ABSTRACT
Objective:
The aim of this systematic review was to evaluate the clinical implications of continuous glucose monitoring (CGM) among patients with diabetes mellitus using variables that include glycated hemoglobin (HbA1c), estimated A1c, glucose variability, and users’ perspectives.
Materials and Methods:
This study analyzed 17 articles that were identified and studied according to the research question criteria. PRISMA guidelines were used for identification and screening of the literature. The required data were searched using Medscape, PubMed, PROSPERO, Wiley Library, Scopus, Clinical Trial Registry, and Trip.
Results:
The articles reviewed were on the use of CGM in type 1 and type 2 diabetes mellitus, which showed significant improvement in the levels of HbA1c as compared to non-CGM. The application of CGM on acute sudden onset type of adverse drug reactions (i.e., hypoglycemia) is better than fasting blood sugar or self-monitoring of blood glucose or capillary blood glucose (random blood glucose monitoring). CGM is beneficial for use in patients with type 2 diabetes mellitus including elderly patients as it gives information regarding glucose variability as well as HbA1c levels. The health-care providers require full spectrum of patients’ CGM data to design a better therapeutic plan. However, the patients experienced inconvenience on wearing the device on the body for longer periods. The findings also stated the fact that more education and training is required for the patients to interpret their own glycemic data using CGM and modify their lifestyle accordingly. Use of CGM along with HbA1c has also been used to achieve better glycemic results and it allows the health care professional to guide patients in terms of their glucose level; whether they are hypoglycemic or hyperglycemic, however its use has some controversies that minimize its application.
Conclusion:
The study concluded that CGM has significant potential in the management of not only patients with type 1 diabetes mellitus but also patients with type 2 diabetes mellitus in spite of the few limitations that are being improvised in the upcoming years. However, limited literature of CGM among patients with type 2 diabetes mellitus and pregnant women reduces the practice scope.
KEYWORDS: Continuous glucose monitoring, diabetes mellitus, estimated A1c, glycated hemoglobin, type 1 diabetes mellitus, type 2 diabetes mellitus
INTRODUCTION
Diabetes Mellitus (DM) is one of the most prevalent endocrine disorders worldwide with several short-term and long-term complications including macro- and microvascular disorders.[1] It is estimated that approximately 592 million people will be affected with diabetes mellitus by 2035.[2] The health-disease burden showed increasing trends over the past decade[2] and to improve health-related quality of life, along with diabetic care, extensive monitoring guidelines should also be implemented.[1,2,3,4,5] Literature showed dominant prevalence of type 2 diabetes mellitus (T2DM) over type 1 diabetes mellitus (T1DM) all around the world.[1,4,5]
There are several challenges in the management of diabetes mellitus in the health-care system.[6] The effect and management outcomes based on multifactorial concept including demographic variability, disease and social functioning, and self-care behavior are the strong influencers in treatment plan.[4,5] Disease control or progression based on continuous/frequent blood glucose monitoring[4,5,6] will further facilitate the process of rational prescribing.[3,6] Several studies showed that self-care behavior specific to glucose monitoring plays a vital role in the management of glycemic levels.[2,3,4,5,6]
The continuous glucose monitoring (CGM) has led to a clinical shift in the management of DM.[7] This technique is based on real-time glycemic monitoring, glucose levels trending alerts, condition-related predictive notifications, and the ability to make care plan on glycemic levels without the use of conventional self-monitoring blood glucose (SMBG).[8,9] This automated system has helped patients with T1DM with insulin dosing process; however, its success is based on users’ perspectives to wear such device in terms of easiness, comfortability, cost, and potential benefits over SMBG. It is found that consistent use of CGM provides sustained glycated hemoglobin (HbA1c) reduction of 0.5%–1.2%.[7,8,9]
The clinical implications of CGM and its application in clinical practice are based on the patient-related factors. Several studies have proposed the beneficial outcomes of CGM in the practice but limited to T1DM only. The patients’ and health-care professionals’ perspectives toward the use of device are still undermining. Therefore, this study aimed to evaluate the literature for clinical implication of CGM in different population (i.e., geriatric, adult, and pregnancy) and understand the users’ perspectives on the CGM use compared with standard SMBG and also identify the areas of application to CGM with limited literature.
MATERIALS AND METHODS
Eligibility criteria
Continuous glucose monitoring
CGM is the monitoring parameter for patients with DM.
SMBG
SMBG is also a self-care model for patients with diabetes mellitus but implication and practice are limited to individual behavior.
Clinical implications of CGM
Studies that focused on CGM-based monitoring among patients with diabetes mellitus and evaluate the clinical outcomes in terms of safety and/or efficacy profile.
HbA1c and eA1c
These are the predictive markers in the treatment of diabetes mellitus. Studies evaluating glycated hemoglobin values are considered for systematic review.
Users perceptions/perspectives
Studies evaluating the personnel perception or perspectives toward the use of CGM are also considered in this study.
Literature search
Articles were searched from different databases and individual journal websites including Google Scholar, ScienceDirect, Sagepub, Libertpub, American Diabetes Association (Diabetes Care), Wiley Online Library, EndocrineWeb, touchENDOCRINOLOGY, PROSPERO, Biomed Central (BMC), Diabetes Technology and Therapeutics, The San Diego Union-Tribune, PubMed(ncbi), Clinical Trials List, SCOPUS, and Elsevier.
Search keywords
The following were the search keywords: “CGM (continuous glucose monitoring)”, “safety”, “efficacy”, “type 2 DM”, “comorbidities”, “elderly”, “benefits”, “improved”, “glycemic”, and “outcomes”
Study selection and data extraction
Abstracts and conference proceedings were screened to exclude that do not involve in the following inclusion criteria. The articles should be published between 2017 and 2019. Primary literature would be specific only for T1DM and T2DM. In addition, the literature should also focus on the safety and efficacy of CGM. Also, studies evaluating users (both health-care providers’ and patients’) perspectives were included in the systematic review.
Quality assessment
Mixed-method appraisal tool (MMAT)[10] checklist is a comprehensive tool for quality assessment of different research design articles in the systematic review. It contains five domains on research design, each with five specific item appraisal questions. The appraisal tool consists of four sub-domains; data collection and recording, methodological design, study population and statistical analysis in-between groups.[10]
RESULTS AND DISCUSSION
General data
This systematic review looks into 17 articles that were taken from 2017 to 2019. Five (29%) studies were from the USA, three (17%) studies were from the UK, one from South America, one from Japan, one from India, one from France, one from Italy, one from Belgium, one from Germany, one from Slovenia, and one from Georgia [Supplementary Table 1]. The quality was assessed using MMAT guidelines, according to which 11 (64%) studies were low-risk and 6 (35%) were mid-risk studies [Figure 1].
Supplementary Table 1: Systematic review of studies included in the study
| S.No. | Author and year of publication | Study design | No. of participants with methods | Objective | Variable | Conclusion |
|---|---|---|---|---|---|---|
| 1 | Anders L. Carlson and others (2017) | Meta analysis, single-arm, open-label study | 1) 100 TD2 patients were taken who were not on insulin. Observation study done for 12 weeks | 1) Studies of Efficacy and HbA1c Reduction. | HbA1c, SMBG | CGM offers patients with T2DM additional information about their glycemic control beyond the HbA1c. |
| 2) 367 patients being studied using observational trials. | 2) Studies of Hypoglycemia | |||||
| 2 | David Rodbard (2017) | Meta analysis | 22 randomized clinical trials been analyzed | can improve quality of glycemic control, reduce risk of hypoglycemia, and permit selection of lower target levels for mean glucose andHbA1c. | HbA1c | CGM (including flash glucose monitoring) systems are safe and effective in both type 1 and type 2 diabetes and |
| 3 | Bruce W. Bode and Tadej Battelino (2019) | open-label, parallel, randomized controlled trial/reterospective | 300 patients using randomized control trials. | To determine the effectiveness of additional use of masked CGM in pregnancies complicated by insulin requiring diabetes compared to using traditional methods such as SMBG | CGM, SMBG | In insulin-requiring diabetes in pregnancy, use of intermittent masked CGM every6 weeks did not reduce the risk of macrosomia or other pregnancy outcomes. HbA1c also did not change. |
| 4 | Peter Adolfsson and others (2018) | Case-control study | compiled a list of general guidelines for matching patients with the CGM system that may best meet their needs. | Explores the strengths and limitations of each approach (either real-time CGM or intermittently scanned CGM) and provides guidance to healthcare professionals in selecting the CGM type that is most appropriate to the individual needs of their patients. | HbA1c,CV,CGM, SMBG | Both isCGM and rtCGM offer clear advantages over SMBG by providing considerably more robust and useable information. |
| 5 | Tadej Battelin (2018) | Observational study | 515 adult patients were taken using prospective observational study | To observe the safety and efficacy of CGM in routine day- to-day home | HbA1c,CGM | As expected, HbA1c, at 4, 8, and 12 months, CGM, continuous glucose monitoring; Hb, hemoglobin; RCT, randomized controlled trial; T2DM, type 2 diabetes. |
| 6 | Thomas Danne and others (2017) | Consensus/ Recommenations. | (ATTD) Congress convened an international panel of physicians, researchers, and individuals with diabetes who are expert in CGM technologies to discuss how CGM results can affect outcomes. | Use CGM to overcome the limitations of HbA1c | HbA1c,CGM,SMBG, eA1C | CGM should be used in clinical trials of new drugs and devices for diabetes treatment because HbA1c alone is insufficient and does not consider intra- and interday glycemic excursions that may lead to acute events (such as hypoglycemia) or postprandial hyperglycemia, |
| 7 | Richard M. Bergenstal and others. (2018) | Prospective study | 387 individuals divided in two groups. 315 patients with type 1 diabetes and 72 with type 2 diabetes Prospective method is used | This Prospective address why a new name for eA1C was needed, why GMI was selected as the new name, how GMI is calculated, how to understand and explain GMI if one chooses to use GMI as a tool in diabetes education or management. | eA1c. Mean glucose concentration | CGM used in TD1>TD2, those using insulin, and pregnant women. changing the name from eA1C to GMI provides a useful measure for connecting CGM. metrics to laboratory A1C and reinforces. the need for ongoing diabetes management and patient and health care professional engagement. |
| 8 | David L. Levitt and others. (2017) | Observational study | 124 medical ICU patients (24 DM, 100 non-DM) using observational study method. | Studies have not focused on hospitalized patients with type 1 diabetes mellitus, the population most likely to benefit from inpatient CGM. This article reviews inpatient CGM glycemic outcomes in the non-ICU and ICU setting. | CGM, ICU CGM and non-ICU CGM | CGM detects hypoglycemia at a greater frequency than capillary BG testing. The studies discussed in this review do not evaluate glycemic variability in the context of inpatient CGM. In the ICU, CGM may not improve glycemic outcomes when patients are receiving intravenous insulin administration, which already requires frequent glucose monitoring. |
| 9 | Martina Vettorett and others. (2018) | Prospective study | No clinical trials conducted | extend CGM utilization beyond diabetes patients, for example, to subjects with prediabetes or even healthy individuals. | HBA1c | use of CGM sensors will certainly grow significantly in the next years when accuracy is improved, approval from regulatory bodies. the need to provide evidence of their clinical safety and utility, must be carefully addressed. |
| 10 | Laure de Decker and others. (2017) | Cross-sectional cohort study | A total of 1552 elderly (age > 80 years old) patients with T2DM were recruited in a Nationwide cross- sectional study. | Aimed to determine whether a high burden of comorbidities is associated with hypoglycemia in very old patients with T2DM. | SMBG, CCI | The occurrence of hypoglycemic events is very common in older patients with diabetes. real-life study is that a high burden of comorbidities, measured by CCI, is an independent predictor of hypoglycemia in this population. Daily SMBG was positively associated with hypoglycemia. |
| 11 | Ramzi A. and others (2017) | Systematic review | 1441 patients with T1DM selected randomly and observational study was being conducted. | The advantages and limitation of HbA1c are discussed together with the clinical importance of hypoglycemia and glycemic variability. | HBA1C, SMBG, CGM, GV | Development of an algorithm for data analysis will further help in interpreting glucose data and make CGM/ FCGM more user friendly for both patients and HCP. The type of diabetes patient likely to benefit from CGM/ FCGM has not been fully characterized with current evidence, |
| 12 | John R. Petrie and others (2017) | RCT | 322 adults and children (8 years of age) were randomized to receive one of three different CGM devices. | To compare the effectiveness of CGM with SBGM and to discuss the limitations of CGM | HBA1C, CGM, SMBG, HBA1C (in iCGM) | Great progress has been made in CGM technology in recent years (10), but several barriers remain that prevent it from reaching its full potential either as a method for improving glycemic control in diabetes. |
| 13 | Takahiro Ishikawa, Masaya Koshizaka and many others. January 2018 | retrospective study | 170 patients aged ≥65 years with type 2 diabetes using univariate analysis | incidence of type 2 diabetes is higher in elderly patients relationship between hypoglycemia and diabetes treatments to identify risk factors for hypoglycemia | Hypoglycemia | Geriatric population exceeding 62 with diabetes type 2, higher glucose variability and lower average glucose levels indicate a greater risk of hypoglycemia. |
| 14 | Jothydev Kesavadev and others 2017 | Ambulatory glucose profile (AGP | Retrospective analysis been done with type 1 and type 2 diabetic patients | development of more and more advanced technologies such as continuous glucose monitoring and flash glucose monitoring | CGM | At the end of the article, they concluded that the new technologies need improvement |
| 15 | Ana María Gómez and others 2017 | REAL-Time System (Medtronic) vs multiple daily injection (MDI) hence this was a comparative study | 54 T1DM patients with baseline A1c of (8.0%±1.3%), observational study | latest-generation sensors are more accurate and sensitive for hypoglycemia, improving adherence to self-monitoring | Flash CGM | They are talking about new developments in association with technologies whereas SAPT being the best one except it is very expensive |
| 16 | J. Lawton and many others 2018 | comprehensive study | The sample comprised 12 participants aged 16+ years, three participants aged 13–15 years and nine parents were interviewed which were then analyzed. | direction and rate of change of blood glucose levels | Problems with CGM | Users are encouraged to use these sensors while they made sure that it is well known to them how to use, tune, optimize it on their own without any external help. |
| 17 | James S. Krinsley and his collegues 2017 | Randomized controlled trial | 63 patients with isolated brain injury using prospective trails | Glucose management in intensive care unit (ICU) | Glucose monitoring and CGM | Hyperglycemia and hypoglycemia |
*ATTD the Advanced Technologies & Treatments for Diabetes
*CCI Charlson Comorbidity Index
Figure 1.
Preferred reporting items for systematic reviews and Meta-analyses (PRISMA) diagram of systematic review
Clinical themes
In this systematic review, some of the things discussed are the discrepancy between calculated estimated A1c (eA1c) and HBA1c, how CGM is user friendly and is less time-consuming than conventional methods for blood glucose monitoring, and how pregnant women with diabetes mellitus also benefit from CGM [Table 1].
Table 1.
Content and subtheme analysis of systematic review
| Headings | Specific questions |
|---|---|
| 1. Clinical relevance of HbA1c in monitoring | (a) HbA1c limit should be set person to person rather than a generalized limit. |
| (b) Use A1c and other variables along with CGM for more accurate results. | |
| (c) HbA1c values have limitations. | |
| 2. Clinical controversies of HbA1c | (a) Discrepancy b/w calculated eA1c and HbA1c. (b) SMBG fails to detect nocturnal and asymptomatic hypoglycemia (sporadic nature). |
| (c) Hypoglycemia is not regularly measured when you are using HBA1c. | |
| 3. CGM used in TD1 than TD2 | (a) Use of CGM in patients with TD1 causes a major decrease in the HbA1c levels. |
| (b) Lack of studies for TD2: More real-time data needed because helpful for TD2. | |
| 4. Limitations of CGM in clinical practice | (c) Insufficient data for adjustment of treatment regimen. |
| (d) For patients on oral therapy or insulin therapy, hypoglycemia measurement is EVEN MORE important. | |
| (e) CGM is expensive, hence limited use. | |
| 5. Patient perception on CGM | (f) Patient inconvenience with SMBG vs. CGM. |
| (g) CGM is user friendly. | |
| (h) CGM is less time consuming. | |
| 6. Future implications of CGM | (i) Pregnant women with diabetes mellitus also benefit from CGM. |
| (j) Comorbidities should be considered: Not enough studies on comorbidities. | |
| (k) May be used for TD2 for therapy decisions. | |
| (l) How involved insurance companies will be? | |
| 7. Glucose variability | Literature reported |
HbA1c = glycated hemoglobin, CGM = continuous glucose monitoring, TD1 = type 1 diabetes mellitus, TD2 = type 2 diabetes mellitus, eA1c = estimated A1C, SMBG = self-monitoring of blood glucose
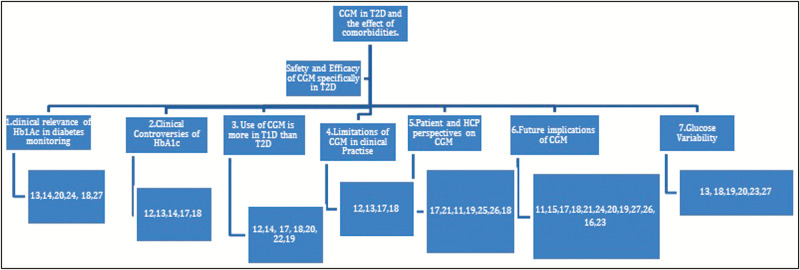
The review addresses “Clinical relevance of HBA1c in monitoring,” “Clinical controversies of HB1Ac,” “CGM used in TD1 than TD2,” “Limitations of CGM in clinical practice,” “Patient and HCPs perception on CGM,” “Future implications of CGM,” and “Glucose variability” [Figure 2].
Figure 2.
Systematic review blueprint
Clinical relevance of glycated hemoglobin
HbA1c is the classic method for assessing the glucose levels.[11,12,13] Most organizations that have been assessing glucose levels using this method have set a recommended target of 7.0% (53 mmol/mol) for adults and 7.5% (58 mmol/mol) for children; however, some other organizations suggest a different target value for HbA1c such as 6.5% for adults as well as for children.[12] Nonetheless, all the organizations agree that HbA1c limit should be set person to person rather than a generalized limit.[13] Considering the importance of HBA1c value and the increased use of CGM has introduced a new term eA1c, which is supposed to be an equivalent to HBA1c (laboratory generated); it uses the mean glucose data from CGM or SBGM to produce a value. However, due to lack of agreement between the lab A1C and eA1c, U.S. Food and Drug Administration (US FDA) suggested changing the nomenclature of eA1c to Glucose Management Indicator (GMI) and also derived a formula to convert the CGM readings of mean blood glucose to GMI.[14] The formula that is used to calculate the GMI is derived from a regression line by plotting a graph of “mean glucose concentration” on the x-axis and “simultaneously measured A1C” on y-axis. The very first study to generate eA1c value was A1c-derived average glucose study conducted in 2006–2007.[14] A more recent study by Beck et al.[14] generated an equation which was validated later by HypoDE study. The equation was GMI (%) = 3.31+0.02392x (mean glucose in mg/dL).
Real-time continuous glucose monitoring (RT-CGM) was carried out, in which patients received various therapies that involved diet, lifestyle, and different combinations of antihyperglycemic therapies including basal insulin therapy.[26] As the trial proceeded, HbA1c reduction was observed despite of the lower doses given to the patients.
RT-CGM study was considered as it will avoid the energy burnout of T2DM people, hence less chances of error in the results, even to those people who use insulin and along all this HbA1c reduction is observed.[26] Major changes are seen in those whose HbA1c values were high at baseline.[26] However, when this study was carried out on a mixed T1DM and T2DM, there were no significant results for HbA1c.[26]
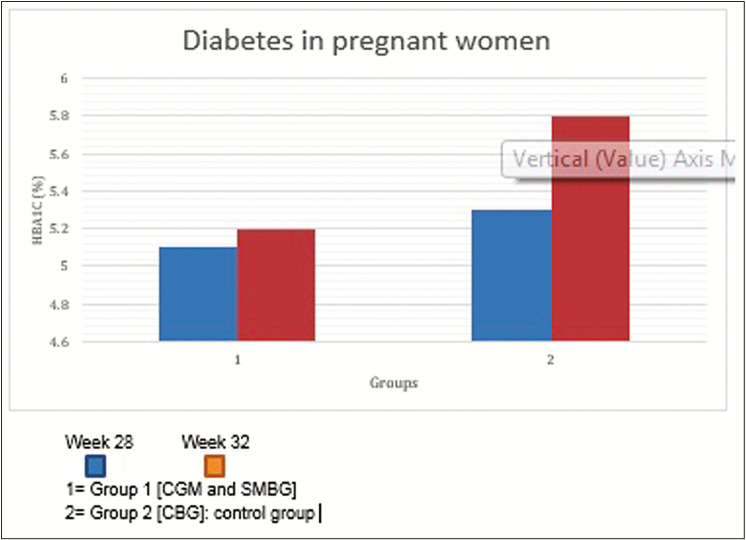
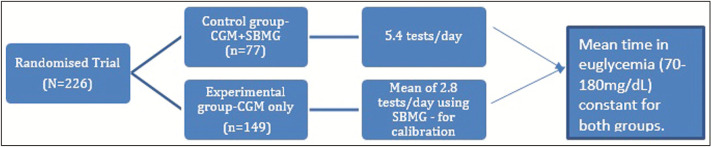
Another study was run on a sample of population consisting of 50 units. These units were 50 pregnant females who were divided into two groups, 25 females in each group where CGM, SMBG, and capillary blood glucose were observed in order to check how these variables affect and how much they affect the HbA1c concentration. These females were kept under study from the beginning of their pregnancy and also, they were to take their values three times a week and the results recorded are shown in Figure 3.[19]
Figure 3.
Continuous glucose monitoring (CGM) implication in pregnant women vs. self-monitoring of blood glucose (SBGM) for adverse drug reaction. As shown in the above the graph, the increase in Hb1Ac level in Group 1 (CGM and SMBG) is almost insignificant, whereas in Group 2 (control group) the increase in Hb1Ac level is approximately 0.5%
Clinical controversies of glycated hemoglobin
The conventional method for evaluating glycemic control has been the measurement of HbA1c. Although this method is still being used, there are several drawbacks of using it. Intra- and interday glycemic excursions are not considered[13] that give rise to acute events such as hypoglycemia and postprandial hyperglycemia, which remains undetected on a daily basis. Moreover, it is an erratic measure in the case of anemic patients,[13] iron deficiency,[13] hemoglobinopathies,[13] and pregnancy.[13] HbA1c values are also questionable due to racial differences as it affects the accuracy.[13] Another extensively used method for blood glucose monitoring to detect the hypoglycemia, which is undetected by HbA1c,[7] is SBGM. It provides a single “point-in-time” measurement.[12,13] Although patients particularly on insulin therapy are at a greater risk of experiencing hypoglycemia, frequent testing is required which may or may not be performed by the patient.[13,17] There are chances of misreporting,[17] and it also does not recognize nocturnal and asymptomatic hypoglycemia,[13] which is why SBGM is also not a very good method for achieving glycemic control.
eA1c is another way of evaluating glycemic control when newer technologies such as CGM and FLASH are used but there is usually a discrepancy between the laboratory (HBA1c) and the eA1c, which makes this value unreliable and insufficient to be used for safe and effective clinical management.[14]
As the use of CGM becomes significant, this inconsistency in comparison of HBA1c and eA1c leads to a new term being introduced by members of the Center for Devices and Radiological Health (CDRH), a division of the U.S. FDA, who is responsible for the regulation of medical devices, “GMI”.[14]
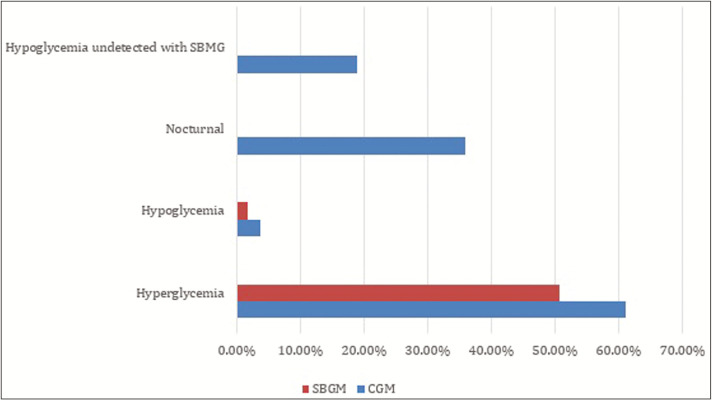
The disagreement between HBA1c and eA1c will be overcome by considering other measurements such as fructosamine, glycated albumin, and SMBG along with HbA1c and the use of GMI, when sufficient data are available [Figure 4].
Figure 4.
Comparison of continuous glucose monitoring (CGM) glucose monitoring (CGM) outcome
Pazos-Couselo et al.[18] completed an observational, prospective study of 63 patients with T2DM on insulin in which the patients were asked to take two SMBG readings for 8 weeks and in the 9th week they wore blinded CGM and the results clearly showed that CGM is an important tool for detecting hypoglycemia and nocturnal hypoglycemia, which are undetectable by SBGM.
Continuous glucose monitoring used in type 1 diabetes mellitus and in type 2 diabetes mellitus
CGM is considered to be a very useful method to help patients who have T1DM or TD2 and also in pregnant women as it shows patterns in hypoglycemia, hyperglycemia, and glucose variability (GV).[14,19] The CGM data therefore provide information regarding the variables aforementioned and record the interstitial glucose every 5min, which would help the doctors in making strategies to help the patients with diabetes mellitus manage their glucose levels.[17,21]
CGM helps clinicians and patients decide how lifestyle changes such as diet, exercise, and stress management can help in managing diabetes mellitus. It is observed that the use of CGM in patients with TD1 causes a major decrease in the HbA1c levels compared to TD2 for which more evidence is required. CGM is beneficial in the use of TD2 including elderly patients as it gives information regarding GV as well as HbA1c levels, although it is advised to be used in a limited manner. This is mainly because TD2 patients are more likely to benefit from insulin therapy (as in TD2 insulin is not made or is made abnormally) or lifestyle modifications, whereas frequent glucose monitoring is required more in TD1 (insulin is produced in a minimal amount) due to which CGM is preferred in TD1 than TD2.[18]
A most important clinical use of CGM is to evaluate the results and compare different types of treatment in both patients with T1DM and T2DM; it is clinically beneficial not only in patients with T1DM but also in patients with T2DM. Patients with T2DM for whom basal–bolus insulin therapy is essential are considered as almost the same as patients with T1DM. Patients with Hirata’s disease (anti-insulin antibodies) and inconsistent glycemic levels benefit the most from CGM.[21]
CGM is more helpful for patients with TD1 than TD2 especially in those patients who use insulin and for pregnant women as it reduces both important factors: hypoglycemia and hyperglycemia, whereas in TD2 no significant change in the reduction of HbA1c levels was observed. In addition, more evidence or clinical trials are required to confirm that CGM is beneficial in TD2.[12,14,17]
Limitations of continuous glucose monitoring in clinical practice
CGM gives a very thorough idea of the glucose changes compared to the traditional methods such as SBMG; however, it is a relatively new technology and requires more clinical data to be proven safe and accurate. The limitations that contribute to CGM’s limited use at present are as follows [Figure 5]:
Figure 5.
Randomized controlled trial (RCT) comparison between self-monitoring of blood glucose (SBMG) and continuous
(1) The lack of internationally accepted standards for CGM system performance unlike older devices.[13] For example, the mean absolute relative difference MARD values that provide an average of the absolute error still does not have an exact threshold for rendering the CGM device as accurate.[13]
(2) They are not affordable[12] to be set up in clinics and it requires HCPs that need to be aware of multiple software and should be capable of understanding the complex nature of the tool,[18] hence limiting the accessibility to patients who could be receiving benefits from it.[17,18]
(3) Need for calibration of CGM systems still remains an issue as it relies on SMBG for calibration[17] and this adds up to one of the crucial reasons for hesitancy of using CGM alone to make treatment decisions.[17]
Research gap
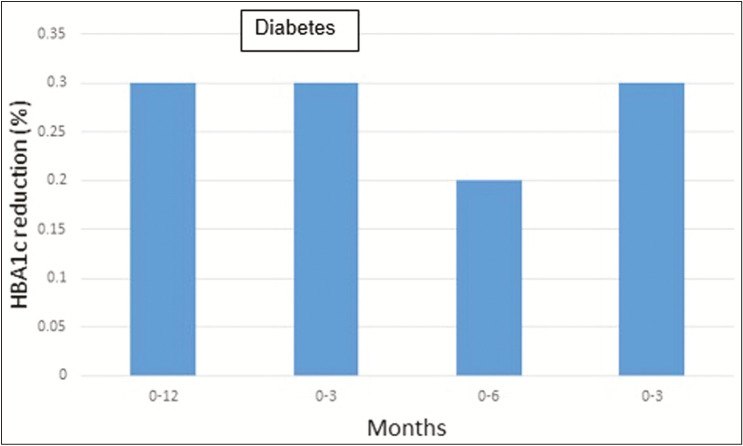
More clinical trials can be performed for TD2 and for pregnant women.[17] More research will be conducted especially for patients with TD2 regarding optimal frequency, timing, and duration of CGM [Figure 6].[18]
Figure 6.
Comparison of glycated hemoglobin (HbA1c) reduction among type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM): (1) the study conducted between 515 patients with T1DM from 0 to 12 months showed Hb1Ac reduction of 0.3%,[22] (2) the study conducted between 25 patients with T1DM from 0 to 3 months showed Hb1Ac reduction of 0.3%,[17] (3) the study conducted between 224 patients with from 0 to 6 months showed Hb1Ac reduction of 0.2%,[18] and (4) the study conducted between 515 patients with T1DM from 0 to 3 months showed Hb1Ac reduction of 0.3%[20]
Future implications of continuous glucose monitoring
CGM has been around since mid-1970s and was commercially available by 1999; however, they are clinically still in its infancy.[23] According to many studies, there are insufficient data on TD2 so for its more abundant use in future, more studies need to be carried out on that. Furthermore, comparison and evaluation of new therapeutic agents is one of the main applications for patients with TD1 and TD2.[18,21] In addition, these systems may also be used later by individuals affected by prediabetes and may also be used by weight-loss programs for obese people and athletes.[11]
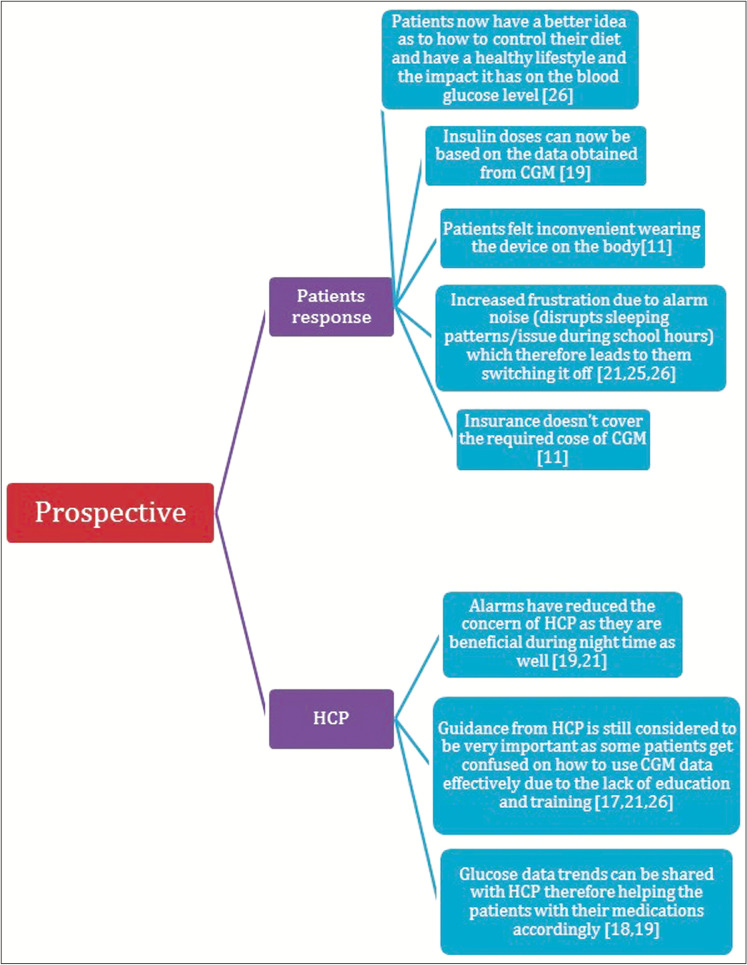
In addition, the target population for CGM is changing focus from just self-monitoring for diabetics to older patients who have comorbidities, intensive care unit (ICU) patients, inpatient care, and pregnant women with T1DM or gestational DM. Figure 7 showed the user and healthcare perspectives on CGM.
Figure 7.
Users perspectives on continuous glucose monitoring (CGM)
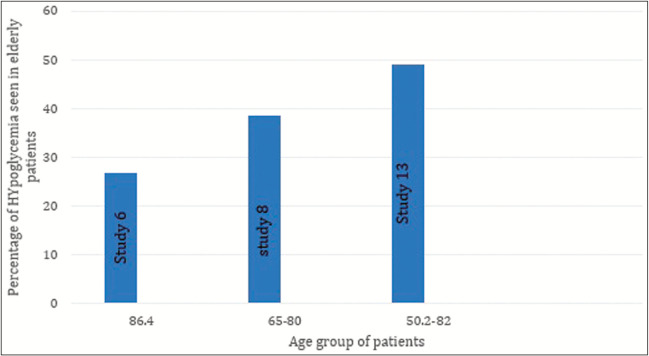
Elderly patients who are diabetics and have comorbidities are at greater risk for a hypoglycemic attack and this must be prevented as it may lead to cognitive impairment,[23] falling, stroke, and even death[16,22] and CGM is useful in this case as it provides close glucose monitoring.[22,24]
Figure 8 shows data from 3 different studies that suggests that above the age of 50, elderly are at a greater risk of experiencing hypoglycemia.
Figure 8.
Risk of hypoglycemia among geriatrics
For ICU patients, who may not be diabetics, it is debatable if glucose monitoring must be performed. However, a study shows reduced mortality in cases such as stress-related hyperglycemia[26] as use of CGM allows rapid adjustability of insulin infusions as the changes in glucose concentrations are more easily identified.[25]
Inpatient hypoglycemia is more easily detected in insulin-treated patients as it allows better supervision because the alerts are sent to the nursing stations through a monitoring device.[15,19] Due to the ongoing improvement in accuracy of these tools, use in hospitalized patients seems to be promising.[21]
There are some but not enough data present for pregnancy-related use of CGM; it has been reported to enhance the glycemic control[17] of the mother but does not reduce the risk of macrosomia, which means that the fetus is significantly larger; this is a common complication in women with diabetes mellitus.[20,21] Moreover, better neonatal outcomes are also observed when CGM in used in mothers with TD1.[19,20]
Lastly, CGM is also clinically useful when it comes to dealing with other conditions such as diabetes mellitus associated with cystic fibrosis, excessive glycemic excursions seen following bariatric surgery, evaluation of hypoglycemia related to insulinoma, Hirata’s disease (anti-insulin autoantibodies), and rare conditions attributable to autoantibodies to the insulin receptor.[21]
Due to such diverse uses of CGM, insurance companies should be more likely to cover for these technologies and provide access to more patients in need; if they realize that the added expense is beneficial for their members and decreases diabetic costs long term.[18,23]
Glucose variability
GV refers to the sudden fluctuations of the blood glucose levels throughout the day including hyperglycemia and hypoglycemia. As it is constantly changing, measuring and understanding it is complex.[13] Increased GV is also a marker for cardiovascular disease[13]; increased GV is consistently associated with mortality in the intensive care unit[13] as well as older patients with T2DM with lower glucose levels or greater GV increases the risk of hypoglycemia.[22] CGM is the best tool to measure glycemic variability[18] as it measures the interstitial glucose levels very frequently and this has reported reduction in incidence of hypoglycemia, hyperglycemia and hence GV.[19,26] Another study also reported that six of eight measures of GV were markedly reduced after 26 weeks of use of CGM.[21] In addition, the study also indicates that GV was progressively reduced as the number of days of CGM used per week increased.[21]
CONCLUSION
This study suggested that although HbA1c is known as the “gold standard” of glycemic control, there are several limitations to this method which is why HCPs are becoming more and more hesitant in using only HbA1c for diabetes mellitus management. SBGM is another useful method but renders insufficient data as it only gives a single “point in time measurement” of blood glucose level hence hypoglycemia or hyperglycemia may go unnoticed, which may be life threatening. CGM is proved to have a substantial role in the management of DM with poor glucose tolerance. It is a safe and effective method for T1DM and T2DM, and it should be used to tailor therapies for the patients. Although the calibration and interpretation of the device still needs improvement, it is still a very convenient tool for people with diabetes mellitus as it is less time-consuming, easy to use and they do not frequently have to be worried about taking readings as with traditional methods such as SBGM.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Gillani SW, Syed Sulaiman SA, Mohammad Abdul MI, Baig MR. Combined effect of metformin with ascorbic acid versus acetyl salicylic acid on diabetes-related cardiovascular complication: a 12-month single blind multicenter randomized control trial. Cardiovasc Diabetol. 2017;16:1–13. doi: 10.1186/s12933-017-0584-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.International Diabetes Federation. Executive summary IDF diabetes atlas. 6th ed. 2013. [Last accessed on 2019 Oct 10]. (updated on 2014). Available from: www.idf.org/sites/default/files/EN_6E_Atlas_Exec_Sum_1.pdf .
- 3.Mehrnoosh K, Ozra T-M, Mostafa Q, Farshad F, Peyvand A, Bagher L. Effect of vitamins C and E on insulin resistance in diabetes: a meta-analysis study. Eur J Clin Invest. 2015;45:1161–74. doi: 10.1111/eci.12534. [DOI] [PubMed] [Google Scholar]
- 4.Tabatabaei-Malazy O, Larijani B, Abdollahi M. A systematic review of in vitro studies conducted on effect of herbal products on secretion of insulin from Langerhans islets. J Pharm Pharm Sci. 2012;15:447–66. doi: 10.18433/j32w29. [DOI] [PubMed] [Google Scholar]
- 5.Gillani SW, Ansari IA, Zaghloul HA, Sulaiman SAS, Rathore HA, Baig MR, et al. Predictors of health-related quality of life among patients with type II diabetes mellitus who are insulin users: a multidimensional model. Curr Ther Res. 2019;90:53–60. doi: 10.1016/j.curtheres.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grey M, Jaser SS, Whittemore R, Jeon S, Lindemann E. Coping skills training for parents of children with type 1 diabetes: 12-month outcomes. Nurs Res. 2011;60:173–81. doi: 10.1097/NNR.0b013e3182159c8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergenstal RM, Tamborlane WV, Ahmann A, Buse JB, Dailey G, Davis SN, et al. STAR 3 Study Group Sensor-augmented pump therapy for A1C reduction (STAR 3) study: results from the 6-month continuation phase. Diabetes Care. 2011;34:2403–5. doi: 10.2337/dc11-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The JDRF Continuous Glucose Monitoring Study Group. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359:1464–76. doi: 10.1056/NEJMoa0805017. [DOI] [PubMed] [Google Scholar]
- 9.Hermanides J, Nørgaard K, Bruttomesso D, Mathieu C, Frid A, Dayan CM, et al. Sensor-augmented pump therapy lowers HbA(1c) in suboptimally controlled type 1 diabetes: a randomized controlled trial. Diabet Med. 2011;28:1158–67. doi: 10.1111/j.1464-5491.2011.03256.x. [DOI] [PubMed] [Google Scholar]
- 10.Hong QN, Pluye P, Fàbregues S, Bartlett G, Boardman F, Cargo M, et al. Mixed methods appraisal tool [MMAT] version 2018 (Registration of Copyright [#1148552]) Montréal, Canada: Canadian Intellectual Property Office; 2019. [Last accessed on 2019 Oct 15]. Available from: http://mixedmethodsappraisaltoolpublic.pbworks.com/w/file/fetch/127916259/MMAT_2018_criteria-manual_2018-08-01_ENG.pdf . [Google Scholar]
- 11.Vettoretti M, Cappon G, Acciaroli G, Facchinetti A, Sparacino G. Continuous glucose monitoring: current use in diabetes management and possible future applications. J Diabetes Sci Technol. 2018;12:1064–71. doi: 10.1177/1932296818774078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petrie JR, Peters AL, Bergenstal RM, Holl RW, Fleming GA, Heinemann L. Improving the clinical value and utility of CGM systems: issues and recommendations. Diabetes Care. 2017;40:1614–21. doi: 10.2337/dci17-0043. [DOI] [PubMed] [Google Scholar]
- 13.Danne T, Nimri R, Battelino T, Bergenstal RM, Close KL, DeVries JH, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40:1631–40. doi: 10.2337/dc17-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bergenstal RM, Beck RW, Close KL, Grunberger G, Sacks DB, Kowalski A, et al. Glucose Management Indicator [GMI]: A new term for estimating A1C from continuous glucose monitoring. Diabetes Care. 2018;41:2275–80. doi: 10.2337/dc18-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levitt DL, Silver KD, Spanakis EK. Inpatient continuous glucose monitoring and glycemic outcomes. J Diabetes Sci Technol. 2017;11:1028–35. doi: 10.1177/1932296817698499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Decker L, Hanon O, Boureau A-S, Chapelet G, Dibon C, Pichelin M, et al. Association between hypoglycemia and the burden of comorbidities in hospitalized vulnerable older diabetic patients: a cross-sectional, population-based study. Diabetes Ther. 2017;8:1405–13. doi: 10.1007/s13300-017-0319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ajjan RA. How can we realize the clinical Benefits of continuous glucose monitoring. Diabetes Technol Ther. 2018;19:1–11. doi: 10.1089/dia.2017.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carlson AL, Mullen DM, Bergenstal RM. Clinical use of continuous glucose monitoring in adults with type 2 diabetes. Diabetes Technol Ther. 2017;19:1–10. doi: 10.1089/dia.2017.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruce WB, Battelino T. Continuous glucose monitoring in 2018. Diabetes Technol Ther. 2019;21:1–19. doi: 10.1089/dia.2019.2502. [DOI] [PubMed] [Google Scholar]
- 20.Adolfsson P, Parkin CG, Thomas A, Krinelke LG. Selecting the appropriate continuous glucose monitoring system––a practical approach. Eur Endocrinol. 2018;14:24–9. doi: 10.17925/EE.2018.14.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.David R. Continuous glucose monitoring: a review of recent studies demonstrating improved glycemic outcomes. Diabetes Technol Ther. 2017;19:1–18. doi: 10.1089/dia.2017.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takahiro I, Masaya K, Yoshiro M, Minoru T, Yoshiharu T, Toshihiro S, et al. Continuous glucose monitoring reveals hypoglycemia risk in elderly patients with type 2 diabetes mellitus. J Diabetes Investig. 2018;9:69–74. doi: 10.1111/jdi.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jothydev K, Lakshmy R, Gopika K. Glucose monitoring technologies–– complementary or competitive? Role of continuous glucose monitoring versus FLASH glucose monitoring versus self-monitoring of blood glucose. J Diabetol. 2017;8:61–7. [Google Scholar]
- 24.Gómez AM, Henao Carrillo DC, Muñoz Velandia OM. Devices for continuous monitoring of glucose: update in technology devices. Med Devices (Auckl) 2017;10:215–24. doi: 10.2147/MDER.S110121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lawton J, Blackburn M, Allen J, Campbell F, Elleri D, Leelarathna L, et al. Patients’ and caregivers’ experiences of using continuous glucose monitoring to support diabetes self-management: qualitative study. BMC Endocr Disord. 2018;18:12. doi: 10.1186/s12902-018-0239-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.James S, Krinsley J, Geoffrey C, Jan G, Johan M, Schultz MJ, et al. Continuous glucose monitoring in the ICU: clinical considerations and consensus. Crit Care. 2017;21:19. doi: 10.1186/s13054-017-1784-0. [DOI] [PMC free article] [PubMed] [Google Scholar]