Abstract
Objective
Telomeres are protective sequences of DNA capping the ends of chromosomes that shorten over time. Leukocyte telomere length (LTL) is posited to reflect the replicative history of cells and general systemic aging of the organism. Chronic stress exposure leads to accelerated LTL shortening, which has been linked to increased susceptibility to and faster progression of aging-related diseases. This study examined longitudinal associations between LTL and experiences of racial discrimination, a qualitatively unique source of minority psychosocial stress, among African Americans.
Method
Data are from 391 African Americans in the Coronary Artery Risk Development in Young Adults (CARDIA) Telomere Ancillary Study. We examined the number of domains in which racial discrimination was experienced in relation to LTL collected in Years 15 and 25 (Y15: 2000/2001; Y25: 2010/2011). Multivariable linear regression examined if racial discrimination was associated with LTL. Latent change score analysis (LCS) examined changes in racial discrimination and LTL in relation to one another.
Results
Controlling for racial discrimination at Y15, multivariable linear regression analyses indicated that racial discrimination at Y25 was significantly associated with LTL at Y25. This relationship remained robust after adjusting for LTL at Y15 (b = −.019, p = .015). Consistent with this finding, LCS revealed that increases in experiences of racial discrimination were associated with faster 10-year LTL shortening (b = −.019, p = .015).
Conclusions
This study adds to evidence that racial discrimination contributes to accelerated physiologic weathering and health declines among African Americans through its impact on biological systems, including via its effects on telomere attrition.
Keywords: African Americans, racial discrimination, leukocyte telomere length
Chronic psychosocial stress contributes to physiologic weathering and premature declines in health due to the burden placed on biological systems, as indexed by health indicators including several biomarkers that reflect elevated disease risk (Geronimus, Hicken, Keene, & Bound, 2006; Harris & Schorpp, 2018; McEwen & Stellar, 1993; McEwen, 1998). One such measure that has been posited to reflect this process is telomere length. Telomeres are repetitive sequences of DNA that cap the ends of chromosomes, and have an important role in supporting chromosomal stability (Buxton et al., 2014; Mason, Schuller, & Skordalakes, 2011; Riethman, 2008). Telomere attrition occurs during cell replication, resulting in shorter length on average over time and an inverse association with chronological age (Aviv, Valdes, & Spector, 2006; Müezzinler, Zaineddin, & Brenner, 2013). Studies suggest that both psychosocial and physiologic stressors can accelerate telomere shortening (Cherkas et al., 2006; Epel et al., 2004, 2006; Simon et al., 2006). Accordingly, telomere length has been posited to be a biological marker of replicative history and a cumulative indicator of “wear and tear” at the cellular level (Geronimus et al., 2010; Monaghan, 2010). Leukocyte telomere length (LTL) in particular has emerged as a marker of immune system aging as well as general systemic aging of the organism, and has been associated with several aging-related health outcomes, including cardiovascular diseases, metabolic syndrome, osteoporosis and osteoarthritis, cognitive decline, and mortality (Blackburn, Epel, & Lin, 2015).
Among African Americans, racism is a psychosocial stressor that contributes to well-documented disparities in health, including the incidence and severity of multiple adverse disease outcomes, which may be driven by accelerated biological aging (Chae et al., 2014; Chae, Nuru-Jeter, Lincoln, & Francis, 2011). Compared to other facets of racism, racial discrimination has been most commonly studied in relation to risk factors for poor health. Although there is some variation in how scholars define discrimination, it is often conceptualized as the direct interpersonal experience of unfair treatment because of membership in a particular social group (Krieger, 2000). Racial discrimination specifically has been studied in relation to biological precursors of clinical disease outcomes (Berger & Sarnyai, 2015; Paradies et al., 2015). Studies have found associations between racial discrimination and glucocorticoids, proinflammatory cytokines, and other markers of inflammation (Williams, 2018). One study found that everyday discrimination was associated with C-reactive protein prospectively over a 7-year period in a racially diverse sample of women, particularly among those who were nonobese (Beatty Moody, Matthews, Bromberger, & Brown, 2014). Additionally, African American adolescents reporting consistently high levels of racial discrimination over time were found to have greater allostatic load, a composite measure of multisystem dysregulation (Brody et al., 2014). Another study found significant effects of discrimination on trajectories of allostatic load among women; higher allostatic load among African American women was partially explained by greater discrimination (Upchurch et al., 2015). Recent research has found that racial discrimination may also be associated with patterns of DNA methylation indicative of epigenetic aging (Brody, Miller, Yu, Beach, & Chen, 2016). The presence of biological stress mediators, particularly those related to inflammation and oxidative stress, may lead to telomere shortening. Cellular senescence and apoptosis associated with critically short telomere length also induces an inflammatory response (Monaghan, 2010; O’Donovan et al., 2011). Accordingly, a compelling pathway through which racial discrimination may become biologically embedded is through its effects on the telomere maintenance system (Chae et al., 2014, 2011; Geronimus et al., 2010; Liu & Kawachi, 2017).
Factors tied to racism contribute to racial disparities in the onset and progression of aging-related diseases via chronic activation of the physiologic stress response over time (Chae et al., 2011; Williams & Mohammed, 2013). LTL among African Americans may in part reflect the accumulation of racism-related stress across the life course. Data from the Health and Retirement Study indicate that reporting high levels of discrimination is associated with shorter salivary telomere length specifically among African Americans but not Whites (Lee, Kim, & Neblett, 2017; Liu & Kawachi, 2017). Another study of African American midlife men reported an interactive effect between racial discrimination and implicit in-group racial bias, with those reporting high levels of racial discrimination and holding an anti-Black bias having the shortest LTL, although there was no main effect of racial discrimination (Chae et al., 2014). These and other cross-sectional studies provide suggestive evidence that racial discrimination may have detrimental consequences for telomere shortening (Beatty Moody et al., 2019; Pantesco et al., 2018).
Some studies on race and telomere length have found no racial differences in telomere length or in fact longer telomeres among African Americans compared to Whites, likely due to pressures stemming from selection bias over time (Hamad, Tuljapurkar, & Rehkopf, 2016). However, other studies have suggested that African Americans undergo a faster rate of telomere shortening compared to Whites (Brown, Needham, & Ailshire, 2017; Lynch et al., 2016). A cross-sectional study found a significant interaction between race and chronological age in predicting LTL, with a steeper inverse association between chronological age and LTL among African Americans (Hunt et al., 2008). Other prospective cohort research has supported the observation that African Americans may have longer telomere length at birth but experience more rapid telomere shortening compared to Whites (Rewak et al., 2014). Collectively, this previous research suggests that identifying factors associated with telomere attrition may be particularly important for understanding biological processes underlying increasing disease vulnerability among African Americans. Longitudinal research examining changes in LTL may be particularly meaningful in elucidating the causes of worsening physiologic deterioration among African Americans and mechanisms involved in the production of racial disparities in health. The rate of LTL attrition over time may in part be informed by the embodiment of psychosocial stress. The current study is the first to our knowledge to explicitly examine changing experiences of racial discrimination in relation to LTL shortening among African Americans over time.
Method
Study Design and Sample
Data from this study are from the Coronary Artery Risk Development in Young Adults (CARDIA) Study, a prospective cohort study of African American and White adults. The study design and procedures of the CARDIA Study have been previously described in detail (Friedman et al., 1988; Hughes et al., 1987). Baseline data were first collected in 1985/1986. Briefly, participants were recruited from four metropolitan areas (Birmingham, AL; Chicago, IL; Minneapolis, MN; and Oakland, CA) using stratified random sampling procedures to obtain equal numbers of participants by race, gender, age subgroup (18–24 and 25–30 years of age), and education (less than and more than 12 years of education). The CARDIA Telomere Ancillary Study assayed banked blood specimens in a subsample who participated in Year 15 (Y15; 2000/2001) to Year 25 (Y25; 2010/2011). The current study is restricted to African American participants (n = 410).
Measures
Leukocyte telomere length
We examined LTL measured in Y15 and Y25. At each wave, the T/S ratio (number of repeats of a specific telomere sequence to a reference single-copy gene as compared with a reference DNA sample) was obtained using a quantitative polymerase chain reaction assay (Cawthon, 2002; Lin et al., 2010). In the current study, T/S ratios were converted to kilobase pairs (kbp) using the formula (3274 + (2413 × T/S)/1000; Cawthon, 2002).
Racial discrimination
Racial discrimination was assessed in Y15 and Y25 using an earlier seven-item situation version of the Experiences of Discrimination (EOD) measure (Krieger, Smith, Naishadham, Hartman, & Barbeau, 2005). The EOD is a widely used and validated measure of racial discrimination (Krieger et al., 2005; Paradies et al., 2015). The seven-item version used in CARDIA included an item assessing racial discrimination “at home,” which is not part of the current EOD and was not included in the current analyses. The remaining six-item set asked participants whether they had “ever experienced discrimination, been prevented from doing something, or been hassled or made to feel inferior” due to their “race or color” in the following contexts: (a) at school; (b) getting a job; (c) getting housing; (d) at work; (e) getting medical care; and (f) on the street or in a public setting. Three items in the EOD that were not included in the CARDIA version assessed racial discrimination in “getting service in a store or restaurant,” “getting credit, bank loans, or a mortgage,” and “from the police or in the courts.” Consistent with other studies that have examined the EOD, in CARDIA as well as in other data sets, we examined the sum of the number of situations in which racial discrimination was experienced (Borrell et al., 2007; Chae et al., 2014; Krieger et al., 2005), which ranged from 0 to 6.
Sociodemographic variables
Demographic characteristics included gender reported at baseline (women = 1 vs. men = 0); and measured at Y15, age in years and relationship status as a categorical variable: married (referent), never married, and divorced, separated, or widowed. Socioeconomic variables measured at Y15 were education (high school or less = 0 vs. more than high school = 1), and total combined family income (1 = less than $5,000, 9 = $100,000 and greater). Health variables at Y15 were smoking status, coded as never (referent), former, and current; and body mass index (kg/m2) calculated continuously based on height and weight measured by trained study staff.
Statistical Analysis
We examined cross-sectional relationships between racial discrimination and LTL at both Y15 and Y25 using multivariable linear regression. Cross-wave models examining LTL at Y25 included racial discrimination at both Y15 and Y25, and LTL at Y15 as predictors.
We utilized latent change score (LCS) analysis to explicitly model the change in LTL by change in racial discrimination. This approach confers several advantages: LCS allows us to index within-person changes in exposure to racial discrimination and to examine this in relation to their change in LTL; it does not conflate change scores with main effects; and also takes into account characteristics associated with change scores (de Haan, Prinzie, Sentse, & Jongerling, 2018). We specifically examined the relationship between the change in LTL and change in racial discrimination between Y15 (t = 0) and Y25 (t = 1).
The observed racial discrimination score of a participant, n, at each time point, t, is expressed by X[t, n], which is determined by the true score (latent score), x[t, n] and measurement error, e[t, n]. The latent change in racial discrimination, Δxn, is the difference in the latent racial discrimination scores between the two time points, x[1, n] − x[0, n]. Based on this equation, the latent racial discrimination score of a participant at Y25 is:
Similarly, the latent LTL of a participant at Y25 is:
The latent change in racial discrimination was indexed by racial discrimination at Y15, demographic, socioeconomic, and health characteristics, and the number of years elapsed between waves. The latent change in LTL was indexed by the latent change in racial discrimination, adjusting for LTL and racial discrimination at Y15, and demographic, socioeconomic, and health characteristics, as well as the number of years elapsed between waves.
Ten participants were excluded because they did not have valid LTL in either Y15 or Y25; nine additional participants with outlying LTL values (greater than three standard deviations from the mean) were not included in analyses, resulting in a total analytic sample size of n = 391. This sample size has been shown to be sufficient to detect meaningful effect sizes associated with racial discrimination in multivariable linear regression analyses of LTL (Chae et al., 2014, 2016). The current sample size also meets the minimum recommendation of 200 for structural equation modeling (Kline, 2011).
All analyses were conducted using Mplus 7.4 (Muthén & Muthén, 1998–2015). Continuous covariates were centered (age, income, BMI). All covariates were declared as endogenous variables by estimating their variances in the MODEL command (Muthén, 2009). Missing data was handled using full information maximum likelihood (FIML), a preferred method that uses all available information in the data to generate estimations (Graham, 2009).
Ethical Approval
All study protocols and procedures for the CARDIA Study were approved by the institutional review boards of each field center (Birmingham, AL: University of Alabama, Birmingham, School of Medicine; Chicago, IL: Northwestern University, Feinberg School of Medicine; Minneapolis, MN: University of Minnesota, School of Public Health; and Oakland, CA: Kaiser Permanente, Division of Research). All participants provided signed informed consent. The CARDIA Telomere Ancillary Study was approved by the University of California, San Francisco Committee on Human Research.
Results
Participant characteristics are shown in Table 1. The mean age of participants at Y15 was 39.74 years (SD = 3.86). The sample was predominantly women (74.4%) and working (86.7%); had approximately equal numbers of those with a high school degree or less (47.8%) versus more than a high school degree (44.2%); and those with annual household income less than $50,000 (54.4%) versus $50,000 or more (45.6%). LTL shortened on average from 5.62 kbp (SD = 0.45) to 4.98 kbp (SD = 0.27; approximately 64 bp/year) over the 10-year period between Y15 and Y25. The change in LTL ranged from −1.92 kbp to 0.21 kbp. There were eight participants (2%) for whom LTL values increased, and one participant whose value did not change.
Table 1.
Characteristics of African Americans in the Coronary Artery Risk Development in Young Adults (CARDIA) Telomere Ancillary Study, Year 15 (2000/2001) and Year 25 (2010/2011)
| Characteristic | n (%) or M (SD) | N | % missing |
|---|---|---|---|
| Telomere length, kbp, Y15, M (SD) | 5.62 (.45) | 391 | 0% |
| Telomere length, kbp, Y25, M (SD) | 4.98 (.27) | 391 | 0% |
| Racial discrimination, Y15, M (SD) | 2.28 (1.88) | 391 | 0% |
| Racial discrimination, Y25, M (SD) | 1.79 (1.87) | 391 | 0% |
| Age, M (SD) | 39.74 (3.86) | 391 | 0% |
| Years elapsed Y15 to Y25, M (SD) | 9.93 (.32) | 391 | 0% |
| Gender, n (%) | 391 | 0% | |
| Women | 291 (74.4) | ||
| Men | 100 (25.6) | ||
| Education, n (%) | 360 | 8% | |
| High school or less | 187 (47.8) | ||
| More than high school | 173 (44.2) | ||
| Household income, n (%) | 384 | 2% | |
| <$25,000 | 91 (23.7) | ||
| $25,000–$49,999 | 118 (30.7) | ||
| $50,000+ | 92 (24.0) | ||
| $75,000+ | 83 (21.6) | ||
| Work status, n (%) | 389 | 1% | |
| Working | 339 (86.7) | ||
| Not working | 50 (12.8) | ||
| Marital status, n (%) | 387 | 1% | |
| Married | 176 (45.5) | ||
| Divorced, separated, widowed | 102 (26.4) | ||
| Never married | 109 (28.2) | ||
| Smoking, n (%) | 390 | 0% | |
| Never | 250 (64.1) | ||
| Former | 45 (11.5) | ||
| Current | 95 (24.4) | ||
| Body Mass Index,a M (SD) | 30.49 (6.59) | 390 | 0% |
Note. N = 391. Education, household income, employment, marital status, smoking, and body mass index measured at Y15. Sum of categories may not total 391 due to missing data. kbp = kilobase pairs; M = mean; SD = standard deviation; Y15 = Year 15; Y25 = Year 25.
Body mass index calculated as (weight in kilograms)/(height in meters)2.
Racial discrimination was most frequently reported “on the street or in a public setting,” followed by “at work” and “getting a job” in both Y15 (59.1, 50.8, and 44.0%, respectively) and in Y25 (46.5, 40.5, and 37.3%, respectively). Reports of racial discrimination decreased overall, from a mean of 2.28 (SD = 1.88) in Y15 to 1.79 (SD = 1.87) in Y25. However, 22.0% of participants reported greater experiences of racial discrimination in Y25 than in Y15, compared to 35.8% who reported no change, and 42.2% who reported less racial discrimination. Additional sample characteristics are shown in Table 1.
Correlations between study variables are shown in Table 2, which were derived in Mplus using FIML to generate estimates using all available information in the entire sample. No significant bivariate relationships were found between racial discrimination measured at Y15/Y25 and LTL at either time point.
Table 2.
Correlations Between Measures of Participant Characteristics Among African Americans in the Coronary Artery Risk Development in Young Adults (CARDIA) Telomere Ancillary Study, Year 15 (2000/2001) and Year 25 (2010/2011)
| Variable | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Telomere Length, kbp, Y15 | .552*** | .054 | .030 | −.083 | .042 | .030 | .135** | .016 | −.021 | .008 | −.098* | −.010 |
| 2. Telomere Length, kbp, Y25 | .048 | −.063 | −.024 | −.007 | .074 | .030 | −.026 | −.049 | .017 | −.059 | −.047 | |
| 3. Racial Discrimination, Y15 | .594*** | .100* | .001 | −.034 | .185** | .159** | −.008 | .080 | −.011 | −.026 | ||
| 4. Racial Discrimination, Y25 | .099* | −.048 | .002 | .175** | .172** | .088 | .033 | −.037 | .015 | |||
| 5. Age | .035 | .068 | .074 | .010 | −.052 | −.024 | .094 | −.002 | ||||
| 6. Years elapsed Y15 to Y25 | −.149** | .031 | .115* | −.032 | .017 | −.070 | −.060 | |||||
| 7. Women (1)/Men (0) | .139** | −.148** | −.029 | −.068 | −.036 | .152** | ||||||
| 8. >(1) vs. ≤High School (0) | .225*** | .103* | .093 | −.127* | −.064 | |||||||
| 9. Household Income | .251*** | .470*** | −.204*** | −.094 | ||||||||
| 10. Working (1)/Not Working (0) | .041 | −.121* | .042 | |||||||||
| 11. Married (1)/Not Married (0)a | −.071 | .070 | ||||||||||
| 12. Smoker (1)/Non-Smoker (0)b | −.124* | |||||||||||
| 13. Body Mass Indexc | ||||||||||||
Note. N = 391. Age, education, household income, work status, marital status, smoking, and body mass index measured at Year 15. kbp = kilobase pairs; Y15 = Year 15; Y25 = Year 25.
Not married included those never married and divorced, separated, or widowed.
Non-smokers included never smokers and former smokers.
Body mass index calculated as (weight in kilograms)/(height in meters)2.
p < .05.
p < .01.
p < .001.
We did not find evidence of a significant cross-sectional relationship between Y15 racial discrimination and Y15 LTL (b = .009, 95% confidence interval (CI) [−.017, .034], p = .504). Results from multivariable linear regression analyses examining Y25 LTL are presented in Table 3. When examined in separate models, neither racial discrimination in Y15 (Model 1: b = .006, 95% CI [−.017, .034], p = .392) nor in Y25 (Model 2: b = −.009, 95% CI [−.024, .005], p = .208) were significantly associated with LTL in Y25. However, when including both in the model, Y25 racial discrimination was significantly associated with Y25 LTL (Model 3: b = −.019, 95% CI [−.038, −.001], p = .040), with greater reports of racial discrimination at Y25 being associated with shorter LTL. Further adjusting for LTL at Y15, the magnitude of this association remained unchanged (Model 4: b = −.019, 95% CI [−.035, −.004], p = .015).
Table 3.
Multivariable Linear Regression Examining Leukocyte Telomere Length at Year 25 (2010/2011) Among African Americans in the Coronary Artery Risk Development in Young Adults (CARDIA) Telomere Ancillary Study
| Model 1: LTL, Year 15 |
Model 2: LTL, Year 25 |
Model 3: LTL, Year 25 |
Model 4: LTL, Year 25 |
|||||
|---|---|---|---|---|---|---|---|---|
| Variable | b | 95% CI | b | 95% CI | b | 95% CI | b | 95% CI |
| Racial discrimination, Y15 | .006 | [−.017, .034] | .017 | [−.001, .035] | .015 | [>.000, .029] | ||
| Racial discrimination, Y25 | −.009 | [−.024, .005] | −.019 | [−.038, −.001]* | −.019 | [−.035, −.004]* | ||
| Telomere length, Y15 | .326 | [.280, .373]*** | ||||||
| Age | −.003 | [−.010, .005] | −.002 | [−.009, .005] | −.002 | [−.009, .005] | .001 | [−.004, .007] |
| Years elapsed Y15 to Y25 | .005 | [−.077, .087] | .001 | [−.082, .084] | −.001 | [−.084, .082] | −.021 | [−.091, .048] |
| Women vs. Men | .047 | [−.018, .112] | .044 | [−.021, .109] | .048 | [−.016, .112] | .042 | [−.010, .095] |
| > High school vs. ≤ high school | .008 | [−.052, .068] | .018 | [−.043, .078] | .011 | [−.049, .071] | −.028 | [−.079, .022] |
| Household income | −.006 | [−.023, .011] | −.004 | [−.021, .013] | −.005 | [−.022, .012] | −.003 | [−.018, .012] |
| Not working vs. working | −.031 | [−.102, .039] | −.030 | [−.101, .040] | −.024 | [−.094, .047] | −.007 | [−.068, .053] |
| Relationship status (ref: Married) | ||||||||
| Divorced, separated, widowed | −.016 | [−.086, .055] | −.011 | [−.081, .060] | −.012 | [−.083, .058] | −.021 | [−.084, .042] |
| Never married | −.031 | [−.102, .041] | −.032 | [−.103, .039] | −.026 | [−.097, .045] | −.017 | [−.077, .043] |
| Body Mass Indexa | −.003 | [−.007, .001] | −.003 | [−.007, .001] | −.003 | [−.007, .002] | −.002 | [−.006, .001] |
| Former vs. never smoker | .060 | [−.023, .143] | .067 | [−.016, .151] | .061 | [−.023, .145] | .054 | [−.011, .120] |
| Current vs. never smoker | −.034 | [−.098, .030] | −.032 | [−.096, .032] | −.034 | [−.098, .030] | −.008 | [−.065, .048] |
Note. N = 391. Age, education, household income, work status, marital status, smoking, and body mass index measured at Year 15. CI = confidence interval; Y15 = Year 15; Y25 = Year 25.
Body mass index calculated as (weight in kilograms)/(height in meters)2.
p < .05.
p < .001.
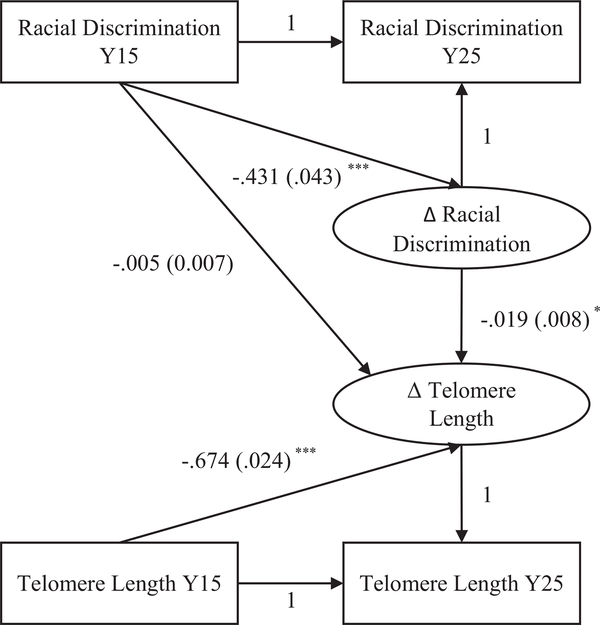
Latent Change Score Analyses
Results from LCS analyses predicting the latent change in LTL are illustrated in Figure 1. Model fit was acceptable: CFI = .915, RMSEA = .053, SRMR = .038 (cut-off values for satisfactory model fit are: CFI > .90, RMSEA < .08, SRMR < .08; Kline, 2011). Results indicated that greater racial discrimination at Y15 was associated with less increases in reports of discrimination from Y15 to Y25 (b = −.431, 95% CI [−.515, −.347], p < .001). Those with longer LTL at Y15 had more telomere shortening (b = −.674, 95% CI [−.721, −.627], p < .001). Racial discrimination at Y15 was not significantly associated with the change in telomere length (b = −.005, 95% CI [−.019, .009], p = .482). However, consistent with linear regression findings, there was a significant association between the latent change in racial discrimination and the latent change in LTL (b = −.019, 95% CI [−.035, −.004], p = .015). Experiencing racial discrimination in more domains was associated with more LTL shortening.
Figure 1.
Structural equation model of latent change in discrimination and telomere length among African Americans in the Coronary Artery Risk Development in Yong Adults (CARDIA) Study (n = 391). * p < .05. ** p < .01. *** p < .001.
Sensitivity Analyses
Several post hoc sensitivity analyses were conducted. First, we examined the full sample of 410 participants without restriction to those with valid LTL data at both Y15 and Y25. The association between the latent change in racial discrimination and latent change in LTL remained the same (b = −.019). Supplementary analyses were conducted excluding 26 participants who reported cancer or HIV, two serious health conditions that can impact the interpretation of LTL values. Additional models were specified controlling for the presence of chronic disease at Y15 and Y25. Across these analyses, results remained unchanged. Additional analyses were conducted taking into account time-varying covariates, further adjusting for Y25 variables that showed a low correlation with Y15 values (r < .70): work status, being divorced, and quitting smoking. Including both Y15 and Y25 measures of these covariates did not result in substantively different conclusions; the association between the latent change in racial discrimination and the latent change in LTL remained the same.
Additional analyses using alternative coding for Y25 racial discrimination were also explored. At both Y15 and Y25, participants were asked if they had “ever” experienced racial discrimination in any of six domains; however, the overall mean reports of racial discrimination decreased between the two time periods, suggesting recall bias in retrospective reports. To address this concern, Y25 racial discrimination scores were recoded to reflect whether racial discrimination was assessed in a new domain that was not previously reported in Y15. Analyses examining incident racial discrimination reported in Y25 yielded substantively similar results. In LCS analyses, the relationship between the latent change in racial discrimination and the latent change in LTL in fact increased from b = −.019 (95% CI [−.035, −.004], p = .015) to b = −.027 (95% CI [−.054, −.001], p = .042).
We also examined the functional form of the association between racial discrimination change scores and changes in LTL by recoding racial discrimination into quartiles; we found that increasing discrimination categories showed successively greater LTL shortening. Furthermore, testing a quadratic effect of change in racial discrimination on the latent change in LTL showed nonsignificant results. These post hoc analyses suggested that modeling a linear association between changes in racial discrimination and LTL was appropriate.
Discussion
Previous cross-sectional research examining associations between discrimination and telomere length have been equivocal. Studies using data from the Health and Retirement Study have reported significant inverse associations between salivary telomere length and both major discrimination events and everyday discrimination (Lee et al., 2017; Liu & Kawachi, 2017). However, this research was conducted in an older sample of African Americans (Mean age = 70 years) using a measure of discrimination that did not distinguish between experiences that were racially motivated versus attributed to another factor. In general, among African Americans in midlife there is greater evidence for interactive rather than main effects of racial discrimination on telomere length. Similar to the CARDIA Study, the Healthy Aging in Neighborhoods of Diversity across the Life Span (HANDLS) Study recruited a midlife sample and administered the race version of the EOD. Studies using the HANDLS data have not reported main associations of racial discrimination, but rather significant interactions with gender and socioeconomic status in relation to telomere length (Beatty Moody et al., 2019; Pantesco et al., 2018). Other research using cross-sectional data from a sample of African American men in midlife detected significant interactions with psychosocial variables, but did not find evidence for significant main effects of racial discrimination in examining telomere length (Chae et al., 2014, 2016).
In accordance with these prior studies, we did not find evidence for cross-sectional associations between racial discrimination and LTL; however, we did find a significant relationship when examining a cross-wave model. Specifically, reporting experiences of racial discrimination in more domains was associated with shorter LTL when adjusting for earlier reports of racial discrimination; this relationship persisted after controlling for baseline LTL. These results were supported by LCS analyses that modeled the change in racial discrimination in relation to changes in LTL. Results indicated that each additional domain in which racial discrimination was experienced was associated with approximately 19 bp greater LTL shortening. This finding may be contextualized in light of a systematic review that found estimates of annual loss of approximately 20–30 bp in most large cross-sectional studies (Müezzinler et al., 2013).
This study is the first to our knowledge to examine the association between changes in LTL and racial discrimination longitudinally among African Americans. Our study advances scientific directions by explicitly modeling LTL shortening, which may be specifically relevant to changing experiences of racial discrimination in midlife. Findings from this study suggest that experiencing racial discrimination in greater domains has detrimental effects on the telomere maintenance system, which may contribute to racial disparities in health. Several studies have found that African Americans experience faster telomere shortening compared to Whites, and that any initial biological advantage of longer telomeres among African Americans are outweighed by social adversities across the life course (Hamad et al., 2016; Rewak et al., 2014). Our study suggests that experiencing racial discrimination in more settings is a source of social adversity that may result in biological tolls. Such experiences may be particularly pertinent to midlife. In Y15 of CARDIA, the mean age of participants was approximately 40 years old. Some types of experiences of racial discrimination may emerge over the subsequent 10-year period, such as in employment, housing, and medical care contexts, and may be more applicable to this age cohort compared to earlier developmental periods when racial discrimination in other domains (e.g., school) might be more salient. Accumulating experiences of racial discrimination in this midlife age range may result in stress-related cell aging.
Results from this study are concordant with other findings on the embodiment of minority stress and experiences of racial discrimination (Krieger, 2012). Racial discrimination reinforces unjust patterns in residential segregation and hampers socioeconomic mobility, subsequently increasing exposure to health-damaging neighborhood conditions and diminishing access to protective resources that can impact LTL (Massey et al., 2018; Needham et al., 2014; Park, Verhoeven, Cuijpers, Reynolds, & Penninx, 2015). Studies have consistently linked racial discrimination to mental health outcomes, such as depression and psychological distress, which have been linked to shortening telomeres (Berger & Sarnyai, 2015; Blackburn et al., 2015). Recent studies indicate that racial discrimination is also associated with a range of other health concerns, including sleep problems and cardiometabolic risk, which have also been associated with shorter LTL (Cribbet et al., 2014; Fuller-Rowell et al., 2017; Goosby, Straley, & Cheadle, 2017; Mazidi, Kengne, Sahebkar, & Banach, 2018). Racial discrimination may more directly compromise health by eliciting a cascade of biochemical responses associated with physiologic arousal (Chae et al., 2011; Clark, Anderson, Clark, & Williams, 1999; Korous, Causadias, & Casper, 2017). Repeated psychosocial insults have potential to cause dysregulation of biological systems involved in the stress response, resulting in a chronic heightened proinflammatory state associated with LTL shortening (Geronimus et al., 2010; Monaghan, 2010). Findings from this study contribute to increasing documentation of negative biological sequelae associated with psychosocial stress for African Americans.
Our findings advance the literature on racial discrimination and health outcomes in several ways. Research on the association between racial discrimination and LTL has heretofore been cross-sectional (Beatty Moody et al., 2019; Chae et al., 2014, 2016; Lee et al., 2017; Liu & Kawachi, 2017; Pantesco et al., 2018). The present study extends this line of research to a relatively large sample of African Americans in midlife, a developmental period characterized by diverging health trajectories, and when stressful life experiences may manifest in signs of biological dysregulation (House, Lantz, & Herd, 2005; Lachman, Teshale, & Agrigoroaei, 2015). Accordingly, identifying risk factors for LTL shortening during this time may yield insight into causes of racial disparities in health. We tested this association over a 10-year period in order to help deduce a temporal relationship between racial discrimination and LTL. The LCS approach allowed us to study the dynamic relationship between changes in these measures, which more robustly supports causal inferences in observational research.
Despite strengths related to the longitudinal design of this study, some caveats related to the unique characteristics of the CARDIA cohort should be noted. Because this was a largely urban sample from four specific metropolitan areas, caution should be taken in generalizing findings to other geographic regions. It is also important to note the uneven gender distribution, especially in light of prior research suggesting that African American women and men may differ in their appraisals of racial discrimination and physiologic responses to such experiences (Kershaw et al., 2016; Lewis & Van Dyke, 2018). Furthermore, findings from this study may be particular to African Americans in midlife. We assessed how experiencing racial discrimination in additional societal domains specifically during this developmental period is related to telomere shortening; however, future research that takes into account earlier experiences during childhood, adolescence, and young adulthood will be important to advancing a more comprehensive developmental perspective on biological consequences of racial discrimination during the life course (Brody et al., 2014, 2016).
Another limitation is related to the study design and analytic method we employed. Using two waves of data, we were able to correlate change in our primary exposure and outcome of interest, but were limited in examining trajectories or sequential changes in racial discrimination and LTL. As a result, inferences regarding the causal direction of the associations we found are more susceptible to alternative explanations. Although examining 10-year change in LTL in our study represents a significant advancement in research on this topic, using more data points will be an important forthcoming step. In addition, because of disagreement on methods to quantify biological aging, in the future it will be important to examine changes in racial discrimination in relation to changes in other genomic and physiologic processes (Belsky et al., 2018).
The CARDIA measure of racial discrimination is largely limited to specific domains, and does not assess more routine experiences, such as instances of being treated with less courtesy or respect in everyday interactions. It is calculated as the sum of the number of domains in which racial discrimination is experienced, and not necessarily an indicator of frequency, chronicity, intensity, or appraisals. However, the situation version of the EOD has been widely used in epidemiologic studies documenting the prevalence of racial discrimination, and has also been used to examine associations across a wide range of health-related outcomes (e.g., Borrell, Kiefe, Diez-Roux, Williams, & Gordon-Larsen, 2013; Cunningham et al., 2012, 2013; Mustillo et al., 2004; Sims et al., 2012; Subramanyam et al., 2012). Other studies have also used measures of racial discrimination, which like the EOD, assess whether participants had ever experienced discrimination in specific contexts using a yes–no response format (Borrell et al., 2010; Dominguez, Dunkel-Schetter, Glynn, Hobel, & Sandman, 2008; Harris et al., 2012; Kessler, Mickelson, & Williams, 1999; Williams et al., 2008), including a recent study that examined racial discrimination in relation to telomere length (Pantesco et al., 2018). In their validation survey, Krieger and colleagues (2005) also reported that the situation version of the EOD was highly correlated with the frequency version (r = .90), as well as strong associations with other commonly used measures of discrimination in a diverse cohort of working class adults.
Additionally, although the explicit focus on racial discrimination allows for more direct inferences about this specific form of unfair treatment, it may not capture those experiences that are not clearly attributable to race or which are motivationally ambiguous. Conceptually important moderators, including dimensions of racial identity that have been shown to influence self-reports of discrimination, were also not examined (Chae et al., 2017). Future studies may integrate a broader panel of measures related to racial discrimination in addition to assessments of other racism-related constructs. In addition, examining potential mediators, such as other forms of psychosocial stress (e.g., financial stress, general stress) and mental health channels, may help to further elucidate mechanisms linking racial discrimination and LTL.
Despite these limitations, results of this study contribute to evidence of the deleterious consequences of racial discrimination at the cellular level, and also point to racism as an important contributor to enduring racial disparities in health. Findings from our study also have relevance to understanding the impact that the current sociopolitical climate may have on the health of racial minority groups in the United States and the embodiment of racism at the population level (Krieger, Huynh, Li, Waterman, & Van Wye, 2018; Pew Research Center, 2019). Our study supports previous findings suggesting that LTL is sensitive to racial discrimination, and that it may be one pathway through which racism-related factors contribute to increased disease risk and accelerated declines in health among African Americans. The negative effect of racial discrimination on the telomere maintenance system may be a mechanism underlying racial disparities across multiple aging-related diseases. Results from this study suggest that addressing health inequities will require efforts to curtail endemic racial discrimination.
Acknowledgments
This article has been reviewed by CARDIA for scientific content and consistency of data interpretation with previous CARDIA publications. David H. Chae conceptualized this study and held primary responsibility for data interpretation and writing. Yijie Wang conducted the analyses. Connor D. Martz, Natalie Slopen, Tiffany Yip, Nancy E. Adler, Thomas E. Fuller-Rowell, Gene H. Brody, and Erica C. Spears assisted with review of the literature, writing, and the discussion of findings. Jue Lin, Karen A. Matthews, Eli Puterman, and Elissa S. Epel were involved in acquiring the data. All authors participated in editing and approving the manuscript. Jue Lin is a cofounder of Telomere Diagnostics, Inc., and serves on its scientific advisory board. The company plays no role in the current study. No other potential interest disclosures were reported by the authors of this paper. This work was supported by the National Institute on Aging (NIA) of the National Institutes of Health (NIH; K01AG041787 to David H. Chae). The Coronary Artery Risk Development in Young Adults (CARDIA) Study is supported by contracts HHSN268201300025C, HHSN268201300026C, HHSN268201300027C, HHSN268201300028C, HSN268201300029C, and HSN268200900041C from the National Heart, Lung, and Blood Institute (NHLBI), the Intramural Research Program of the NIA, and an intra-agency agreement between NIA and NHLBI (AG0005). The telomere ancillary study was funded by the MacArthur Foundation SES and Health Network. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NHLBI, or MacArthur Foundation.
Contributor Information
David H. Chae, Department of Human Development and Family Studies, Auburn University
Connor D. Martz, Department of Human Development and Family Studies, Auburn University.
Tiffany Yip, Department of Psychology, Fordham University.
Thomas E. Fuller-Rowell, Department of Human Development and Family Studies, Auburn University
Karen A. Matthews, Department of Psychology, University of Pittsburgh
Erica C. Spears, Department of Health Behavior and Health Systems, University of North Texas Health Science Center
Yijie Wang, Department of Human Development and Family Studies, Michigan State University.
Natalie Slopen, Department of Epidemiology and Biostatistics, University of Maryland.
Nancy E. Adler, Department of Psychiatry, University of California, San Francisco
Jue Lin, Department of Biochemistry and Biophysics, University of California, San Francisco.
Gene H. Brody, Department of Human Development and Family Science, University of Georgia
Eli Puterman, School of Kinesiology, University of British Columbia.
Elissa S. Epel, Department of Psychiatry, University of California, San Francisco
References
- Aviv A, Valdes AM, & Spector TD (2006). Human telomere biology: Pitfalls of moving from the laboratory to epidemiology. International Journal of Epidemiology, 35, 1424–1429. 10.1093/ije/dyl169 [DOI] [PubMed] [Google Scholar]
- Beatty Moody DL, Leibel DK, Darden TM, Ashe JJ, Waldstein SR, Katzel LI, … Zonderman AB (2019). Interpersonal-level discrimination indices, sociodemographic factors, and telomere length in African-Americans and Whites. Biological Psychology, 141, 1–9. 10.1016/j.biopsycho.2018.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty Moody DL, Matthews KA, Bromberger JT, & Brown C (2014). Everyday discrimination prospectively predicts inflammation across 7 years in racially diverse midlife women: Study of Women’s Health Across the Nation. Journal of Social Issues, 70, 298–314. 10.1111/josi.12061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky DW, Moffitt TE, Cohen AA, Corcoran DL, Levine ME, Prinz JA, … Caspi A (2018). Eleven telomere, epigenetic clock, and biomarker-composite quantifications of biological aging: Do they measure the same thing? American Journal of Epidemiology, 187, 1220–1230. 10.1093/aje/kwx346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger M, & Sarnyai Z (2015). “More than skin deep”: Stress neurobiology and mental health consequences of racial discrimination. Stress, 18, 1–10. 10.3109/10253890.2014.989204 [DOI] [PubMed] [Google Scholar]
- Blackburn EH, Epel ES, & Lin J (2015). Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science, 350, 1193–1198. 10.1126/science.aab3389 [DOI] [PubMed] [Google Scholar]
- Borrell LN, Diez-Roux AV, Jacobs DR Jr., Shea S, Jackson SA, Shrager S, & Blumenthal RS (2010). Perceived racial/ethnic discrimination, smoking and alcohol consumption in the Multi-Ethnic Study of Atherosclerosis (MESA). Preventive Medicine, 51, 307–312. 10.1016/j.ypmed.2010.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrell LN, Jacobs DR Jr., Williams DR, Pletcher MJ, Houston TK, & Kiefe CI (2007). Self-reported racial discrimination and substance use in the Coronary Artery Risk Development in Adults Study. American Journal of Epidemiology, 166, 1068–1079. 10.1093/aje/kwm180 [DOI] [PubMed] [Google Scholar]
- Borrell LN, Kiefe CI, Diez-Roux AV, Williams DR, & Gordon-Larsen P (2013). Racial discrimination, racial/ethnic segregation, and health behaviors in the CARDIA study. Ethnicity & Health, 18, 227–243. 10.1080/13557858.2012.713092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody GH, Lei M-K, Chae DH, Yu T, Kogan SM, & Beach SRH (2014). Perceived discrimination among African American adolescents and allostatic load: A longitudinal analysis with buffering effects. Child Development, 85, 989–1002. 10.1111/cdev.12213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody GH, Miller GE, Yu T, Beach SRH, & Chen E (2016). Supportive family environments ameliorate the link between racial discrimination and epigenetic aging: A replication across two longitudinal cohorts. Psychological Science, 27, 530–541. 10.1177/0956797615626703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L, Needham B, & Ailshire J (2017). Telomere length among older U.S. adults: Differences by race/ethnicity, gender, and age. Journal of Aging and Health, 29, 1350–1366. 10.1177/0898264316661390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton JL, Suderman M, Pappas JJ, Borghol N, McArdle W, Blakemore AIF, … Pembrey M (2014). Human leukocyte telomere length is associated with DNA methylation levels in multiple subtelomeric and imprinted loci. Scientific Reports, 4, 4954 10.1038/srep04954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon RM (2002). Telomere measurement by quantitative PCR. Nucleic Acids Research, 30(10), e47 10.1093/nar/30.10.e47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae DH, Epel ES, Nuru-Jeter AM, Lincoln KD, Taylor RJ, Lin J, … Thomas SB (2016). Discrimination, mental health, and leukocyte telomere length among African American men. Psychoneuroendocrinology, 63, 10–16. 10.1016/j.psyneuen.2015.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae DH, Nuru-Jeter AM, Adler NE, Brody GH, Lin J, Blackburn EH, & Epel ES (2014). Discrimination, racial bias, and telomere length in African-American men. American Journal of Preventive Medicine, 46, 103–111. 10.1016/j.amepre.2013.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae DH, Nuru-Jeter AM, Lincoln KD, & Francis DD (2011). Conceptualizing racial disparities in health: Advancement of a socio-psychobiological approach. Du Bois Review, 8, 63–77. 10.1017/S1742058X11000166 [DOI] [Google Scholar]
- Chae DH, Powell WA, Nuru-Jeter AM, Smith-Bynum MA, Seaton EK, Forman TA, … Sellers R (2017). The role of racial identity and implicit racial bias in self-reported racial discrimination: Implications for depression among African American men. Journal of Black Psychology, 43, 789–812. 10.1177/0095798417690055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherkas LF, Aviv A, Valdes AM, Hunkin JL, Gardner JP, Surdulescu GL, … Spector TD (2006). The effects of social status on biological aging as measured by white-blood-cell telomere length. Aging Cell, 5, 361–365. 10.1111/j.1474-9726.2006.00222.x [DOI] [PubMed] [Google Scholar]
- Clark R, Anderson NB, Clark VR, & Williams DR (1999). Racism as a stressor for African Americans. A biopsychosocial model. American Psychologist, 54, 805–816. 10.1037/0003-066X.54.10.805 [DOI] [PubMed] [Google Scholar]
- Cribbet MR, Carlisle M, Cawthon RM, Uchino BN, Williams PG, Smith TW, … Light KC (2014). Cellular aging and restorative processes: Subjective sleep quality and duration moderate the association between age and telomere length in a sample of middle-aged and older adults. Sleep, 37, 65–70. 10.5665/sleep.3308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham TJ, Berkman LF, Kawachi I, Jacobs DR Jr., Seeman TE, Kiefe CI, & Gortmaker SL (2013). Changes in waist circumference and body mass index in the U.S. CARDIA cohort: Fixed-effects associations with self-reported experiences of racial/ethnic discrimination. Journal of Biosocial Science, 45, 267–278. 10.1017/S0021932012000429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham TJ, Seeman TE, Kawachi I, Gortmaker SL, Jacobs DR, Kiefe CI, & Berkman LF (2012). Racial/ethnic and gender differences in the association between self-reported experiences of racial/ethnic discrimination and inflammation in the CARDIA cohort of 4 U.S. communities. Social Science & Medicine, 75, 922–931. 10.1016/j.socscimed.2012.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haan A, Prinzie P, Sentse M, & Jongerling J (2018). Latent difference score modeling: A flexible approach for studying informant discrepancies. Psychological Assessment, 30, 358–369. 10.1037/pas0000480 [DOI] [PubMed] [Google Scholar]
- Dominguez TP, Dunkel-Schetter C, Glynn LM, Hobel C, & Sandman CA (2008). Racial differences in birth outcomes: The role of general, pregnancy, and racism stress. Health Psychology, 27, 194–203. 10.1037/0278-6133.27.2.194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, & Cawthon RM (2004). Accelerated telomere shortening in response to life stress. Proceedings of the National Academy of Sciences of the United States of America, 101, 17312–17315. 10.1073/pnas.0407162101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Lin J, Wilhelm FH, Wolkowitz OM, Cawthon R, Adler NE, … Blackburn EH (2006). Cell aging in relation to stress arousal and cardiovascular disease risk factors. Psychoneuroendocrinology, 31, 277–287. 10.1016/j.psyneuen.2005.08.011 [DOI] [PubMed] [Google Scholar]
- Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR Jr., … Savage PJ (1988). CARDIA: Study design, recruitment, and some characteristics of the examined subjects. Journal of Clinical Epidemiology, 41, 1105–1116. 10.1016/0895-4356(88)90080-7 [DOI] [PubMed] [Google Scholar]
- Fuller-Rowell TE, Curtis DS, El-Sheikh M, Duke AM, Ryff CD, & Zgierska AE (2017). Racial discrimination mediates race differences in sleep problems: A longitudinal analysis. Cultural Diversity & Ethnic Minority Psychology, 23, 165–173. 10.1037/cdp0000104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geronimus AT, Hicken M, Keene D, & Bound J (2006). “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. American Journal of Public Health, 96, 826–833. 10.2105/AJPH.2004.060749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geronimus AT, Hicken MT, Pearson JA, Seashols SJ, Brown KL, & Cruz TD (2010). Do U.S. Black women experience stress-related accelerated biological aging? Human Nature, 21, 19–38. 10.1007/s12110-010-9078-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goosby BJ, Straley E, & Cheadle JE (2017). Discrimination, sleep, and stress reactivity: Pathways to African American-White cardiometabolic risk inequities. Population Research and Policy Review, 36, 699–716. 10.1007/s11113-017-9439-z [DOI] [Google Scholar]
- Graham JW (2009). Missing data analysis: Making it work in the real world. Annual Review of Psychology, 60, 549–576. 10.1146/annurev.psych.58.110405.085530 [DOI] [PubMed] [Google Scholar]
- Hamad R, Tuljapurkar S, & Rehkopf DH (2016). Racial and socioeconomic variation in genetic markers of telomere length: A cross-sectional study of U.S. older adults. EBioMedicine, 11, 296–301. 10.1016/j.ebiom.2016.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM, & Schorpp KM (2018). Integrating biomarkers in social stratification and health research. Annual Review of Sociology, 44, 361–386. 10.1146/annurev-soc-060116-053339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris R, Cormack D, Tobias M, Yeh L-C, Talamaivao N, Minster J, & Timutimu R (2012). Self-reported experience of racial discrimination and health care use in New Zealand: Results from the 2006/07 New Zealand Health Survey. American Journal of Public Health, 102, 1012–1019. 10.2105/AJPH.2011.300626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- House JS, Lantz PM, & Herd P (2005). Continuity and change in the social stratification of aging and health over the life course: Evidence from a nationally representative longitudinal study from 1986 to 2001/2002 (Americans’ Changing Lives Study). The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 60(Special Issue 2), S15–S26. 10.1093/geronb/60.Special_Issue_2.S15 [DOI] [PubMed] [Google Scholar]
- Hughes GH, Cutter G, Donahue R, Friedman GD, Hulley S, Hunkeler E, … Wagenknecht L (1987). Recruitment in the Coronary Artery Disease Risk Development in Young Adults (CARDIA) Study. Controlled Clinical Trials, 8(4, Suppl. 1), 68–73. 10.1016/0197-2456(87)90008-0 [DOI] [PubMed] [Google Scholar]
- Hunt SC, Chen W, Gardner JP, Kimura M, Srinivasan SR, Eckfeldt JH, … Aviv A (2008). Leukocyte telomeres are longer in African Americans than in Whites: The National Heart, Lung, and Blood Institute Family Heart Study and the Bogalusa Heart Study. Aging Cell, 7, 451–458. 10.1111/j.1474-9726.2008.00397.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kershaw KN, Lewis TT, Diez-Roux AV, Jenny NS, Liu K, Penedo FJ, & Carnethon MR (2016). Self-reported experiences of discrimination and inflammation among men and women: The multiethnic study of atherosclerosis. Health Psychology, 35, 343–350. 10.1037/hea0000331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Mickelson KD, & Williams DR (1999). The prevalence, distribution, and mental health correlates of perceived discrimination in the United States. Journal of Health and Social Behavior, 40, 208–230. 10.2307/2676349 [DOI] [PubMed] [Google Scholar]
- Kline RB (2011). Convergence of structural equation modeling and multilevel modeling In Williams M & Vogt WP (Eds.), Handbook of methodological innovation (pp. 562–589). Thousand Oaks, CA: Sage; 10.4135/9781446268261.n31 [DOI] [Google Scholar]
- Korous KM, Causadias JM, & Casper DM (2017). Racial discrimination and cortisol output: A meta-analysis. Social Science & Medicine, 193, 90–100. 10.1016/j.socscimed.2017.09.042 [DOI] [PubMed] [Google Scholar]
- Krieger N (2000). Discrimination in health In Berkman LF & Kawachi I (Eds.), Social epidemiology (pp. 36–75). New York, NY: Oxford University Press. [Google Scholar]
- Krieger N (2012). Methods for the scientific study of discrimination and health: An ecosocial approach. American Journal of Public Health, 102, 936–944. 10.2105/AJPH.2011.300544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger N, Huynh M, Li W, Waterman PD, & Van Wye G (2018). Severe sociopolitical stressors and preterm births in New York City: 1 September 2015 to 31 August 2017. Journal of Epidemiology and Community Health, 72, 1147–1152. 10.1136/jech-2018-211077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger N, Smith K, Naishadham D, Hartman C, & Barbeau EM (2005). Experiences of discrimination: Validity and reliability of a self-report measure for population health research on racism and health. Social Science & Medicine, 61, 1576–1596. 10.1016/j.socscimed.2005.03.006 [DOI] [PubMed] [Google Scholar]
- Lachman ME, Teshale S, & Agrigoroaei S (2015). Midlife as a pivotal period in the life course: Balancing growth and decline at the crossroads of youth and old age. International Journal of Behavioral Development, 39, 20–31. 10.1177/0165025414533223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DB, Kim ES, & Neblett EW (2017). The link between discrimination and telomere length in African American adults. Health Psychology, 36, 458–467. 10.1037/hea0000450 [DOI] [PubMed] [Google Scholar]
- Lewis TT, & Van Dyke ME (2018). Discrimination and the health of African Americans: The potential importance of intersectionalities. Current Directions in Psychological Science, 27, 176–182. 10.1177/0963721418770442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Epel E, Cheon J, Kroenke C, Sinclair E, Bigos M, … Blackburn E (2010). Analyses and comparisons of telomerase activity and telomere length in human T and B cells: Insights for epidemiology of telomere maintenance. Journal of Immunological Methods, 352, 71–80. 10.1016/j.jim.2009.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SY, & Kawachi I (2017). Discrimination and telomere length among older adults in the United States. Public Health Reports, 132, 220–230. 10.1177/0033354916689613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch SM, Peek MK, Mitra N, Ravichandran K, Branas C, Spangler E, … Riethman H (2016). Race, ethnicity, psychosocial factors, and telomere length in a multicenter setting. PLoS ONE, 11, e0146723 10.1371/journal.pone.0146723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason M, Schuller A, & Skordalakes E (2011). Telomerase structure function. Current Opinion in Structural Biology, 21, 92–100. 10.1016/j.sbi.2010.11.005 [DOI] [PubMed] [Google Scholar]
- Massey DS, Wagner B, Donnelly L, McLanahan S, Brooks-Gunn J, Garfinkel I, … Notterman DA (2018). Neighborhood disadvantage and telomere length: Results from the Fragile Families Study. The Russell Sage Foundation Journal of the Social Sciences, 4, 28–42. 10.7758/RSF.2018.4.4.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazidi M, Kengne AP, Sahebkar A, & Banach M (2018). Telomere length is associated with cardiometabolic factors in U.S. adults. Angiology, 69, 164–169. 10.1177/0003319717712860 [DOI] [PubMed] [Google Scholar]
- McEwen BS (1998). Protective and damaging effects of stress mediators. The New England Journal of Medicine, 338, 171–179. 10.1056/NEJM199801153380307 [DOI] [PubMed] [Google Scholar]
- McEwen BS, & Stellar E (1993). Stress and the individual. Mechanisms leading to disease. Archives of Internal Medicine, 153, 2093–2101. 10.1001/archinte.1993.00410180039004 [DOI] [PubMed] [Google Scholar]
- Monaghan P (2010). Telomeres and life histories: The long and the short of it. Annals of the New York Academy of Sciences, 1206, 130–142. 10.1111/j.1749-6632.2010.05705.x [DOI] [PubMed] [Google Scholar]
- Müezzinler A, Zaineddin AK, & Brenner H (2013). A systematic review of leukocyte telomere length and age in adults. Ageing Research Reviews, 12, 509–519. 10.1016/j.arr.2013.01.003 [DOI] [PubMed] [Google Scholar]
- Mustillo S, Krieger N, Gunderson EP, Sidney S, McCreath H, & Kiefe CI (2004). Self-reported experiences of racial discrimination and Black–White differences in preterm and low-birthweight deliveries: The CARDIA Study. American Journal of Public Health, 94, 2125–2131. 10.2105/AJPH.94.12.2125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén L (2009). Missing on x-variables. Retrieved from Mplus Missing Data Modeling website: http://www.statmodel.com/discussion/messages/22/4448.html [Google Scholar]
- Muthén LK, & Muthén BO (1998–2015). Mplus user’s guide (7th ed.). Los Angeles, CA: Author. [Google Scholar]
- Needham BL, Carroll JE, Diez-Roux AV, Fitzpatrick AL, Moore K, & Seeman TE (2014). Neighborhood characteristics and leukocyte telomere length: The Multi-Ethnic Study of Atherosclerosis. Health & Place, 28, 167–172. 10.1016/j.healthplace.2014.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan A, Pantell MS, Puterman E, Dhabhar FS, Blackburn EH, Yaffe K, … Epel ES (2011). Cumulative inflammatory load is associated with short leukocyte telomere length in the Health, Aging and Body Composition Study. PLoS ONE, 6, e19687 10.1371/journal.pone.0019687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantesco EJ, Leibel DK, Ashe JJ, Waldstein SR, Katzel LI, Liu HB, … Beatty Moody DL (2018). Multiple forms of discrimination, social status, and telomere length: Interactions within race. Psychoneuroendocrinology, 98, 119–126. 10.1016/j.psyneuen.2018.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradies Y, Ben J, Denson N, Elias A, Priest N, Pieterse A, … Gee G (2015). Racism as a determinant of health: A systematic review and meta-analysis. PLoS ONE, 10, e0138511 10.1371/journal.pone.0138511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M, Verhoeven JE, Cuijpers P, Reynolds CF III, & Penninx BWJH (2015). Where you live may make you old: The association between perceived poor neighborhood quality and leukocyte telomere length. PLoS ONE, 10, e0128460 10.1371/journal.pone.0128460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pew Research Center. (2019). Views on race in America 2019. Retrieved from https://www.pewsocialtrends.org/2019/04/09/race-in-america-2019/
- Rewak M, Buka S, Prescott J, De Vivo I, Loucks EB, Kawachi I, … Kubzansky LD (2014). Race-related health disparities and biological aging: Does rate of telomere shortening differ across Blacks and Whites? Biological Psychology, 99, 92–99. 10.1016/j.biopsycho.2014.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riethman H (2008). Human telomere structure and biology. Annual Review of Genomics and Human Genetics, 9, 1–19. 10.1146/annurev.genom.8.021506.172017 [DOI] [PubMed] [Google Scholar]
- Simon NM, Smoller JW, McNamara KL, Maser RS, Zalta AK, Pollack MH, … Wong K-K (2006). Telomere shortening and mood disorders: Preliminary support for a chronic stress model of accelerated aging. Biological Psychiatry, 60, 432–435. 10.1016/j.biopsych.2006.02.004 [DOI] [PubMed] [Google Scholar]
- Sims M, Diez-Roux AV, Dudley A, Gebreab S, Wyatt SB, Bruce MA, … Taylor HA (2012). Perceived discrimination and hypertension among African Americans in the Jackson Heart Study. American Journal of Public Health, 102(Suppl. 2), S258–S265. 10.2105/AJPH.2011.300523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanyam MA, Diez-Roux AV, Hickson DA, Sarpong DF, Sims M, Taylor HA Jr., … Wyatt SB (2012). Subjective social status and psychosocial and metabolic risk factors for cardiovascular disease among African Americans in the Jackson Heart Study. Social Science & Medicine, 74, 1146–1154. 10.1016/j.socscimed.2011.12.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upchurch DM, Stein J, Greendale GA, Chyu L, Tseng C-H, Huang M-H, … Seeman T (2015). A longitudinal investigation of race, socioeconomic status, and psychosocial mediators of allostatic load in midlife women: Findings from the Study of Women’s Health Across the Nation. Psychosomatic Medicine, 77, 402–412. 10.1097/PSY.0000000000000175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR (2018). Stress and the mental health of populations of color: Advancing our understanding of race-related stressors. Journal of Health and Social Behavior, 59, 466–485. 10.1177/0022146518814251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR, Gonzalez HM, Williams S, Mohammed SA, Moomal H, & Stein DJ (2008). Perceived discrimination, race and health in South Africa. Social Science & Medicine, 67, 441–452. 10.1016/j.socscimed.2008.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR, & Mohammed SA (2013). Racism and health I: Pathways and scientific evidence. American Behavioral Scientist, 57, 1152–1173. 10.1177/0002764213487340 [DOI] [PMC free article] [PubMed] [Google Scholar]