Abstract
Voltage-gated sodium channels (Navs) initiate the action potential waveforms in excitable cells. The molecular mechanisms controlling this process have been actively debated. New prokaryotic Nav structures by Wisedchaisri et al. have completed our understanding of the molecular conformations required for cellular electrical signaling, and provide key templates for research to examine eukaryotic Navs.
Keywords: sodium channel, voltage-gated ion channel, voltage-dependent channel gating, membrane protein, resting state, gating charge
Hodgkin, Huxley, and Katz, 67 years ago, measured the current–voltage relationship in the membrane of the giant axon and concluded that an influx of sodium ions across the membrane was responsible for generating the ‘action potential’ – the means by which fast electrical signaling occurs in excitable cells such as neurons and myocytes [1]. Hodgkin and Huxley ultimately won the 1963 Nobel Prize in Physiology or Medicine ‘for their discoveries concerning the ionic mechanisms involved in excitation and inhibition in the peripheral and central portions of the nerve cell membrane’ and inspired generations of scientists to study the molecular mechanism of electrical signaling [2].
It is well-understood that transmembrane proteins called voltage gated sodium channels (Navs) are responsible for selectively conducting sodium ions that drive the first phase of the action potential. Navs open their ion-conducting pore or ‘gate’ in response to membrane depolarization. The steep voltage-dependence of sodium channel opening is preceded by outward movement of positively charged amino acids (arginines or lysines), called ‘gating charges’ found in the voltage sensor domain (VSD) (Figure 1) [2]. The VSD transitions from the resting to the activated state when the membrane potentials shift from negative to depolarized voltages. Here, the state of the VSD is physically linked to the opening of the sodium ion-conducting pore domain [3]. The molecular mechanism responsible for how the VSD changes structure in response to voltage has been extensively studied using experimental, structural, and computational modeling approaches. Most results support a sliding helix model in which gating charges sequentially form ion pairs with anionic residues within the VSD as it transitions from the resting to the activated state 4, 5. However, a complete view of the VSD activation cycle has remained elusive. Although several crystallographic and cryo-electron microscopic (cryo-EM) Nav structures have been captured in activated states, the resting state has escaped determination 3, 6. This is partly because the resting state is a high-energy conformation that depends on a negative membrane potential to hold the VSD in the deactivated position and the ion-conducting pore in the closed state. Because current methodologies can only capture ion channel structures at zero millivolts, the resting state has remained the missing first step.
Figure 1. Comparison of Bacterial NavAb Channel Structures in the Resting and Activated States.
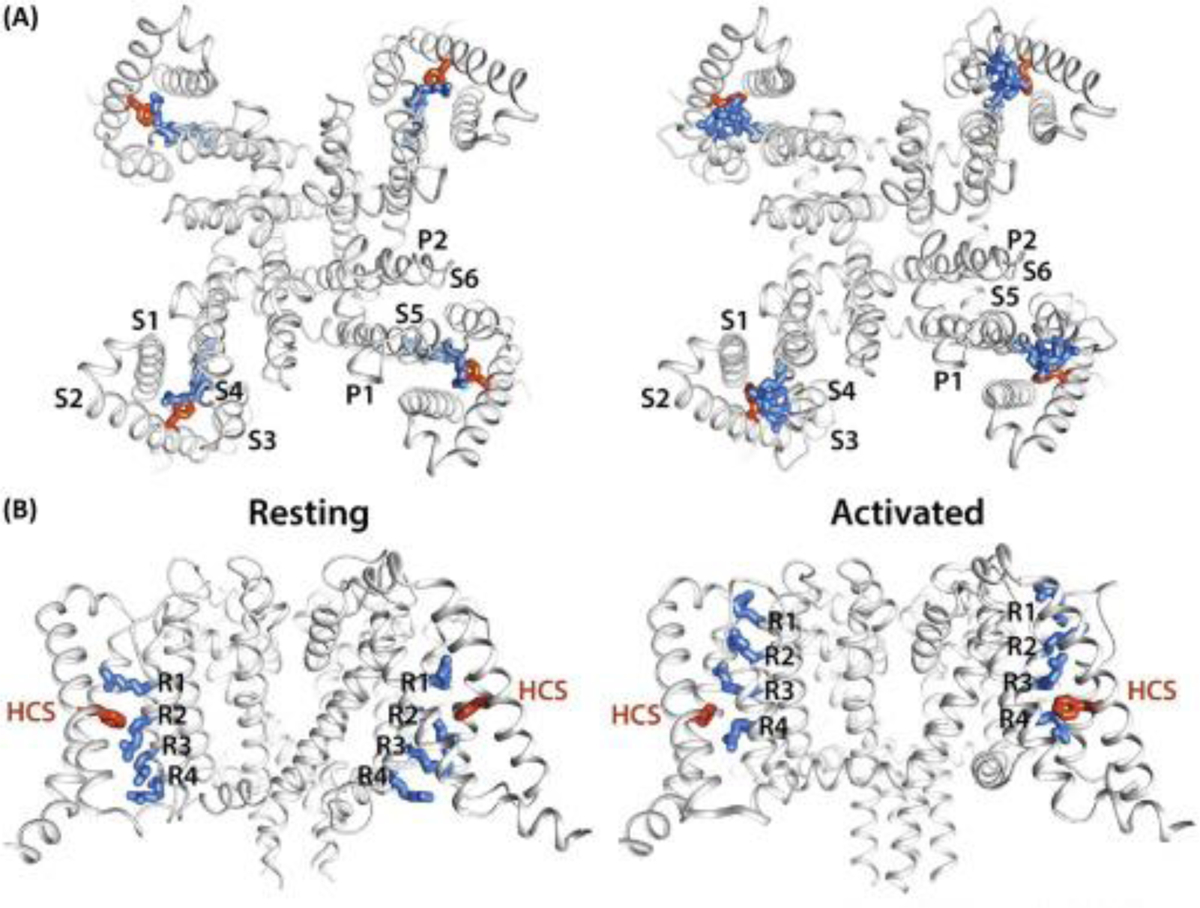
(A) Extracellular view of the resting and activated states. Bacterial voltage-gated sodium channels (Navs) consist of four identical subunits, each containing six transmembrane segments (S1 through S6). The voltage-sensing domain (VSD) is formed by S1–S4, where S4 contains the four arginines that carry the gating charges (shown as blue sticks). The hydrophobic constriction site (HCS) residue F56 (in S2) is shown in stick representation and is colored in orange. The pore domain (PD) is formed by S5 and S6, and the membrane-reentrant P1 and P2 helices containing loop that forms the selectivity filter. (B) Transmembrane view of the resting and activated states. The gating charge-carrying arginines (R1–R4) in transmembrane segment S4 and HCS residue F56 are shown in stick representation and are colored blue and orange, respectively. Structures were rendered using the CHIMERA program at the University of California San Francisco (www.cgl.ucsf.edu/chimera/).
However, recent work published by Wisedchaisri and colleagues tackles this challenge head-on. They solved the structure of a Nav from the bacteria Arcobacter butzleri (NavAb), in the resting/closed state at 4 Å overall resolution using a clever approach [7]. The authors introduced several mutations into NavAb (called the KAV mutant channel) that shifted its voltage-dependence by several hundred millivolts, and trapped the VSD in the deactivated position, by creating a disulfide bond between G94C (in S3–S4) and Q150C (in S5). Although efforts to crystallize this channel were unsuccessful, the authors linked the N-terminus of NavAb to the C-terminal helix of maltose-binding protein and solved the channel structure using cryo-EM. The VSD of the resulting channel was stabilized in a resting state, without the need for a membrane potential [7]. Notably, the structure of the NavAb VSD in the resting state is reminiscent of the Rosetta-modeled resting-state VSD structure of a bacterial Nav from Bacillus halodurans (NaChBac), and is validated by the electrophysiology results presented by Wisedchaisri et al. as well as by previous work to capture disulfide bridge interactions from functional channels in real-time [8].
Further, the work also captured the NavAb channel structure in an activated state using a disulfide bridge between V100C (in S4) and Q150C (in S5) [7]. Comparison of the NavAb channel structures in the resting (G94C–Q150C) and activated (V100C–Q150C) states revealed that S4 has a 310 helix conformation that orients all gating charges on the same face of the helix toward S2 during the gating cycle. From the resting to the activated states, the VSD transfers three gating charges across the hydrophobic constriction site (HCS) while moving S4 ∼11.5 Å in the plane of the membrane – features that are most consistent with the sliding helix model proposed more two decades ago (Figure 1) [4].
Since the discoveries of Hodgkin, Huxley, and Katz, this epic story continues – the end is really the beginning. Given that structural determination of most of the prokaryotic Nav gating-cycle steps is now complete, new questions loom for the more complex gating mechanism of eukaryotic Navs [9]. Although some of this work has already begun, the findings from the homotetrameric prokaryotic Navs still need to be compared with heterotetrameric eukaryotic Navs [10]. More specifically, what are the precise conformational changes in each of the four homologous VSDs and the pore in eukaryotic Navs during channel opening, and during fast and slow inactivation? Ultimately, these research pursuits will bring a greater understanding of the molecular dysregulation caused by Nav mutations, and will facilitate the rational design of novel therapeutics for the control of pain, epilepsy, arrhythmia, and other Nav related diseases.
References
- 1.Hodgkin AL, et al. Measurement of current–voltage relations in the membrane of the giant axon of Loligo, J. Physiol, 116 (1952), pp. 424–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong CM, Bezanilla F Charge movement associated with the opening and closing of the activation gates of the Na channels J. Gen. Physiol, 63 (1974), pp. 533–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sula A, et al. The complete structure of an activated open sodium channel, Nat. Commun, 8 (2017), Article 14205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Catterall WA Molecular properties of voltage-sensitive sodium channels Annu. Rev. Biochem, 55 (1986), pp. 953–985 [DOI] [PubMed] [Google Scholar]
- 5.Guy HR, Seetharamulu P Molecular model of the action potential sodium channel, Proc. Natl. Acad. Sci. U. S. A, 83 (1986), pp. 508–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Payandeh J, et al. The crystal structure of a voltage-gated sodium channel, Nature, 475 (2011), pp. 353–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wisedchaisri G, et al. Resting-state structure and gating mechanism of a voltage-gated sodium channel Cell, 178 (2019), pp. 993–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yarov-Yarovoy V, et al. Structural basis for gating charge movement in the voltage sensor of a sodium channel, Proc. Natl. Acad. Sci. U. S. A, 109 (2012), pp. E93–E102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chanda B, Bezanilla F Tracking voltage-dependent conformational changes in skeletal muscle sodium channel during activation, J. Gen. Physiol, 120 (2002), pp. 629–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen H, et al. Structure of a eukaryotic voltage-gated sodium channel at near-atomic resolution Science, 355 (2017) [DOI] [PubMed] [Google Scholar]


