Abstract
Background:
Arthritis pain is primarily managed by nonsteroidal anti-inflammatory drugs (NSAIDs), such as diclofenac. Topical diclofenac gel is limited in efficacy due to its limited penetration through the skin. This study investigates the use of a multihour, wearable, localized, sonophoresis transdermal drug delivery device for the penetration enhancement of diclofenac through the skin.
Materials & methods:
A commercially available, sustained acoustic medicine (sam®) ultrasound device providing 4 h, 1.3 W, 132 mW/cm2, 3 MHz ultrasound treatment was evaluated for increasing the drug delivery of diclofenac gel through a human skin model and was compared with standard of care topical control diclofenac gel.
Results:
Sonophoresis of the diclofenac gel for 4 h increases diclofenac delivery by 3.8× (p < 0.01), and penetration by 32% (p < 0.01).
Conclusion:
Sustained acoustic medicine can be used as a transdermal drug-delivery device for nonsteroidal anti-inflammatory drugs.
Keywords: : acoustic streaming, arthritis, continuous ultrasound, inflammation, pain management, physical therapy, sonophoresis, sustained acoustic medication, transdermal drug delivery
Arthritis is one of the most prevalent medical conditions affecting 54 million people in the USA and costs the US economy approximately $304 billion annually in medical expenses and lost wages [1–3]. Per-person medical costs attributed to an average of $11,052 per year for arthritis patients. There are multiple kinds of arthritis, with osteoarthritis and rheumatoid arthritis being the two primary forms [1–3]. It is the disease of the whole joint, which involves activation of catabolic enzymes, nitric oxide synthase-2, cyclooxygenases-(COX)-2, degradation of the matrix through matrix metalloproteinases (MMP-1, MMP-3 and MMP-13), a disintegrin and metalloproteinase with thrombospondin-1 domains 4 and 5 and activation of cytokines, such as (IL-6, IL-1β and TNF-α), leading to the destruction of the total joint and chronic pain [4–7].
Currently, there is no cure for arthritis and arthritis-induced pain. The pain is managed through weight loss, physical therapy, hot/cold therapy, ultrasound therapy, compression therapy and either systemic or topical nonsteroidal anti-inflammatory drugs (NSAIDs) [4,8,9]. Ultimately most patients with moderate-to-severe arthritis progress onto surgical procedures to repair and/or replace the damaged joint [10]. NSAIDs are broadly used for pain management and have short-term efficacy. The long-term application of orally administered NSAIDs has adverse effects on multiple organs, including the kidney, liver, heart and intestinal tract [11]. The topical application of NSAIDs reduces the systemic risks but has reduced efficacy due to limited penetration through the skin [12]. Diclofenac is one of the most common NSAIDs used [13,14]. It inhibits COX-1 and -2, the underlying expression of prostaglandin-2 and thromboxane synthesis approximately 3–1000× more than other NSAIDs [15]. Furthermore, inhibition of COX-1 and COX-2 downregulate pain sensitization through the L-arginine/nitric oxide/cyclic guanosine monophosphate pathway [13,14]. Recent meta-analyses have shown that diclofenac is more effective, relative to other NSAIDs, such as ketoprofen, ibuprofen and piroxicam [9,15,16].
Ultrasound is a high-frequency (nonionizing radiation) mechanical wave with a frequency above 20 kHz (not audible to the human ear). Ultrasound has medical applications for therapeutic and diagnostics purposes. The application of ultrasound varies with intensity and frequency of the ultrasound signal. Therapeutic ultrasound has mechano-transductive, thermal, and cavitation-based effects [17–20]. Mechano-transduction leads to matrix regeneration and activation of multiple molecular and cellular pathways [17,18]. Thermal effects lead to increased tissue plasticity and blood flow to enhance nutrient transport, vasodilation and removal of cellular waste products [19]. The pressure changes due to acoustic waves, allow ultrasound to induce cavitation effects in the cell membrane, increasing cell permeability and the potential for drug delivery [20,21]. Continuous ultrasound also creates a convective phenomenon of acoustic streaming; the bulk flow of fluids and molecules in the propagation path of the ultrasonic wave [22]. Acoustic streaming increases drug delivery in phantom muscle and brain tissues [23].
The sonophoresis mechanism of ultrasound uses thermal, cavitational and streaming properties as well as continuous low-intensity ultrasound that can be used as a potential tool to enhance localized transdermal drug delivery to treat arthritis [24]. Application of topical NSAIDs, along with ultrasound stimulation, can potentially increase the delivery of NSAIDs to the joint. While there have been many studies that have shown the efficacy of sonophoresis in preclinical settings [25], their transition into clinical practice has proven difficult, since daily sonophoresis treatment is cost-prohibitive, as treatment is delivered in a healthcare providers office [21,24,25]. There is a medical need to develop a sonophoresis technology that is portable and easy-to-use, to deliver NSAIDs.
Sustained acoustic medicine (sam®) is a US FDA-cleared class-II prescription home-use medical device that delivers continuous 3 MHz, 1.3 W, 132 mW/cm2 ultrasound for 4 h [26–28]. The device is applied to the treatment area through an ultrasonic coupling patch with high-viscosity ultrasound coupling gel (Figure 1). Throughout a 4-h treatment, the sam device delivers 18,760 Joules of energy, which is significantly higher than any other commercially available devices, generating vigorous diathermy and increased healing rates [19]. sam has established clinical efficacy to reduce pain and improve function for soft tissue injuries, spasms, strains, joint arthritis and back pain [26–28]. The ultrasound parameters of sam are well suited for sonophoresis drug delivery, since studies have shown that continuous ultrasound has a better drug delivery performance relative to pulsed ultrasound [29]. sam's multihour treatment increases skin permeability and tissue regeneration along with diathermic effects to reduce arthritis-associated pain [30]. Furthermore, the wearable and home-use design of sam allows it to be used outside of the clinical environment for daily therapy. sam increases the permeability of skin to allow deeper penetration of the drug. This becomes a potential approach for the treatment of localized pathology like arthritis, as sam enhances localized drug delivery while decreasing adverse effects of the systemic application of NSAIDs.
Figure 1. . Sustained acoustic medicine transdermal drug-delivery system.
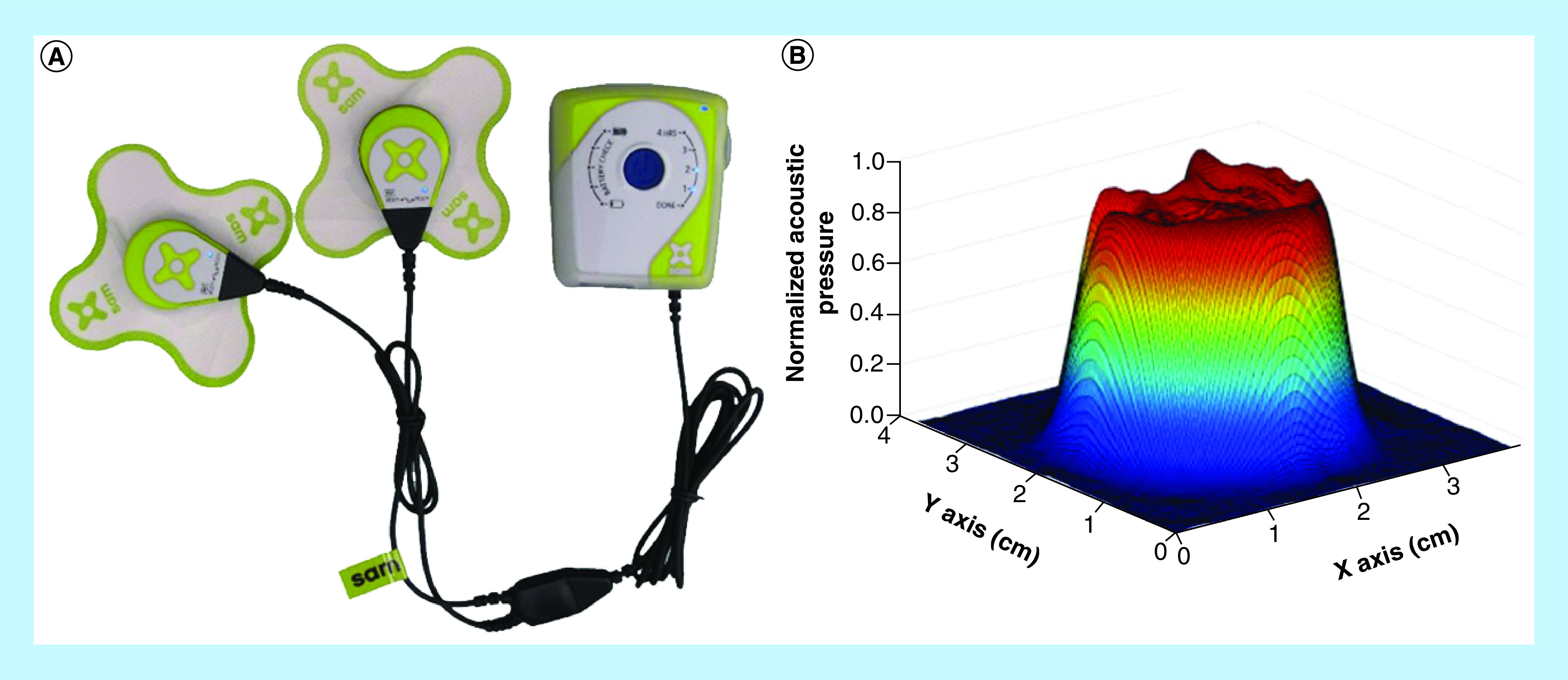
(A) Sustained acoustic medicine ultrasound system, two ultrasound generators approximately 2.5 cm diameter, connected to the ultrasonic coupling patch, delivering constant ultrasound signal at 3 MHz, 1.3 W and 132 mW/cm2 intensity. The entire system is approximately the size of a cell phone. (B) Ultrasonic 3D beam scan of pressure field 3 mm from the face of an ultrasound generator. The pressure field is uniform with a beam nonuniformity ratio less than 2:1, and an active radiating surface area of 5 cm2.
Furthermore, the anti-inflammatory properties of ultrasound have been well studied. Ultrasound downregulates IL-1β, NF-κB and Iκ-βα to reduce the rate of cartilage and bone degradation, due to inflammation [31,32]. Emerging evidence also suggests that ultrasound slows down the inflammatory degeneration of arthritic joints and associated pain with the inflammatory environment. The localized application of sam treatment has little or no adverse effects making it a useful drug delivery device for arthritis. The objective of this study is to evaluate the application of sam as an NSAID sonophoresis device for the potential application in arthritis associated pain management.
Materials & methods
Ultrasonic pressure field distribution & beam scan
The commercially available sam treatment device is a fully integrated closed-loop device. Operating off 3.7 volts direct current power, containing parallel low-impedance ultrasound generation circuitry, dual transducer with coupling interface, closed-loop feedback control monitoring coupling, energy-delivered and temperature all housed in a welded acrylonitrile butadiene styrene shell. The device is powered by a rechargeable lithium-polymer cell and coupled to the treatment area with an ultrasonic coupling patch (Figure 1A). The sam device delivers ultrasound at 3 MHz, 1.3 W (0.65 W per transducer) and 132 mW/cm2 intensity. Each ultrasound transducer generator has an active radiating area of 5 cm2, a beam nonuniformity ration of less than 2:1 (Figure 1B) and a 10° divergent lens made from medical grade polymethyl pentene to spread the ultrasonic energy entering the application site. The ultrasound transducer spreads over the tissue to maximize acoustic streaming while preventing focal regions, hotspots and the formation of standing waves inside the tissue. The divergent ultrasound field, natural reflection and scattering in the tissue increases the treatment volume of each generator to be approximately 58 cm3 and up to 5-cm deep inside the tissue. Internal ultrasonic and temperature control at the transducer-circuit interface provides real-time echogenic feedback to assure the device is appropriately coupled to the application target.
Preparation of the sam device & diclofenac ultrasound gel patches
A 3M™ science applied to life custom, biocompatible, nonwoven material was selected as the material to create the sonophoresis delivery patch, to secure the sam ultrasound system in place. Spun-lace polypropylene material provided welding compatibility to a custom polypropylene containment reservoir that housed the drug in a sealed compartment. The containment reservoir was designed with a break-away tab to allow for easy insertion and removal of the ultrasound generator, as shown in Figure 1. The containment reservoir holds 3 ml of gel when the ultrasound generator is secured into the device and attached to the treatment location. The containment reservoir was optimized for the insertion of the ultrasound generator and containment of the ultrasound coupling agent. During the design process, the containment reservoir developed ±5 N of force for insertion and removal of the ultrasound device measured with a pull and insertion force load-cell. The removal of the adhesive patch force is approximately 2 N, measured by the peel test as described by Mohammed et al. [33]. The containment reservoir was filled with 1% diclofenac gel, formulated using diclofenac sodium [34,35] All components were tested for biocompatibility and stability.
Testing ultrasonic coupling & diathermy profile of the sam diclofenac gel patch versus commercial gel patch
Bovine muscle samples (n = 12) were harvested from the local butcher (3.5 cm × 10 cm × 15 cm, 1000 g ± 60 g, 20°C), and divided into two groups: diclofenac gel patch (n = 6) and commercial gel patch (n = 6). Samples were wrapped in cellophane to maintain the moisture content of the tissue. A 3-cm hole was cut in the cellophane to apply the coupling gel for evaluation. Needle thermocouples Type K with hypodermic tips (Oakton 072297B-K, Oakton Instruments, IL, USA) were inserted at 1, 2 and 5 cm below the surface of the tissue and centered below the coupling to record temperatures using an Omega Data Logger (OM-DAQPRO-5300, Omega Engineering, CT, USA). Temporal coupling and the diathermic profile of the diclofenac gel patch was measured, side by side, in comparison with the commercial gel patch over a 4-h treatment cycle.
Testing NSAID drug delivery with the sam device
Skin and tissue phantoms were used to assess the penetration depth of 1% diclofenac ultrasound gel with the sam device over a 4-h treatment period. Active sam treatment was compared side-by-side with a nonactive device as a topical control. Strat-M Membrane (EMD Millipore, MA, USA) transdermal skin and an established experimental technique for concentration/penetration mapping through thirty 1-mm thick 32-mm diameter polyethylene oxide (PEO) (Hydress, Alliqua Biomedical, Inc., PA, USA) tissue hydrogel phantoms (n = 3) were used to mimic human tissue (human skin and tissue analog) [36,37]. PEO discs were stacked into a 30-mm pile and fitted into a polyurethane Franz-cell with a 32-mm diameter and 30-mm depth (Figure 3A). Before placing into the Franz-cell, PEO discs were soaked in deionized, degassed water and stacked into groups of ten smoothly to ensure there were no air bubbles between the discs while filling the Franz-cell (Figure 3B). Strat-M Membrane was applied to the top of the Franz cell chamber, holding the hydrogel stack in place. The 47-mm diameter Strat-M Membrane was large enough to seal the entire top of the Franz-cell and was sandwiched between the PEO discs on the bottom and diclofenac gel patch + sam device on the top. The presectioned 1-mm thick PEO hydrogel discs in the 30 mm stack provided an efficient means of profiling diclofenac drug delivery across the skin analog, including penetration and concentration through visualization techniques. It was ensured that there were no air gaps between the ultrasound transducer, diclofenac gel, Strat-M membrane and hydrogel discs. The set up was stimulated for 4 h with active sam (3 MHz, 132 mw/cm2, continuous ultrasound) and an in-active device (Figure 3C).
Figure 3. . Transdermal drug delivery experimental setup with sustained acoustic medicine device.
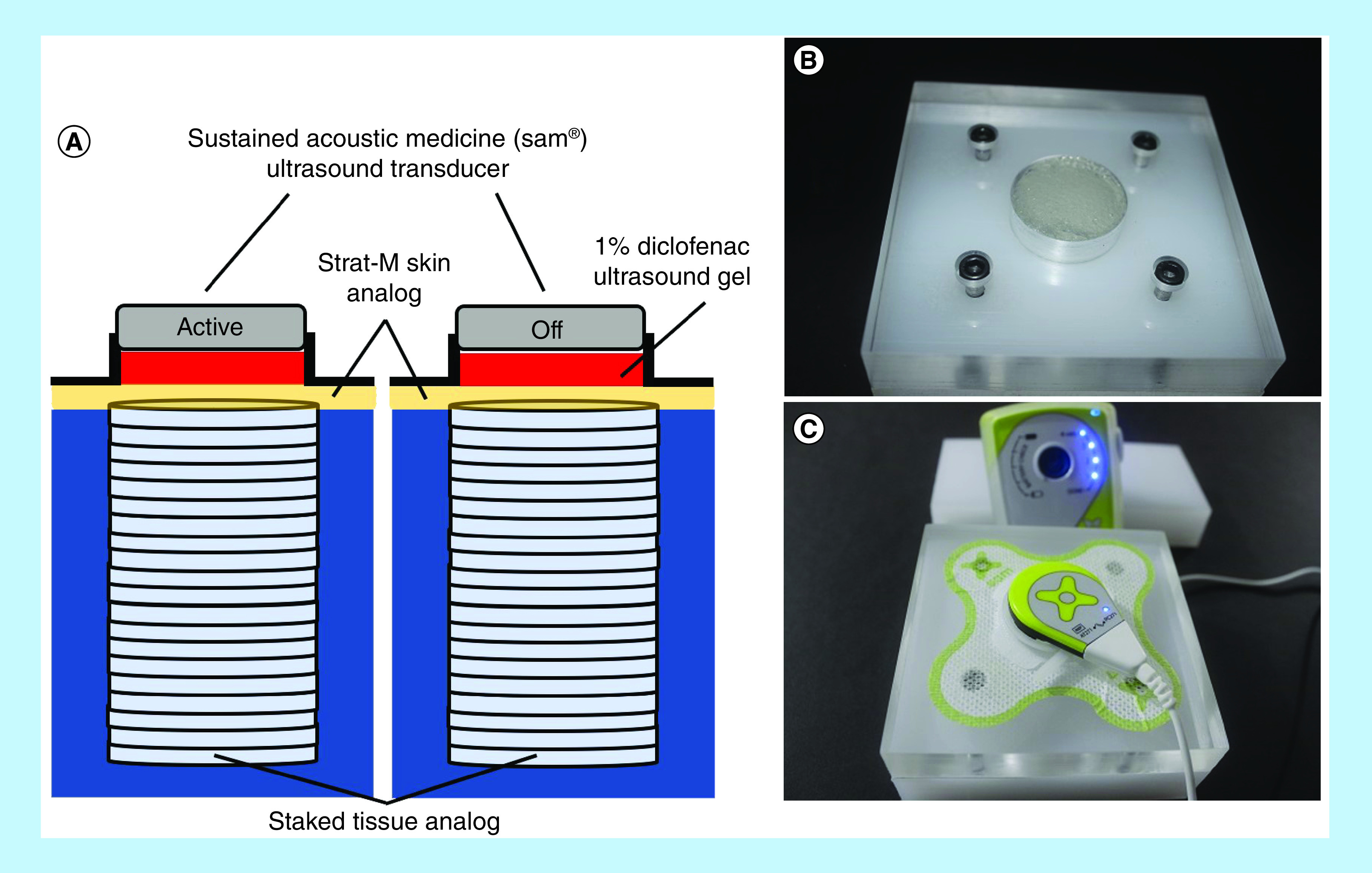
(A) Depiction of Franz-cell setup with sustained acoustic medicine (sam®) active and off/control ultrasound generators. The 1% diclofenac ultrasound gel shown in red is captured between the ultrasound transducer and Strat-M membrane with a coupling patch and reservoir. Beneath the membrane, a stack of 30 polyethylene oxide layered hydrogels mimic human tissue to measure active versus off/control sam for drug delivery. (B) Experimental sam test chambers with hydrogel layers filled, and (C) with a 1% diclofenac ultrasound coupling patch and active sam device.
Visualizing the transport of diclofenac & sam-enhanced diclofenac delivery
After 4 h of sam stimulation, the hydrogel discs were individually collected with a tweezer and immersed in a 0.1% iron (III) chloride solution for 90 s. This resulted in the formation of a ferri-phenol complex and rapid coloration of the hydrogel discs, the discs were immersed for an extended time to ensure completion of the reaction [38]. The discs from the respective chamber were placed in a series and imaged with a digital camera. ImageJ was used to quantify the amount of drug penetration through PEO hydrogel discs and normalized by the area covered by the stain [39,40]. The depth of penetration was quantified through numbers of hydrogels with positive a stain. The data were averaged and presented with standard deviation. Student t-test was used to test statistical significance with p ≤ 0.05 considered as a significant difference.
Results
sam device shows constant uniform stimulation over its surface
The sam device delivers a constant 2.5-cm diameter acoustic signal across the transducer surface, ensuring consistent and leveled drug delivery across the skin analog and through the hydrogel stack, as shown in Figure 1.
sam does not change diathermic profile with diclofenac ultrasound coupling gel patch
sam stimulation shows a significant gradual increase in temperature over 240 min (4 h) and a consistent temperature drop poststimulation of sam. Diclofenac ultrasound gel (1%) provided clinically relevant >8, >6, >2°C modulation at 1-, 2- and 5-cm tissue depths, respectively, and showed no significant change in diathermic properties compared with the control group (Figure 2A & B). sam stimulation provides significant diathermic effects enabling the increase in permeability and comparative ANOVA analysis of the ultrasound gel coupling patch with a 1% diclofenac gel coupling patch showing no significant change in diathermic profiles through the depth of bovine tissue (Figure 2).
Figure 2. . Sustained acoustic medicine ultrasound coupling and diathermic measurements.
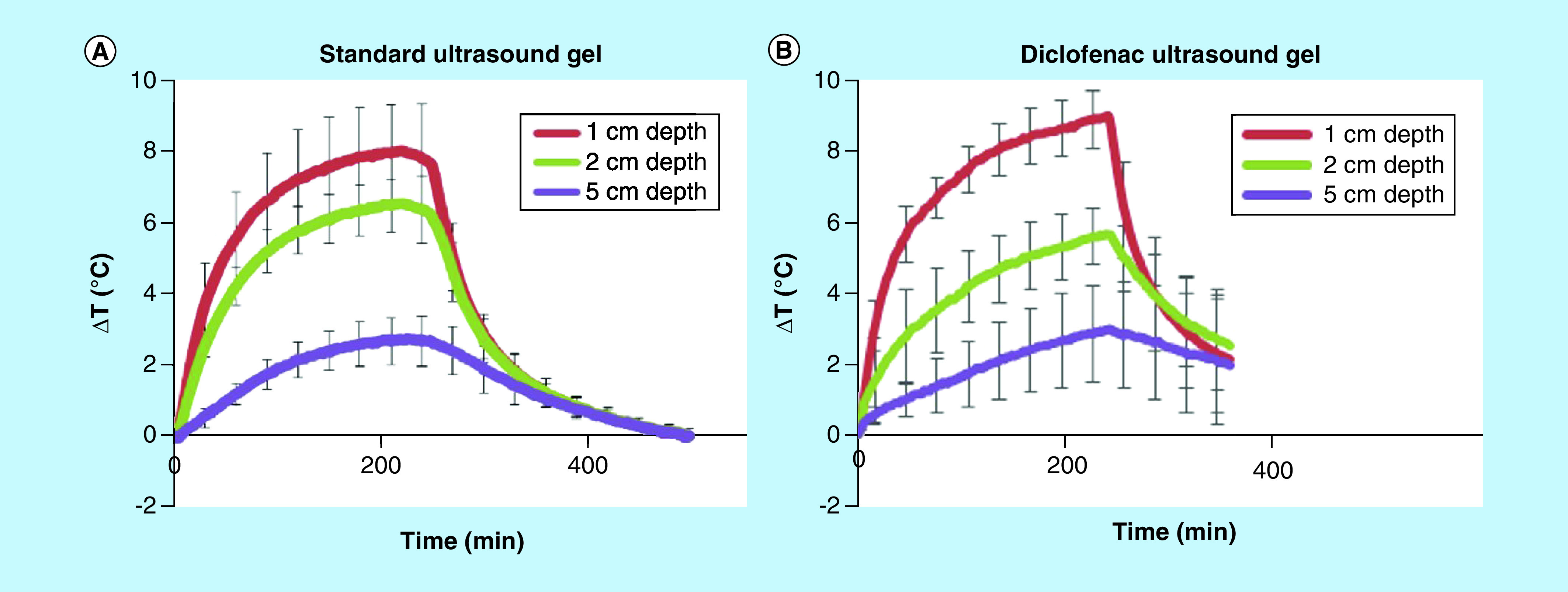
(A) Temporal diathermic profile of sustained acoustic medicine stimulation with standard ultrasound gel and (B) 1% diclofenac coupling gel showing no statistical differences at 1-, 2- or 5-cm measurement depths. Both standard and diclofenac ultrasound gels provide excellent coupling of ultrasound into deep muscle tissue.
ΔT: Change in temperature.
sam treatment enhances drug transport through skin analog
Active sam treatment improved the relative transmission of diclofenac by 3.8-fold ± 0.18 (p < 0.01) relative to the 4 h of control (Figure 4C). Diclofenac delivered with sam showed significantly deeper penetration through 21 ± 1 tissue discs (Figure 4B) relative to 16 ± 1 discs in the control group (Figure 4A) (p < 0.01). sam increased diclofenac penetration by 32% for 5 mm more than the control group (p < 0.01). Active sam treatment provided a more significant increase in both penetration and the total quantity of NSAID delivered through the skin analog.
Figure 4. . Increased transdermal drug delivery with sustained acoustic medicine ultrasound treatment.
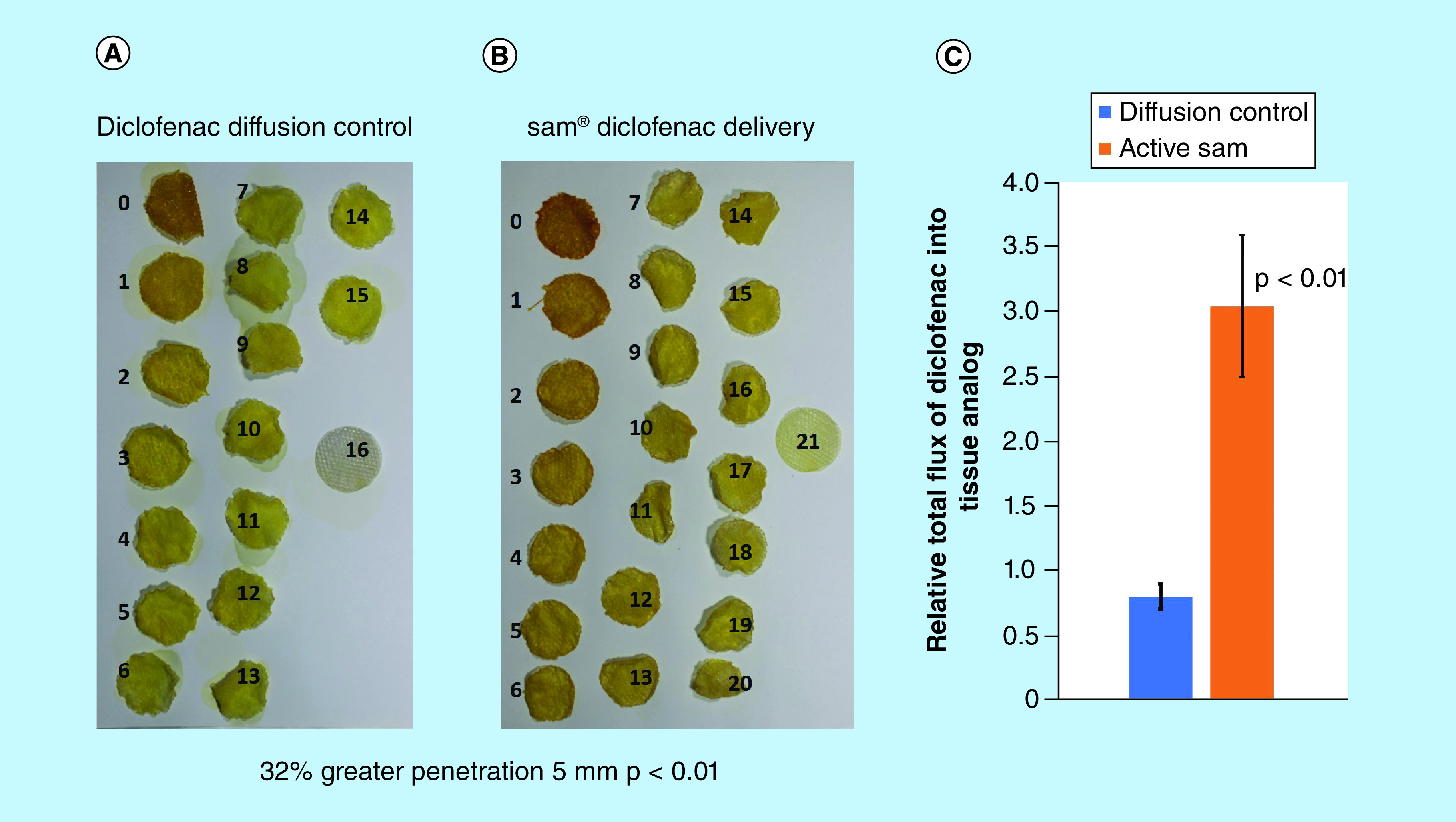
(A) Diffusion control group. (B) sam-treated group show penetration of dye through more than 21 hydrogel discs (32% greater penetration, +5 mm) while the control group show dye penetration through approximately 16 hydrogel discs (p < 0.01). (C) Active sam treatment group (orange) show 3.8 ± 0.18-fold increase in diclofenac flux relative to control group (blue) (p < 0.01).
sam: Sustained acoustic medicine.
Discussion
Arthritis is the pathology of the full joint, which leads to degeneration of cartilage, meniscus, tendon and bone due to a highly inflammatory environment over a long time [4]. COX-1/2 pathway regulates the most common inflammatory cytokines involved in the degradation of joints, which are known for joint destruction but also because of underlying pain associated with arthritis. NSAIDs such as ibuprofen, ketoprofen, salicylate, naproxen and diclofenac have been used for pain management along with physical therapy [11,15]. Diclofenac has shown the most efficacy, as COX-1/2 inhibitors lead to the downregulation of prostaglandin-2 and associated inflammatory cytokines such as us IL-1β, TNF α, along with MMP 13, a disintegrin and metalloproteinase with thrombospondin-1 domains 4 and 5 [13,14,34,41]. Systemic application of long-term use NSAIDs can cause severe damage to the kidney, liver and gastrointestinal complications, while only providing short-term pain relief. The topical application of NSAIDs reduces the systemic adverse effects of NSAIDs and significantly reduces their efficacy due to limited penetration through the skin [8,9,12,16,42]. Various methodologies have been applied to increase localized permeability, limited efficacy and/or high adverse events.
Sonophoresis has shown to be a potentially useful mechanism for localized transdermal drug delivery. Feiszthuber et al. showed an increase in the pectinaceous delivery of insulin in the porcine skin model [43]. Similarly, Jabbari et al. [44] used a portable ultrasound device to deliver insulin in vivo rat model. The parameters used to test the efficacy of ultrasound as a transdermal drug delivery device have varied through different studies. Aldwaikat and Alarjah used diclofenac 1 and 20 MHZ varying amplitudes, times and duty cycles to study the effects of changing ultrasound parameters on the drug-delivery efficacy in skin cultures [45]. The data from the study show that 20 kHz 20% amplitude for 5 min with 100 duty cycle has the most significant efficiency. More recent studies have also used a dual transducer approach to increase thermal and cavitational effects [46]. Some research has studied ultrasound as a combination mechanism with other mechanisms such as electroporation, nanoparticles and microneedles, to improve the efficacy of transdermal drug delivery [24,47–49]. Huang et al. used the ultrasound-mediated microbubble approach to enhance diclofenac transdermal drug delivery in the rat arthritis model [50,51]. Similarly, Liao et al. has shown significantly high transdermal diclofenac delivery in the rheumatoid arthritis rat model [34,35].
Furthermore, studies have shown that ultrasound has regenerative properties leading to regeneration by increasing glycosaminoglycan and collagen II levels in the cartilage matrix [32]. Meta-analysis has demonstrated that diclofenac is potentially the most effective NSAID in pain management of knee arthritis [15,34,41]. This study uses a commercially available FDA class-II sam medical device; a wearable, multihour ultrasound device to enhance the drug delivery of diclofenac using a tissue analog mimicking human skin. sam-ultrasound-treated diclofenac sample shows 3.8-fold more delivery of NSAID and 5 mm (32%) increase in deeper penetration through the skin analog, relative to the control group. The quantification of the data confirms the penetration of the drug deeper through the tissue analog.
The sam device provides a continuous ultrasound signal with 3 MHz, 0.65 W and 132 mW/cm2 intensity that generates constant mechanical signals, penetrating layers of skin along with diathermic effects leading to the increase of the permeability of the skin. Previous studies have shown that continuous ultrasound has better sonophoresis abilities than pulsed ultrasound, furthermore, multihour therapy ensures complete penetration of the drug [29,52–54]. The device application with topical NSAIDs, specifically topical diclofenac or diclofenac patches, makes it a potential treatment for pain management for arthritis patients. The delivery of diclofenac is localized because low intensity continuous ultrasound is being applied at a certain area (5 cm2) with 10° divergence, resulting in a localized acoustic signal and skin permeability. Deeper and sustained drug delivery ensures improved pain management of arthritis by downregulating cytokines and potentially enhancing cartilage regeneration.
The 1% diclofenac ultrasound gel does not significantly change the diathermic properties of sam treatment with standard gel. Side by side comparisons demonstrated similar temporal heating profiles at 1-, 2- and 5-cm tissue depths. Diathermic stability confirms that the 1% diclofenac ultrasound gel does not alter the therapeutic effectiveness of sam device applied to the skin. Fraz-cell experiments using a human skin analog applied the acoustic streaming to increase the penetration of diclofenac through the layers of skin analog, showing that sam device provides significantly deeper penetration of diclofenac. The current study uses a skin analog, which implies the benefits of acoustic streaming to increase the penetration of diclofenac through the skin analog. Application of sam in vivo skin will further enhance its efficacy as previous studies have shown that ultrasound increases skin permeability through thermal and cavitational effects.
Furthermore, the application of ultrasound increases skin loosening, the blood supply to the skin and roots of hair follicles, allowing drug penetration and accessibility deeper into the skin tissue, which is typically not assessable to topical applications of the drug. sam diathermic changes will lead to increased skin permeability, loosening and overall porosity for the drug to penetrate deeper. sam uses a sonophoresis mechanism to improve drug delivery as a portable, easy to use, multihour wearable device.
The improved delivery of diclofenac may clinically provide enhanced inhibition of inflammatory cytokines, increased drug availability in the joint space through acoustic streaming and overall tissue oxygenation, leading to reduced osteoarthritis associated pain and increase mobility. Furthermore, retaining the anabolic abilities of ultrasound alone may help slow down the degradation of cartilage during disease progression. Our previous studies have clinically shown the efficacy of sam as a pain management device. Collectively, this study shows the efficiency of the commercially available sam device as a drug delivery sonophoresis system for drugs such as diclofenac.
Conclusion
Arthritis is a localized, degenerative disease. The systemic application of NSAIDs over a long period can have severe consequences to multiple organs, thus, there is a need for a localized therapy to reduce arthritis induced pain. sam is an FDA-approved medical device for pain management, and the current study shows the sonophoretic application of sam using a 1% diclofenac ultrasound patch in the human skin analog. The data show an increase in temperature, increasing localized vasodilation, oxygenation and drug exchange, as well as increased drug penetration due to the increase in skin porosity and acoustic streaming. Collectively all these properties can improve targeted drug delivery, essential for localized pathology like arthritis.
Future perspective
Arthritis is a localized pathology and long-term application of NSAIDs has adverse effects. Currently, there is no effective cure for arthritis and there is a need to develop technology that can specifically target arthritis joints. The novel approach of sam sonophoresis therapy is to provide a medical device that is portable, more efficient and convenient. The proprietary design uses a unique, low impedance approach to produce medically relevant acoustic pressures with reduced energy consumption. The miniaturized transducer and circuit technology allow the ultra-efficient, low impedance electro-acoustic conversion to the smallest size of any currently available device with the ability to deliver localized NSAIDs through the skin. In future experiments, the rate of penetration can be quantified by stimulating hydrogel for multiple periods and comparing the rate of penetration with and without sam stimulation. Relative to other drug-delivery devices, this sam – 1% diclofenac provides multiple hours of in-home, easy-to-use, sonophoresis targeting inflammatory COX-1 and COX-2 pathways. sam device has already been approved by FDA for pain management and 1% diclofenac has shown high efficacy in arthritis, as combination therapy can significantly enhance the effectiveness of NSAIDs and reduce their adverse systemic effects. The future use of this device can allow patients to use the sonophoresis of drugs in the convenience of their home.
Summary points.
Sustained acoustic medicine (sam®) is a novel sonophoresis system, 100% duty cycle, 3 MHz, 1.3 W and 132 mW/cm2 intensity multihour wearable device.
3D ultrasound beam mapping of sam shows consistent uniform acoustic pressure over the surface of the transducer with a 2:1 beam nonuniformity ratio.
Stimulating bovine tissue with sam for 4 h increases tissue temperature >8°C with a gradual increase in temperature and no change in diathermic profile, when 1% diclofenac ultrasound gel is compared with a standard ultrasound coupling gel.
sam transdermal driven diclofenac drug showed increased delivery 3.8× (p < 0.01) and penetration 32% (p < 0.01) into and through the skin analog compared with the control.
sam 1% diclofenac patch is a potential localized drug-delivery system to target arthritis and other painful conditions with limited or no systemic risks.
Acknowledgment
Dr Lewis Sr supervised the research and the paper was written prior to Dr Lewis Sr passing away.
Footnotes
Financial & competing interests disclosure
The funding for this research was provided by NIH (AG061985). The authors worked in collaboration with ZetrOZ at the ZetrOZ research facility as part of the internship program with the University of Cincinnati. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: • of interest
- 1.Barbour KE, Moss S, Croft JB. et al. Geographic variations in arthritis prevalence, health-related characteristics, and management – United States, 2015. MMWR Surveil. l Summ. 67(4), 1–28 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbour KE, Helmick CG, Boring M, Brady TJ. Vital signs: prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation – United States, 2013–2015. MMWR Morb. Mortal. Wkly. Rep. 66(9), 246–253 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hootman JM, Helmick CG, Barbour KE, Theis KA, Boring MA. Updated projected prevalence of self-reported doctor-diagnosed arthritis and arthritis-attributable activity limitation among US adults, 2015–2040. Arthritis Rheumatol. 68(7), 1582–1587 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mobasheri A, Saarakkala S, Finnila M, Karsdal MA, Bay-Jensen AC, van Spil WE. Recent advances in understanding the phenotypes of osteoarthritis. F1000Res 1–8, 8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Neill TW, Felson DT. Mechanisms of osteoarthritis (OA) pain. Curr. Osteoporos. Rep. 16(5), 611–616 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen J, Abu-Amer Y, O'Keefe RJ, McAlinden A. Inflammation and epigenetic regulation in osteoarthritis. Connect. Tissue Res. 58(1), 49–63 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vila S. Inflammation in osteoarthritis. P. R. Health Sci. J. 36(3), 123–129 (2017). [PubMed] [Google Scholar]
- 8.Maniar KH, Jones IA, Gopalakrishna R, Vangsness CT ., Jr Lowering side effects of NSAID usage in osteoarthritis: recent attempts at minimizing dosage. Expert Opin. Pharmacother. 19(2), 93–102 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Zeng C, Wei J, Persson MSM. et al. Relative efficacy and safety of topical non-steroidal anti-inflammatory drugs for osteoarthritis: a systematic review and network meta-analysis of randomised controlled trials and observational studies. Br. J. Sports Med. 52(10), 642–650 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abramoff B, Caldera FE. Osteoarthritis: pathology, diagnosis, and treatment options. Med. Clin. North Am. 104(2), 293–311 (2020). [DOI] [PubMed] [Google Scholar]
- 11.Crofford LJ. Use of NSAIDs in treating patients with arthritis. Arthritis Res. Ther. 15(Suppl. 3), S2 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar L, Verma S, Singh M, Chalotra T, Utreja P. Advanced drug delivery systems for transdermal delivery of non-steroidal anti-inflammatory drugs: a review. Curr. Drug Deliv. 15(8), 1087–1099 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Atzeni F, Masala IF, Sarzi-Puttini P. A review of chronic musculoskeletal pain: central and peripheral effects of diclofenac. Pain Ther. 7(2), 163–177 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burian M, Tegeder I, Seegel M, Geisslinger G. Peripheral and central antihyperalgesic effects of diclofenac in a model of human inflammatory pain. Clin. Pharmacol. Ther. 74(2), 113–120 (2003). [DOI] [PubMed] [Google Scholar]
- 15.van Walsem A, Pandhi S, Nixon RM, Guyot P, Karabis A, Moore RA. Relative benefit-risk comparing diclofenac to other traditional non-steroidal anti-inflammatory drugs and cyclooxygenase-2 inhibitors in patients with osteoarthritis or rheumatoid arthritis: a network meta-analysis. Arthritis Res. Ther. 17, 66 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.da Costa BR, Reichenbach S, Keller N. et al. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet 390(10090), e21–e33 (2017). [DOI] [PubMed] [Google Scholar]; • Application of diclofenac as prevalent nonsteroidal anti-inflammatory drug (NSAID) for arthritis.
- 17.Dalecki D. Mechanical bioeffects of ultrasound. Annu. Rev. Biomed. Eng. 6, 229–248 (2004). [DOI] [PubMed] [Google Scholar]
- 18.Louw TM, Budhiraja G, Viljoen HJ, Subramanian A. Mechanotransduction of ultrasound is frequency dependent below the cavitation threshold. Ultrasound Med. Biol. 39(7), 1303–1319 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rigby JH, Taggart RM, Stratton KL, Lewis GK, Jr, Draper DO. Intramuscular heating characteristics of multihour low-intensity therapeutic ultrasound. J. Athl. Train. 50(11), 1158–1164 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.ter Haar G. Therapeutic ultrasound. Eur. J. Ultrasound 9(1), 3–9 (1999). [DOI] [PubMed] [Google Scholar]
- 21.Pitt WG, Husseini GA, Staples BJ. Ultrasonic drug delivery – a general review. Expert Opin. Drug Deliv. 1(1), 37–56 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dong Z, Delacour C, Mc Carogher K, Udepurkar AP, Kuhn S. Continuous ultrasonic reactors: design, mechanism and application. Materials (Basel). 13(2), (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zderic V. Ultrasound-enhanced drug and gene delivery: a review. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2008, 4472 (2008). [DOI] [PubMed] [Google Scholar]
- 24.Seah BC, Teo BM. Recent advances in ultrasound-based transdermal drug delivery. Int. J. Nanomedicine 13, 7749–7763 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polat BE, Blankschtein D, Langer R. Low-frequency sonophoresis: application to the transdermal delivery of macromolecules and hydrophilic drugs. Expert Opin. Drug Deliv. 7(12), 1415–1432 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Potential application of ultrasound in transdermal drug delivery.
- 26.Best TM, Wilk KE, Moorman CT, Draper DO. Low intensity ultrasound for promoting soft tissue healing: a systematic review of the literature and medical technology. Intern. Med. Rev. (Wash D C). 2(11), 1–9 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Draper DO, Castel JC, Castel D. Rate of temperature increase in human muscle during 1 MHz and 3 MHz continuous ultrasound. J. Orthop. Sports Phys. Ther. 22(4), 142–150 (1995). [DOI] [PubMed] [Google Scholar]
- 28.Langer MD, Lewis GK., Jr Sustained acoustic medicine: a novel long duration approach to biomodulation utilizing low intensity therapeutic ultrasound. Proc. SPIE. Int. Soc. Opt. Eng. 1–14, 9467 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang JH, Kim DK, Yun MY, Kim TY, Shin SC. Transdermal delivery system of triamcinolone acetonide from a gel using phonophoresis. Arch. Pharm. Res. 29(5), 412–417 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Stratton K, Taggart R, Lewis GK. Long duration ultrasound facilitates delivery of a therapeutic agent. J. Acoust. Soc. Am. 4(136), 1 (2014). [Google Scholar]
- 31.Ebisawa K, Hata K, Okada K. et al. Ultrasound enhances transforming growth factor beta-mediated chondrocyte differentiation of human mesenchymal stem cells. Tissue Eng. 10(5–6), 921–929 (2004). [DOI] [PubMed] [Google Scholar]
- 32.Uddin SM, Qin YX. Enhancement of osteogenic differentiation and proliferation in human mesenchymal stem cells by a modified low intensity ultrasound stimulation under simulated microgravity. PLoS ONE 8(9), e73914 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mohammed IK, Charalambides MN, Kinloch AJ. Modelling the interfacial peeling of pressure-sensitive adhesives. J. Non-Newtonian Fluid. 222, 141–150 (2014). [Google Scholar]
- 34.Liao AH, Chung HY, Chen WS, Yeh MK. Efficacy of combined ultrasound-and-microbubbles-mediated diclofenac gel delivery to enhance transdermal permeation in adjuvant-induced rheumatoid arthritis in the rat. Ultrasound Med. Biol. 42(8), 1976–1985 (2016). [DOI] [PubMed] [Google Scholar]
- 35.Liao AH, Chuang HC, Chung HY. Efficacy of ultrasound mediated microbubbles in diclofenac gel to enhance transdermal permeation in rheumatoid arthritis induced rat. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2015, 3521–3524 (2015). [DOI] [PubMed] [Google Scholar]
- 36.Suksaeree J, Siripornpinyo P, Chaiprasit S. Formulation, characterization, and in vitro evaluation of transdermal patches for inhibiting crystallization of mefenamic acid. J. Drug Deliv. 2017, 7358042 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gupta SP, Jain SK. Development of matrix-membrane transdermal drug delivery system for atenolol. Drug Deliv. 11(5), 281–286 (2004). [DOI] [PubMed] [Google Scholar]
- 38.Banerjee S, Haldar BC. Constitution of ferri-phenol complex in solution. Nature 165(4208), 1012 (1950). [DOI] [PubMed] [Google Scholar]
- 39.Roy S, Kumar Jain A, Lal S, Kini J. A study about color normalization methods for histopathology images. Micron 114, 42–61 (2018). [DOI] [PubMed] [Google Scholar]
- 40.Gavrilovic M, Azar JC, Lindblad J. et al. Blind color decomposition of histological images. IEEE Trans. Med. Imaging 32(6), 983–994 (2013). [DOI] [PubMed] [Google Scholar]
- 41.Verhoeven F, Totoson P, Marie C. et al. Diclofenac but not celecoxib improves endothelial function in rheumatoid arthritis: a study in adjuvant-induced arthritis. Atherosclerosis 266, 136–144 (2017). [DOI] [PubMed] [Google Scholar]
- 42.Malfait AM, Schnitzer TJ. Towards a mechanism-based approach to pain management in osteoarthritis. Nat. Rev. Rheumatol. 9(11), 654–664 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feiszthuber H, Bhatnagar S, Gyongy M, Coussios CC. Cavitation-enhanced delivery of insulin in agar and porcine models of human skin. Phys. Med. Biol. 60(6), 2421–2434 (2015). [DOI] [PubMed] [Google Scholar]
- 44.Jabbari N, Asghari MH, Ahmadian H, Mikaili P. Developing a commercial air ultrasonic ceramic transducer to transdermal insulin delivery. J. Med. Signals Sens. 5(2), 117–122 (2015). [PMC free article] [PubMed] [Google Scholar]
- 45.Aldwaikat M, Alarjah M. Investigating the sonophoresis effect on the permeation of diclofenac sodium using 3D skin equivalent. Ultrason. Sonochem. 22, 580–587 (2015). [DOI] [PubMed] [Google Scholar]
- 46.Schoellhammer CM, Srinivasan S, Barman R. et al. Applicability and safety of dual-frequency ultrasonic treatment for the transdermal delivery of drugs. J. Control. Release 202, 93–100 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zorec B, Becker S, Rebersek M, Miklavcic D, Pavselj N. Skin electroporation for transdermal drug delivery: the influence of the order of different square wave electric pulses. Int. J. Pharm. 457(1), 214–223 (2013). [DOI] [PubMed] [Google Scholar]
- 48.Hamidi M, Azadi A, Rafiei P. Hydrogel nanoparticles in drug delivery. Adv. Drug Deliv. Rev. 60(15), 1638–1649 (2008). [DOI] [PubMed] [Google Scholar]
- 49.Al-Qallaf B, Das DB. Optimizing microneedle arrays for transdermal drug delivery: extension to non-square distribution of microneedles. J. Drug Target. 17(2), 108–122 (2009). [DOI] [PubMed] [Google Scholar]
- 50.Huang D, Sun M, Bu Y. et al. Microcapsule-embedded hydrogel patches for ultrasound responsive and enhanced transdermal delivery of diclofenac sodium. J. Mater. Chem. B 7(14), 2330–2337 (2019). [DOI] [PubMed] [Google Scholar]
- 51.Huang B, Dong WJ, Yang GY, Wang W, Ji CH, Zhou FN. Dendrimer-coupled sonophoresis-mediated transdermal drug-delivery system for diclofenac. Drug Des. Devel. Ther. 9, 3867–3876 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meshali M, Abdel-Aleem H, Sakr F, Nazzal S, El-Malah Y. Effect of gel composition and phonophoresis on the transdermal delivery of ibuprofen: in vitro and in vivo evaluation. Pharm. Dev. Technol. 16(2), 93–101 (2011). [DOI] [PubMed] [Google Scholar]
- 53.Park SR, Park SH, Jang KW. et al. The effect of sonication on simulated osteoarthritis. Part II: alleviation of osteoarthritis pathogenesis by 1 MHz ultrasound with simultaneous hyaluronate injection. Ultrasound Med. Biol. 31(11), 1559–1566 (2005). [DOI] [PubMed] [Google Scholar]
- 54.Levy D, Kost J, Meshulam Y, Langer R. Effect of ultrasound on transdermal drug delivery to rats and guinea pigs. J. Clin. Invest. 83(6), 2074–2078 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]


