Abstract
Leucine-rich repeat kinase 2 (LRRK2) encodes a 2527-amino acid (aa) protein composed of multiple functional domains, including a Ras of complex proteins (ROC)-type GTP-binding domain, a carboxyl terminal of ROC (COR) domain, a serine/threonine protein kinase domain, and several repeat domains. LRRK2 is genetically involved in the pathogenesis of both sporadic and familial Parkinson’s disease (FPD). Parkinson’s disease (PD) is the second most common neurodegenerative disorder, manifesting progressive motor dysfunction. PD is pathologically characterized by the loss of dopaminergic neurons in the substantia nigra pars compacta, and the presence of intracellular inclusion bodies called Lewy bodies (LB) in the remaining neurons. As the most frequent PD-causing mutation in LRRK2, G2019S, increases the kinase activity of LRRK2, an abnormal increase in LRRK2 kinase activity is believed to contribute to PD pathology; however, the precise biological functions of LRRK2 involved in PD pathogenesis remain unknown. Although biochemical studies have discovered several substrate proteins of LRRK2 including Rab GTPases and tau, little is known about whether excess phosphorylation of these substrates is the cause of the neurodegeneration in PD. In this review, we summarize latest findings regarding the physiological and pathological functions of LRRK2, and discuss the possible molecular mechanisms of neurodegeneration caused by LRRK2 and its substrates.
Keywords: leucine rich repeat kinase, neurodegeneration, Parkinsons disease, rab
Introduction
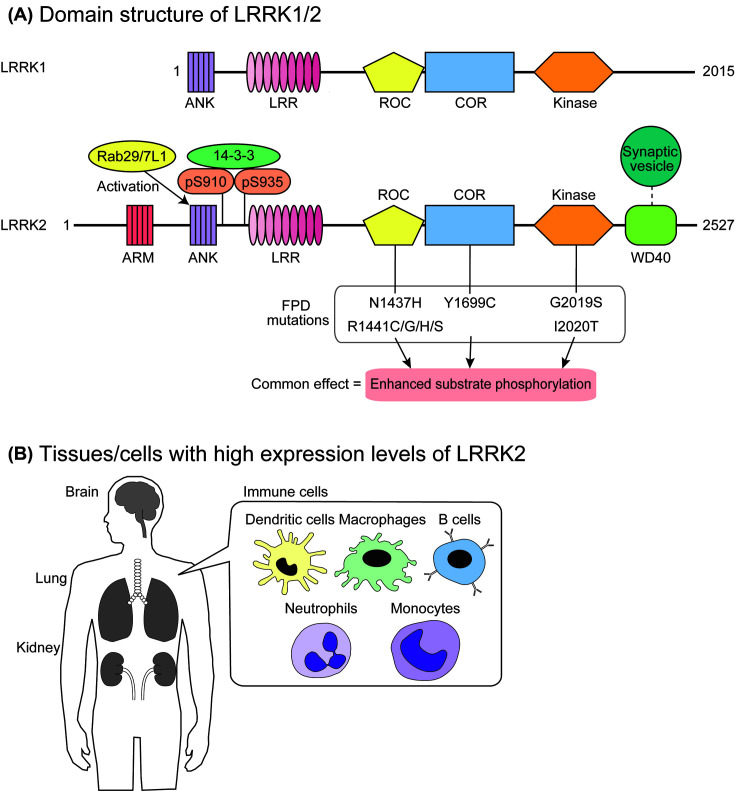
Leucine-rich repeat kinase 2 (LRRK2) is a large protein consisting of 2527 amino acids (aa), harboring multiple functional domains, including a Ras of complex proteins (ROC)-type GTP-binding domain, a carboxyl terminal of ROC (COR) domain, and a serine/threonine protein kinase domain (Figure 1A). Proteins harboring both ROC and COR domains are categorized into the ROCO protein family, and LRRK2 was first described in the literature as Roco2 [1]. Most ROCO proteins have a kinase domain and some repeat domains besides the ROC and COR tandem domains [1]. There is a paralog of LRRK2 in the mammalian genome, LRRK1, which produces a protein product with a similar domain structure to that of LRRK2 (Figure 1A).
Figure 1. Structure and expression of LRRK2.
(A) The domain structure of LRRK1/2. Both LRRK1 and LRRK2 are composed of an ROC-type GTP-binding domain, a COR domain, a serine/threonine protein kinase domain, and several repeat domains. Eight pathogenic mutations found in LRRK2 are shown below the domain structure of LRRK2, which increase the levels of substrate phosphorylation in vivo. (B) LRRK2 is highly expressed in the brain, lungs, kidneys, and immune cells, including dendritic cells, macrophages, B cells, neutrophils, and monocytes.
LRRK2 has been implicated in the pathogenesis of Parkinson’s disease (PD). PD is one of the most common neurodegenerative diseases, and selectively affects dopaminergic neurons in the midbrain [2]. A pathological hallmark of PD is inclusion bodies called Lewy bodies (LBs), in which the major component is aggregated α-synuclein [3–5]. Although in most PD patients the disease is sporadic, there are several families in which PD is inherited, and this type of PD is called familial PD (FPD). The genetic linkage of the PARK8 locus on chromosome 12q12 with one of the autosomal dominant forms of FPD was first identified in 2002 in a large Japanese family with multiple affected generations [6] (OMIM entry 607060), and several missense mutations in the LRRK2 gene were subsequently identified from PARK8-linked FPD in 2004 [7,8]. To date, eight missense mutations (i.e. N1437H, R1441C/G/H/S, Y1699C, G2019S, and I2020T) in LRRK2 have been confirmed to be pathogenic [9] (Figure 1A). Moreover, several genome-wide association studies (GWAS) have demonstrated that single nucleotide polymorphisms located near the LRRK2 locus are associated with the risk of sporadic PD [10–13]. Thus, mounting evidence suggests that LRRK2 plays an important role in the pathogenesis of PD.
In addition to dopaminergic neuronal loss, deposition of aggregated α-synuclein is one of the pathological hallmarks of PD brain. There have been a number of papers describing the histopathology of autopsied PD patients carrying LRRK2 mutations [14–20]. However, the α-synuclein pathology was surprisingly variable amongst mutation carriers, ranging from pure nigral degeneration without α-synuclein deposition, to massive deposition of α-synuclein in the cerebral cortex in addition to the midbrain. Thus, it remains unclear whether LRRK2 directly plays a role in the process of α-synuclein aggregation and deposition. Of note, LRRK2 has also been genetically implicated in several immunological disorders, namely inflammatory bowel diseases (IBDs), including Crohn’s disease and ulcerative colitis [21–23], Hansen’s disease (leprosy) [24,25], and systemic lupus erythematosus [26]. Collectively, given the unique domain structure of LRRK2 and its potential importance in the pathogenesis of several incurable diseases, it has received a large amount of attention from researchers. In this review, we systematically summarize our current understanding of the physiological roles of LRRK2 in the brain as well as in peripheral tissues, and discuss the possible mechanisms of how abnormalities in LRRK2 functions lead to the neurodegeneration observed in PD.
Physiological roles of LRRK2
Structure and function of domains in LRRK2
LRRK2 consists of 13 armadillo repeats (ARM; aa 49–657), 7 ankyrin repeats (ANK; aa 673–862), 14 leucine-rich repeats (LRR; aa 983-1319), an ROC domain (aa 1335–1515), a COR domain (aa 1515–1845), a protein kinase domain (aa 1859–2138), and 7 WD40 repeats (aa 2142–2498) [27–29] (Figure 1A). Amongst the FPD mutations, five mutations (i.e. N1437H, R1441C/G/H/S) are in the ROC domain, whereas Y1699C is in the COR domain, and G2019S and I2020T are in the kinase domain. In this section, we summarize the structure and function of these accessory domains apart from the kinase domain which will be discussed in a subsequent section in more detail.
The precise function of the ARM domains in LRRK2 is unclear. Based on a structural analysis of LRRK2 using cryo-electron microscopy (cryo-EM) and single particle analysis, LRRK2 forms a homodimer [30]. However, the ARM domain of LRRK2 extended out of the dimer core. Because ARM domains of other proteins (e.g. β-catenin) are involved in the protein–protein interaction [31], it might function in the association with other LRRK2-interacting proteins.
Amino acid substitutions of conserved leucines in the ANK domain of LRRK2 diminished the Rab29/7L1-mediated activation of LRRK2 [32]. Considering that the ANK domain in VPS9-domain ankyrin repeat protein (VARP) directly interacts with Rab32 [33], which is one of the closest homologs of Rab29/7L1, the ANK domain of LRRK2 might also directly interact with Rab29/7L1 (Figure 1A). It was also suggested that the ANK domain of LRRK2 intramolecularly interacts with the kinase domain of LRRK2 [34]. Guaitoli and colleagues [34] found that the model structure of the ANK and kinase domains of LRRK2 were similar to that of the kinase domain of cyclin-dependent kinase 6 (CDK6) and the inhibitory ankyrin domain of inhibitor of CDK4 (INK4) [35], suggesting that the ankyrin domain of LRRK2 is involved in the inhibition of its kinase activity.
The function of the LRR domain of LRRK2 is also not well understood. In a structure model of LRRK2, the LRR domain was on the surface of the LRRK2 dimer, suggesting the potential involvement of this domain in protein–protein interaction [34]. The amino-terminal region of the LRR domain in LRRK2 is known to be highly phosphorylated at Ser860, 910, 935, 955, and 973, amongst which the phosphorylations at Ser910 and Ser935 have been shown to be essential to the interaction with 14-3-3 proteins [36] (Figure 1A). Kinase(s) responsible for these phosphorylations remain elusive, whereas protein phosphatase 1 has been proposed as the responsible phosphatase for these sites [37]. However, it has been repeatedly shown that LRRK2 lacking the ARM, ANK, and LRR domains retains its intrinsic phosphotransferase activity in an in vitro system using peptide substrates [38,39]. Thus, it is clear that these domains are not involved in the intrinsic phosphotransferase activity of LRRK2.
In contrast with the other three repeat domains mentioned above, the WD40 domain seems to be essential to the intrinsic phosphotransferase activity of LRRK2 as LRRK2 lacking the WD40 domain did not phosphorylate the peptide substrate in an in vitro system [38]. An electron microscopic analysis has demonstrated that the WD40 domain of LRRK2 show doughnut-shaped particles, which is consistent with the typical structure of WD40 domains [40]. The authors also identified a large number of synaptic vesicle-associated proteins interacting with the WD40 domain of LRRK2 by MS, suggesting that the WD40 domain of LRRK2 plays an important role for controlling synaptic vesicle trafficking (Figure 1A).
The ROC domain of LRRK2 harbors motifs well-conserved amongst GTP-binding proteins. We and others have showed that GTP binding to the ROC domain of LRRK2 is essential to its intrinsic phosphotransferase activity [41,42]. It has also been shown that an LRRK2 mutant lacking the GTP-binding activity (i.e. T1348N) fails to phosphorylate Rab10, a physiological substrate of LRRK2, indicating that GTP binding to LRRK2 is required for eliciting its downstream signaling [32]. It is hypothesized that the ROC domain interacts with the kinase domain to activate it in a similar manner to the activation of Raf kinases by Ras [43]. In support of this notion, the FPD mutations in the ROC domain decrease its GTP-hydrolyzing activity and result in the increase in the GTP-bound form of LRRK2, thereby leading to the activation of the LRRK2 kinase activity [44,45]. In ROCO proteins, the ROC domain is always followed by a COR domain, forming a ROC-COR tandem domain [1], which suggests an important role of the COR domain related to the ROC domain. The existence of an FPD mutation (i.e. Y1699C) in the COR domain further underscores the importance of the COR domain in the LRRK2 function.
Of note, LRRK1, a paralog of LRRK2 with the similar ROC-COR-kinase domain structure, lacks the ARM and WD40 domains [27,46], which might result in differentiating the functions of two proteins from each other.
Tissue distribution of LRRK2
LRRK2 is physiologically expressed in various tissues, with the highest expression in the brain, lung, and kidney [47] (Figure 1B). LRRK2 is ubiquitously expressed throughout the brain, but it has been suggested that its expression is relatively low in the substantia nigra and the ventral tegmental area of the midbrain where dopaminergic neurons are abundant [48–50]. In more recent studies using a defined set of specific antibodies against LRRK2, West and colleagues [52] found the highest expression of LRRK2 in the striatum, particularly in medium spiny neurons [51]. They also demonstrated the expression of LRRK2 in dopaminergic neurons in the substantia nigra. Although these studies using rodent brains were adequately performed using the brains of Lrrk2 knockout (KO) mice as negative controls, the distribution of LRRK2 in the human brain remains largely unknown. In situ hybridization, quantitative real-time PCR, and immunohistochemical analysis have demonstrated that LRRK2 is expressed in regions affected in PD brains including the striatum and substantia nigra [53–55].
Subcellular distribution of LRRK2
As LRRK2 does not contain obvious transmembrane domains, it is expected to be a cytosolic protein. Indeed, in cultured cells overexpressing LRRK2, LRRK2 is distributed throughout the cytoplasm but is not observed in the nucleus [56]. However, biochemical fractionation as well as careful immunocytochemical studies have suggested that LRRK2 also localizes to organelle membranes [56–59]. Immunoelectron microscopic analysis demonstrated that LRRK2 also localizes to various membranous structures [48]. Given the recent findings of the involvement of LRRK2 in membrane trafficking, it is possible that LRRK2 shuttles between the cytosol and membrane to regulate intracellular trafficking [60,61]. Intriguingly, Eguchi and colleagues [62] have recently discovered that LRRK2 is physiologically recruited on to the membranes of stressed lysosomes to maintain their homeostasis in co-operation with Rab29/7L1, Rab8, and Rab10. Moreover, LRRK2 often adopts a punctate localization at the perikarya, particularly when cells are treated with inhibitors for LRRK2 [63]. Kalogeropulou and colleagues [64] have demonstrated that this punctate structure is co-localized with p62/sequestosome-1, a ubiquitin-binding protein, although the nature of this punctate structure as well as whether this structure can be observed under physiological conditions remains unknown. The loss of phosphorylation at Ser910 and Ser935 of LRRK2, which are responsible for its interaction with 14-3-3 proteins, causes this translocation [63]. Although the biological significance of this phosphorylation-dependent translocation remains unknown, mouse embryonic fibroblasts (MEFs) endogenously expressing LRRK2 harboring the non-phosphorylated mutants S910A and S935A demonstrated reduced phosphorylation of Rab10 compared with wild-type MEFs [65]. Taken together, it would be reasonable to speculate that the phosphorylation at Ser910 and Ser935 promotes relocalization of LRRK2 to a subcellular compartment where LRRK2 efficiently phosphorylates Rab10. Identification of upstream kinase(s) responsible for the phosphorylation at Ser910 and Ser935 of LRRK2 will be important to elucidate the signaling pathway regulating the phosphorylation of Rab10.
Physiological functions of LRRK2: implications from loss-of-function models
Several Lrrk2 KO mouse/rat lines have been generated to date and have been extensively characterized to analyze the physiological roles of LRRK2 [66–73]. All these reports agree that the Lrrk2 KO rodents are viable, fertile, and survive up to their usual lifespan with no obvious motor phenotypes, whereas they reproducibly found morphological abnormalities in various peripheral tissues, including the lungs and kidneys. Some reports described a significant increase in the number of hippocampal neuroblasts in Lrrk2 KO animals, suggesting a role of LRRK2 in adult neurogenesis [72,74]. Collectively, these results indicate that LRRK2 does not play an essential role in animal survival, and that the loss-of-function of LRRK2 does not cause neurodegeneration, at least in rodents.
Morphological abnormalities have been reproducibly observed in the kidneys and lungs of some Lrrk2 KO rodents [68,70,71,73,75]. The kidneys of Lrrk2 KO animals show macroscopic enlargement and discoloration. Histological analyses demonstrated age-dependent accumulation of lipid-containing autofluorescent granules called lipofuscin and the autophagy substrate p62, in the renal tubules [68,70,73]. As lipofuscin granules are composed of peroxidized unsaturated fatty acids and are formed in lysosomes in aged animals, including humans [76], these results suggested that LRRK2 plays an important role in the autophagy-lysosomal pathway protecting renal tubules from age-dependent oxidative stress. The lungs of Lrrk2 KO animals show specific enlargement of lamellar bodies in alveolar epithelial type II (ATII) cells [70,73,75]. ATII cells are specialized in the synthesis and secretion of pulmonary surfactant, which is released into the alveolar lumen upon the exocytosis of lamellar bodies [77]. Lamellar bodies display a unique concentric lamellar structure under the electron microscope and are thought to be a lysosome-related organelle, as they contain lysosomal markers [78]. Taken together, the peripheral phenotypes of Lrrk2 KO animals suggest that LRRK2 deficiency compromises the homeostasis of lysosome-related organelles.
LRRK2 has a closely related ortholog known as LRRK1. To analyze whether LRRK1 can compensate for the loss of LRRK2 function, Giaime and colleagues [79] generated KO mice deficient in both Lrrk1 and Lrrk2 (Lrrk1/2 double KO (DKO)) and discovered that the Lrrk1/2 DKO mice show significant age-dependent neurodegeneration in areas relevant to PD. Immunohistochemical and biochemical analyses of Lrrk1/2 DKO mice demonstrated an impairment of the autophagy-lysosomal pathway. These results imply that LRRK1 and LRRK2 have redundant functions in protecting neurons from degeneration. Toyofuku and colleagues [80] have generated Lrrk1 KO mice, and they showed that Lrrk1 KO mice exhibited neonatal lethality within 24 h after birth presumably due to lysosomal dysfunction. They further revealed that LRRK1 spatiotemporally regulates inactivation of Rab7A, thereby promoting the fusion of endocytic and autophagic components to lysosomes. LRRK1 has also been implicated in the endosomal trafficking of epidermal growth factor (EGF) receptors [81]. Collectively, these data suggest that LRRK1 and LRRK2 have redundant functions in the autophagy-lysosomal pathway. However, comparative proteomic analyses on the interactomes of LRRK1 and LRRK2 demonstrated little overlap between the two [82,83], which suggested that LRRK1 and LRRK2 might have distinct functions. Moreover, Taylor and colleagues [84] have shown that LRRK1 variations are not a frequent cause of PD by sequencing the LRRK1 gene in FPD patients. Nevertheless, further investigation would be required for clarifying the differences and similarities between the functions of LRRK1 and LRRK2.
LRRK2 might possess a scaffolding function through interaction with other proteins, as LRRK2 has multiple protein–protein interaction domains. Herzig and colleagues [70] generated knock-in mice harboring a kinase-dead (KD) mutation (D1994S) in LRRK2. These KD mice showed essentially the same phenotypes as the Lrrk2 KO mice regarding kidney phenotypes, although they did not show any lung phenotypes. However, the expression levels of full-length LRRK2 were significantly decreased in the kidneys of KD mice. This observation again raised the possibility that reduction in the expression levels of LRRK2, but not the loss of its kinase activity, caused the kidney phenotype in D1994S knock-in mice. However, animals administered with LRRK2 inhibitors showed phenotypic changes at the kidneys and lungs in a similar manner to those observed in KO mice as discussed below. Thus, the scaffolding function of LRRK2, if any, does not seem to play an essential role in these phenotypes.
There have also been a number of reports describing the outcomes of LRRK2 inhibitor treatment in rodents [85,86]. Fell and colleagues utilized MitoPark [87] mice, which lack mitochondrial transcription factor A specifically in dopaminergic neurons, and showed the progressive degeneration of the nigrostriatal dopamine system. Although chronic oral administration of MLi-2, one of the most potent and selective inhibitors for LRRK2 [88], to MitoPark mice did not modify the progression of their behavioral and neurochemical phenotypes, MLi-2 administered mice showed similar morphological changes in the kidneys and lungs as have been observed in Lrrk2 KO mice [85]. Andersen and colleagues chronically administered another potent LRRK2 inhibitor, PFE-360 [89], into rats and observed similar changes in the kidneys but not in the lungs [86]. These results suggested that the kinase activity of LRRK2 is responsible at least for the kidney phenotypes observed in Lrrk2 KO mice. Fuji and colleagues [90] tested the peripheral effects of LRRK2 inhibitors in non-human primates. They administered GNE-7915, another potent LRRK2 inhibitor [91], to cynomolgus monkeys via oral gavage and found enlargement of lamellar bodies in lung ATII cells, similar to that observed in Lrrk2 KO lungs. There were no morphological changes observed in the kidneys or in the brain in this monkey model.
The physiological consequences of the peripheral phenotypes observed in the loss-of-function models of LRRK2 have also been studied. Lrrk2 KO mice were found to have proteinuria at the age of 18 months, which was not observed at the age of 2 months although they noticed the morphological changes including microvacuolization in Lrrk2 KO kidneys even at the age of 6 weeks [70]. These mice also manifested breathing difficulties. In non-human primates administered with an LRRK2 inhibitor, a decrease in di-22:6-BMP, a biomarker of lysosomal dysregulation was observed [90]. Overall, the administration of LRRK2 inhibitors caused adverse effects due to peripheral inhibition, although a preliminary study suggested that the peripheral changes are reversible and do not cause pulmonary dysfunction [73]. However, there was a 16.5-month interval between the onset of morphological changes in the kidneys and the physiological dysfunction (i.e. proteinuria) in Lrrk2 KO mice [70]. Thus, adverse effects on physiology caused by LRRK2 inhibitors might occur after several years of treatment also in humans. Future studies on the molecular mechanisms of these peripheral effects will help establish safer therapeutics based on LRRK2 inhibition.
Substrate phosphorylation by LRRK2: downstream signaling
Since the discovery of the pathogenic mutations in LRRK2, a large amount of effort has been put into the identification of the physiological substrates of LRRK2. However, until recently, most substrates of LRRK2 reported are phosphorylated only in in vitro experimental settings [92]. For example, Jaleel and colleagues [38] identified the ERM family of proteins, including ezrin, moesin, and radixin, as in vitro substrates of LRRK2. Although the in vitro phosphorylation of ERM proteins by LRRK2 is reproducibly observed and the peptide sequence surrounding the phosphorylation site is now widely used as a peptide substrate for LRRK2 (i.e. LRRKtide; NH2–RLGRDKYK(T)LRQIRQ–COOH, where (T) stands for the threonine phosphorylated by LRRK2), the phosphorylation of ERM proteins by LRRK2 under physiological conditions has never been described. Nevertheless, LRRK2 phosphorylates serine or threonine, but not tyrosine, in substrate proteins as well as in substrate peptides [93], indicating that LRRK2 is a Ser/Thr kinase.
There is mounting evidence supporting the idea that LRRK2 phosphorylates tau. As GWAS of sporadic PD patients have repeatedly identified risk polymorphisms in the MAPT locus encoding tau proteins [11,12,94,95], and because PD patients with LRRK2 mutations sometimes show tau accumulation [19,96,97], tau is thought to be one of the most pathologically relevant substrates. However, researchers have not yet reached a consensus regarding whether or not tau is physiologically phosphorylated by LRRK2. Screening for LRRK2 substrates have been carried out using fibroblast cells with low levels of tau expression [60]. Now that extensively validated research toolkits, such as knock-in mice harboring disease mutations, as well as highly specific brain-penetrable inhibitors for LRRK2 are available, it is important to address this question using mouse brains or primary neurons.
Apart from the physiological relevance, in vitro kinase assay experiments using artificial substrates of LRRK2 have provided important clues to elucidate the effects of the pathogenic mutations in LRRK2. Using the well-known non-specific substrate myelin-basic protein, West and colleagues [56] demonstrated that the most frequent pathogenic mutation, G2019S, increases the kinase activity of LRRK2 by two to three fold. However, the other mutations failed to reproducibly increase the in vitro kinase activity of LRRK2.
After several painstaking phosphoproteomic analyses, Steger and colleagues [60] finally identified physiologically relevant LRRK2 substrates, which were Rab proteins, including Rab8A/B and Rab10, and these were confirmed by a subsequent report [98]. To our surprise, whereas the G2019S mutation moderately increased the phosphorylation of Rab proteins in vivo, all other mutations, such as R1441G, also increased their phosphorylation by 10–20 fold [60,65]. The reason why the mutations other than G2019S increase LRRK2 kinase activity only in vivo remains unknown. This could be because the in vitro kinase assay lacks essential factor(s) required for the activation of LRRK2 by FPD mutations, or alternatively, FPD mutant forms of LRRK2 in vivo are more readily recruited to the place where LRRK2 becomes activated, resulting in higher levels of substrate phosphorylation (as discussed below).
Another intriguing LRRK2 substrate identified so far is p62/sequestosome-1 [64]. Kalogeropulou and colleagues [64] have shown that p62 binds to the ARM domain of LRRK2, and LRRK2 phosphorylates p62 at Thr138. p62 is a component of α-synuclein aggregates in PD brains [99]. It possesses a ubiquitin-binding domain as well as an LC3 interaction region (LIR), which sequestrates ubiquitinated proteins to autophagosomes for degradation. Park and colleagues [100] have also shown that p62 interacts with LRRK2 through the ARM/ANK domains of LRRK2. Given the involvement of LRRK2 in the autophagy-lysosomal pathway, it would be important to explore whether the p62 phosphorylation by LRRK2 plays a role in this pathway.
Details of the phosphorylation of Rab proteins by LRRK2
In the initial report by Steger and colleagues [60], they proposed that the phosphorylation of Rab proteins by LRRK2 inhibits their interaction with GDP-dissociation inhibitors (GDIs) and guanine nucleotide exchange factors (GEFs), which has been reproduced in a subsequent study [101]. GDIs are essential for extracting GDP-bound forms of Rab proteins from their acceptor membranes, to recycle them back to donor membranes, and GEFs are required for the facilitation of GDP-GTP exchange (reviewed in [102]). Therefore, preventing Rab proteins from interacting with GEFs/GDIs should maintain them in their GDP-bound forms on the membrane.
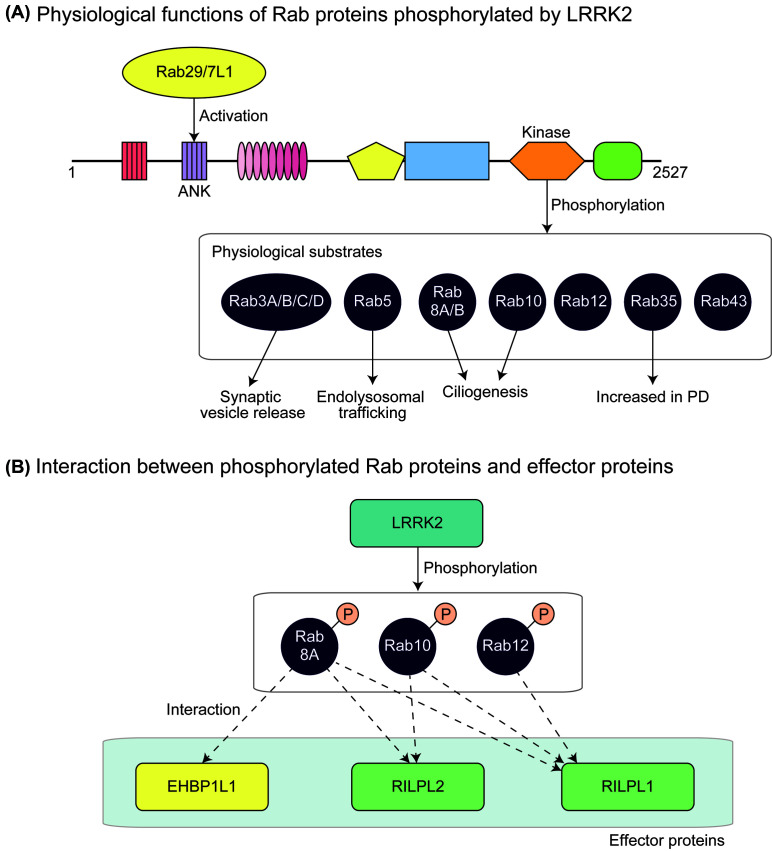
Steger and colleagues [61] further elucidated that at least ten members of the Rab GTPase family, namely Rab3A/B/C/D, Rab8A/B, Rab10, Rab12, Rab35, and Rab43, are endogenously phosphorylated by LRRK2 under physiological conditions (Figure 2). Interestingly, these Rab proteins are closely associated with each other, as suggested by genomic analyses [103]. Importantly, the authors identified Rab-interacting lysosomal protein (RILP)-like 1 (RILPL1) and RILPL2 as specific interactors for p-Rab8A, Rab10, and Rab12. RILPL1 and RILPL2 belong to the RILP family sharing the amino-terminal RILP-homology 1 (RH1) and the carboxyl-terminal RILP-homology 2 (RH2) domains. As Rab8A/B, Rab10, and RILPL1/2 are reported to regulate primary ciliogenesis [104–106], they hypothesized that LRRK2 regulates primary ciliogenesis through the phosphorylation of these Rab proteins. Indeed, they showed that cells expressing FPD mutants of LRRK2 are deficient in primary ciliogenesis [61] (Figure 2). In a recent paper by Eguchi and colleagues [62], EH domain-binding protein 1 (EHBP1) and EHBP1-like protein 1 (EHBP1L1) were identified as effector proteins responsible for maintaining the homeostasis of stressed lysosomes. This is consistent with a previous finding showing that EHBP1L1 also binds to Rab8A in a phosphorylation-dependent manner [61]. It was also reported that, in hepatocytes, EHBP1 binds to both active Rab10 and EH domain-containing 2 (EHD2) to form Rab10–EHBP1–EHD2 complex under autophagy-stimulating conditions [107]. This complex is involved in engulfment of lipid droplets (LDs) by autophagic membrane. Therefore, it would be interesting to explore the possibility that LRRK2 regulates the engulfment of LDs via Rab10 phosphorylation. Collectively, it is tempting to speculate that LRRK2 recruited by Rab29/7L1 on to lysosomal membranes recruits and phosphorylates Rab8A/B and Rab10, thereby facilitating the interaction with their effector proteins, which might play a vital role on maintaining the homeostasis of lysosomes. In the future study, it is critical to pinpoint the responsible effector protein whose function is impaired in PD.
Figure 2. Physiological functions of Rab proteins phosphorylated by LRRK2.
(A) Rab29/7L1 acts upstream of LRRK2, leading to its activation. Other Rab proteins are then endogenously phosphorylated by LRRK2. Rab3A/B/C/D are predominantly expressed in the brain and are involved in synaptic vesicle release. Rab8A/B and Rab10 are involved in endolysosomal trafficking. Rab8A/B and Rab10 have been shown to regulate primary ciliogenesis. Increased levels of Rab35 were detected in serum samples of PD patients compared with healthy controls, suggesting that Rab35 is involved in the pathogenesis of PD. (B) Phosphorylated Rab8A/B, Rab10, and Rab12 interact with RILPL1, whereas phosphorylated Rab8A and Rab10, but not Rab12, interact with RILPL2. It has also been shown that phosphorylated Rab8A binds to EHBP1L1.
The phenotypes of KO mice of substrate Rab proteins are particularly important for discussing their physiological roles and the potential effects of their phosphorylation. Mice lacking Rab10 were embryonically lethal at embryonic day 9.5 (E9.5). Embryos at E7.5 showed approximately 20% reduction in proliferating cells and large vacuoles in the cytoplasm, suggesting a marked inhibition of endosomal trafficking [108]. Mice lacking Rab8a showed microvillus atrophy, microvillus inclusions, and enlarged lysosomes in intestinal epithelial cells at 3 weeks of age, and died within 5 weeks after birth [109]. These phenotypes might be attributable to the mislocalization of apical proteins to lysosomes. DKO mice lacking both Rab8a and Rab8b showed essentially the same but exaggerated phenotypes compared with Rab8a single-KO mice, suggesting the redundant function of Rab8A and Rab8B [106]. Collectively, these results suggest that Rab8A/8B and Rab10 function in the endolysosomal pathway, which is consistent with the phenotypes observed in Lrrk2 KO mice.
Both Rab8A/8B and Rab10 are reportedly involved in primary ciliogenesis [104], and Rab8a/8b DKO cells showed a significant deficiency in primary ciliogenesis only when Rab10 was knocked down by RNAi [106]. Indeed, Steger and colleagues [61] reported that treatment of MEF with an LRRK2 inhibitor increased the number of ciliated cells. Given the proposed inhibitory function of Rab phosphorylation by LRRK2, these results are consistent with the view that LRRK2 plays an inhibitory role in ciliogenesis by phosphorylating the responsible Rab proteins. However, whether involvement of LRRK2 in ciliogenesis has any relevance to neurodegeneration in PD remains to be elucidated.
Rab3 has four isoforms, Rab3A, Rab3B, Rab3C, and Rab3D. Rab3A, Rab3B, and Rab3C are predominantly expressed in the brain and endocrine pituitary, whereas Rab3D is absent from the brain but is expressed in the endocrine pituitary, exocrine glands, and adipose tissues [110]. Schluter and colleagues [111] generated Rab3 quadruple KO (QKO) mice lacking all four isoforms. Rab3 QKO mice developed normally and were born alive but died shortly after birth because of respiratory failure. Cultured neurons from Rab3 QKO mice showed a significant impairment in the release probability at synapses. To elucidate the effects of Rab3 phosphorylation by LRRK2 in neurons, it would be important to investigate whether cultured neurons from Lrrk2 KO mice show a similar phenotype.
Although mice lacking Rab12 have not been reported in the literature to our knowledge, Rab12 KO mice have been generated by The International Mouse Phenotyping Consortium (IMPC) (https://www.mousephenotype.org/data/genes/MGI:894284). The IMPC website does not report an overt phenotype of Rab12 KO mice in its phenotype database. In contrast, homozygous Rab35 KO mice generated by the IMPC (https://www.mousephenotype.org/data/genes/MGI:1924657) are apparently embryonically lethal, while some eye phenotypes in heterozygous KO mice.
Whereas little is known about the causal association between Rab phosphorylation by LRRK2 and neurodegeneration in PD, Jeong and colleagues [112] found that the overexpression of Rab1A T75A mutant, Rab3C T94A mutant, as well as Rab35 T72A and T72D mutants caused TUNEL-positive apoptosis in primary neurons. The overexpression of Rab35 T72A and T72D mutants also caused neurodegeneration in mouse brains [112]. These results suggested that the phosphorylation of Rab35 plays an important role in the neurodegeneration observed in PD. Interestingly, in serum samples of PD patients, the levels of Rab35 were increased compared with healthy controls and patients with other neurodegenerative diseases (i.e. progressive supranuclear palsy (PSP) and multiple system atrophy) [113], further suggesting the possible involvement of Rab35 in the pathogenesis of PD.
Regulation of LRRK2 by Rab proteins: upstream signaling events
Whereas Rab proteins are the physiological substrates of LRRK2, there has been accumulating evidence suggesting that LRRK2 is also regulated by Rab proteins. Amongst the genetic risk factors for idiopathic PD identified in GWAS was the genomic region including the RAB29 (also known as RAB7L1) locus [10,11,13,95,114], and this region is assigned as PARK16 (OMIM entry 613164). The association between PARK16 and PD risk was subsequently validated in other ethnic populations [115], and in some reports, polymorphisms in the RAB29 promoter region were found to be associated with PD risk [116], further underscoring the importance of RAB29 in the pathogenesis of PD.
Meanwhile, MacLeod and colleagues [117] discovered a significant similarity between the transcriptome signatures of individuals harboring a PD risk allele either in RAB29 or LRRK2, implicating a genetic interaction between these genes. Kuwahara and colleagues [118] further provided genetic evidence suggesting that in Caenorhabditis elegans, RAB29 acts upstream of LRRK2. Importantly, they also showed that Rab29 KO mice demonstrated abnormal lysosomal pathology in the kidneys, which is identical with the phenotype observed in Lrrk2 KO mouse kidneys, supporting that the Rab29/7L1-LRRK2 axis also exists in mammals. Furthermore, there were reportedly no abnormalities in the brains of Rab29 KO mice [118], suggesting that abnormal activation of the Rab29/7L1-LRRK2 axis is involved in the neurodegeneration occurring in PD.
More recently, three independent reports by Liu and colleagues [119], Purlyte and colleagues [32], and Fujimoto and colleagues [120] described that Rab29/7L1 activates LRRK2 in cells. They showed that Rab29/7L1 recruits LRRK2 to the trans-Golgi network (TGN), possibly via interaction with the ANK domain of LRRK2. They also showed that LRRK2 p-Rab29/7L1 on both Ser71 and Thr72, which apparently abolished the ability of Rab29/7L1 to activate LRRK2, suggesting negative feedback regulation of LRRK2 [32]. As the FPD mutant forms of LRRK2 were activated more robustly by Rab29/7L1 compared with wild-type LRRK2, the FPD mutant forms of LRRK2 have been hypothesized to show enhanced localization to the TGN, thereby becoming more readily activated by Rab29/7L1. Thus, genetic and biochemical evidence suggests that LRRK2 and Rab29/7L1 operate a common signaling pathway, and overactivation of this pathway is thought to be involved in the pathogenesis of PD.
Physiological roles of LRRK2 in immune cells
In addition to the brain and peripheral tissues, LRRK2 is also highly expressed in immune cells, including B lymphocytes, monocytes, and neutrophils [121,122] (Figure 1B). In tissue culture models, LRRK2 protein expression was confirmed in murine bone-marrow derived macrophages, bone-marrow derived dendritic cells, the murine macrophage cell line RAW264.7, and the human monocytic cell line THP-1 [123–125]. It has also been reported that the expression of LRRK2 is induced in response to interferon-γ treatment in THP-1 cells as well as in human peripheral blood mononuclear cells, suggesting that LRRK2 plays a role in the innate immune system [123].
Notably, LRRK2 is genetically implicated in the pathogenesis of IBDs, including Crohn’s disease and ulcerative colitis [21–23]. In these reports, a genomic region containing the LRRK2 and MUC19 loci was identified as a susceptibility locus for IBD. Recently, Hui and colleagues [126] reported that variations in the LRRK2 locus tend to have similar effects on the odds ratio of Crohn’s disease and PD. Thus, these results not only suggest the involvement of LRRK2 in the innate immune system but imply a common mechanism underlying IBD and PD [126].
Liu and colleagues [124] investigated the roles of LRRK2 in a mouse model of acute colitis induced by dextran sulphate sodium (DSS), which depends on the innate immune response. Lrrk2 KO mice showed more severe response to DSS in their colons, including inflammatory infiltrates, thickened walls, and disruption of mucosal structures [124]. Another report from Zhang and colleagues [127] described the enhanced susceptibility of Lrrk2 KO mice to intestinal infection, and suggested a possible role of LRRK2 in maintaining symbiosis with commensal bacteria to control intestinal infection. They found that in Paneth cells in the mouse intestine, LRRK2 localized to the membrane of dense core vesicles (DCVs), which store bactericidal substances, including lysozymes, and secrete them into the intestinal lumen upon infection. Importantly, lysozymes were missorted to lysosomes and degraded in Paneth cells from Lrrk2 KO mice, leading to a deficiency of bactericidal activity. These results are in-line with the hypothesis that LRRK2 functions in regulating endolysosomal trafficking. Indeed, they found Rab10 as well as Rab2A localized to DCVs, although the involvement of phosphorylation-dependent regulation of Rab proteins is unclear at this stage. Although the molecular mechanisms are not well understood, these reports at least suggest that LRRK2 plays a role in the innate immune response.
Activation of microglia is a hallmark in the brain pathology of neurodegenerative diseases, including Alzheimer disease (AD) and PD (reviewed in [128]). Microglia are thought to be responsible for phagocytic activity in the brain (reviewed in [129]). LRRK2 has been shown to be expressed in activated microglia induced by an intracranial injection of lipopolysaccharide (LPS) to mouse brains [130]. LRRK2 is also expressed in primary cultured murine microglial cells [131]. Lrrk2 KO primary microglia showed an attenuated inflammatory response to LPS treatment [132]. Daher and colleagues [133] showed that in rat brains, the administration of LPS as well as adeno-associated virus-mediated overexpression of human α-synuclein caused the selective degeneration of dopaminergic neurons in the substantia nigra, which was attenuated in Lrrk2 KO rats. They also showed that, in the brains of Lrrk2 KO rats, a smaller number of CD68-positive myeloid cells, including macrophages, monocytes, and microglia, were observed in the lesioned substantia nigra, suggesting an attenuated activation of the inflammatory response in Lrrk2 KO rats. Consistent with this finding, primary microglia cultured from Lrrk2 KO mice showed decreased interleukin 1β (IL-1β) production in response to LPS [132], which has also been observed in Lrrk2 KO macrophages [134]. In this report by Liu and colleagues, they showed that the activation of inflammasomes containing NLR family CARD domain-containing protein 4 (NLRC4) in response to the infection of pathogens was insufficient in Lrrk2 KO macrophages, leading to the reduced production of IL-1β [134]. Although the precise mechanisms of how LRRK2 regulates the activation of NLRC4-containing inflammasomes remain unclear, they suggested that the phosphorylation at Ser533 of NLRC4 by LRRK2 might be involved in this process.
Given the pathological evidence that inflammation plays an important role in the pathogenesis of PD (reviewed in [135]), it is possible to hypothesize that LRRK2 plays an important role in the innate immune response to pathogens under healthy conditions, but once overactivated, it has a deleterious effect on neurons through abnormal activation of the microglial inflammatory response.
Pathological roles of LRRK2
Histopathology of FPD patients harboring LRRK2 mutations
Neuropathological studies on PD patients harboring LRRK2 mutations have demonstrated surprisingly diverse pathology amongst patients, which has been summarized in a review by Schneider and Alcalay [136]. So far, many neuropathological studies have been conducted on autopsy samples from PD patients with LRRK2 mutations, including N1437H [18], R1441C [8,14], R1441G [16,20], Y1699C [15], G2019S [97,137–142], and I2020T [17,19]. Although a loss of dopaminergic neurons in the substantia nigra was commonly observed amongst patients with LRRK2 mutations, the accumulation of phosphorylated α-synuclein (e.g. LB and Lewy neurites), which is another hallmark pathology of PD, was not always observed [16,17]. Moreover, some patients showed an accumulation of phosphorylated tau as well as Transactive Response DNA binding protein 43 kDa (TDP-43). Although Kalia and colleagues [143] reported that LB pathology is more frequently associated with the G2019S mutation compared with the other mutations, Vilas and colleagues [97] reported two cases of G2019S carriers that did not show LB pathology and instead showed the accumulation of phosphorylated tau in astrocytes and neurons, which is a pathology consistent with PSP. Such diverse pathology was sometimes observed within the same family (e.g. a family with the R1441C mutation reported in [144], and a family with the I2020T mutation reported in [19]), suggesting that the pathological manifestations of PD patients harboring mutations in LRRK2 can be modulated by other genetic or environmental factors.
LRRK2 is genetically involved in the pathogenesis of both sporadic and FPD. Although FPD mutations in LRRK2 abnormally increase the phosphorylation of substrate Rab proteins, whether the phosphorylation of LRRK2 substrates is increased in sporadic PD has not been clarified. Maio and colleagues [145] recently reported that the LRRK2 kinase activity is increased in dopaminergic neurons in the brains of patients with idiopathic PD compared with healthy controls using a newly developed proximity ligation assay. Importantly, an immunohistochemical analysis demonstrated dramatically up-regulated Rab10 phosphorylation in PD brains, although the sample size was relatively small (eight controls compared with seven PDs). These results would encourage researchers to utilize the levels of Rab10 phosphorylation in peripheral samples for diagnosing as well as tracking PD. Recently, Fan and colleagues [122] reported the usefulness of peripheral blood neutrophils for quantitating the levels of Rab10 phosphorylation in human samples. This discovery opened up the possibility of investigating changes in Rab10 phosphorylation in PD patients, although a more high-throughput assay (e.g. ELISA) rather than immunoblotting would be required for undertaking a large-scale comparison between healthy elderly individuals and PD patients.
Animals expressing FPD mutant forms of LRRK2
Since the discovery of pathogenic mutations in LRRK2 that are linked with FPD, researchers have generated transgenic animals overexpressing LRRK2 in the brain or knock-in mice harboring disease-linked mutations in their endogenous Lrrk2 gene, in the hope that such animals would model the disease and be useful for elucidating the molecular mechanisms of the disease (summarized in [146]). Transgenic rodents developed using bacterial artificial chromosomes (BACs) carrying wild-type or mutant forms of either the human or murine LRRK2 gene showed a subtle but significant reduction in the level of striatal dopamine, although they failed to show nigral neurodegeneration or LB-like pathology [147–149]. Of note, these reports consistently described an increased staining of phosphorylated tau in the striatum.
Besides BAC-based transgenic animals, cDNA-based transgenic mice in which transgene expression is driven by an artificial promoter have been generated (summarized in [146]). Transgenic mice overexpressing G2019S LRRK2 under the cytomegalovirus enhancer and platelet-derived growth factor-β chain promoter showed significant loss of dopaminergic neurons at 12–16 months of age [150], which was not observed at younger ages [151]. On the other hand, another transgenic line overexpressing G2019S LRRK2 in dopaminergic neurons under the Pitx3 promoter in a tetracycline-dependent manner did not show nigral neurodegeneration, but again, they showed impaired release of dopamine in the striatum [152]. Considering the absence of neurodegeneration in BAC transgenic mouse lines, it has been controversial as to whether the phenotypes of cDNA-based transgenic mice have relevance to the pathophysiology of PD [146].
In this sense, knock-in mice harboring a mutation linked with FPD should be more relevant to physiological conditions in terms of the expression levels and the place where the transgenes are expressed. To date, none of the generated knock-in mice showed age-dependent neurodegeneration. However, again, Tong and colleagues [153] showed impaired dopaminergic transmission in R1441C knock-in mice and suggested impaired function of the dopamine D2 receptor. Furthermore, a more recent report by Yue and colleagues [154] described a significant reduction in the extracellular dopamine induced by amphetamine as well as an increase in the staining of phosphorylated tau in the striatum of G2019S knock-in mice. Overall, these results suggest that FPD mutant forms of LRRK2 impair dopaminergic transmission, although the underlying molecular mechanisms remain unknown.
In addition to the neuron-associated phenotypes, microglia-associated phenotypes have also been reported in LRRK2 transgenic rodents. BAC-based transgenic rats overexpressing human G2019S LRRK2 were more susceptible to LPS-induced nigral degeneration [155]. A larger number of CD68-positive myeloid cells were observed in the degenerated area in the brains of rats injected with LPS compared with non-transgenic rats. These results are consistent with the previous report from the same group showing opposite effects in Lrrk2 KO mice [130]. However, such augmented responses to LPS were not observed in the recently reported BAC-based transgenic mice overexpressing human G2019S LRRK2 [156], and hence the effects of the overexpression of FPD mutant forms of LRRK2 on microglia remain controversial.
Molecular mechanisms of neurodegeneration by LRRK2: hypotheses
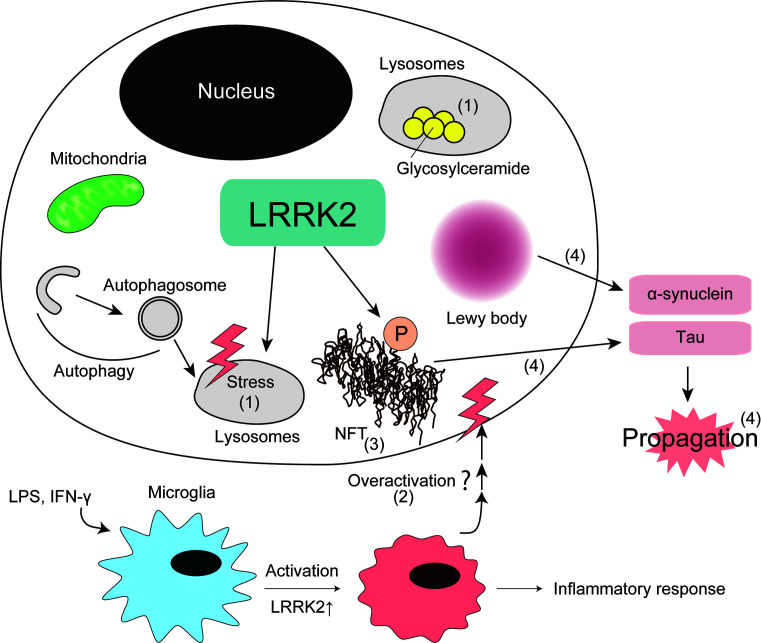
In this section, we summarize hypotheses of how dysfunction of LRRK2 leads to neurodegeneration in PD based on the current understanding of physiological and pathological roles of LRRK2 (Figure 3).
Figure 3. Pathological function of LRRK2 in PD.
Possible molecular mechanisms of PD pathology caused by LRRK2 based on the literature. (1) Lysosomal dysfunction, (2) microglia overactivation, (3) tau accumulation, and (4) prion-like propagation of aggregation-prone proteins.
Lysosomal dysfunction
Considering that Lrrk2 KO mice showed peripheral phenotypes associated with lysosomal dysfunction, it is reasonable to hypothesize that LRRK2 has some lysosome-associated functions also in neurons. Although neurons of Lrrk2 KO mice appear to be normal, Lrrk1/2 DKO mice showed age-dependent neurodegeneration and lysosomal dysfunction [79], suggesting that the lysosome-associated functions of LRRK2 is redundant, and can be compensated by LRRK1 in neurons. In this sense, it would be interesting to explore the possibility that there is a common lysosome-associated signaling pathway regulated by both LRRK1 and LRRK2 in neurons. In addition, considering the autosomal-dominant inheritance of LRRK2 mutations, which suggests a gain-of-function mechanism, it would be important to investigate whether the overactivation of LRRK2 also causes the dysfunction of lysosomes.
The involvement of lysosomal dysfunction in the pathogenesis of PD is also supported by two genetic findings. First, loss-of-function mutations in the ATP13A2 gene, which is also called PARK9, have been found in families with a rare hereditary form of juvenile-onset parkinsonism, called Kufor–Rakeb syndrome (reviewed in [157]). ATP13A2 is a lysosomal P-type ATPase [158], and a cell study has suggested that ATP13A2 is involved in the homeostasis of intracellular Zn2+ [159]. As compromising Zn2+ homeostasis leads to impaired lysosomal function, it has been strongly suggested that PARK9-type parkinsonism is caused by lysosomal dysfunction.
Another line of evidence of lysosomal involvement in the pathogenesis of sporadic PD is from etiological studies of Gaucher’s disease (GD), showing a high prevalence of PD in GD patients [160]. GD is an autosomal recessive lysosomal storage disorder caused by mutations in the GBA gene encoding glucocerebrosidase (GCase). GCase is a lysosomal enzyme responsible for the degradation of glucocerebroside to glucose and ceramide. GBA mutations decrease GCase activity, leading to the accumulation of glucocerebroside in lysosomes, which in turn compromises lysosomal activity (reviewed in [161]). More recently, Sidransky and colleagues [162] undertook a large-scale genetic study and reported a strong association between GBA mutations and sporadic PD.
Collectively, it is most likely that the overactivation of LRRK2 compromises lysosomal homeostasis, thereby leading to the neurodegeneration observed in PD.
Overactivation of microglia
There have been a large number of reports describing an increase in blood cytokine levels in PD patients as well as in PD model animals (reviewed in [163]), suggesting that inflammation plays a role in the pathogenesis of PD. Given that the expression of LRRK2 has been shown to be up-regulated in response to LPS and inflammatory cytokines, it is likely that LRRK2 is involved in the innate immune response in the brain as well as peripheral tissues.
Given that Lrrk2 KO mice show vulnerability to experimentally induced colitis as well as to intestinal infection, LRRK2 might play an important role in maintaining the innate immune system active in peripheral tissues. LRRK2 may also play a role in activating microglia in the brain, as suggested by rodent models injected with LPS to elicit neuroinflammation. Therefore, although systemic activation of LRRK2 might be beneficial outside the brain in terms of protection from bacterial infection, it also causes the overactivation of microglia in the brain, which can somehow cause deleterious effects on neurons. Indeed, it was reported very recently that chronic inflammation in the periphery can modulate neuropathology in the brain in a mouse model of AD [164]. Therefore, maintaining normal LRRK2 activity might be important for balancing the peripheral demand for innate immunity and the overactivation of microglia in the brain.
Abnormal tau accumulation
Accumulation of abnormally phosphorylated tau, a microtubule-associated protein, is a hallmark pathology of several neurological disorders, including AD, frontotemporal dementia, Pick disease, and corticobasal degeneration (reviewed in [165]). Although GWAS indicate that tau is also involved in the pathogenesis of sporadic PD besides α-synuclein and LRRK2 [11,13], its precise role has not been well understood.
As mentioned above, a number of reports have described that LRRK2 promotes tau phosphorylation in the brain in BAC-based transgenic mice as well as in knock-in mice of FPD mutant forms of LRRK2. Whereas the direct phosphorylation of tau by recombinant LRRK2 in an in vitro assay has been observed [166], some reports suggested that LRRK2 phosphorylates tau in the presence of tubulin or microtubules [167,168]. Other reports proposed that LRRK2 directly interacts with GSK-3β via the LRRK2 kinase domain, and this interaction enhances the kinase activity of GSK-3β [169]. In this report, the phosphorylated GSK-3β phosphorylates tau at Ser396. tau phosphorylation at Ser396 was elevated in cells overexpressing LRRK2 G2019S compared with those overexpressing WT LRRK2. More recently, Ohta and colleagues [170] also showed that induced pluripotent stem (iPS) cells established from PD patients carrying I2020T mutation showed activation of GSK-3β and high levels of tau phosphorylation. Lin and colleagues [171] have reported that, in fruitfly neurons, overexpression of LRRK2 G2019S promoted the recruitment of activated Shaggy, a fly homolog of GSK-3β, to dendrites, leading to accumulation of hyperphosphorylated tau in dendrites and dendrite degeneration. Shanley and colleagues [172] have found that LRRK2 strongly binds to tau in vitro and that LRRK2 is co-immunoprecipitated with tau and Cdk5, the latter of which is one of the major protein kinases phosphorylating tau besides GSK-3β. Based on these observations, they suggested that LRRK2 might serve as a scaffold protein, facilitating the phosphorylation by Cdk5. It has also been shown that microtubule affinity-regulating kinase 1 (MARK1) also directly interacts with LRRK2 and phosphorylated by LRRK2 at Thr215 and Ser219 in vitro and in cells. MARK1 plays an important role in regulating microtubule stability through phosphorylation of microtubule-bound tau [173]. Collectively, it remains possible that LRRK2 recruits or activates tau kinases, such as GSK-3β, Cdk5, and MARK1, to indirectly promote tau phosphorylation. Therefore, it remains controversial as to whether LRRK2 directly or indirectly promotes tau phosphorylation.
Some, but not all PD patients harboring LRRK2 mutations manifested tau accumulation in the brain [15,19]. However, tau pathology is also found in healthy elderly controls [174], which makes it difficult to conclude the causal association between the tau accumulation observed in PD patients harboring LRRK2 mutations and neurodegeneration.
Prion-like propagation of PD-associated proteins
It has been experimentally established that intracellularly aggregated proteins, including α-synuclein and tau, are transferred from cell to cell (reviewed in [175]). This transmission is generally called ‘prion-like propagation’, as the spreading of abnormally accumulated proteins in the brain resembles prion diseases (reviewed in [176]).
It has been pointed out that the histopathological manifestations in the brains of PD patients harboring FPD mutations in LRRK2 are pleiotropic, ranging from pure nigral degeneration without α-synuclein deposition [16,17,20], to dementia with LB (DLB)-like widespread α-synuclein pathology [8,15]. Although the mechanism underlying this prion-like propagation of α-synuclein is still controversial, it would be reasonable to conceive that the process involves intracellular trafficking for transporting aggregated proteins out of cells and for taking them up into cells. Therefore, it is possible that LRRK2 is involved in the propagation mechanisms, given its potential role in the regulation of Rab proteins. The first simple but fundamental experiment would be to investigate the propagation of α-synuclein and in Lrrk2 KO mice, as well as in mice treated with LRRK2 inhibitors. If the loss-of-function of LRRK2 attenuates the propagation, then identification of responsible substrates of LRRK2 would be the next step. Considering the proposed functional redundancy between LRRK1 and LRRK2 in the brain, it would also be interesting to test these hypotheses in Lrrk1/2 DKO mice.
Conclusion
This review aimed at providing a systematic overview of the current understanding of the physiological functions of LRRK2, to discuss how LRRK2 causes PD. Although it has been 14 years since the discovery of the pathogenic mutations in LRRK2, research on the physiological functions of LRRK2 has only just begun, particularly in the context of its involvement in intracellular membrane trafficking. Further investigation into the details of the functions of LRRK2 should be conducted to establish mechanism-based therapies for PD, which currently remains incurable.
Acknowledgements
We thank our current and past laboratory members for helpful discussions.
Abbreviations
- AD
Alzheimer disease
- ATII
alveolar epithelial type II
- BAC
bacterial artificial chromosome
- COR
carboxyl terminal of ROC
- DCV
dense core vesicle
- DKO
double knockout
- DSS
dextran sulphate sodium
- EHBP1
EH domain-binding protein 1
- EHBP1L1
EHBP1-like protein 1
- EHD2
EH domain-containing 2
- FPD
familial Parkinson’s disease
- GCase
glucocerebrosidase
- GD
Gaucher’s disease
- GDI
GDP-dissociation inhibitor
- GEF
guanine nucleotide exchange factor
- GWAS
genome-wide association study
- IBD
inflammatory bowel disease
- IL-1β
interleukin 1β
- IMPC
International Mouse Phenotyping Consortium
- KD
kinase-dead
- KO
knockout
- LB
Lewy body
- LPS
lipopolysaccharide
- LRRK1/2
leucine-rich repeat kinase 1/2
- MEF
mouse embryonic fibroblast
- NLRC4
NLR family CARD domain-containing protein 4
- PD
Parkinson’s disease
- PSP
progressive supranuclear palsy
- QKO
quadruple KO
- RILP
Rab-interacting lysosomal protein
- RILPL1/2
RILP-like protein 1/2
- ROC
Ras of complex protein
Contributor Information
Genta Ito, Email: genta@mol.f.u-tokyo.ac.jp.
Taisuke Tomita, Email: taisuke@mol.f.u-tokyo.ac.jp.
Author contribution
M.A., G.I., and T.T. wrote the paper.
Competing interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was supported in part by the Grants-in-Aid for Scientific Research (A) [grant number 15H02492 (to T.T.)]; the Grants-in-Aid for Scientific Research (C) [grant number 17K08265 (to G.I.)]; the Challenging Exploratory Research [grant number 16K15229 to (T.T.)] from the Japan Society for the Promotion of Science, and Scientific Research on Innovative Areas from the Ministry of Education, Culture, Sports, Science and Technology [grant number 26111705 (to T.T.)]; the Uehara Memorial Foundation; and the Mitsubishi Foundation [grant number 32].
References
- 1.Bosgraaf L. and van Haastert P.J.M. (2003) Roc, a Ras/GTPase domain in complex proteins. Biochim. Biophys. Acta 1643, 5–10 10.1016/j.bbamcr.2003.08.008 [DOI] [PubMed] [Google Scholar]
- 2.Sveinbjornsdottir S. (2016) The clinical symptoms of Parkinson’s disease. J. Neurochem. 139, 318–324 10.1111/jnc.13691 [DOI] [PubMed] [Google Scholar]
- 3.Fahn S. (2003) Description of Parkinson’s disease as a clinical syndrome. Ann. N.Y. Acad. Sci. 991, 1–14 10.1111/j.1749-6632.2003.tb07458.x [DOI] [PubMed] [Google Scholar]
- 4.Spillantini M.G., Crowther R.A., Jakes R., Hasegawa M. and Goedert M. (1998) alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with lewy bodies. Proc. Natl. Acad. Sci. U.S.A. 95, 6469–6473 10.1073/pnas.95.11.6469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baba M., Nakajo S., Tu P.-H., Tomita T., Nakaya K., Lee V.M.-Y. et al. (1998) Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson’s disease and dementia with Lewy bodies. Am. J. Pathol. 152, 879–884 [PMC free article] [PubMed] [Google Scholar]
- 6.Funayama M., Hasegawa K., Kowa H., Saito M., Tsuji S. and Obata F. (2002) A new locus for Parkinson’s disease (PARK8) maps to chromosome 12p11.2-q13.1. Ann. Neurol. 51, 296–301 10.1002/ana.10113 [DOI] [PubMed] [Google Scholar]
- 7.Paisán-Ruíz C., Jain S., Evans E.W., Gilks W.P., Simón J., van der Brug M. et al. (2004) Cloning of the gene containing mutations that cause PARK8-linked Parkinson’s disease. Neuron 44, 595–600 10.1016/j.neuron.2004.10.023 [DOI] [PubMed] [Google Scholar]
- 8.Zimprich A., Biskup S., Leitner P., Lichtner P., Farrer M.J., Lincoln S. et al. (2004) Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron 44, 601–607 10.1016/j.neuron.2004.11.005 [DOI] [PubMed] [Google Scholar]
- 9.Cookson M.R. (2015) LRRK2 pathways leading to neurodegeneration. Curr. Neurol. Neurosci. Rep. 15, 10.1007/s11910-015-0564-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Satake W., Nakabayashi Y., Mizuta I., Hirota Y., Ito C., Kubo M. et al. (2009) Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson’s disease. Nat. Genet. 41, 1303–1307 10.1038/ng.485 [DOI] [PubMed] [Google Scholar]
- 11.Simón-Sánchez J., Schulte C., Bras J.M., Sharma M., Gibbs J.R., Berg D. et al. (2009) Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat. Genet. 41, 1308–1312 10.1038/ng.487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klein C. and Ziegler A. (2011) Imputation of sequence variants for identification of genetic risks for Parkinson’s disease: a meta-analysis of genome-wide association studies. Lancet 377, 641–649 10.1016/S0140-6736(10)62345-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nalls M.a., Pankratz N., Lill C.M., Do C.B., Hernandez D.G., Saad M. et al. (2014) Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson’s disease. Nat. Genet. 56, 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wszolek Z., Pfeiffer R. and Tsuboi Y. (2004) Autosomal dominant parkinsonism associated with variable synuclein and tau pathology. Neurology 62, 1619–1622 10.1212/01.WNL.0000125015.06989.DB [DOI] [PubMed] [Google Scholar]
- 15.Khan N.L., Jain S., Lynch J.M., Pavese N., Abou-Sleiman P.M., Holton J.L. et al. (2005) Mutations in the gene LRRK2 encoding dardarin (PARK8) cause familial Parkinson’s disease: clinical, pathological, olfactory and functional imaging and genetic data. Brain 128, 2786–2796 10.1093/brain/awh667 [DOI] [PubMed] [Google Scholar]
- 16.Martí-Massó J.-F.F., Ruiz-Martínez J., Bolaño M.J., Ruiz I., Gorostidi A., Moreno F. et al. (2009) Neuropathology of Parkinson’s disease with the R1441G mutation in LRRK2. Mov. Disord. 24, 1998–2001 10.1002/mds.22677 [DOI] [PubMed] [Google Scholar]
- 17.Hasegawa K., Stoessl A.J., Yokoyama T., Kowa H., Wszolek Z.K. and Yagishita S. (2009) Familial parkinsonism: study of original Sagamihara PARK8 (I2020T) kindred with variable clinicopathologic outcomes. Parkinsonism Relat. Disord. 15, 300–306 10.1016/j.parkreldis.2008.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Puschmann A., Englund E., Ross O.A., Vilariño-Güell C., Lincoln S.J., Kachergus J.M. et al. (2012) First neuropathological description of a patient with Parkinson’s disease and LRRK2 p.N1437H mutation. Parkinsonism Relat. Disord. 18, 332–338 10.1016/j.parkreldis.2011.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ujiie S., Hatano T., Kubo S.-I., Imai S., Sato S., Uchihara T. et al. (2012) LRRK2 I2020T mutation is associated with tau pathology. Parkinsonism Relat. Disord. 18, 819–823 10.1016/j.parkreldis.2012.03.024 [DOI] [PubMed] [Google Scholar]
- 20.Vilas D., Gelpi E., Aldecoa I., Grau O., Rodriguez-Diehl R., Jaumà S. et al. (2018) Lack of central and peripheral nervous system synuclein pathology in R1441G LRRK2-associated Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry, 10.1136/jnnp-2018-318473 [DOI] [PubMed] [Google Scholar]
- 21.Barrett J.C., Hansoul S., Nicolae D.L., Cho J.H., Duerr R.H., Rioux J.D. et al. (2008) Genome-wide association defines more than 30 distinct susceptibility loci for Crohn’s disease. Nat. Genet. 40, 955–962 10.1038/ng.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franke A., McGovern D.P.B., Barrett J.C., Wang K., Radford-Smith G.L., Ahmad T. et al. (2010) Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nat. Genet. 42, 1118–1125 10.1038/ng.717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Umeno J., Asano K., Matsushita T., Matsumoto T., Kiyohara Y., Iida M. et al. (2011) Meta-analysis of published studies identified eight additional common susceptibility loci for Crohn’s disease and ulcerative colitis. Inflamm. Bowel Dis. 17, 2407–2415 10.1002/ibd.21651 [DOI] [PubMed] [Google Scholar]
- 24.Zhang F.-R., Huang W., Chen S.-M., Sun L.-D., Liu H., Li Y. et al. (2009) Genome wide association study of leprosy. N. Engl. J. Med. 361, 2609–2618 10.1056/NEJMoa0903753 [DOI] [PubMed] [Google Scholar]
- 25.Fava V.M., Manry J., Cobat A., Orlova M., Van Thuc N., Ba N.N. et al. (2016) A missense LRRK2 variant is a risk factor for excessive inflammatory responses in leprosy. PLoS Negl. Trop. Dis. 10, 1–14 10.1371/journal.pntd.0004412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y.M., Zhou X.J., Cheng F.J., Qi Y.Y., Hou P., Zhao M.H. et al. (2017) Autophagy-related gene LRRK2 is likely a susceptibility gene for systemic lupus erythematosus in northern Han Chinese. Oncotarget 8, 13754–13761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Civiero L., Vancraenenbroeck R., Belluzzi E., Beilina A., Lobbestael E., Reyniers L. et al. (2012) Biochemical characterization of highly purified leucine-rich repeat kinases 1 and 2 demonstrates formation of homodimers. PLoS ONE 7, e43472 10.1371/journal.pone.0043472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mills R.D., Mulhern T.D., Cheng H.-C. and Culvenor J.G. (2012) Analysis of LRRK2 accessory repeat domains: prediction of repeat length, number and sites of Parkinson’s disease mutations. Biochem. Soc. Trans. 40, 1086–1089 10.1042/BST20120088 [DOI] [PubMed] [Google Scholar]
- 29.Vancraenenbroeck R., Lobbestael E., Weeks S.D., Strelkov S.V, Baekelandt V., Taymans J.-M. et al. (2012) Expression, purification and preliminary biochemical and structural characterization of the leucine rich repeat namesake domain of leucine rich repeat kinase 2. Biochim. Biophys. Acta 1824, 450–460 10.1016/j.bbapap.2011.12.009 [DOI] [PubMed] [Google Scholar]
- 30.Sejwal K., Chami M., Rémigy H., Vancraenenbroeck R., Sibran W., Sütterlin R. et al. (2017) Cryo-EM analysis of homodimeric full-length LRRK2 and LRRK1 protein complexes. Sci. Rep., 1–12, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peifer M., Berg S. and Reynolds A.B. (1994) A repeating amino acid motif shared by proteins with diverse cellular roles. Cell 76, 789–791 [DOI] [PubMed] [Google Scholar]
- 32.Purlyte E., Dhekne H.S., Sarhan A.R., Gomez R., Lis P., Wightman M. et al. (2018) Rab29 activation of the Parkinson’s disease‐associated LRRK2 kinase. EMBO J. 37, 1–18 10.15252/embj.201798099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hesketh G.G., Pérez-Dorado I., Jackson L.P., Wartosch L., Schäfer I.B., Gray S.R. et al. (2014) VARP is recruited on to endosomes by direct interaction with retromer, where together they function in export to the cell surface. Dev. Cell 29, 591–606 10.1016/j.devcel.2014.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guaitoli G., Raimondi F., Gilsbach B.K., Gómez-Llorente Y., Deyaert E., Renzi F. et al. (2016) Structural model of the dimeric Parkinson’s protein LRRK2 reveals a compact architecture involving distant interdomain contacts. Proc. Natl. Acad. Sci. U.S.A. 113, E4357–E4366 10.1073/pnas.1523708113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jeffrey P.D., Tong L. and Pavletich N.P. (2000) Structural basis of inhibition of CDK - cyclin complexes by INK4 inhibitors. Genes Dev. 14, 3115–3125 10.1101/gad.851100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nichols R.J., Dzamko N., Morrice N.A., Campbell D.G., Deak M., Ordureau A. et al. (2010) 14-3-3 binding to LRRK2 is disrupted by multiple Parkinson’s disease-associated mutations and regulates cytoplasmic localization. Biochem. J. 430, 393–404 10.1042/BJ20100483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lobbestael E., Zhao J., Rudenko I.N., Beylina A., Gao F., Wetter J. et al. (2013) Identification of protein phosphatase 1 as a regulator of the LRRK2 phosphorylation cycle. Biochem. J. 10.1042/BJ20121772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jaleel M., Nichols R.J., Deak M., Campbell D.G., Gillardon F., Knebel A. et al. (2007) LRRK2 phosphorylates moesin at threonine-558: characterization of how Parkinson’s disease mutants affect kinase activity. Biochem. J. 405, 307–317 10.1042/BJ20070209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kamikawaji S., Ito G. and Iwatsubo T. (2009) Identification of the autophosphorylation sites of LRRK2. Biochemistry 48, 10963–10975 10.1021/bi9011379 [DOI] [PubMed] [Google Scholar]
- 40.Piccoli G., Onofri F., Cirnaru M.D., Kaiser C. J.O., Jagtap P., Kastenmüller A. et al. (2014) LRRK2 binds to neuronal vesicles through protein interactions mediated by its C-terminal WD40 domain. Mol. Cell. Biol. 10.1128/MCB.00914-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ito G., Okai T., Fujino G., Takeda K., Ichijo H., Katada T. et al. (2007) GTP binding is essential to the protein kinase activity of LRRK2, a causative gene product for familial Parkinson’s disease. Biochemistry 46, 1380–1388 10.1021/bi061960m [DOI] [PubMed] [Google Scholar]
- 42.West A.B., Moore D.J., Choi C., Andrabi S.A., Li X., Dikeman D. et al. (2007) Parkinson’s disease-associated mutations in LRRK2 link enhanced GTP-binding and kinase activities to neuronal toxicity. Hum. Mol. Genet. 16, 223–232 10.1093/hmg/ddl471 [DOI] [PubMed] [Google Scholar]
- 43.Vojtek A.B., Hollenberg S.M. and Cooper J.A. (1993) Mammalian Ras interacts directly with the serine/threonine kinase raf. Cell 74, 205–214 10.1016/0092-8674(93)90307-C [DOI] [PubMed] [Google Scholar]
- 44.Li X., Tan Y.-C., Poulose S., Olanow C.W., Huang X.-Y. and Yue Z. (2007) Leucine-rich repeat kinase 2 (LRRK2)/PARK8 possesses GTPase activity that is altered in familial Parkinson’s disease R1441C/G mutants. J. Neurochem. 103, 238–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lewis P.A., Greggio E., Beilina A., Jain S., Baker A.K. and Cookson M.R. (2007) The R1441C mutation of LRRK2 disrupts GTP hydrolysis. Biochem. Biophys. Res. Commun. 357, 668–671 10.1016/j.bbrc.2007.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Civiero L. and Bubacco L. (2012) Human leucine-rich repeat kinase 1 and 2: intersecting or unrelated functions? Biochem. Soc. Trans. 40, 1095–1101 10.1042/BST20120123 [DOI] [PubMed] [Google Scholar]
- 47.Giasson B.I., Covy J.P., Bonini N.M., Hurtig H.I., Farrer M.J., Trojanowski J.Q. et al. (2006) Biochemical and pathological characterization of Lrrk2. Ann. Neurol. 59, 315–322 10.1002/ana.20791 [DOI] [PubMed] [Google Scholar]
- 48.Biskup S., Moore D.J., Celsi F., Higashi S., West A.B., Andrabi S.A. et al. (2006) Localization of LRRK2 to membranous and vesicular structures in mammalian brain. Ann. Neurol. 60, 557–569 10.1002/ana.21019 [DOI] [PubMed] [Google Scholar]
- 49.Taymans J.-M., Van den Haute C. and Baekelandt V. (2006) Distribution of PINK1 and LRRK2 in rat and mouse brain. J. Neurochem. 98, 951–961 10.1111/j.1471-4159.2006.03919.x [DOI] [PubMed] [Google Scholar]
- 50.Higashi S., Moore D.J., Colebrooke R.E., Biskup S., Dawson V.L., Arai H. et al. (2007) Expression and localization of Parkinson’s disease-associated leucine-rich repeat kinase 2 in the mouse brain. J. Neurochem. 100, 368–381 10.1111/j.1471-4159.2006.04246.x [DOI] [PubMed] [Google Scholar]
- 51.Davies P., Hinkle K.M., Sukar N.N., Sepulveda B., Mesias R., Serrano G. et al. (2013) Comprehensive characterization and optimization of leucine rich repeat kinase 2 (LRRK2) monoclonal antibodies. Biochem. J. 453, 101–113 10.1042/BJ20121742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.West A.B., Cowell R.M., Daher J.P.L., Moehle M.S., Hinkle K.M., Melrose H.L. et al. (2014) Differential LRRK2 expression in the cortex, striatum, and substantia nigra in transgenic and nontransgenic rodents. J. Comp. Neurol. 522, 2465–2480 10.1002/cne.23583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miklossy J., Arai T., Guo J.P., Klegeris A., Yu S., McGeer E.G. et al. (2006) LRRK2 expression in normal and pathologic human brain and in human cell lines. J. Neuropathol. Exp. Neurol. 65, 953–963 10.1097/01.jnen.0000235121.98052.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Higashi S., Biskup S., West A.B., Trinkaus D., Dawson V.L., Faull R.L.M. et al. (2007) Localization of Parkinson’s disease-associated LRRK2 in normal and pathological human brain. Brain Res. 1155, 208–219 10.1016/j.brainres.2007.04.034 [DOI] [PubMed] [Google Scholar]
- 55.Sharma S., Bandopadhyay R., Lashley T., Renton A.E.M., Kingsbury A.E., Kumaran R. et al. (2011) LRRK2 expression in idiopathic and G2019S positive Parkinson’s disease subjects: a morphological and quantitative study. Neuropathol. Appl. Neurobiol. 37, 777–790 10.1111/j.1365-2990.2011.01187.x [DOI] [PubMed] [Google Scholar]
- 56.West A.B., Moore D.J., Biskup S., Bugayenko A., Smith W.W., Ross C.A. et al. (2005) Parkinson’s disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc. Natl. Acad. Sci. U.S.A. 102, 16842–16847 10.1073/pnas.0507360102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gloeckner C.J., Kinkl N., Schumacher A., Braun R.J., O’Neill E., Meitinger T. et al. (2006) The Parkinson disease causing LRRK2 mutation I T is associated with increased kinase activity Hum. Mol. Genet. 15, 2020–232 10.1093/hmg/ddi439 [DOI] [PubMed] [Google Scholar]
- 58.Hatano T., Kubo S.-I., Imai S., Maeda M., Ishikawa K., Mizuno Y. et al. (2007) Leucine-rich repeat kinase 2 associates with lipid rafts. Hum. Mol. Genet. 16, 678–690 10.1093/hmg/ddm013 [DOI] [PubMed] [Google Scholar]
- 59.Ito G. and Iwatsubo T. (2012) Re-examination of the dimerization state of leucine-rich repeat kinase 2: predominance of the monomeric form. Biochem. J. 441, 987–994 10.1042/BJ20111215 [DOI] [PubMed] [Google Scholar]
- 60.Steger M., Tonelli F., Ito G., Davies P., Trost M., Vetter M. et al. (2016) Phosphoproteomics reveals that Parkinson’s disease kinase LRRK2 regulates a subset of Rab GTPases. Elife 5, e12813, 10.7554/eLife.12813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Steger M., Diez F., Dhekne H.S., Lis P., Nirujogi R.S., Karayel O. et al. (2017) Systematic proteomic analysis of LRRK2-mediated Rab GTPase phosphorylation establishes a connection to ciliogenesis. Elife 6, e31012 10.7554/eLife.31012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eguchi T., Kuwahara T., Sakurai M., Komori T., Fujimoto T., Ito G. et al. (2018) LRRK2 and its substrate Rab GTPases are sequentially targeted onto stressed lysosomes and maintain their homeostasis. Proc. Natl. Acad. Sci. U.S.A. 115, E9115–E9124 10.1073/pnas.1812196115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dzamko N., Deak M., Hentati F., Reith A.D., Prescott A.R., Alessi D.R. et al. (2010) Inhibition of LRRK2 kinase activity leads to dephosphorylation of Ser(910)/Ser(935), disruption of 14-3-3 binding and altered cytoplasmic localization. Biochem. J. 430, 405–413 10.1042/BJ20100784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kalogeropulou A.F., Zhao J., Bolliger M.F., Memou A., Narasimha S., Molitor T.P. et al. (2018) p62/SQSTM1 is a novel leucine rich repeat kinase 2 (LRRK2) substrate that enhances neuronal toxicity. Biochem. J. 475, 1271–1293 10.1042/BCJ20170699 [DOI] [PubMed] [Google Scholar]
- 65.Ito G., Katsemonova K., Tonelli F., Lis P., Baptista M., Shpiro N. et al. (2016) Phos-tag analysis of Rab10 phosphorylation by LRRK2: a powerful assay for assessing kinase function and inhibitors. Biochem. J. 473, 2671–2685 10.1042/BCJ20160557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lin X., Parisiadou L., Gu X.-L., Wang L., Shim H., Sun L. et al. (2009) Leucine-rich repeat kinase 2 regulates the progression of neuropathology induced by Parkinson’s-disease-related mutant alpha-synuclein. Neuron 64, 807–827 10.1016/j.neuron.2009.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Andres-Mateos E., Mejias R., Sasaki M., Li X., Lin B.M., Biskup S. et al. (2009) Unexpected lack of hypersensitivity in LRRK2 knock-out mice to MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine). J. Neurosci. 29, 15846–15850 10.1523/JNEUROSCI.4357-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tong Y., Yamaguchi H., Giaime E., Boyle S., Kopan R., Kelleher R.J. et al. (2010) Loss of leucine-rich repeat kinase 2 causes impairment of protein degradation pathways, accumulation of alpha-synuclein, and apoptotic cell death in aged mice. Proc. Natl. Acad. Sci. U.S.A. 107, 9879–9884 10.1073/pnas.1004676107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dachsel J.C., Behrouz B., Yue M., Beevers J.E., Melrose H.L. and Farrer M.J. (2010) A comparative study of Lrrk2 function in primary neuronal cultures. Parkinsonism Relat. Disord. 16, 650–655 10.1016/j.parkreldis.2010.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Herzig M.C., Kolly C., Persohn E., Theil D., Schweizer T., Hafner T. et al. (2011) LRRK2 protein levels are determined by kinase function and are crucial for kidney and lung homeostasis in mice. Hum. Mol. Genet. 20, 4209–4223 10.1093/hmg/ddr348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hinkle K.M., Yue M., Behrouz B., Dächsel J.C., Lincoln S.J., Bowles E.E. et al. (2012) LRRK2 knockout mice have an intact dopaminergic system but display alterations in exploratory and motor co-ordination behaviors. Mol. Neurodegener. 7, 25 10.1186/1750-1326-7-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Paus M., Kohl Z., Ben Abdallah N.M.-B., Galter D., Gillardon F. and Winkler J. (2013) Enhanced dendritogenesis and axogenesis in hippocampal neuroblasts of LRRK2 knockout mice. Brain Res. 1497, 85–100 10.1016/j.brainres.2012.12.024 [DOI] [PubMed] [Google Scholar]
- 73.Baptista M.A.S., Dave K.D., Frasier M.a., Sherer T.B., Greeley M., Beck M.J. et al. (2013) Loss of leucine-rich repeat kinase 2 (LRRK2) in rats leads to progressive abnormal phenotypes in peripheral organs. PLoS ONE 8, e80705 10.1371/journal.pone.0080705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schulz C., Paus M., Frey K., Schmid R., Kohl Z., Mennerich D. et al. (2011) Leucine-rich repeat kinase 2 modulates retinoic acid-induced neuronal differentiation of murine embryonic stem cells. PLoS ONE 6, e20820 10.1371/journal.pone.0020820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miklavc P., Ehinger K. and Thompson K. (2014) Surfactant secretion in LRRK2 knock-out rats: changes in lamellar body morphology and rate of exocytosis. PLoS ONE 9, 10.1371/journal.pone.0084926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brunk U.T. and Terman A. (2002) Lipofuscin: mechanisms of age-related accumulation and influence on cell function. Free Radic. Biol. Med. 33, 611–619 10.1016/S0891-5849(02)00959-0 [DOI] [PubMed] [Google Scholar]
- 77.Schmitz G. and Müller G. (1991) Structure and function of lamellar bodies, lipid-protein complexes involved in storage and secretion of cellular lipids. J. Lipid Res. 32, 1539–1570 [PubMed] [Google Scholar]
- 78.Weaver T.E., Na C.-L. and Stahlman M. (2002) Biogenesis of lamellar bodies, lysosome-related organelles involved in storage and secretion of pulmonary surfactant. Semin. Cell Dev. Biol. 13, 263–270 10.1016/S1084952102000551 [DOI] [PubMed] [Google Scholar]
- 79.Giaime E., Tong Y., Wagner L.K., Yuan Y., Huang G. and Shen J. (2017) Age-dependent dopaminergic neurodegeneration and impairment of the autophagy-lysosomal pathway in LRRK-deficient mice. Neuron 96, 796–807 10.1016/j.neuron.2017.09.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Toyofuku T., Morimoto K., Sasawatari S. and Kumanogoh A. (2015) LRRK1 regulates autophagy through turning on the TBC1D2-dependent Rab7 inactivation. Mol. Cell. Biol. 10.1128/MCB.00085-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hanafusa H., Ishikawa K., Kedashiro S., Saigo T., Iemura S.-I., Natsume T. et al. (2011) Leucine-rich repeat kinase LRRK1 regulates endosomal trafficking of the EGF receptor. Nat. Commun. 2, 158 10.1038/ncomms1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reyniers L., Del Giudice M.G., Civiero L., Belluzzi E., Lobbestael E., Beilina A. et al. (2014) Differential protein protein interactions of LRRK1 and LRRK2 indicate roles in distinct cellular signaling pathways. J. Neurochem. 131, 239–250 10.1111/jnc.12798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tomkins J.E., Dihanich S., Beilina A., Ferrari R., Ilacqua N., Cookson M.R. et al. (2018) Comparative protein interaction network analysis identifies shared and distinct functions for the human ROCO proteins. Proteomics 18, e1700444 10.1002/pmic.201700444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Taylor J.P., Hulihan M.M., Kachergus J.M., Melrose H.L., Lincoln S.J., Hinkle K.M. et al. (2007) Leucine-rich repeat kinase 1: a paralog of LRRK2 and a candidate gene for Parkinson’s disease. Neurogenetics 8, 95–102 10.1007/s10048-006-0075-8 [DOI] [PubMed] [Google Scholar]
- 85.Fell M.J., Mirescu C., Basu K., Cheewatrakoolpong B., DeMong D.E., Ellis J.M. et al. (2015) MLi-2, a potent, selective and centrally active compound for exploring the therapeutic potential and safety of LRRK2 kinase inhibition. J. Pharmacol. Exp. Ther. 355, 397–409 10.1124/jpet.115.227587 [DOI] [PubMed] [Google Scholar]
- 86.Andersen M.A., Wegener K.M., Larsen S., Badolo L., Smith G.P., Jeggo R. et al. (2018) PFE-360-induced LRRK2 inhibition induces reversible, non-adverse renal changes in rats. Toxicology 395, 15–22 10.1016/j.tox.2018.01.003 [DOI] [PubMed] [Google Scholar]
- 87.Ekstrand M.I., Terzioglu M., Galter D., Zhu S., Hofstetter C., Lindqvist E. et al. (2007) Progressive parkinsonism in mice with respiratory-chain-deficient dopamine neurons. Proc. Natl. Acad. Sci. U.S.A. 104, 1325–1330 10.1073/pnas.0605208103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Scott J.D., DeMong D.E., Greshock T.J., Basu K., Dai X., Harris J. et al. (2017) Discovery of a 3-(4-Pyrimidinyl) indazole (MLi-2), an orally available and selective leucine-rich repeat kinase 2 (LRRK2) Inhibitor that reduces brain kinase activity. J. Med. Chem. 60, 2983–2992 10.1021/acs.jmedchem.7b00045 [DOI] [PubMed] [Google Scholar]
- 89.Baptista M.A.S., Merchant K., Bryce D., Ellis M., Estrada A.A., Galatsis P. et al. (2015) LRRK2 kinase inhibitors of different structural classes induce abnormal accumulation of lamellar bodies in type II pneumocytes in non-human primates but are reversible and without pulmonary functional consequences workflow and study design, https://www.michaeljfox.org/foundation/publication-detail.html?id=594&category=9 (Accessed 7 October 2018)
- 90.Fuji R.N., Flagella M., Baca M., Baptista M.A.S., Brodbeck J., Chan B.K. et al. (2015) Effect of selective LRRK2 kinase inhibition on nonhuman primate lung. Sci. Transl. Med. 7, 1–13 10.1126/scitranslmed.aaa3634 [DOI] [PubMed] [Google Scholar]
- 91.Estrada A.a., Liu X., Baker-Glenn C., Beresford A., Burdick D.J., Chambers M. et al. (2012) Discovery of highly potent, selective, and brain-penetrable leucine-rich repeat kinase 2 (LRRK2) small molecule inhibitors. J. Med. Chem. 55, 9416–9433 10.1021/jm301020q [DOI] [PubMed] [Google Scholar]
- 92.Lobbestael E., Baekelandt V. and Taymans J.-M. (2012) Phosphorylation of LRRK2: from kinase to substrate. Biochem. Soc. Trans. 40, 1102–1110 10.1042/BST20120128 [DOI] [PubMed] [Google Scholar]
- 93.Pungaliya P.P., Bai Y., Lipinski K., Anand V.S., Sen S., Brown E.L. et al. (2010) Identification and characterization of a leucine-rich repeat kinase 2 (LRRK2) consensus phosphorylation motif. PLoS ONE 5, e13672 10.1371/journal.pone.0013672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Edwards T.L., Scott W.K., Almonte C., Burt A., Powell E.H., Beecham G.W. et al. (2010) Genome-Wide association study confirms SNPs in SNCA and the MAPT region as common risk factors for parkinson disease. Ann. Hum. Genet. 74, 97–109 10.1111/j.1469-1809.2009.00560.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Spencer C.C.A., Plagnol V., Strange A., Gardner M., Paisan-Ruiz C., Band G. et al. (2011) Dissection of the genetics of Parkinson’s disease identifies an additional association 5′ of SNCA and multiple associated haplotypes at 17q21. Hum. Mol. Genet. 20, 345–353 10.1093/hmg/ddq469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wszolek Z.K., Pfeiffer R.F., Tsuboi Y., Uitti R.J., McComb R.D., Stoessl A.J. et al. (2004) Autosomal dominant parkinsonism associated with variable synuclein and tau pathology. Neurology 62, 1619–1622 10.1212/01.WNL.0000125015.06989.DB [DOI] [PubMed] [Google Scholar]
- 97.Vilas D., Sharp M., Gelpi E., Genís D., Marder K.S., Cortes E. et al. (2018) Clinical and neuropathological features of progressive supranuclear palsy in Leucine rich repeat kinase (LRRK2) G2019S mutation carriers. Mov. Disord. 33, 335–338 10.1002/mds.27225 [DOI] [PubMed] [Google Scholar]
- 98.Thirstrup K., Dachsel J., Oppermann F.S., Williamson D.S., Smith G.P., Fog K. et al. (2017) Selective LRRK2 kinase inhibition reduces phosphorylation of endogenous Rab10 and Rab12 in human peripheral mononuclear blood cells. Sci. Rep. 7, 10300 10.1038/s41598-017-10501-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kuusisto E., Salminen A. and Alafuzoff I. (2001) Ubiquitin-binding protein p62 is present in neuronal and glial inclusions in human tauopathies and synucleinopathies. Neuroreport 12, 2085–2090 10.1097/00001756-200107200-00009 [DOI] [PubMed] [Google Scholar]
- 100.Park S., Han S., Choi I., Kim B., Park S.P., Joe E.H. et al. (2016) Interplay between leucine-rich repeat kinase 2 (LRRK2) and p62/SQSTM-1 in selective autophagy. PLoS ONE 11, 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Madero-Pérez J., Fdez E., Fernández B., Lara Ordóñez A.J., Blanca Ramírez M., Romo Lozano M. et al. (2017) Cellular effects mediated by pathogenic LRRK2: homing in on Rab-mediated processes. Biochem. Soc. Trans. 45, 147–154 10.1042/BST20160392 [DOI] [PubMed] [Google Scholar]
- 102.Stenmark H. (2009) Rab GTPases as coordinators of vesicle traffic. Nat. Rev. Mol. Cell Biol. 10, 513–525 10.1038/nrm2728 [DOI] [PubMed] [Google Scholar]
- 103.Klöpper T.H., Kienle N., Fasshauer D. and Munro S. (2012) Untangling the evolution of Rab G proteins: implications of a comprehensive genomic analysis. BMC Biol. 10, 71 10.1186/1741-7007-10-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lim Y.S., Chua C.E.L. and Tang B.L. (2011) Rabs and other small GTPases in ciliary transport. Biol. Cell 103, 209–221 10.1042/BC20100150 [DOI] [PubMed] [Google Scholar]
- 105.Schaub J.R. and Stearns T. (2013) The Rilp-like proteins Rilpl1 and Rilpl2 regulate ciliary membrane content. Mol. Biol. Cell 24, 453–464 10.1091/mbc.e12-08-0598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sato T., Iwano T., Kunii M., Matsuda S., Mizuguchi R., Jung Y. et al. (2014) Rab8a and Rab8b are essential for several apical transport pathways but insufficient for ciliogenesis. J. Cell Sci. 127, 422–431 10.1242/jcs.136903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li Z., Schulze R.J., Weller S.G., Krueger E.W., Schott M.B., Zhang X. et al. (2016) A novel Rab10-EHBP1-EHD2 complex essential for the autophagic engulfment of lipid droplets. Sci. Adv. 2, e1601470 10.1126/sciadv.1601470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lv P., Sheng Y., Zhao Z., Zhao W., Gu L., Xu T. et al. (2015) Targeted disruption of Rab10 causes early embryonic lethality. Protein Cell 6, 463–467 10.1007/s13238-015-0150-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sato T., Mushiake S., Kato Y., Sato K., Sato M., Takeda N. et al. (2007) The Rab8 GTPase regulates apical protein localization in intestinal cells. Nature 448, 366–369 10.1038/nature05929 [DOI] [PubMed] [Google Scholar]
- 110.Schlüter O.M., Khvotchev M., Jahn R. and Südhof T.C. (2002) Localization versus function of Rab3 proteins: evidence for a common regulatory role in controlling fusion. J. Biol. Chem. 277, 40919–40929 10.1074/jbc.M203704200 [DOI] [PubMed] [Google Scholar]
- 111.Schluter O.M., Schmitz F., Jahn R., Rosenmund C. and Sudhof T.C. (2004) A complete genetic analysis of neuronal Rab3 function. J. Neurosci. 24, 6629–6637 10.1523/JNEUROSCI.1610-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jeong G.R., Jang E., Bae J.R., Jun S., Kang H.C., Park C. et al. (2018) Dysregulated phosphorylation of Rab GTPases by LRRK2 induces neurodegeneration. Mol. Neurodegen. 13, 8 10.1186/s13024-018-0240-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chiu C., Yeh T., Lai S., Weng Y., Huang C., Cheng Y. et al. (2016) Increased Rab35 expression is a potential biomarker and implicated in the pathogenesis of Parkinson’s disease. Oncotarget 7, 54215–54227 10.18632/oncotarget.11090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tucci A., Nalls M.a., Houlden H., Revesz T., Singleton A.B., Wood N.W. et al. (2010) Genetic variability at the PARK16 locus. Eur. J. Hum. Genet. 18, 1356–1359 10.1038/ejhg.2010.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gan-Or Z. (2012) Association of sequence alterations in the putative promoter of RAB7L1 with a reduced Parkinson disease risk. Arch. Neurol. 69, 105 10.1001/archneurol.2011.924 [DOI] [PubMed] [Google Scholar]
- 116.Khaligh A., Goudarzian M., Moslem A., Mehrtash A., Jamshidi J., Darvish H. et al. (2017) RAB7L1 promoter polymorphism and risk of Parkinson’s disease; a case-control study. Neurol. Res. 39, 468–471 10.1080/01616412.2017.1297558 [DOI] [PubMed] [Google Scholar]
- 117.MacLeod D.A., Rhinn H., Kuwahara T., Zolin A., Di Paolo G., McCabe B.D. et al. (2013) RAB7L1 interacts with LRRK2 to modify intraneuronal protein sorting and Parkinson’s disease risk. Neuron 77, 425–439 10.1016/j.neuron.2012.11.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kuwahara T., Inoue K., D’Agati V.D., Fujimoto T., Eguchi T., Saha S. et al. (2016) LRRK2 and RAB7L1 coordinately regulate axonal morphology and lysosome integrity in diverse cellular contexts. Sci. Rep. 6, 29945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu Z., Bryant N., Kumaran R., Beilina A., Abeliovich A., Cookson M.R. et al. (2018) LRRK2 phosphorylates membrane-bound Rabs and is activated by GTP-bound Rab7L1 to promote recruitment to the trans-Golgi network. Hum. Mol. Genet. 27, 385–395 10.1093/hmg/ddx410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fujimoto T., Kuwahara T., Eguchi T., Sakurai M., Komori T. and Iwatsubo T. (2018) Parkinson’s disease-associated mutant LRRK2 phosphorylates Rab7L1 and modifies trans-Golgi morphology. Biochem. Biophys. Res. Commun. 495, 1708–1715 10.1016/j.bbrc.2017.12.024 [DOI] [PubMed] [Google Scholar]
- 121.Thévenet J., Pescini Gobert R., Hooft van Huijsduijnen R., Wiessner C. and Sagot Y.J. (2011) Regulation of LRRK2 expression points to a functional role in human monocyte maturation. PLoS ONE 6, e21519 10.1371/journal.pone.0021519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fan Y., Howden A.J., Sarhan A.R., Lis P., Ito G., Martinez T.N. et al. (2017) Interrogating Parkinson’s disease LRRK2 kinase pathway activity by assessing Rab10 phosphorylation in human neutrophils. Biochem. J. 475, 23–44 10.1042/BCJ20170803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gardet A., Benita Y., Li C., Sands B.E., Ballester I., Stevens C. et al. (2010) LRRK2 is involved in the IFN-gamma response and host response to pathogens. J. Immunol. 185, 5577–5585 10.4049/jimmunol.1000548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu Z., Lee J., Krummey S., Lu W., Cai H. and Lenardo M.J. (2011) The kinase LRRK2 is a regulator of the transcription factor NFAT that modulates the severity of inflammatory bowel disease. Nat. Immunol. 12, 1063–1070 10.1038/ni.2113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dzamko N., Inesta-Vaquera F., Zhang J., Xie C., Cai H., Arthur J. S.C. et al. (2012) The IkappaB kinase family phosphorylates the Parkinson’s disease kinase LRRK2 at Ser935 and Ser910 during Toll-like receptor signaling. PLoS ONE 7, e39132 10.1371/journal.pone.0039132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hui K.Y., Fernandez-Hernandez H., Hu J., Schaffner A., Pankratz N., Hsu N.-Y. et al. (2018) Functional variants in the LRRK2 gene confer shared effects on risk for Crohn’s disease and Parkinson’s disease. Sci. Transl. Med. 10, eaai7795 10.1126/scitranslmed.aai7795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang Q., Pan Y., Yan R., Zeng B., Wang H., Zhang X. et al. (2015) Commensal bacteria direct selective cargo sorting to promote symbiosis. Nat. Immunol. 16, 918–926 10.1038/ni.3233 [DOI] [PubMed] [Google Scholar]
- 128.Perry V.H., Nicoll J.A.R. and Holmes C. (2010) Microglia in neurodegenerative disease. Nat. Rev. Neurol. 6, 193–201 10.1038/nrneurol.2010.17 [DOI] [PubMed] [Google Scholar]
- 129.Sierra A., Abiega O., Shahraz A. and Neumann H. (2013) Janus-faced microglia: beneficial and detrimental consequences of microglial phagocytosis. Front. Cell. Neurosci. 7, 1–22 10.3389/fncel.2013.00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Moehle M.S., Webber P.J., Tse T., Sukar N., Standaert D.G., DeSilva T.M. et al. (2012) LRRK2 inhibition attenuates microglial inflammatory responses. J. Neurosci. 32, 1602–1611 10.1523/JNEUROSCI.5601-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gillardon F., Schmid R. and Draheim H. (2012) Parkinson’s disease-linked leucine-rich repeat kinase 2(R1441G) mutation increases proinflammatory cytokine release from activated primary microglial cells and resultant neurotoxicity. Neuroscience 208, 41–48 10.1016/j.neuroscience.2012.02.001 [DOI] [PubMed] [Google Scholar]
- 132.Russo I., Berti G., Plotegher N., Bernardo G., Filograna R., Bubacco L. et al. (2015) Leucine-rich repeat kinase 2 positively regulates inflammation and down-regulates NF-κB p50 signaling in cultured microglia cells. J. Neuroinflammation 12, 230 10.1186/s12974-015-0449-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Daher J.P.L., Volpicelli-Daley L.a, Blackburn J.P., Moehle M.S. and West A.B. (2014) Abrogation of α-synuclein-mediated dopaminergic neurodegeneration in LRRK2-deficient rats. Proc. Natl. Acad. Sci. U.S.A. 2014, 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liu W., Liu X., Li Y., Zhao J., Liu Z., Hu Z. et al. (2017) LRRK2 promotes the activation of NLRC4 inflammasome during Salmonella Typhimurium infection. J. Exp. Med. 214, 3051–3066 10.1084/jem.20170014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hirsch E.C. and Hunot S. (2009) Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol. 8, 382–397 10.1016/S1474-4422(09)70062-6 [DOI] [PubMed] [Google Scholar]
- 136.Schneider S.A. and Alcalay R.N. (2017) Neuropathology of genetic synucleinopathies with parkinsonism: review of the literature. Mov. Disord. 32, 1504–1523 10.1002/mds.27193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rajput A., Dickson D.W., Robinson C.A., Ross O.A., Dächsel J.C., Lincoln S.J. et al. (2006) Parkinsonism, Lrrk2 G2019S, and tau neuropathology. Neurology 67, 1506–1508 10.1212/01.wnl.0000240220.33950.0c [DOI] [PubMed] [Google Scholar]
- 138.Dächsel J.C., Ross O.A., Mata I.F., Kachergus J., Toft M., Cannon A. et al. (2007) Lrrk2 G2019S substitution in frontotemporal lobar degeneration with ubiquitin-immunoreactive neuronal inclusions. Acta Neuropathol. 113, 601–606 10.1007/s00401-006-0178-1 [DOI] [PubMed] [Google Scholar]
- 139.Silveira-Moriyama L., Guedes L.C., Kingsbury A., Ayling H., Shaw K., Barbosa E. et al. (2008) Hyposmia in G2019S LRRK2-related parkinsonism: clinical and pathologic data. Neurology 71, 1021–1026 10.1212/01.wnl.0000326575.20829.45 [DOI] [PubMed] [Google Scholar]
- 140.Gaig C., Ezquerra M., Martí M.J., Valldeoriola F., Muñoz E., Lladó A. et al. (2008) Screening for the LRRK2 G2019S and codon-1441 mutations in a pathological series of parkinsonian syndromes and frontotemporal lobar degeneration. J. Neurol. Sci. 270, 94–98 10.1016/j.jns.2008.02.010 [DOI] [PubMed] [Google Scholar]
- 141.Poulopoulos M., Cortes E., Vonsattel J.-P.G., Fahn S., Waters C., Cote L.J. et al. (2012) Clinical and pathological characteristics of LRRK2 G2019S patients with PD. J. Mol. Neurosci. 47, 139–143 10.1007/s12031-011-9696-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mamais A., Manzoni C., Nazish I., Arber C., Sonustun B., Wray S. et al. (2018) Analysis of macroautophagy related proteins in G2019S LRRK2 Parkinson’s disease brains with Lewy Body pathology. Brain Res. 1701, 75–84 10.1016/j.brainres.2018.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kalia L.V., Lang A.E., Hazrati L.N., Fujioka S., Wszolek Z.K., Dickson D.W. et al. (2015) Clinical correlations with lewy body pathology in LRRK2-related Parkinson disease. JAMA Neurol. 72, 100–105 10.1001/jamaneurol.2014.2704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wszolek Z.K., Pfeiffer B., Fulgham J.R., Parisi J.E., Thompson B.M., Uitti R.J. et al. (1995) Western Nebraska family (family D) with autosomal dominant parkinsonism. Neurology 45, 502–505 10.1212/WNL.45.3.502 [DOI] [PubMed] [Google Scholar]
- 145.Maio R.D., Hoffman E.K., Rocha E.M., Keeney M.T., Sanders L.H., Miranda B.R.D. et al. (2018) LRRK2 activation in idiopathic Parkinson’s disease. Sci. Transl. Med. 10, eaar5429 10.1126/scitranslmed.aar5429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Volta M. and Melrose H. (2017) LRRK2 mouse models: dissecting the behavior, striatal neurochemistry and neurophysiology of PD pathogenesis. Biochem. Soc. Trans. 45, 113–122 10.1042/BST20160238 [DOI] [PubMed] [Google Scholar]
- 147.Li Y., Liu W., Oo T.F., Wang L., Tang Y., Jackson-Lewis V. et al. (2009) Mutant LRRK2(R1441G) BAC transgenic mice recapitulate cardinal features of Parkinson’s disease. Nat. Neurosci. 12, 826–828 10.1038/nn.2349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Li X., Patel J.C., Wang J., Avshalumov M.V., Nicholson C., Buxbaum J.D. et al. (2010) Enhanced striatal dopamine transmission and motor performance with LRRK2 overexpression in mice is eliminated by familial Parkinson’s disease mutation G2019S. J. Neurosci. 30, 1788–1797 10.1523/JNEUROSCI.5604-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Melrose H.L., Dächsel J.C., Behrouz B., Lincoln S.J., Yue M., Hinkle K.M. et al. (2010) Impaired dopaminergic neurotransmission and microtubule-associated protein tau alterations in human LRRK2 transgenic mice. Neurobiol. Dis. 40, 503–517 10.1016/j.nbd.2010.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chen C.-Y., Weng Y.-H., Chien K.-Y., Lin K.-J., Yeh T.-H., Cheng Y.-P. et al. (2012) (G2019S) LRRK2 activates MKK4-JNK pathway and causes degeneration of SN dopaminergic neurons in a transgenic mouse model of PD. Cell Death Differ. 19, 1623–1633 10.1038/cdd.2012.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chou J.S., Chen C.Y., Chen Y.L., Weng Y.H., Yeh T.H., Lu C.S. et al. (2014) (G2019S) LRRK2 causes early-phase dysfunction of SNpc dopaminergic neurons and impairment of corticostriatal long-term depression in the PD transgenic mouse. Neurobiol. Dis. 68, 190–199 10.1016/j.nbd.2014.04.021 [DOI] [PubMed] [Google Scholar]
- 152.Liu G., Sgobio C., Gu X., Sun L., Lin X., Yu J. et al. (2015) Selective expression of Parkinson’s disease-related Leucine-rich repeat kinase 2 G2019S missense mutation in midbrain dopaminergic neurons impairs dopamine release and dopaminergic gene expression. Hum. Mol. Genet. 24, 5299–5312 10.1093/hmg/ddv249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tong Y., Pisani A., Martella G., Karouani M., Yamaguchi H., Pothos E.N. et al. (2009) R1441C mutation in LRRK2 impairs dopaminergic neurotransmission in mice. Proc. Natl. Acad. Sci. U.S.A. 106, 14622–14627 10.1073/pnas.0906334106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yue M., Hinkle K.M., Davies P., Trushina E., Fiesel F.C., Christenson T.A. et al. (2015) Progressive dopaminergic alterations and mitochondrial abnormalities in LRRK2 G2019S knock-in mice. Neurobiol. Dis. 78, 172–195 10.1016/j.nbd.2015.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Moehle M.S., Daher J. P.L., Hull T.D., Boddu R., Abdelmotilib H.A., Mobley J. et al. (2015) The G2019S LRRK2 mutation increases myeloid cell chemotactic responses and enhances LRRK2 binding to actin-regulatory proteins. Hum. Mol. Genet. 24, 4250–4267 10.1093/hmg/ddv157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Litteljohn D., Rudyk C., Dwyer Z., Farmer K., Fortin T. and Hayley S. (2018) The impact of murine LRRK2 G2019S transgene overexpression on acute responses to inflammatory challenge. Brain Behav. Immun. 67, 246–256 10.1016/j.bbi.2017.09.002 [DOI] [PubMed] [Google Scholar]
- 157.Park J.-S., Blair N.F. and Sue C.M. (2015) The role of ATP13A2 in Parkinson’s disease: clinical phenotypes and molecular mechanisms. Mov. Disord. 30, 770–779 10.1002/mds.26243 [DOI] [PubMed] [Google Scholar]
- 158.Podhajska A., Musso A., Trancikova A., Stafa K., Moser R., Sonnay S. et al. (2012) Common pathogenic effects of missense mutations in the P-type ATPase ATP13A2 (PARK9) associated with early-onset parkinsonism. PLoS ONE 7, e39942 10.1371/journal.pone.0039942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tsunemi T. and Krainc D. (2014) Zn2+ dyshomeostasis caused by loss of ATP13A2/PARK9 leads to lysosomal dysfunction and alpha-synuclein accumulation. Hum. Mol. Genet. 23, 2791–2801 10.1093/hmg/ddt572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Neudorfer O., Giladi N., Elstein D., Abrahamov A., Turezkite T., Aghai E. et al. (1996) Occurrence of Parkinson’s syndrome in type I Gaucher disease. QJM 89, 691–694 10.1093/qjmed/89.9.691 [DOI] [PubMed] [Google Scholar]
- 161.Sidransky E. (2012) Gaucher disease: insights from a rare Mendelian disorder. Discov. Med. 14, 273–281 [PMC free article] [PubMed] [Google Scholar]
- 162.Sidransky E., Nalls M.A., Aasly J.O., Aharon-Peretz J., Annesi G., Barbosa E.R. et al. (2009) Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N. Engl. J. Med. 361, 1651–1661 10.1056/NEJMoa0901281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dzamko N., Geczy C.L. and Halliday G.M. (2015) Inflammation is genetically implicated in Parkinson’s disease. Neuroscience 302, 89–102 10.1016/j.neuroscience.2014.10.028 [DOI] [PubMed] [Google Scholar]
- 164.Wendeln A.-C., Degenhardt K., Kaurani L., Gertig M., Ulas T., Jain G. et al. (2018) Innate immune memory in the brain shapes neurological disease hallmarks. Nature 556, 332–338, 10.1038/s41586-018-0023-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ballatore C., Lee V.M.Y. and Trojanowski J.Q. (2007) Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat. Rev. Neurosci. 8, 663–672 10.1038/nrn2194 [DOI] [PubMed] [Google Scholar]
- 166.Bailey R.M., Covy J.P., Melrose H.L., Rousseau L., Watkinson R., Knight J. et al. (2013) LRRK2 phosphorylates novel tau epitopes and promotes tauopathy. Acta Neuropathol. 10.1007/s00401-013-1188-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kawakami F., Yabata T., Ohta E., Maekawa T., Shimada N., Suzuki M. et al. (2012) LRRK2 phosphorylates tubulin-associated tau but not the free molecule: LRRK2-mediated regulation of the tau-tubulin association and neurite outgrowth. PLoS ONE 7, e30834 10.1371/journal.pone.0030834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hamm M., Bailey R., Shaw G., Yen S.-H., Lewis J. and Giasson B.I. (2015) Physiologically relevant factors influence tau phosphorylation by leucine-rich repeat kinase 2. J. Neurosci. Res. 93, 1567–1580 10.1002/jnr.23614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kawakami F., Shimada N., Ohta E., Kagiya G., Kawashima R., Maekawa T. et al. (2014) Leucine-rich repeat kinase 2 regulates tau phosphorylation through direct activation of glycogen synthase kinase-3β. FEBS J. 281, 3–13 10.1111/febs.12579 [DOI] [PubMed] [Google Scholar]
- 170.Ohta E., Nihira T., Uchino A., Imaizumi Y., Okada Y., Akamatsu W. et al. (2015) I2020T mutant LRRK2 iPSC-derived neurons in the Sagamihara family exhibit increased Tau phosphorylation through the AKT/GSK-3ß signaling pathway. Hum. Mol. Genet. 24, 4879–4900 10.1093/hmg/ddv212 [DOI] [PubMed] [Google Scholar]
- 171.Lin C.-H., Tsai P.-I., Wu R.-M. and Chien C.-T. (2010) LRRK2 G2019S mutation induces dendrite degeneration through mislocalization and phosphorylation of tau by recruiting autoactivated GSK3ß. J. Neurosci. 30, 13138–13149 10.1523/JNEUROSCI.1737-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Shanley M.R., Hawley D., Leung S., Zaidi N.F., Dave R., Schlosser K.a. et al. (2015) LRRK2 facilitates tau phosphorylation through strong interaction with tau and cdk5. Biochemistry 54, 5198–5208 10.1021/acs.biochem.5b00326 [DOI] [PubMed] [Google Scholar]
- 173.Krumova P., Reyniers L., Meyer M., Lobbestael E., Stauffer D., Gerrits B. et al. (2015) Chemical genetic approach identifies microtubule affinity-regulating kinase 1 as a leucine-rich repeat kinase 2 substrate. FASEB J. 1–13 [DOI] [PubMed] [Google Scholar]
- 174.Bennett D.A., Schneider J.A., Arvanitakis Z., Kelly J.F., Aggarwal N.T., Shah R.C. et al. (2006) Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology 66, 1837–1844 10.1212/01.wnl.0000219668.47116.e6 [DOI] [PubMed] [Google Scholar]
- 175.Jucker M. and Walker L.C. (2013) Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature 501, 45–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Goedert M., Clavaguera F. and Tolnay M. (2010) The propagation of prion-like protein inclusions in neurodegenerative diseases. Trends Neurosci. 33, 317–325 10.1016/j.tins.2010.04.003 [DOI] [PubMed] [Google Scholar]