Abstract
Both Human T-lymphotropic virus type 1 (HTLV-1) and hepatitis C virus (HCV) are endemic in Brazil. In Salvador, the capital of the state of Bahia, 2% and 1.5% of the general population is infected with HTLV-1 or HCV. This study aimed to estimate the prevalence and the distribution of HTLV/HCV coinfection in Bahia. This cross-sectional study was conducted at the Central Laboratory of Public Health for the state of Bahia (LACEN-BA). All samples in the LACEN database submitted to serological testing for anti-HCV (chemiluminescence) and anti-HTLV-1/2 (chemiluminescence/ELISA and Western blot) from 2004 to 2013 were included. Infection rate was expressed as the number of infected individuals per 100,000 inhabitants in a given municipality; municipalities were grouped by microregion for further analysis. A total of 120,192 samples originating from 358 of the 417 municipalities in Bahia (85.8%) were evaluated. The overall HCV coinfection rate in HTLV-positive was 14.31% [2.8 (ranging from 0.4 to 8.0) per 100,000 inhabitants.] Twenty-one (5%) of the municipalities reported at least one case of HTLV/HCV coinfection. Most cases (87%) were concentrated in three microregions (Salvador: 79%, Ilhéus/Itabuna: 5%, Porto Seguro: 3%). Coinfection occurred more frequently in males (51%) with a mean age of 59 [(IQR): 46–59] years. HTLV/HCV coinfection in the state of Bahia was more frequently found among males living in the microregions of Salvador, Ilhéus/Itabuna and Porto Seguro, all of which are known to be endemic for HTLV infection.
Introduction
Both human T-Lymphotropic virus (HTLV) and hepatitis C virus (HCV) are transmitted by parenteral exposure to contaminated blood or blood products [1–3]. In addition, HTLV can be transmitted sexually [4] and vertically from mother-to-child, predominantly through breastfeeding [5, 6]. HTLV type 1 (HTLV-1) is endemic in several parts of the world, with an estimated 5 to 10 million people harboring this virus [7]. In Brazil, the prevalence of HTLV-1 varies according to geographic location, with the North and Northeast regions being the most affected [8]. Recently, it was reported that HTLV-1 is widespread throughout the state of Bahia, with at least 130,000 individuals infected with this virus [9]. While HCV infection affects around 2.5% of the world's population (177.5 million adults), a population-based study in Brazil focusing on all 26 state capitals and the Federal District found an overall HCV seroprevalence of 1.38% [10]; in Salvador, 1.5% of the general population is estimated to be infected with HCV [11].
In some regions endemic for HTLV infection, such as Asia and sub-Saharan Africa, the prevalence of HTLV/HCV coinfection in urban areas can reach up to 28% [12–15]. However, this coinfection has not been reported in Ethiopian rural areas [16]. In Europe, where the rate of HTLV infection in the general population is below 0.1% [7], although no reports of HTLV-1/HCV coinfection have been published to date [17, 18], HTLV-2/HCV coinfection was reportedly found in injecting drug users [19].
Brazil is endemic for both HTLV and HCV, and the presence of coinfection has been rarely reported in HCV patients undergoing treatment, as well as in blood donors, especially those in Southeastern Brazil. The prevalence of HTLV in individuals infected with HCV ranges from 5.3% in São Paulo [20] to 7.5% in Rio de Janeiro [21]. In addition, in blood donors, HCV was found in 35.9% of first-time HTLV-positive blood donors [22].
Contradictory clinical outcomes in the course of HTLV/HCV coinfection have been reported in Brazil and Japan. A better prognosis was described in coinfected Brazilian individuals who presented higher levels of Th1-type cytokines and CD4+ T lymphocytes, as well as lower hepatic fibrosis and alanine aminotransferase (ALT) [23–26]. Conversely, a Japanese study involving coinfected individuals described higher viral loads, a more rapid progression to hepatocellular carcinoma and a decreased response to treatment with interferon [15, 27–30]. In light of considerations regarding the influence of HTLV/HCV coinfection on the outcome of either infection and a lack of epidemiologic studies, notably in the Brazilian Northeast macroregion, the present study aimed to determine the rate of coinfection throughout the state of Bahia and map the geographical distribution of cases over a 10-year period.
Materials and methods
Ethics statement
The Institutional Review Board (IRB) for Human Research at the Gonçalo Moniz Institute of the Oswaldo Cruz Foundation (Salvador, Bahia, Brazil) provided ethical approval to conduct this study (CAAE number 22478813.7.0000.0040). In order to maintain patient information confidentiality, data were fully were anonymized so that the researchers do not have access to patient’s individual information avoiding the need for verbal or written consent.
Study area
The present study was carried out in the state of Bahia, Brazil, the fourth largest state in terms of population size (15,203,934 inhabitants), and the fifth-largest in terms of area: 564.722,611 km2. Bahia is comprised of 417 municipalities, which are grouped into 32 microregions and seven mesoregions according to economic and social similarities by the Brazilian National Institute of Geography and Statistics (IBGE) (http://www.ibge.gov.br).
Study design
The present ecological retrospective study was using data obtained from the Central Laboratory of Public Health of Bahia (LACEN-BA), which is responsible for the laboratory analysis of infectious disease surveillance throughout the state. The population served by LACEN mainly consists of individuals who exhibit symptoms of infectious disease, pregnant women and individuals referred by blood blanks, the prison system or public health units distributed throughout the state of Bahia. Individuals were included if submitted to serological testing for both HTLV and HCV, either concomitantly or in isolation, from 2004 and 2013. Any indeterminate confirmatory test results were excluded from the present analysis.
Laboratory testing
Serology for HTLV was performed at LACEN using the Murex HTLV-1/2 kit (DiaSorin SpA, Dartford, UK) from 2004 to 2008, the anti-HTLV-1/2 Sym Solution kit (Symbiosis Diagnostica LTDA, Leme, Brazil) from 2009 to 2010 and by microparticle CLIA chemiluminescence (Architect rHTLV-1/2, Abbott Diagnostics Division, Wiesbaden, Germany) from 2011 on. Confirmatory Western blotting (HTLV Blot 2.4, Genelabs Diagnostics, Singapore) was performed for all samples presenting seroreactivity. Serology for HCV was performed at LACEN by microparticle enzyme immunoassay (MEIA; AxSYM Anti-HCV Abbott Diagnostics Division, Illinois, USA) from 2004 to 2007, and thereafter by chemiluminescent microparticle immunoassay (Architect Anti-HCV, Abbott Diagnostics Division, Wiesbaden, Germany). With respect to molecular testing, RT-PCR (AMPLICOR MONITOR®, Roche Molecular Systems, Branchburg, NJ, USA) was employed in accordance with the manufacturer's specifications. Genotyping was performed by analyzing the highly conserved 5’ untranslated region using the Linear Array Hepatitis C Virus Genotyping Test (LiPA—Line Probe Assay—Roche Diagnostics, USA), following the manufacturer’s guidelines. This assay allows for the determination of six genotypes and subtypes (1a, 1b, 2, 2a, 2b, 3, 3a, 4, 4c, 5, 5a and 6).
Data analysis
The SMART LAB laboratory management system was used to extract data from all serological HTLV and HCV tests performed throughout the study period, considering all individuals who were submitted to at least one HTLV and one HCV test. To avoid duplication, the most recent available serological results were considered. Each individual’s unique registration number was considered as the key variable. The resulting database was validated using the R software package and analyzed with STATA v13.0. With regard to age, median and interquartile range (IQR) intervals were calculated and individuals were grouped accordingly. The prevalence of HTLV / HCV coinfection was determined by taking into account the presence of HTLV and HCV antibodies. The presence of anti-HTLV indicates infection, while the presence of anti-HCV might indicate exposure and / or infection. The rate of coinfection the primary outcome and was expressed as the number of individuals infected per 100,000 inhabitants. Digital maps detailing municipalities and microregions were obtained from the IBGE cartographic database in shapefile (.shp) format, and reformatted and analyzed using TerraView version 4.2 software freely provided by the National Institute for Space Research (www.dpi.inpe.br/terraview).
Results
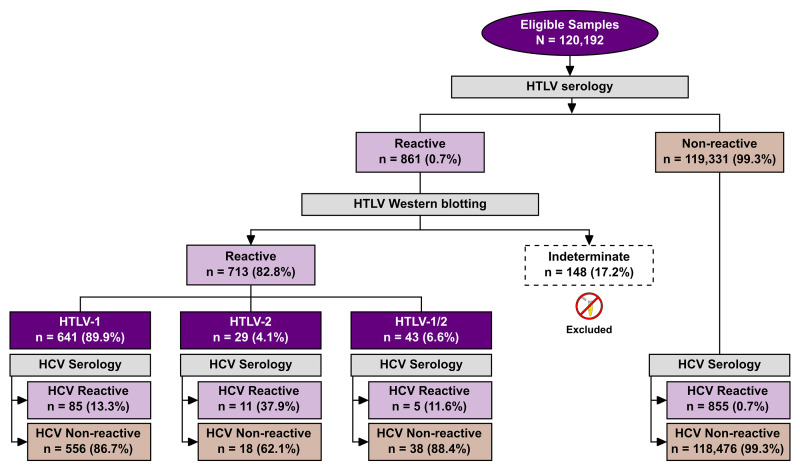
A total of 120,192 individuals were submitted to both anti-HCV and anti-HTLV 1/2 serology sometime from 2004 and 2013 (S1 Table). The median age of the studied population was 40 years [interquartile range (IQR): 25–41 years] and the female:male ratio was 7:1. The 861 HTLV-positive samples submitted to confirmatory Western blotting produced 713 [0.6%, 95%CI: 0.55–0.64, (713/120,192)] results with seroreactivity for HTLV: 641 (90%) were positive for HTLV-1, 29 (4.0%) for HTLV-2, and 43 (6%) were positive for both HTLV-1 and HTLV-2. The 148 (17.2%) indeterminate serologies were excluded from all further analysis (Fig 1).
Fig 1. Flow chart detailing the classification protocols employed in the studied population to determine HTLV and HCV infection status.
HCV infection was detected in 14.2% (101/713; 95% CI: 11.9–17.1) of the HTLV-positive individuals, versus 0.72% (855/119,331; 95% CI: 0.67–0.77) of the HTLV-negative samples, p< 0.0001 (OR: 22.9; CI: 18.3–28.5) (Fig 1). With regard to coinfection, HCV infection was detected mainly among HTLV-2 (37.9%) infected individuals, followed by HTLV-1 (13.3%) and HTLV-1/2 (11.6%). The HTLV infection prevalence in the 120,192 individuals submitted to both anti-HCV and anti-HTLV-1/2 was substantially higher in women than in man subjects (75.6% vs. 24.4%, p < 0.001). The coinfection rate was more frequent in men (~30%, 52/174; p< 0.0001) compared to women (~9%, 49/534). The median age of cases de HTLV/HCV was 59 years [interquartile range (IQR): 46–59 years]. No age was registered for 16 coinfected individuals (Table 1). The genotype of HCV was identified in 31 out of 101 coinfected individuals. Gentotypes 1 (87%) and 3 (13%) were the most frequently identified.
Table 1. Profile of HTLV/HCV coinfection in municipalities located in Bahia, Brazil from 2004 to 2013.
| Municipality | Population* | Microregion | # performed tests (%Female) | # cases | Age | %Female | Rate** |
|---|---|---|---|---|---|---|---|
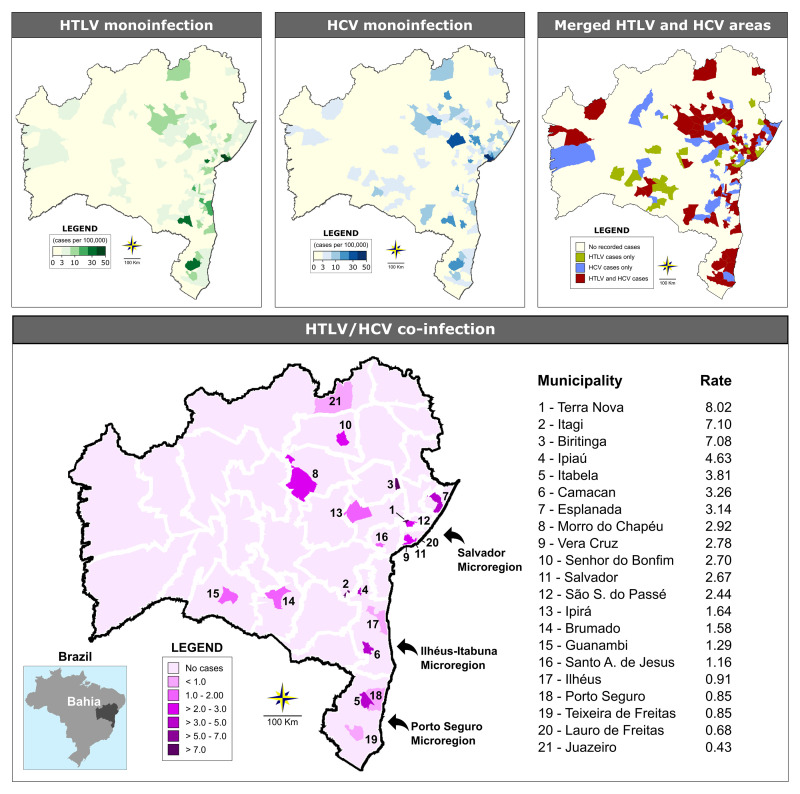
| Terra Nova | 12,467 | Catu | 224 (98) | 1 | 46 | 0 | 8.02 |
| Itagi | 14,084 | Jequié | 151 (87) | 1 | 48 | 100 | 7.10 |
| Biritinga | 14,129 | Serrinha | 580 (97) | 1 | 32 | 100 | 7.08 |
| Ipiaú | 43,169 | Ilhéus/Itabuna | 1,496 (91) | 2 | 57 | 0 | 4.63 |
| Itabela | 26,228 | Porto Seguro | 323 (93) | 1 | 47 | 0 | 3.81 |
| Camacan | 30,677 | Ilhéus/Itabuna | 647 (70) | 1 | 66 | 0 | 3.26 |
| Esplanada | 31,852 | Entre Rios | 517 (98) | 1 | 47 | 100 | 3.14 |
| Morro do Chapéu | 34,276 | Jacobina | 1,464 (92) | 1 | 27 | 100 | 2.92 |
| Vera Cruz | 35,951 | Salvador | 231 (94) | 1 | 56 | 100 | 2.78 |
| Senhor do Bonfim | 73,955 | Senhor do Bonfim | 1,016 (74) | 2 | 30 | 50 | 2.70 |
| Salvador | 2,920,679 | Salvador | 30,001 (81) | 78 | 54£ | 44.8 | 2.67 |
| São Sebastião do Passé | 40,972 | Catu | 140 (86) | 1 | 39 | 100 | 2.44 |
| Ipirá | 60,891 | Feira de Santana | 2,831 (93) | 1 | 50 | 0 | 1.64 |
| Brumado | 63,391 | Brumado | 276 (93) | 1 | 40 | 100 | 1.58 |
| Guanambi | 77,691 | Guanambi | 595 (89) | 1 | 51 | 0 | 1.29 |
| Santo Antônio de Jesus | 86,014 | Santo Antônio de Jesus | 1,644 (68) | 1 | 51 | 100 | 1.16 |
| Ilhéus | 219,927 | Ilhéus/Itabuna | 201 (56) | 2 | NA | 50 | 0.91 |
| Porto Seguro | 117,402 | Porto Seguro | 306 (86) | 1 | 60 | 100 | 0.85 |
| Teixeira de Freitas | 121,268 | Porto Seguro | 1,181 (85) | 1 | 63 | 100 | 0.82 |
| Lauro de Freitas | 147,661 | Salvador | 2,619 (87) | 1 | 52 | 100 | 0.68 |
| Juazeiro | 234,082 | Juazeiro | 93 (71) | 1 | 33 | 100 | 0.43 |
| TOTAL | 101 | 2.8 |
Mean age (years)
*Mean population from 2008 to 2009 (www.ibge.gov.br).
** No. of cases per 100,000 inhabitants.
£ Mean age calculated from data available for 64 individuals.
Out of the 417 state municipalities, 358 (85.8%) sent samples to LACEN at some point during the study period. Information regarding sample origin was absent for 1.2% of the analyzed samples. The overall rate of HTLV/HCV coinfection was estimated at 2.8 per 100,000 inhabitants (range from 0.43 to 8.02 per 100,000 inhabitants). At least one case of HTLV or HCV infection was reported in 135 and 123 of the municipalities, respectively (Fig 2). Regarding the geographical distribution of coinfected HTLV/HCV cases, 21 (5.03%) municipalities presented at least one occurrence of coinfection. The majority of cases were concentrated in just three microregions: Salvador (80/101 cases), Ilhéus/Itabuna (5/101 cases) and (Porto Seguro (3/101 cases) (Table 1 and Fig 2). In the Salvador microregion, 78 out of 80 cases of coinfection with both HTLV and HCV were concentrated the municipality of Salvador, followed by the municipalities of Ilhéus and Ipiaú (both located in Ilhéus/Itabuna microregion) with two identified cases each (Table 1).
Fig 2. Geographic distribution of HTLV and HCV cases among the municipalities in the state of Bahia from 2004 and 2013.
Microregions are delimited by white lines. The three microregions with the highest concentrations of coinfection cases are highlighted by arrows.
Discussion
The present study represents the first analysis of HTLV/HCV coinfection performed in the entire state of Bahia, which is considered endemic for both HTLV and HCV infections [11, 31]. A total of 14.2% of the HTLV-infected individuals investigated herein were found to be coinfected with HCV, resulting in an overall coinfection prevalence of 2.8/100,000 inhabitants. Although isolated infection with HTLV or HCV was disseminated throughout the state’s microregions, the majority of HTLV/HCV coinfection cases clustered in just three microregions: Salvador (79% of cases), Ilhéus/Itabuna (5% of cases) and Porto Seguro (3% of cases). Of note, the Salvador microregion, which boasts a population of more than 3.4 million inhabitants, had the largest absolute number of coinfected individuals. This result was expected, since population-based studies conducted in this city reported that around 2% and 1.5% of individuals are infected with HTLV-1 and HCV, respectively [11, 31]. Salvador and the neighboring municipalities located around the Baía de Todos os Santos (Bay of All Saints) comprise a region that was historically heavily engaged in slave trading from the 16th to 19th centuries. Nowadays, the local population is mainly of African descent. The economy is based on commercial, service, and industrial activities. In the Ilhéus-Itabuna and Porto Seguro microregions, both located in the southernmost region of the state, tourism and commercial activities are essential to the local economy. Recently, these two microregions were highlighted as significant foci of HTLV-1 infection [9].
The present study sought to determine the prevalence of HCV infection in a sample of HTLV-infected individuals. In Brazil, epidemiological data on the rate of HTLV/HCV coinfection remains scarce. Only three studies that estimated the prevalence of HTLV/HCV coinfection were recovered in the scientific literature: one in the city of São Paulo and one in the city of Rio de Janeiro, both studies evaluated the coinfection of HTLV / HCV in individuals undergoing HCV treatment [20, 21]. The third study was performed in the city of Ribeirão Preto (São Paulo) and evaluated for 11 years the seroprevalence of HTLV and co-infections with other sexually transmitted diseases such as HIV, syphilis and HBV [22]. In the first two studies, the denominator was the number of HCV cases, in the last one the denominator was the number of HTLV cases. Our study is similar to studies conducted in Japan, in which the prevalence of HTLV / HCV coinfection was estimated in HTLV endemic areas [12, 15] and in donor donors blood from Ribeirão Preto [22]. In contrast to our analysis, previous studies have evaluated HTLV infection in individuals with hepatitis C who received treatment [20, 21]. We found that HTLV-positive individuals presented a significantly higher prevalence for anti-HCV and also had a higher probability of acquiring hepatitis C (OR: 22.9) compared to HTLV-negative individuals. Similar results were obtained on Iki Island in Japan, an endemic region for both viruses [12]. However, this difference was not observed in a study conducted in two southern Japanese villages, where HTLV and HCV are endemic [15].
Throughout the study period, the testing methodology for both HTLV and HCV was updated from ELISA to chemiluminescence assays. Nonetheless, both tests offer similarly high sensitivity and specificity. Recent studies evaluating the performance of these methods indicate improved accuracy using chemiluminescence, both in HTLV [32] and HCV serology [33], i.e. both can be effectively used for serological screening in the laboratory diagnosis of HTLV and HCV. Chemiluminescence is mainly used by the laboratories that analyze a large number of samples, as this technique reduces time needed to produce results and minimizes errors in analysis.
In the present study, males over 50 years were predominantly found to be coinfected with HTLV and HCV, which is consistent with reports from other studies [20, 34, 35]. Moreover, it is known that the prevalence of HTLV-1 infection increases with age [31]. The advanced age of coinfected individuals was expected, since both HTLV and HCV have a long asymptomatic course, and the diagnosis of these infections often only occurs when chronic complications are present. In addition, since screening for both viruses only became mandatory at blood banks beginning in 1993, prior to this period, poor sterilization in the reuse of glass syringes was frequent, which contributed to increased transmission of HCV [36], and possibly also HTLV. Although the present study did not attempt to determine the routes of infection in co-infected individuals, it is possible that the sharing of needles and syringes was responsible for infection in these cases [10, 20, 34, 37, 38]. In HCV, which is transmitted through exposure to blood, particularly by transfusion, organ transplantation of infected individuals [39], injected drug use is considered the leading risk factor for virus acquisition [10, 40]. In contrast, HTLV-1 infection occurs more frequently by sexual route and breastfeeding, both uncommon in HCV transmission [41].
In the context of HTLV/HCV coinfection, the present study found a higher proportion of HTLV-2 in coinfected individuals. HTLV-2 infection has been mostly reported among injecting drug users in urban areas [8] and in indigenous populations in northern Brazil [42]. Achieving an accurate discrimination between HTLV-1 and HTLV-2 may have prognostic value in the outcome of HCV [20]. Some clinical complications, such as increased HCV load, hepatocyte damage, hepatocarcinogenesis, high levels of alanine aminotransferase (ALT) and increases in the spontaneous production of IL-1, IL-2 and IFN-γ, have been more frequently reported in individuals coinfected with HTLV-1/HCV than those with HTLV-2/HCV [23, 25, 26, 29, 34, 40, 43].
Regarding molecular testing, out of 101 HTLV/HCV-coinfected individuals included, only 40 records contained information regarding molecular investigation (RNA-HCV), and 31 of these were submitted to HCV genotyping. The Brazilian Ministry of Health protocol recommends genotyping for patients who are initiating treatment, as well as those undergoing treatment who present resistance to antivirals [44]. With respect to the profile of HCV circulating genotypes, the present study detected the presence of Genotypes 1 and 3, in 31 coinfected individuals. The most prevalent genotype was type 1 (83%). Accordingly, the HCV genotypes 1 and 3 are the most prevalent worldwide, accounting for about 46.2 and 30.1% of infections, respectively [45]. However, a low number of HCV genotype samples were tested, possibly leading to a misinterpretation of results, since the HCV clearance rate may vary in HTLV infected persons and by genotype.
The main limitation of the present study was the use of non-random sampling, which resulted in a predominance of females. In the state of Bahia, serological HTLV screening for pregnant women is compulsory since 2011, which could thusly contribute to a higher proportion of women in the samples analyzed. However, the robust representation of cases reported throughout the state’s municipalities, as well as the absolute number of individuals analyzed, provide an overview of the circulation of HTLV and HCV in the state. Another limitation is using anti-HCV serology instead HCV-RNA measurement to determine the prevalence of infection. Different of HTLV, infection by HCV may be spontaneously cleared and treated. However, anti-HCV is the most common marker of hepatitis C infection, used to estimate its prevalence globally. In Brazil, it is one of the markers used to report cases of HCV infection in the SINAN [44]. Other studies previously conducted to estimate the prevalence of hepatitis C infection in Brazil and worldwide have also used anti-HCV for this purpose [8, 10, 46]. In the present study, we had information on RNA-HCV data for 40 HTLV/HCV-coinfected individuals. Of these, 32 had detectable viral load.
In conclusion, the present study evaluated a large number of individuals in Bahia, considered one of the states in Brazil most affected by HTLV infection. We found that at least 14% of the individuals infected with HTLV also harbor HCV. Coinfection was concentrated in males who resided in the microregions of Salvador, Ilhéus-Itabuna and Porto Seguro, all considered hotspots for HTLV infection, despite HTLV and HCV being widespread throughout the state. It is our hope that these findings will provide support for the implementation of preventive measures against the spread of these viruses, especially in areas where higher rates of HTLV/HCV coinfection were described. Moreover, as the presence of HTLV can negatively impact the course of HCV infection, the surveillance of active cases should be a priority in order to provide early treatment. The identification, control and prevention of the main risk factors associated with HTLV/HCV coinfection can lead to efficacious actions in a variety of epidemiological contexts specific to each affected region.
Supporting information
(XLSX)
Acknowledgments
We thank Andris K. Walter for providing English language revision and manuscript copyediting assistance.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by the Coordination of Superior Level Staff Improvement-Brazil (CAPES) - Finance Code 001 and National Foundation for the Development of Private Higher Education (FUNADESP), grants 9600140 and 9600141. Maria Fernanda R. Grassi and Bernardo Galvão-Castro are research fellows of CNPq (process no. 304811/2017-3 and 311054/2014-5, respectively).
References
- 1.Sullivan MT, Williams AE, Fang CT, Grandinetti T, Poiesz BJ, Ehrlich GD. Transmission of human T-lymphotropic virus types I and II by blood transfusion. A retrospective study of recipients of blood components (1983 through 1988). The American Red Cross HTLV-I/II Collaborative Study Group. Arch Intern Med. 1991; 151: 2043–2048. 10.1001/archinte.1991.00400100115019 . [DOI] [PubMed] [Google Scholar]
- 2.Manns A, Wilks RJ, Murphy EL, Haynes G, Figueroa JP, Barnett M, et al. A prospective study of transmission by transfusion of HTLV-I and risk factors associated with seroconversion. Int J Cancer. 1992; 51: 886–891. 10.1002/ijc.2910510609 . [DOI] [PubMed] [Google Scholar]
- 3.Alter MJ. Prevention of spread of hepatitis C. Hepatology. 2002; 36: S93–S98. 10.1053/jhep.2002.36389 . [DOI] [PubMed] [Google Scholar]
- 4.Nunes D, Boa-Sorte N, Grassi MF, Taylor GP, Teixeira MG, Barreto ML, et al. HTLV-1 is predominantly sexually transmitted in Salvador, the city with the highest HTLV-1 prevalence in Brazil. PLoS One. 2017; 12:e0171303 10.1371/journal.pone.0171303 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bittencourt AL, Dourado I, Filho PB, Santos M, Valadao E, Alcantara LC, et al. Human T-cell lymphotropic virus type 1 infection among pregnant women in northeastern Brazil. J Acquir Immune Defic Syndr. 2001; 26: 490–494. 10.1097/00126334-200104150-00016 . [DOI] [PubMed] [Google Scholar]
- 6.Li HC, Biggar RJ, Miley WJ, Maloney EM, Cranston B, Hanchard B, et al. Provirus load in breast milk and risk of mother-to-child transmission of human T lymphotropic virus type I. J Infect Dis. 2004; 190: 1275–1278. Epub 2004 Aug 30. 10.1086/423941 . [DOI] [PubMed] [Google Scholar]
- 7.Gessain A, Cassar O. Epidemiological aspects and world distribution of HTLV-1 infection. Front Microbiol. 2012; 3: 388 10.3389/fmicb.2012.00388 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Catalan-Soares B, Carneiro-Proietti AB, Proietti FA, Interdisciplinary HRG. Heterogeneous geographic distribution of human T-cell lymphotropic viruses I and II (HTLV-I/II): serological screening prevalence rates in blood donors from large urban areas in Brazil. Cad Saude Publica. 2005; 21: 926–931. Epub 2005 May 2. 10.1590/s0102-311x2005000300027 . [DOI] [PubMed] [Google Scholar]
- 9.Pereira FM, Almeida MC, Santos FL, Carreiro RP, Regis-Silva CG, Galvão-Castro B, et al. Evidence of new highly endemic clusters of human T-cell lymphotropic viral infection (HTLV) in Bahia, Brazil. Front Microbiol. 2019; 10: 1002 10.3389/fmicb.2019.01002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pereira LM, Martelli CM, Moreira RC, Merchan-Hamman E, Stein AT, Cardoso MR, et al. Prevalence and risk factors of Hepatitis C virus infection in Brazil, 2005 through 2009: a cross-sectional study. BMC Infect Dis. 2013; 13: 60 10.1186/1471-2334-13-60 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zarife MA, Silva LK, Silva MB, Lopes GB, Barreto ML, Teixeira Mda G, et al. Prevalence of hepatitis C virus infection in north-eastern Brazil: a population-based study. Trans R Soc Trop Med Hyg. 2006; 100: 663–668. Epub 2005 Dec 28. 10.1016/j.trstmh.2005.09.009 . [DOI] [PubMed] [Google Scholar]
- 12.Nakashima K, Hayashi J, Hirata M, Kashiwagi S, Eda F. Hepatitis C virus infection on Iki Island, Japan, an area endemic for human T-lymphotropic virus type-I. A preliminary study in patients at clinics or hospitals. J Epidemiol. 1994; 4: 17–23. 10.2188/jea.4.17. [DOI] [Google Scholar]
- 13.Ziaee M, Namaei MH, Azarkar G. The prevalence of HTLV-1 and its co-Infection with HCV, HBV and HIV in hemophilic patients. Pak J Med Sci. 2015; 31: 1246–1249. 10.12669/pjms.315.7888 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prasetyo AA, Dirgahayu P, Sari Y, Hudiyono H, Kageyama S. Molecular epidemiology of HIV, HBV, HCV, and HTLV-1/2 in drug abuser inmates in central Javan prisons, Indonesia. J Infect Dev Ctries. 2013; 7: 453–467. 10.3855/jidc.2965 . [DOI] [PubMed] [Google Scholar]
- 15.Boschi-Pinto C, Stuver S, Okayama A, Trichopoulos D, Orav EJ, Tsubouchi H, et al. A follow-up study of morbidity and mortality associated with hepatitis C virus infection and its interaction with human T lymphotropic virus type I in Miyazaki, Japan. J Infect Dis. 2000; 181: 35–41. 10.1086/315177 . [DOI] [PubMed] [Google Scholar]
- 16.Ramos JM, Belda S, Reyes F, Rodriguez JC, Royo G, Gutierrez F. Prevalence of HIV, HBV, HCV, HTLV and Treponema pallidum among patients attending a rural hospital in Southern Ethiopia. J Clin Virol. 2012; 53: 268–269. Epub 2011 Dec 30. 10.1016/j.jcv.2011.12.004 . [DOI] [PubMed] [Google Scholar]
- 17.Vallejo A, Loza E, Mateos M. Absence of HTLV-1/2 infection among HCV- infected patients with no HIV-1/2 infection in Spain. J Clin Virol. 2015; 64: 72–73. Epub 2015 Jan 14. 10.1016/j.jcv.2015.01.010 . [DOI] [PubMed] [Google Scholar]
- 18.Kucharska M, Inglot M, Szymczak A, Rymer W, Zalewska M, Malyszczak K, et al. Co-infection of the hepatitis C virus with other blood-borne and hepatotropic viruses among hemophilia patients in Poland. Hepat Mon. 2016; 16: e35658 10.5812/hepatmon.35658 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de la Fuente L, Toro C, Soriano V, Brugal MT, Vallejo F, Barrio G, et al. HTLV infection among young injection and non-injection heroin users in Spain: prevalence and correlates. J Clin Virol. 2006; 35: 244–249. Epub 2005 Sep 6. 10.1016/j.jcv.2005.06.006 . [DOI] [PubMed] [Google Scholar]
- 20.Caterino-de-Araujo A, Alves FA, Campos KR, Lemos MF, Moreira RC. Making the invisible visible: searching for human T-cell lymphotropic virus types 1 and 2 (HTLV-1 and HTLV-2) in Brazilian patients with viral hepatitis B and C. Mem Inst Oswaldo Cruz. 2018; 113: 130–134. 10.1590/0074-02760170307 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maciel AM, Mello CE. Hepatitis C virus and Human T-cell lymphotropic virus co- infection: an epidemiological, clinical and laboratory analysis. Arch Clin Microbiol. 2015; 6: 1–7. [Google Scholar]
- 22.Pinto MT, Rodrigues ES, Malta TM, Azevedo R, Takayanagui OM, Valente VB, et al. HTLV-1/2 seroprevalence and coinfection rate in Brazilian first-time blood donors: an 11-year follow-up. Rev Inst Med Trop Sao Paulo. 2012; 54: 123–129. 10.1590/s0036-46652012000300002 . [DOI] [PubMed] [Google Scholar]
- 23.Bahia F, Novais V, Evans J, Le Marchand C, Netto E, Page K, et al. The impact of human T-cell lymphotropic virus I infection on clinical and immunologic outcomes in patients coinfected with HIV and hepatitis C virus. J Acquir Immune Defic Syndr. 2011; 57: S202–S207. 10.1097/QAI.0b013e31821e9a1e . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abad-Fernández M, Moreno A, Dronda F, del Campo S, Quereda C, Casado JL, et al. Delayed liver fibrosis in HTLV-2-infected patients co-infected with HIV-1 and hepatitis C virus with suppressive antiretroviral therapy. AIDS. 2015; 29: 401–409. 10.1097/QAD.0000000000000555 . [DOI] [PubMed] [Google Scholar]
- 25.Silva MC, Silva CA, Machado GU, Atta A, S MF, Carvalho E, et al. HCV/HTLV coinfection: Does HTLV-1 interfere in the natural history of HCV-related diseases? J Med Virol. 2016; 88: 1967–1972. Epub 2016 Apr 22. 10.1002/jmv.24538 . [DOI] [PubMed] [Google Scholar]
- 26.Espíndola OM, Vizzoni AG, Lampe E, Andrada-Serpa MJ, Araujo AQ, Leite AC. Hepatitis C virus and human T-cell lymphotropic virus type 1 co-infection: impact on liver disease, virological markers, and neurological outcomes. Int J Infect Dis. 2017; 57: 116–122. Epub 2017 Feb 7. 10.1016/j.ijid.2017.01.037 . [DOI] [PubMed] [Google Scholar]
- 27.Kamihira S, Momita S, Ikeda S, Yamada Y, Sohda H, Atogami S, et al. Cohort study of hepatotropic virus and human T lymphotropic virus type-I infections in an area endemic for adult T cell leukemia. Jpn J Med. 1991; 30: 492–497. 10.2169/internalmedicine1962.30.492 . [DOI] [PubMed] [Google Scholar]
- 28.Kishihara Y, Furusyo N, Kashiwagi K, Mitsutake A, Kashiwagi S, Hayashi J. Human T lymphotropic virus type 1 infection influences hepatitis C virus clearance. J Infect Dis. 2001; 184: 1114–1119. Epub 2001 Sep 21. 10.1086/323890 . [DOI] [PubMed] [Google Scholar]
- 29.Hisada M, Chatterjee N, Zhang M, Battjes RJ, Goedert JJ. Increased hepatitis C virus load among injection drug users infected with human immunodeficiency virus and human T lymphotropic virus type II. J Infect Dis. 2003; 188: 891–897. Epub 2003 Sep 9. 10.1086/377585 . [DOI] [PubMed] [Google Scholar]
- 30.Tokunaga M, Uto H, Oda K, Tokunaga M, Mawatari S, Kumagai K, et al. Influence of human T-lymphotropic virus type 1 coinfection on the development of hepatocellular carcinoma in patients with hepatitis C virus infection. J Gastroenterol. 2014; 49: 1567–1577. Epub 2014 Jan 25. 10.1007/s00535-013-0928-5 . [DOI] [PubMed] [Google Scholar]
- 31.Dourado I, Alcantara LC, Barreto ML, da Gloria Teixeira M, Galvão-Castro B. HTLV-I in the general population of Salvador, Brazil: a city with African ethnic and sociodemographic characteristics. J Acquir Immune Defic Syndr. 2003; 34: 527–531. 10.1097/00126334-200312150-00013 . [DOI] [PubMed] [Google Scholar]
- 32.Brito V da S, Santos FLN, Gonçalves NLS, Araujo TH, Nascimento DSV, Pereira FM, et al. Performance of commercially available serological screening tests for human T-cell lymphotropic virus infection in Brazil. J Clin Microbiol. 2018; pii: e00961–18. 10.1128/JCM.00961-18 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naz A, Mukry SN, Naseer I, Shamsi TS. Evaluation of efficacy of serological methods for detection of HCV infection in blood donors: A single centre experience. Pakistan J Med Sci. 2018; 34: 1204–1208. 10.12669/pjms.345.15707 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moreira M, Ramos A, Netto EM, Brites C. Characteristics of co-infections by HCV and HBV among Brazilian patients infected by HIV-1 and/or HTLV-1. Braz J Infect Dis. 2013; 17: 661–666. Epub 2013 Sep 9. 10.1016/j.bjid.2013.04.009 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Milagres FA, Duarte MI, Viso AT, Segurado AC. Hepatitis C virus and human T-lymphotropic virus coinfection: epidemiological, clinical, laboratory and histopathological features. Rev Soc Bras Med Trop. 2009; 42: 363–368. 10.1590/s0037-86822009000400001 . [DOI] [PubMed] [Google Scholar]
- 36.Prati D. Transmission of hepatitis C virus by blood transfusions and other medical procedures: a global review. J Hepatol. 2006; 45: 607–616. Epub 2006 Jul 25. 10.1016/j.jhep.2006.07.003 . [DOI] [PubMed] [Google Scholar]
- 37.Caterino-de-Araujo A, Sacchi CT, Goncalves MG, Campos KR, Magri MC, Alencar WK, et al. Short Communication: current prevalence and risk factors associated with human T lymphotropic virus type 1 and human T lymphotropic virus type 2 infections among HIV/AIDS patients in São Paulo, Brazil. AIDS Res Hum Retroviruses. 2015; 31: 543–549. Epub 2015 Jan 2. 10.1089/AID.2014.0287 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oliveira-Filho AB, Sawada L, Pinto LC, Locks D, Bahia SL, Castro JA, et al. Epidemiological aspects of HCV infection in non-injecting drug users in the Brazilian state of Para, eastern Amazon. Virol J. 2014; 11: 38 10.1186/1743-422X-11-38 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alter M. Epidemiology of hepatitis C virus infection. World J Gastroenterol. 2007; 13: 2436–2441. 10.3748/wjg.v13.i17.2436 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cardoso DF, Souza FV, Fonseca LA, Duarte AJ, Casseb J. Influence of human T-cell lymphotropic virus type 1 (HTLV-1) Infection on laboratory parameters of patients with chronic hepatitis C virus. Rev Inst Med Trop São Paulo. 2009; 51: 325–329. 10.1590/s0036-46652009000600003 . [DOI] [PubMed] [Google Scholar]
- 41.Terrault NA. Sexual activity as a risk factor for hepatitis C. Hepatology. 2002; 36: S99–S105. 10.1053/jhep.2002.36797 . [DOI] [PubMed] [Google Scholar]
- 42.Ishak R, Harrington WJ Jr., Azevedo VN, Eiraku N, Ishak MO, Guerreiro JF, et al. Identification of human T cell lymphotropic virus type IIa infection in the Kayapo, an indigenous population of Brazil. AIDS Res Hum Retroviruses. 1995; 11: 813–821. 10.1089/aid.1995.11.813 . [DOI] [PubMed] [Google Scholar]
- 43.Le Marchand C, Bahia F, Page K, Brites C. Hepatitis C virus infection and spontaneous clearance in HTLV-1 and HIV co-infected patients in Salvador, Bahia, Brazil. Braz J Infect Dis. 2015; 19: 486–491. Epub 2015 Aug 5. 10.1016/j.bjid.2015.06.007 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ministério da Saúde do Brasil. Boletim Epidemiológico—Hepatites virais. Brasília: Ministério da Saúde; 2018. Available: http://www.aids.gov.br.
- 45.Messina JP, Humphreys I, Flaxman A, Brown A, Cooke GS, Pybus OG, et al. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology. 2015; 61: 77–87. Epub 2014 Jul 28 10.1002/hep.27259 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petruzziello A, Marigliano S, Loquercio G, Cacciapuoti C. Hepatitis C virus (HCV) genotypes distribution: an epidemiological up-date in Europe. Infect Agent Cancer. 2016; 11: 53 10.1186/s13027-016-0099-0 . [DOI] [PMC free article] [PubMed] [Google Scholar]