organophosphates have the most effects on the reproductive system.[30] The findings of this research suggested that the administration of Malathion had adverse effects on testis histology and sperm parameters, oxidant–antioxidant imbalance as well as increase in testosterone hormone level. On the contrary, T. terrestris as a natural flavonoid in all doses relieve the diverse effects of Malathion administration, obviously in the male reproductive parameter. It also recovers the cell damage offering by a decrement of MDA and histology evaluation and the rate of oxidation (by calculating the amount of TAC). The results of this study also showed that T. terrestris extract in all doses is able to reduce lipid peroxidation and increase antioxidant capacity in testis tissue. As consistent with these findings, a large amount of studies has shown the antioxidant properties of T. terrestris extract.[16,17]Tribulus terrestris apparently prevents the formation of lipid peroxidation induced by tert-Butyl hydroperoxide in sperm cells. Thus, it appears that T. terrestris extract with its antioxidant properties could reduce MDA and increase TAC in the treatment groups by inhibiting the production of reactive oxygen species. Although the sperms lose a large amount of their cytoplasm during spermatogenesis (lack of antioxidant systems), they seem to have a higher sensitivity to elevated reactive oxygen species (ROS) than somatic cells.[15] The first outcome of ROS attack to membrane structures can be cellular peroxidation in the cell membrane.[29] The use of antioxidants such as T. terrestris to eliminate the free radicals from the cell surroundings can inhibit lipid peroxidation, thereby maintaining the biochemical structure of cells.[30] The findings of Nahid et al.[31] were in line with the results of this study in which the administration of Malathion significantly reduced serum total antioxidant levels, increased lipid peroxidation, decreased the height of germinal epithelium, and reduced the number of primary spermatocytes in male rats. The results of this study showed that all sperm parameters in the Malathion group reduced significantly compared to the sham group. In all T. terrestris and Malathion + T. terrestris groups, a significant increase was observed in all sperm cell parameters compared to the Malathion group. ROS can affect DNA and RNA synthesis in the sperm cell and inhibit their mitochondrial function.[15] Malathion-induced oxidative stress seems to interfere with the differentiation of sperms so that a number of spermatogonia are impaired on the basement membrane and the number of primary and secondary spermatocytes, spermatids, and mature sperms is reduced.[5] The results of Aitken et al.[32] confirmed the findings of this study in which the oxidative stress disrupts spermatogenesis and causes a reduction in the number of spermatogonia, spermatocytes, spermatids, and spermatozoa. Reduced number of sperms in the Malathion group may be due to the direct increase of oxidative stress-induced lipid peroxidation, which may have altered the natural properties of the membrane and consequently result in the loss of sperms transmitted to the epididymis.[5] On the contrary, high levels of ROS cause mitochondrial impairment and consequently release the proapoptotic proteins in the intermembrane space, activate caspases molecules, and reduce the adenosine triphosphate (ATP) synthesis release ROS and also release of calcium from mitochondria into the cytosol, which in turn may lead to activation of apoptosis process.[33] The findings of Selmi et al.[5] is in agreement with the results of this study, indicating that administration of Malathion significantly decreases the testis weight and sperm parameters, and increases sperm DNA damage in comparison with the control group. Elevated free radicals can lead to impairment of Sertoli cells and destruction of cytoplasmic bridges via loss of epithelial cells, thereby decreasing the sperm count and increasing the sperm cell deformity.[34]Tribulus terrestris extract seems to have some biological effects including the inhibitory effects on free radicals, antioxidant properties, and increased amount of anti-oxidative enzymes.[15]Tribulus terrestris extract is also able to stabilize the blood–testis barrier and protect sperm DNA against the oxidative stress induced by free radicals.[23] This research shows a significant decrease in testosterone level in blood serum and diameter of seminiferous tubules in the Malathion group as compared to the sham group. Moreover, T. terrestris extracts in all doses significantly elevate the testosterone level and the height of germinal layer of seminiferous tubules in all groups receiving Malathion plus T. terrestris in comparison with the Malathion group. Organophosphates can disrupt the expression of the steroidogenic acute regulatory protein (StAR).[35] The results of Mojica-Villegas et al.[36] confirmed the findings of this research in which the administration of resveratrol significantly reduced the testosterone in rats. In addition, it seems that organophosphates increase the level of cortisol, which can disrupt the spermatogenesis process.[37] Considering its potent antioxidant properties, T. terrestris has positive effects on hypothalamic–pituitary–gonadal axis, testosterone level, and sperm production and motility. Apparently, the elevated level of ROS due to the administration of Malathion increases lipid peroxidation, which in turn induces atrophy in the germinal layer thickness of seminiferous tubules.[38] Salahshoor et al.[27] showed a reduction in the epithelial volume of seminiferous tubules due to oxidative stress, which is in line with the findings of this study. Tribulus terrestris extract seems to protect lipids against peroxidation, prevents testicular oxidative stress, and plays a role in the production of testicular steroids.[17] The findings of Adaay and Mattar et al.[21] are also in agreement with the results of this study, indicating that the oxidative stress impairs the germinal layer of seminiferous tubules, and T. terrestris improves the height of germinal layer in seminiferous tubules and elevates the testosterone level in groups exposed to oxidative stress.
CONCLUSION
The outcomes of this study show that the Malathion can produce defects in some of male reproductive parameters, although the T. terrestris extract has antioxidant and defensive effect. It was showed that this phytochemical substance induces the elevated quality of some spermatozoa and improves the normal morphology, sperm cell viability, germinal layer seminiferous tubules height, TAC, motility, and count; it also reduces the testis MDA level. Tribulus terrestris extract could be valuable for the treatment of infertile men to enhance male fertility. The antioxidant properties of T. terrestris could be a main reason for its optimistic outcome on reproductive parameters. Supplementary studies are essential to explain its careful mechanism of action.
Acknowledgment
We gratefully acknowledge the Research Council of Kermanshah University of Medical Sciences in 2018 (Grant No. 97499) for the financial support.
Financial support and sponsorship
This study was funded by Research Council of Kermanshah University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Al-Raddadi R, Alwafi O, Shabouni O, Akbar N, Alkhalawi M, Ibrahim A, et al. Seroprevalence of dengue fever and the associated sociodemographic, clinical, and environmental factors in Makkah, Madinah, Jeddah, and Jizan, Kingdom of Saudi Arabia. Acta Trop. 2019;189:54–64. doi: 10.1016/j.actatropica.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 2.Swan SH, Kruse RL, Liu F, Barr DB, Drobnis EZ, Redmon JB, et al. Study for Future Families Research Group Semen quality in relation to biomarkers of pesticide exposure. Environ Health Perspect. 2003;111:1478–84. doi: 10.1289/ehp.6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guimarães ATB, de Oliveira Ferreira R, de Souza JM, da Costa Estrela D, Talvani A, Souza DMS, et al. Evaluating the reproductive toxicology of tannery effluent in male SWISS mice. Sci Total Environ. 2019;648:1440–52. doi: 10.1016/j.scitotenv.2018.08.253. [DOI] [PubMed] [Google Scholar]
- 4.Madzorera T, Sibanda M, Focke W, Madito M, Manyala N. Malathion-filled trilayer polyolefin film for malaria vector control. Mater Sci Eng C Mater Biol Appl. 2019;96:419–25. doi: 10.1016/j.msec.2018.11.057. [DOI] [PubMed] [Google Scholar]
- 5.Selmi S, Rtibi K, Grami D, Sebai H, Marzouki L. Lavandula stoechas essential oils protect against malathion-induces reproductive disruptions in male mice. Lipids Health Dis. 2018;17:253. doi: 10.1186/s12944-018-0891-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fortunato JJ, Feier G, Vitali AM, Petronilho FC, Dal-Pizzol F, Quevedo J. Malathion-induced oxidative stress in rat brain regions. Neurochem Res. 2006;31:671-–8. doi: 10.1007/s11064-006-9065-3. [DOI] [PubMed] [Google Scholar]
- 7.Salahshoor MR. Effects of curcumin on reproductive parameters in male mice. J Clin Res Paramed Sci. 2012;1:31-–7. [Google Scholar]
- 8.Hariri AT, Moallem SA, Mahmoudi M, Hosseinzadeh H. The effect of crocin and safranal, constituents of saffron, against subacute effect of diazinon on hematological and genotoxicity indices in rats. Phytomedicine. 2011;18:499-–504. doi: 10.1016/j.phymed.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Ahmadi S, Bashiri R, Ghadiri-Anari A, Nadjarzadeh A. Antioxidant supplements and semen parameters: an evidence based review. Int J Reprod Biomed (Yazd) 2016;14:729–36. [PMC free article] [PubMed] [Google Scholar]
- 10.Prete CD, Ciani F, Tafuri S, Pasolini MP, Valle GD, Palumbo V, et al. Effect of superoxide dismutase, catalase, and glutathione peroxidase supplementation in the extender on chilled semen of fertile and hypofertile dogs. J Vet Sci. 2018;19:667–75. doi: 10.4142/jvs.2018.19.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valdiglesias V, Pásaro E, Méndez J, Laffon B. In vitro evaluation of selenium genotoxic, cytotoxic, and protective effects: a review. Arch Toxicol. 2010;84:337–51. doi: 10.1007/s00204-009-0505-0. [DOI] [PubMed] [Google Scholar]
- 12.Altuntas I, Kilinc I, Orhan H, Demirel R, Koylu H, Delibas N. The effects of diazinon on lipid peroxidation and antioxidant enzymes in erythrocytes in vitro. Hum Exp Toxicol. 2004;23:9–13. doi: 10.1191/0960327104ht408oa. [DOI] [PubMed] [Google Scholar]
- 13.Sánchez-Peña LC, Reyes BE, López-Carrillo L, Recio R, Morán-Martínez J, Cebrián ME, et al. Organophosphorous pesticide exposure alters sperm chromatin structure in Mexican agricultural workers. Toxicol Appl Pharmacol. 2004;196:108–13. doi: 10.1016/j.taap.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 14.Evenson D, Jost L. Sperm chromatin structure assay for fertility assessment. Curr Protoc Cytom. 2000;13:7–13. doi: 10.1002/0471142956.cy0713s13. [DOI] [PubMed] [Google Scholar]
- 15.Jalili C, Salahshoor MR, Jalili F, Kakaberaei S, Akrami A, Sohrabi M, et al. Therapeutic effect of resveratrol on morphine-induced damage in male reproductive system of mice by reducing nitric oxide serum level. Int J Morphol. 2017;35:1342–7. [Google Scholar]
- 16.Kistanova E, Zlatev H, Karcheva V, Kolev A. Effect of plant Tribulus terrestris extract on reproductive performances of rams. Biotechnol Anim Husb. 2005;21:55–63. [Google Scholar]
- 17.Elahi RK, Asl S, Shahian F. Study on the effects of various doses of Tribulus terrestris extract on epididymal sperm morphology and count in rat. Glob Veterin. 2013;10:13–7. [Google Scholar]
- 18.Grigorova S, Kashamov B, Sredkova V, Surdjiiska S, Zlatev H. Effect of Tribulus terrestris extract on semen quality and serum total cholesterol content in White Plymouth Rock-mini cocks. Biotechnol Anim Husb. 2008;24:139–46. [Google Scholar]
- 19.Ranjithkumar R, Alhadidi Q, Shah ZA, Ramanathan M. Tribulusterine containing Tribulus terrestris extract exhibited neuroprotection through attenuating stress kinases mediated inflammatory mechanism: in vitro and in vivo studies. Neurochem Res. 2019;12:1–5. doi: 10.1007/s11064-019-02768-7. [DOI] [PubMed] [Google Scholar]
- 20.Kamboj P, Aggarwal M, Puri S, Singla SK. Effect of aqueous extract of Tribulus terrestris on oxalate-induced oxidative stress in rats. Indian J Nephrol. 2011;21:154–9. doi: 10.4103/0971-4065.83727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adaay MH, Mattar AG. Effect of aqueous and ethanolic extracts of Tribulus terrestris, Phoenix dactylifera and Nasturtium officinale mixture on some reproductive parameters in male mice. Baghdad Sci J. 2012;9:640–50. [Google Scholar]
- 22.Erasmus N, Solomon MC, Fortuin KA, Henkel RR. Effect of Eurycoma longifolia jack (Tongkat ali) extract on human spermatozoa in vitro. Andrologia. 2012;44:308–14. doi: 10.1111/j.1439-0272.2012.01282.x. [DOI] [PubMed] [Google Scholar]
- 23.Dastjerdi MN, Salahshoor MR, Mardani M, Rabbani M, Hashemibeni B, Gharagozloo M, et al. The apoptotic effects of sirtuin1 inhibitor on the MCF-7 and MRC-5 cell lines. Res Pharm Sci. 2013;8:79–89. [PMC free article] [PubMed] [Google Scholar]
- 24.Zheleva-Dimitrova DI, Obreshkova DA, Nedialkov P. Antioxidant activity of Tribulus terrestris—a natural product in infertility therapy. Int J Pharm Pharm Sci. 2012;4:508–11. [Google Scholar]
- 25.Choudhary N, Goyal R, Joshi SC. Effect of malathion on reproductive system of male rats. J Environ Biol. 2008;29:259–62. [PubMed] [Google Scholar]
- 26.Gauthaman K, Ganesan AP, Prasad RN. Sexual effects of puncturevine (Tribulus terrestris) extract (protodioscin): an evaluation using a rat model. J Altern Complement Med. 2003;9:257–65. doi: 10.1089/10755530360623374. [DOI] [PubMed] [Google Scholar]
- 27.Salahshoor MR, Haghjoo M, Roshankhah S, Makalani F, Jalili C. Effect of thymoquinone on reproductive parameter in morphine-treated male mice. Adv Biomed Res. 2018;7:18. doi: 10.4103/abr.abr_69_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jalili C, Kamani M, Roshankhah S, Sadeghi H, Salahshoor MR. Effect of Falcaria vulgaris extracts on sperm parameters in diabetic rats. Andrologia. 2018;50:e13130. doi: 10.1111/and.13130. [DOI] [PubMed] [Google Scholar]
- 29.Jalili C, Moradi D, Roshankhah S, Salahshoor MR. Effect of pentoxifylline on kidney damage induced by nitrosamine in male rats. Res Pharm Sci. 2019;14:64–73. doi: 10.4103/1735-5362.251854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar CNR, Nayak GH, Biradar SS, Sudhan SM, Karlawad M, Selvan M. Trends of death due to poisoning among females at a tertiary care centre in north Karnataka. Indian J Forensic Med Toxicol. 2019;13:67–71. [Google Scholar]
- 31.Nahid Z, Tavakol HS, Abolfazl GK, Leila M, Negar M, Hamed F, et al. Protective role of green tea on malathion-induced testicular oxidative damage in rats. Asian Pac J Reprod. 2016;5:42–5. [Google Scholar]
- 32.Aitken RJ, Curry BJ. Redox regulation of human sperm function: from the physiological control of sperm capacitation to the etiology of infertility and DNA damage in the germ line. Antioxid Redox Signal. 2011;14:367–81. doi: 10.1089/ars.2010.3186. [DOI] [PubMed] [Google Scholar]
- 33.Valko M, Morris H, Cronin MT. Metals, toxicity and oxidative stress. Curr Med Chem. 2005;12:1161–208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- 34.Ikeda K, Tojo K. Efonidipine, a Ca(2+)-channel blocker, enhances the production of dehydroepiandrosterone sulfate in NCI-H295R human adrenocortical carcinoma cells. Tohoku J Exp Med. 2011;224:63–71. doi: 10.1620/tjem.224.263. [DOI] [PubMed] [Google Scholar]
- 35.da Costa KJ, Gala-García A, Passos JJ, Santos VR, Sinisterra RD, Lanza CR, et al. Testosterone improves the osteogenic potential of a composite in vitro and in vivo. Cell Tissue Res. 2019;12:1–11. doi: 10.1007/s00441-018-2970-3. [DOI] [PubMed] [Google Scholar]
- 36.Mojica-Villegas MA, Izquierdo-Vega JA, Chamorro-Cevallos G, Sánchez-Gutiérrez M. Protective effect of resveratrol on biomarkers of oxidative stress induced by iron/ascorbate in mouse spermatozoa. Nutrients. 2014;6:489–503. doi: 10.3390/nu6020489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Selvage DJ, Rivier C. Importance of the paraventricular nucleus of the hypothalamus as a component of a neural pathway between the brain and the testes that modulates testosterone secretion independently of the pituitary. Endocrinology. 2003;144:594–8. doi: 10.1210/en.2002-220781. [DOI] [PubMed] [Google Scholar]
- 38.Huang F, Ning H, Xin QQ, Huang Y, Wang H, Zhang ZH, et al. Melatonin pretreatment attenuates 2-bromopropane-induced testicular toxicity in rats. Toxicology. 2009;256: 75–82. doi: 10.1016/j.tox.2008.11.005. [DOI] [PubMed] [Google Scholar]