ABSTRACT
Aim:
The aim of this study was to investigate the involvement of peroxisome proliferator-activated receptor gamma (PPAR-γ) in renal protection offered by sinapic acid in cisplatin-induced nephrotoxicity in male rats.
Materials and Methods:
Nephrotoxicity was induced by single dose of cisplatin (5 mg/kg, intraperitoneal [i.p.]) in rats. Cisplatin-induced nephrotoxicity was assessed by measuring serum creatinine, creatinine clearance, urea, uric acid, potassium, magnesium levels, fractional excretion of sodium, and microproteinuria in rats. Superoxide anion generation, thiobarbituric acid reactive substances, myeloperoxidase activity, and reduced glutathione levels were measured to assess oxidative stress in renal tissues. Hematoxylin and eosin stain showed renal histological changes.
Results:
The significant changes in serum and urinary parameters, elevated oxidative stress, and renal histological changes established the induction of nephrotoxicity. Sinapic acid treatment (20 and 40 mg/kg, orally [p.o.]) provides dose-dependent and significant (P < 0.05) nephroprotection against cisplatin-mediated nephrotoxicity in rats. Nephroprotective effect of sinapic acid was abolished by PPAR-γ inhibitor, bisphenol A diglycidyl ether (30 mg/kg, i.p.) in rats.
Conclusion:
It is concluded that PPAR-γ agonism serves as one of the mechanisms in sinapic acid-mediated renoprotection.
KEYWORDS: Bisphenol A diglycidyl ether, cisplatin, nephrotoxicity, oxidative stress, peroxisome proliferator-activated receptor-gamma, renoprotection, sinapic acid
INTRODUCTION
The kidneys are the primary organs responsible for the maintenance of extracellular fluid volume and homoeostasis. As a result of which, kidneys are exposed to harmful substances leading to the damage of renal tissues as well as degeneration of parenchymal cells.[1] Nephrotoxicity is one the most widely recognized side effects related with malignancy chemotherapy in which the urine output reduces to less than half of the normal volume, whereas the level of serum creatinine and other nitrogenous and biochemical wastes is increased in body.[2,3]
Cisplatin is the most successful chemotherapeutic agent used in the treatment of solid tumor. Still, neurotoxicity, ototoxicity, nausea, vomiting, and especially nephrotoxicity are the main restraining factors for the use of cisplatin in treatment of cancer. Among these side effects, the predominance of nephrotoxicity is very high.[4] Cisplatin-induced nephrotoxicity affects nearly 20%–30% patients receiving therapeutic dose.[5] Moreover, the chance of nephrotoxicity increases significantly along with patient age and comorbidities.[6,7,8]
Several studies indicate that cisplatin accumulation in kidneys results in inflammation, increases lipid peroxidation, and reduces the level of antioxidant, mitochondrial dysfunction, along with deoxyribonucleic acid (DNA) adduct formation and activation of apoptotic pathways.[9] Moreover, the accumulation of cisplatin is found to be more than five times in kidney as compared to the blood. As a result of which, the concentration of cisplatin in kidney is very high (resulting in toxicity) as compared to the concentration in the blood which is generally nontoxic. This accumulation of cisplatin is responsible for the development of renal dysfunction and nephrotoxicity.[10,11,12]
Plants are important source of photochemical, which are proven as effective therapeutic agents. Many phytoconstituents such as flavonoids and alkaloids isolated form plants are found to be effective drugs for various disorders. Moreover, several phenolic compounds present in food such as rosemary, oregano, ginger, green tea, and soybean have been found to be significantly effective antioxidant agents.[13] The oxidative stress and apoptotic pathway is an important factor responsible for several diseases. Due to increasing involvement of oxidative stress in pathological conditions, antioxidants and antiapoptotic substances are considered as potential therapeutic agents.[14]
Sinapic acid (3,5-dimethoxy-4-hydroxycinnamic acid) is a hydroxycinnamic acid of phenylpropanoid family that is abundantly present in plant kingdom such as spices, wheat, rice, oil seeds, vegetables, fruits especially citrus fruits, cereals, vinegar, and wine.[15] Moreover, sinapic acid has become a major active component of Chinese traditional remedies.[16] Sinapic acid is proved as an antimicrobial,[17] neuroprotective,[18] antihyperglycemic,[19] anxiolytic,[20] anti-inflammatory,[21] peroxynitrite scavenging,[22] antihypertensive, anticancer, and as an antioxidant agent.[23,24]
Researchers proved the involvement of nuclear receptor peroxisome proliferator-activated receptors (PPARs) especially PPAR-gamma (PPAR-γ) in the transcriptional modulation of diverse cellular functions such as lipid metabolism, inflammation, glucose homeostasis, cell differentiation, and extracellular matrix remodeling.[25] Activated PPAR-γ pathway is proved as an important target in diabetes, obesity, atherosclerosis, hypertension, cancer chemoprevention, and drug-induced nephropathy.[26,27] PPAR-γ expression in glomerulus, medullary thick ascending limb, proximal tubules, and collecting ducts of kidney imparts a significant role in renal metabolism and maintenance of systemic homeostasis.[28] Moreover, PPAR-γ-attenuated glomerulonephritis acts on macrophages to produce anti-inflammatory effects as well as improve the metabolic parameters in type-2 diabetes.[29] PPAR-γ agonists were also reported to possess nephroprotective effects in different animal models via anti-inflammatory, antioxidant, vascular, metabolic, and hemodynamic effects.[30] Several antioxidant and anti-inflammatory natural products are also found to activate peroxisome PPARs; therefore, these compounds may help to overcome drug-induced cytotoxicity by modulating PPAR-γ transduction pathway.[31,32,33]
In preview of previous studies, this study was framed to investigate the protective effect of sinapic acid against cisplatin-induced nephrotoxicity in rats, as well as to explore the role of PPAR-γ in nephroprotection.
MATERIALS AND METHODS
The study was executed as per the instructions of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Environment, Forests and Climate Change, Government of India. In this study, inbred male Wistar albino rats weighing 200–250g were used.[34] The rats were kept on standard rat diet and water ad libitum while exposing to 12-h light and dark cycles. At the end of study, blood samples were withdrawn to measure parameters. After that animals were euthanized under the influence of anesthesia and kidneys were harvested.
Drugs and chemicals
Sinapic acid, cisplatin, bisphenol A diglycidyl ether (BADGE), and other chemicals were obtained from Sigma-Aldrich, Bangalore, India. All other agents used were of analytical grade.
Induction of nephrotoxicity
Animals were divided into five groups and each group comprised six rats. Nephrotoxicity was induced by intraperitoneal single dose of cisplatin (5 mg/kg) on the first day of study. Sinapic acid was dissolved in 0.2% dimethyl sulfoxide (DMSO) and was administered at two dose levels (20 and 40 mg/kg, orally [p.o.]) to rats for 5 days. BADGE was dissolved in minimal volume of ethanol and final volume was made with 0.9% saline. After 4 days, the rats were kept individually in metabolic cages for the next 24h. On the fifth day, rats were removed from metabolic cages; their blood samples were collected and euthanized by cervical dislocation under anesthesia. Serum creatinine, urea, uric acid, magnesium, and potassium levels were estimated in blood samples. The microprotein, sodium, and creatinine levels were also determined in urine samples. A part of renal tissue was fixed with formalin for histopathological studies and a small portion of renal tissue was used for estimation of superoxide anion generation (SAG). The remaining of renal tissue was used to estimate myeloperoxidase (MPO), hydroxyproline, thiobarbituric acid reactive substances (TBARS), reduced glutathione (GSH) levels, tumor necrosis factor-alpha (TNF-α), and interleukin-1 beta (IL-1β).[35,36,37,38,39,40,41,42,43,44]
Experimental protocol
The animals were divided into different groups and each group comprised of six animals.
Group 1 (vehicle control): Vehicle (saline, 10mL/kg, intraperitoneal [i.p.]) for cisplatin was administered on day 1 only. Vehicle (0.2% DMSO in water, 10mL/kg, p.o.) for sinapic acid was administered once daily for 4 days to the male Wistar rats.
Group 2 (disease control): The nephrotoxicity was induced by single dose of cisplatin (5 mg/kg, i.p.) on day 1 to the male Wistar rats.
Group 3 (sinapic acid (20 mg/kg) + cisplatin group): Sinapic acid (20 mg/kg/day, p.o.) was administered to experimental animals for 4 days after administration of single dose of cisplatin (5 mg/kg, i.p.) on day 1.
Group 4 (sinapic acid (40 mg/kg) + cisplatin group): Sinapic acid (40 mg/kg/day) was administered to experimental animals for 4 days after administration of single dose of cisplatin (5 mg/kg, i.p.) on day 1.
Group 5 (sinapic acid (40 mg/kg) + BADGE (30 mg/kg): BADGE (30 mg/kg, i.p.) was administered 30min before treatment of animals with 40 mg/kg/day sinapic acid for consequent 4 days after administration of single dose of cisplatin (5 mg/kg, i.p.) on day 1.
Statistical analysis
Results were expressed as mean ± standard error of mean. The data obtained from various groups were statistically analyzed using one-way analysis of variance followed by Tukey’s multiple range tests. The value of P < 0.05 was considered to be statistically significant.
RESULTS
No significant change was observed in different renal and oxidative parameters in the case of vehicle control group.
Effect of sinapic acid treatment on serum creatinine, creatinine clearance, serum urea, uric acid, and serum potassium and magnesium level
A significant decrease was observed in creatinine clearance level in urine, serum potassium, and serum magnesium levels, whereas serum creatinine, serum urea, and uric acid levels were found to be increased significantly (P < 0.05) in cisplatin (5 mg/kg, i.p.) group as compared to vehicle control group. The treatment with sinapic acid (20 and 40 mg/kg, p.o.) significantly and dose dependently (P < 0.05) increased creatinine clearance rate, serum potassium, and serum magnesium level, whereas it decreased the elevated level of serum creatinine and serum urea, as well as uric acid levels as compared to cisplatin group. The prior treatment with BADGE (30 mg/kg, i.p.) significantly (P < 0.05) abolished the renoprotective effect offered by sinapic acid [Table 1].
Table 1.
Effect of various pharmacological treatments on renal parameters in rats (serum and urine)
| Parameter | Group | ||||
|---|---|---|---|---|---|
| Vehicle control | Cisplatin control (5 mg/kg, i.p.) | Cisplatin + sinapic (20 mg/kg, p.o.) | Cisplatin + sinapic (40 mg/kg, p.o.) | Cisplatin+ sinapic (40 mg/kg, p.o.) + BADGE (30 mg/kg, i.p.) | |
| Serum creatinine (mg/ dL) | 0.56 ± 0.06 | 2.78 ± 0.61a | 1.33 ± 0.25b | 0.87 ± 0.06b | 2.03 ± 0.28c |
| CrCl (mL/min/kg) | 1.28 ± 0.04 | 0.33 ± 0.06a | 0.65 ± 0.04b | 0.92 ± 0.03b | 0.298 ± 0.03c |
| Serum urea (mg/dL) | 31.97 ± 5.20 | 109.95 ± 5.76a | 76.66 ± 4.46b | 55.76 ± 5.02b | 93.05 ± 5.04c |
| Uric acid (mg/dL) | 2.46 ± 0.62 | 4 ± 0.32a | 2.98 ± 0.23b | 2.42 ± 0.42b | 3.59 ± 0.44c |
| Serum potassium (mM/L) | 8.38 ± 0.77 | 4.28 ± 0.36a | 5.64 ± 0.28b | 7.02 ± 0.61b | 4.27 ± 0.33c |
| Serum magnesium (mEq/L) | 3.6 ± 0.36 | 1.53 ± 0.11a | 2.17 ± 0.10b | 3.16 ± 0.22b | 1.48 ± 0.12c |
| FeNa (%) | 0.97 ± 0.12 | 6.35 ± 0.78a | 4.1 ± 0.20b | 3.09 ± 0.23b | 4.57 ± 0.48c |
| Microproteinuria (mg/ day) | 4.33 ± 0.30 | 8.81 ± 0.33a | 6.11 ± 0.23b | 4.86 ± 0.25b | 7.8 ± 0.41c |
BADGE = bisphenol A diglycidyl ether, FeNa = fractional excretion of sodium, i.p. = intraperitoneal, p.o. = orally
Data were expressed as mean ± standard error of mean (n = 6)
aP < 0.05 versus vehicle control group
bP < 0.05 versus cisplatin (5 mg/kg, i.p.)
cP < 0.05 versus cisplatin (5 mg/kg, i.p.) + sinapic acid (40 mg/kg, p.o.)
Effect of sinapic acid treatment on fractional excretion of sodium and microprotein urea
A significant (P < 0.05) increase in fractional excretion of sodium (FeNa) level in serum and microprotein urea level in urine was noticed in the cisplatin group (5 mg/kg, i.p.) as compared with the vehicle control group. Treatment with sinapic acid (20 and 40 mg/kg, p.o.) produced a significant decrease in FeNa and microproteinuria as compared to cisplatin control group. Pretreatment with BADGE (30 mg/kg, i.p.) considerably attenuated the protection produced by sinapic acid [Table 1].
Effect of sinapic acid treatment on hydroxyproline thiobarbituric acid reactive substances, renal superoxide anion generation, myeloperoxidase, and glutathione levels
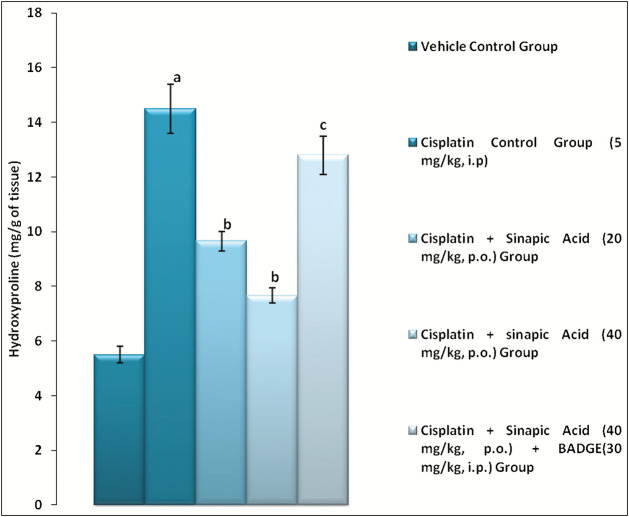
The level of hydroxyproline, renal TBARS, SAG, and MPO activity was found to increase in cisplatin group (5 mg/kg, i.p.), whereas GSH level decreased as compared with the vehicle control group [Figure 1 and Table 2]. Administration of sinapic acid (20 and 40 mg/kg, p.o.) dose dependently produced a significant (P < 0.05) reduction in the hydroxyproline [Figure 1]. Moreover, sinapic acid attenuated cisplatin-induced rise in renal TBARS, SAG, and MPO activity along with raising the renal GSH levels in rats [Table 2]. Pretreatment with BADGE abolished sinapic acid-mediated correction of renal parameters in rats.
Figure 1.
Effect of various treatments on hydroxyproline level in renal tissue. Data were expressed as mean ± standard error of mean (n = 6). aP < 0.05 versus vehicle control group; bP < 0.05 versus cisplatin (5 mg/kg, intraperitoneal [i.p.]); cP < 0.05 versus cisplatin + sinapic acid (40 mg/kg, orally [p.o.])
Table 2.
Effect of various pharmacological treatments on oxidative stress parameters in rats
| Parameter | Group | ||||
|---|---|---|---|---|---|
| Vehicle control | Cisplatin control (5 mg/kg, i.p.) | Cisplatin + sinapic (20 mg/kg, p.o.) | Cisplatin + sinapic 40 mg | Cisplatin + sinapic 40mg + BADGE | |
| TBARS (µM/mg of protein) | 0.45 ± 0.04 | 1.55 ± 0.05a | 0.75 ± 0.04b | 0.58 ± 0.03b | 1.28 ± 0.07c |
| SAG (µM/mg of tissue) | 21.4 ± 0.64 | 65.2 ±1.39a | 45.1 ± 1.24b | 35.2 ± 0.86b | 62.8 ± 1.38c |
| MPO (U/g of tissue) | 1.79 ± 0.52 | 6.91 ± 0.91a | 5.02 ± 2.31b | 2.06 ± 0.63b | 5.24 ± 0.96c |
| GSH (mM/mg of protein) | 16.2 ± 0.41 | 5.35 ± 0.52a | 12.65 ± 0.52b | 14.18 ± 0.33b | 7.82 ± 0.64c |
TBARS = thiobarbituric acid reactive substances, SAG = superoxide anion generation, MPO = myeloperoxidase, GSH = glutathione, BADGE = bisphenol A diglycidyl ether, i.p. = intraperitoneal, p.o. = orally
Data were expressed as mean ± standard error of mean (n = 6)
aP < 0.05 versus vehicle control group
bP < 0.05 versus cisplatin (5 mg/kg, i.p.)
cP < 0.05 versus cisplatin + sinapic acid (40 mg/kg, p.o.)
Effect of sinapic acid treatment on pro-inflammatory cytokine levels
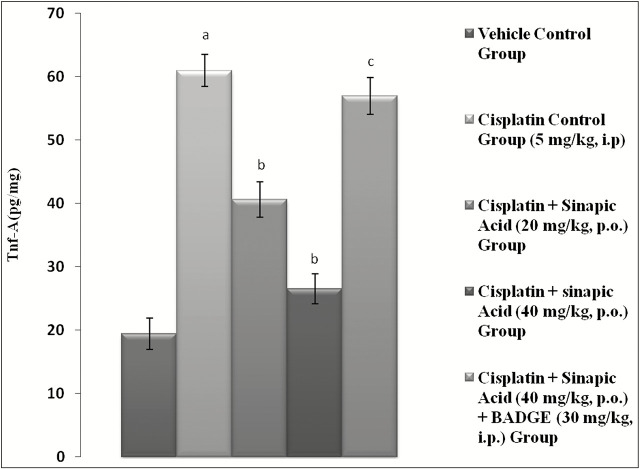
TNF-α and IL-1β level were found to be significantly (P < 0.05) elevated in the cisplatin group (5 mg/kg, i.p.) as compared to vehicle control group indicating inflammation. Administration of sinapic acid dose (20 and 40 mg/kg, p.o.) dose dependently and significantly (P < 0.05) attenuated the elevated levels of TNF-α and IL-1β as compared to cisplatin control group [Figures 2 and 3].
Figure 2.
Effect of various treatments on level of tumor necrosis factor-alpha (TNF-α) in renal tissue. Data were expressed as mean ± standard error of mean (n = 6). aP < 0.05 versus vehicle control group; bP < 0.05 versus cisplatin (5 mg/kg, intraperitoneal [i.p.]); cP < 0.05 versus cisplatin + sinapic acid (40 mg/kg, orally [p.o.])
Figure 3.
Effect of various treatments on level of interleukin-1 beta (IL-1β) in renal tissue. Data were expressed as mean ± standard error of mean (n = 6). aP < 0.05 versus vehicle control group; bP < 0.05 versus cisplatin (5 mg/kg, intraperitoneal [i.p.]); cP < 0.05 versus cisplatin + sinapic acid (40 mg/kg, orally [p.o.])
Effect of sinapic acid treatment on histopathological evaluation
Histopathological examination of renal tissue from normal rat showed normal structure of the glomeruli and tubules (A). Cisplatin control rats showed coagulative necrosis and severe degenerative changes in the tubular lining (B). Administration of sinapic acid (20 and 40 mg/kg, p.o.) resulted in a significant improvement of the overall histopathological picture of the kidneys. The renal tissues of vehicle control group rats stained with hematoxylin and eosin show intact glomerulus surrounded by medulla Bowman capsule, convoluted tubules, loop of Henle, and collecting tubule. The cisplatin control group showed distorted histology such as atrophied glomerulus, collecting tubules showing necrosis, vacuolization, neutrophil accumulation, and loss of normal architecture. Administration of sinapic acid produced significant protection by attenuating the inflammation, vacuolization, and necrosis (C). Treatment with BADGE attenuated the protection provided by sinapic acid against cisplatin-induced nephrotoxicity in rodents (D) [Figure 4].
Figure 4.
Hematoxylin and eosin staining of renal sections at ×200 magnification. (A) Vehicle control. (B) Cisplatin control (5 mg/kg intraperitoneal [i.p.]). (C) Cisplatin + sinapic acid (40 mg/kg, orally [p.o.]). (D) Cisplatin + sinapic acid (40 mg/kg, p.o.) + bisphenol A diglycidyl ether (30 mg/kg, i.p.). The black lines indicates the site of injury
DISCUSSION
The duration and dose-related nephrotoxicity is the main side effect of cisplatin, which confines its clinical use as a chemotherapeutic agent.[2] Slow excretion rate and high accumulation of cisplatin in kidney as compared to blood are the main factors responsible for cisplatin-induced deleterious effects.[45] Cisplatin-induced oxidative stress in renal tissue causes inflammation that produces structural damages, renal dysfunction, and apoptosis, which lead to nephrotoxicity.[46] Cisplatin-induced nephrotoxicity was found to be associated by altered level of MPO, hydroxyproline, TBARS, SAG, and GSH. Several previous studies clearly indicated the role of free radical formation and oxidative stress in cisplatin-induced nephrotoxicity.[47-48,49] Similarly, in this study, levels of MPO, hydroxyproline, and TBARS were found to be elevated, whereas levels of antioxidant enzymes were found to be reduced as compared to normal rats. In this study, the treatment with sinapic acid (20 and 40 mg/kg, p.o.) significantly attenuated the cisplatin-induced altered renal marker levels and increased oxidative stress. These outcomes were in accordance with those obtained in previous studies, which showed that antioxidative potential of sinapic acid has protective activity against cisplatin-induced nephrotoxicity.[50,51,52,53,54]
There are reports those affirming that inflammation plays a vital role in the development of cisplatin-initiated renal damage. Anti-inflammatory agents have been proven as to diminish renal injuries.[55,56] Earlier studies also shown that in the case of cisplatin-induced renal injuries, inflammatory cytokines and chemokine levels are elevated in renal tubular epithelium.[57] TNF-α and IL-1β among the other inflammatory cytokines play a major role to initiate cisplatin-induced inflammatory responses.[58] In inflamed renal parenchyma the IL-1β promotes the influx of circulating monocytes, whereas TNF-α enhances the level of leucocytes in the inflamed renal parenchyma in renal endothelial cells.[59,60,61]
In a study by Ansari,[62] inflammatory cytokine levels (TNF-α and IL-1β) were found to reduce by administration of sinapic acid. In this study, a well-established anti-inflammatory agent,[63] sinapic acid pretreatment (40 mg/kg), significantly downregulated TNF-α and IL-1β levels by averting the inflammatory infiltration and apoptosis of renal tubules in cisplatin nephrotoxicity.
Histopathological examination shown that sinapic acid treatment decreased the neutrophil infiltration and glomerular atrophy, and restored the texture of in renal tissue. We can summarize that the sinapic acid can produce nephroprotective effect by abolishing renal dysfunctioning, oxidative stress, and increased free radicals produced by cisplatin.
The nuclear receptor, PPAR-γ, is mainly involved in storage of fat and controls the metabolism, inflammation in immune cells, and cell proliferation. Substances that are PPAR-γ agonists also show anti-inflammatory, antidiabetic, antifibrotic, antioxidant, and antiapoptotic effects and protective effects against renal ischemia/reperfusion.[47,48,49] Early reports also documented that due to expression of PPAR-γ in renal tissues such as renal microvasculature, glomerulus, proximal, and collecting tubules produce beneficial role in renal disorders by regulation inflammatory mediators into renal tissues, inhibiting the intracellular cell adhesion molecule-1 expression and consequently reducing the oxidative stress level.[32,65,66] Downregulation of PPAR-γ, TNF-α level, and elevation of IL are the indicators of inflammation and elevation of oxidative stress.[33] It was also documented that renal expression of PPAR-γ was reduced by cisplatin,[49,66] whereas Hwang et al.[67] reported that sinapic acid could protect the differentiation potential of stem cells against ultraviolet-A irradiation by upregulation of PPAR- γ. It was also reported that administration of BADGE, a PPAR-γ inhibitor, abolished the antioxidant effect of PPAR-γ agonist, confirming the PPAR-γ mediated protective role of in antioxidant against cisplatin-induced nephrotoxicity.[21,64,67,68] In this study, dose-dependent renoprotective effect of PPAR-γ agonist, sinapic acid, was abolished by BADGE as supported by the histopathological findings.
Hence, as per above discussion, it is can be summarized that the sinapic acid can produce nephroprotective effect in the case of nephrotoxicity caused by cisplatin. Moreover PPAR-γ activation imparts an important role in sinapic acid-mediated nephroprotection against cisplatin-induced nephrotoxicity.
Acknowledgement
We are grateful to the Chitkara College of Pharmacy, Chitkara University, Rajpura, Patiala, Punjab, India, for providing the necessary facilities to carry out the research work.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Ferguson MA, Vaidya VS, Bonventre JV. Biomarkers of nephrotoxic acute kidney injury. Toxicology. 2008;245:182–93. doi: 10.1016/j.tox.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim SY, Moon A. Drug-induced nephrotoxicity and its biomarkers. Biomol Ther (Seoul) 2012;20:268–72. doi: 10.4062/biomolther.2012.20.3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peres LA, da Cunha AD., Jr Acute nephrotoxicity of cisplatin: molecular mechanisms. J Bras Nefrol. 2013;35:332–40. doi: 10.5935/0101-2800.20130052. [DOI] [PubMed] [Google Scholar]
- 4.Miller RP, Tadagavadi RK, Ramesh G, Reeves WB. Mechanisms of cisplatin nephrotoxicity. Toxins (Basel) 2010;2:2490–518. doi: 10.3390/toxins2112490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manohar S, Leung N. Cisplatin nephrotoxicity: a review of the literature. J Nephrol. 2018;31:15–25. doi: 10.1007/s40620-017-0392-z. [DOI] [PubMed] [Google Scholar]
- 6.Yao X, Panichpisal K, Kurtzman N, Nugent K. Cisplatin nephrotoxicity: a review. Am J Med Sci. 2007;334:115–24. doi: 10.1097/MAJ.0b013e31812dfe1e. [DOI] [PubMed] [Google Scholar]
- 7.Wang X, Bonventre JV, Parrish AR. The aging kidney: increased susceptibility to nephrotoxicity. Int J Mol Sci. 2014;15:15358–76. doi: 10.3390/ijms150915358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xavier SK, Haneefa SM, Anand DR, Polo PR, Maheshwari R, Shreedhara CS, et al. Antioxidant and nephroprotective activities of the extract and fractions of Homonoia riparia Lour. Pharmacogn Mag. 2017;13:25–30. doi: 10.4103/0973-1296.197647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jilanchi S, Talebi A, Nematbakhsh M. Cisplatin alters sodium excretion and renal clearance in rats: gender and drug dose related. Adv Biomed Res. 2018;7:54. doi: 10.4103/abr.abr_124_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kohli HS, Bhaskaran MC, Muthukumar T, Thennarasu K, Sud K, Jha V, et al. Treatment-related acute renal failure in the elderly: a hospital-based prospective study. Nephrol Dial Transplant. 2000;15:212–7. doi: 10.1093/ndt/15.2.212. [DOI] [PubMed] [Google Scholar]
- 11.Naughton CA. Drug-induced nephrotoxicity. Am Fam Physician. 2008;78:743–50. [PubMed] [Google Scholar]
- 12.Nagai J, Takano M. Molecular-targeted approaches to reduce renal accumulation of nephrotoxic drugs. Expert Opin Drug Metab Toxicol. 2010;6:1125–38. doi: 10.1517/17425255.2010.497140. [DOI] [PubMed] [Google Scholar]
- 13.Nakatani N. Phenolic antioxidants from herbs and spices. Biofactors. 2000;13:141–6. doi: 10.1002/biof.5520130123. [DOI] [PubMed] [Google Scholar]
- 14.Akomolafe SF, Akinyemi AJ, Anadozie SO. Phenolic acids (gallic and tannic acids) modulate antioxidant status and cisplatin induced nephrotoxicity in rats. Int Sch Res Not. 2014;2014:984709. doi: 10.1155/2014/984709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andreasen MF, Landbo AK, Christensen LP, Hansen A, Meyer AS. Antioxidant effects of phenolic rye (Secale cereale L.) extracts, monomeric hydroxycinnamates, and ferulic acid dehydrodimers on human low-density lipoproteins. J Agric Food Chem. 2001;49:4090–6. doi: 10.1021/jf0101758. [DOI] [PubMed] [Google Scholar]
- 16.Menezes JC, Kamat SP, Cavaleiro JA, Gaspar A, Garrido J, Borges F. Synthesis and antioxidant activity of long chain alkyl hydroxycinnamates. Eur J Med Chem. 2011;46:773–7. doi: 10.1016/j.ejmech.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 17.Maddox CE, Laur LM, Tian L. Antibacterial activity of phenolic compounds against the phytopathogen Xylella fastidiosa. Curr Microbiol. 2010;60:53–8. doi: 10.1007/s00284-009-9501-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim DH, Yoon BH, Jung WY, Kim JM, Park SJ, Park DH, et al. Sinapic acid attenuates kainic acid-induced hippocampal neuronal damage in mice. Neuropharmacology. 2010;59:20–30. doi: 10.1016/j.neuropharm.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 19.Kanchana G, Shyni WJ, Rajadurai M, Periasamy R. Evaluation of antihyperglycemic effect of sinapic acid in normal and streptozotocin-induced diabetes in albino rats. Glob J Pharmacol. 2011;5:33–9. [Google Scholar]
- 20.Yoon BH, Jung JW, Lee JJ, Cho YW, Jang CG, Jin C, et al. Anxiolytic-like effects of sinapic acid in mice. Life Sci. 2007;81:234–40. doi: 10.1016/j.lfs.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Yun KJ, Koh DJ, Kim SH, Park SJ, Ryu JH, Kim DG, et al. Anti-inflammatory effects of sinapic acid through the suppression of inducible nitric oxide synthase, cyclooxygase-2, and proinflammatory cytokines expressions via nuclear factor-kappaB inactivation. J Agric Food Chem. 2008;56:10265–72. doi: 10.1021/jf802095g. [DOI] [PubMed] [Google Scholar]
- 22.Zou Y, Kim AR, Kim JE, Choi JS, Chung HY. Peroxynitrite scavenging activity of sinapic acid (3,5-dimethoxy-4-hydroxycinnamic acid) isolated from Brassica juncea. J Agric Food Chem. 2002;50:5884–90. doi: 10.1021/jf020496z. [DOI] [PubMed] [Google Scholar]
- 23.Hudson EA, Dinh PA, Kokubun T, Simmonds MS, Gescher A. Characterization of potentially chemopreventive phenols in extracts of brown rice that inhibit the growth of human breast and colon cancer cells. Cancer Epidemiol Biomarkers Prev. 2000;9:1163–70. [PubMed] [Google Scholar]
- 24.Kikuzaki H, Hisamoto M, Hirose K, Akiyama K, Taniguchi H. Antioxidant properties of ferulic acid and its related compounds. J Agric Food Chem. 2002;50:2161–8. doi: 10.1021/jf011348w. [DOI] [PubMed] [Google Scholar]
- 25.Guan Y, Breyer MD. Peroxisome proliferator-activated receptors (PPARs): novel therapeutic targets in renal disease. Kidney Int. 2001;60:14–30. doi: 10.1046/j.1523-1755.2001.00766.x. [DOI] [PubMed] [Google Scholar]
- 26.Vitale SG, Lagana AS, Nigro A, La Rosa VL, Rossetti P, Rapisarda AM, et al. Peroxisome proliferator-activated receptor modulation during metabolic diseases and cancers: master and minions. PPAR Res. 2016;2016:1–9. doi: 10.1155/2016/6517313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou Y, Kong X, Zhao P, Yang H, Chen L, Miao J, et al. Peroxisome proliferator-activated receptor-α is renoprotective in doxorubicin-induced glomerular injury. Kidney Int. 2011;79:1302–11. doi: 10.1038/ki.2011.17. [DOI] [PubMed] [Google Scholar]
- 28.Tovar-Palacio C, Torres N, Diaz-Villaseñor A, Tovar AR. The role of nuclear receptors in the kidney in obesity and metabolic syndrome. Genes Nutr. 2012;7:483–98. doi: 10.1007/s12263-012-0295-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kiss-Tóth E, Roszer T. PPARgamma in kidney physiology and pathophysiology. PPAR Res. 2008;2008:183108. doi: 10.1155/2008/183108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ibrahim MA, El-Sheikh AA, Khalaf HM, Abdelrahman AM. Protective effect of peroxisome proliferator activator receptor (PPAR)-α and -γ ligands against methotrexate-induced nephrotoxicity. Immunopharmacol Immunotoxicol. 2014;36:130–7. doi: 10.3109/08923973.2014.884135. [DOI] [PubMed] [Google Scholar]
- 31.Jesse CR, Bortolatto CF, Wilhelm EA, Roman SS, Prigol M, Nogueira CW. The peroxisome proliferator-activated receptor-γ agonist pioglitazone protects against cisplatin-induced renal damage in mice. J Appl Toxicol. 2014;34:25–32. doi: 10.1002/jat.2818. [DOI] [PubMed] [Google Scholar]
- 32.Sivarajah A, Chatterjee PK, Patel NS, Todorovic Z, Hattori Y, Brown PA, et al. Agonists of peroxisome-proliferator activated receptor-gamma reduce renal ischemia/reperfusion injury. Am J Nephrol. 2003;23:267–76. doi: 10.1159/000072088. [DOI] [PubMed] [Google Scholar]
- 33.El-Ashmawy NE, Khedr NF, El-Bahrawy HA, Helal SA. Upregulation of PPAR-γ mediates the renoprotective effect of omega-3 PUFA and ferulic acid in gentamicin-intoxicated rats. Biomed Pharmacother. 2018;99:504–10. doi: 10.1016/j.biopha.2018.01.036. [DOI] [PubMed] [Google Scholar]
- 34.Nematbakhsh M, Pezeshki Z, Eshraghi Jazi F, Mazaheri B, Moeini M, Safari T, et al. Cisplatin-induced nephrotoxicity; protective supplements and gender differences. Asian Pac J Cancer Prev. 2017;18:295–314. doi: 10.22034/APJCP.2017.18.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sehajpal J, Kaur T, Bhatti R, Singh AP. Role of progesterone in melatonin-mediated protection against acute kidney injury. J Surg Res. 2014;191:441–7. doi: 10.1016/j.jss.2014.04.025. [DOI] [PubMed] [Google Scholar]
- 36.Kapil A, Singh JP, Kaur T, Singh B, Singh AP. Involvement of peroxisome proliferator-activated receptor gamma in vitamin D-mediated protection against acute kidney injury in rats. J Surg Res. 2013;185:774–83. doi: 10.1016/j.jss.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 37.Wang HD, Pagano PJ, Du Y, Cayatte AJ, Quinn MT, Brecher P, et al. Superoxide anion from the adventitia of the rat thoracic aorta inactivates nitric oxide. Circ Res. 1998;82:810–8. doi: 10.1161/01.res.82.7.810. [DOI] [PubMed] [Google Scholar]
- 38.Singh R, Singh AP, Singh M, Krishan P. Impact of obesity on hypertension-induced cardiac remodeling: role of oxidative stress and its modulation by gemfibrozil treatment in rats. Free Radic Biol Med. 2011;50:363–70. doi: 10.1016/j.freeradbiomed.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 39.Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78:206–9. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- 40.Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984;87:1344–50. [PubMed] [Google Scholar]
- 41.Niehaus WG, Jr, Samuelsson B. Formation of malonaldehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur J Biochem. 1968;6:126–30. doi: 10.1111/j.1432-1033.1968.tb00428.x. [DOI] [PubMed] [Google Scholar]
- 42.Bergman I, Roy L. Two improved and simplified methods for the spectrophotometric determination of hydroxyproline. Anal. Chem. 1963;35:1961–5. [Google Scholar]
- 43.Kuhad A, Pilkhwal S, Sharma S, Tirkey N, Chopra K. Effect of curcumin on inflammation and oxidative stress in cisplatin-induced experimental nephrotoxicity. J Agric Food Chem. 2007;55:10150–5. doi: 10.1021/jf0723965. [DOI] [PubMed] [Google Scholar]
- 44.More AN, Shah TK, Parab PB, Apte KG. Oroxylum indicum (Linn.) whole stem extract regulates expression of TNFa, IL6, NFkB, P38 MAPK and oxidative status in antitubercular therapy induced hepatotoxicity in Wistar rats. Matters. 2017;3:e201704000014. [Google Scholar]
- 45.Vickers AE, Rose K, Fisher R, Saulnier M, Sahota P, Bentley P. Kidney slices of human and rat to characterize cisplatin-induced injury on cellular pathways and morphology. Toxicol Pathol. 2004;32:577–90. doi: 10.1080/01926230490508821. [DOI] [PubMed] [Google Scholar]
- 46.Abdel Moneim AE, Othman MS, Aref AM. Azadirachta indica attenuates cisplatin-induced nephrotoxicity and oxidative stress. Biomed Res Int. 2014;2014:647131. doi: 10.1155/2014/647131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chirino YI, Pedraza-Chaverri J. Role of oxidative and nitrosative stress in cisplatin-induced nephrotoxicity. Exp Toxicol Pathol. 2009;61:223–42. doi: 10.1016/j.etp.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 48.Pan H, Mukhopadhyay P, Rajesh M, Patel V, Mukhopadhyay B, Gao B, et al. Cannabidiol attenuates cisplatin-induced nephrotoxicity by decreasing oxidative/nitrosative stress, inflammation, and cell death. J Pharmacol Exp Ther. 2009;328:708–14. doi: 10.1124/jpet.108.147181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Michel HE, Menze ET. Tetramethylpyrazine guards against cisplatin-induced nephrotoxicity in rats through inhibiting HMGB1/TLR4/NF-κb and activating nrf2 and PPAR-γ signaling pathways. Eur J Pharmacol. 2019;857:172422. doi: 10.1016/j.ejphar.2019.172422. [DOI] [PubMed] [Google Scholar]
- 50.Niciforovic N, Abramovic H. Sinapic acid and its derivatives: natural sources and bioactivity. Compr Rev Food Sci Food Saf. 2014;13:34–51. doi: 10.1111/1541-4337.12041. [DOI] [PubMed] [Google Scholar]
- 51.Chen C. Sinapic acid and its derivatives as medicine in oxidative stress-induced diseases and aging. Oxid Med Cell Longev. 2016;2016:3571614. doi: 10.1155/2016/3571614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kamel KM, Abd El-Raouf OM, Metwally SA, Abd El-Latif HA, El-sayed ME. Hesperidin and rutin, antioxidant citrus flavonoids, attenuate cisplatin-induced nephrotoxicity in rats. J Biochem Mol Toxicol. 2014;28:312–9. doi: 10.1002/jbt.21567. [DOI] [PubMed] [Google Scholar]
- 53.Mazaheri S, Nematbakhsh M, Bahadorani M, Pezeshki Z, Talebi A, Ghannadi AR, et al. Effects of fennel essential oil on cisplatin-induced nephrotoxicity in ovariectomized rats. Toxicol Int. 2013;20:138–45. doi: 10.4103/0971-6580.117256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang J, Stanley RA, Adaim A, Melton LD, Skinner MA. Free radical scavenging and cytoprotective activities of phenolic antioxidants. Mol Nutr Food Res. 2006;50:996–1005. doi: 10.1002/mnfr.200600072. [DOI] [PubMed] [Google Scholar]
- 55.Kilic U, Kilic E, Tuzcu Z, Tuzcu M, Ozercan IH, Yilmaz O, et al. Melatonin suppresses cisplatin-induced nephrotoxicity via activation of nrf-2/HO-1 pathway. Nutr Metab (Lond) 2013;10:7. doi: 10.1186/1743-7075-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ramesh G, Reeves WB. Salicylate reduces cisplatin nephrotoxicity by inhibition of tumor necrosis factor-alpha. Kidney Int. 2004;65:490–9. doi: 10.1111/j.1523-1755.2004.00413.x. [DOI] [PubMed] [Google Scholar]
- 57.Faubel S, Lewis EC, Reznikov L, Ljubanovic D, Hoke TS, Somerset H, et al. Cisplatin-induced acute renal failure is associated with an increase in the cytokines interleukin (IL)-1beta, IL-18, IL-6, and neutrophil infiltration in the kidney. J Pharmacol Exp Ther. 2007;322:8–15. doi: 10.1124/jpet.107.119792. [DOI] [PubMed] [Google Scholar]
- 58.Ramesh G, Reeves WB. TNF-alpha mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J Clin Invest. 2002;110:835–42. doi: 10.1172/JCI15606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kumar P, Sulakhiya K, Barua CC, Mundhe N. TNF-α, IL-6 and IL-10 expressions, responsible for disparity in action of curcumin against cisplatin-induced nephrotoxicity in rats. Mol Cell Biochem. 2017;431:113–22. doi: 10.1007/s11010-017-2981-5. [DOI] [PubMed] [Google Scholar]
- 60.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11:762–74. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ramseyer VD, Garvin JL. Tumor necrosis factor-α: regulation of renal function and blood pressure. Am J Physiol Renal Physiol. 2013;304:F1231–42. doi: 10.1152/ajprenal.00557.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ansari MA. Sinapic acid modulates nrf2/HO-1 signaling pathway in cisplatin-induced nephrotoxicity in rats. Biomed Pharmacother. 2017;93:646–53. doi: 10.1016/j.biopha.2017.06.085. [DOI] [PubMed] [Google Scholar]
- 63.Zeng X, Zheng J, Fu C, Su H, Sun X, Zhang X, et al. A newly synthesized sinapic acid derivative inhibits endothelial activation in vitro and in vivo. Mol Pharmacol. 2013;83:1099–108. doi: 10.1124/mol.112.084368. [DOI] [PubMed] [Google Scholar]
- 64.Singh JP, Singh AP, Bhatti R. Explicit role of peroxisome proliferator-activated receptor gamma in gallic acid-mediated protection against ischemia-reperfusion-induced acute kidney injury in rats. J Surg Res. 2014;187:631–9. doi: 10.1016/j.jss.2013.11.1088. [DOI] [PubMed] [Google Scholar]
- 65.Singh AP, Singh AJ, Singh N. Pharmacological investigations of Punica granatum in glycerol-induced acute renal failure in rats. Indian J Pharmacol. 2011;43:551–6. doi: 10.4103/0253-7613.84971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Helmy MM, Helmy MW, El-Mas MM. Additive renoprotection by pioglitazone and fenofibrate against inflammatory, oxidative and apoptotic manifestations of cisplatin nephrotoxicity: modulation by PPARs. PLoS One. 2015;10:e0142303. doi: 10.1371/journal.pone.0142303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hwang YS, Park SH, Kang M, Oh SW, Jung K, Park YS, et al. Stemness and differentiation potential-recovery effects of sinapic acid against ultraviolet-A-induced damage through the regulation of p38 MAPK and NF-κb. Sci Rep. 2017;7:909. doi: 10.1038/s41598-017-01089-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meng Q, Wong YT, Chen J, Ruan R. Age-related changes in mitochondrial function and antioxidative enzyme activity in fischer 344 rats. Mech Ageing Dev. 2007;128:286–92. doi: 10.1016/j.mad.2006.12.008. [DOI] [PubMed] [Google Scholar]