Abstract
Purpose:
Hypofractionation in the setting of postmastectomy radiation (PMRT) is not currently the standard of care in most countries. Here we present a 5-year update of our multi-institutional, phase 2 prospective trial evaluating a novel 15-day hypofractionated PMRT regimen.
Methods and Materials:
Patients were enrolled to receive 3.33 Gy daily to the chest wall (or reconstructed breast) and regional lymphatics in 11 fractions with an optional 4-fraction mastectomy scar boost. The primary endpoint was freedom from grade 3 or higher late non–reconstruction-related radiation toxicities. Toxicities were scored using Common Terminology Criteria for Adverse Events v4.0. Secondary endpoints included local and locoregional recurrence rates, cosmesis, and reconstruction complications.
Results:
After enrolling 69 patients with stage II-IIIa breast cancer, 67 women were eligible for analysis. At a median follow up of 54 months, there were no acute or late grade 3 and 4 nonreconstruction reported toxicities. The grade 2 or greater late toxicity rate was only 12% and comprised grade 2 pain, fatigue, and lymphedema that persisted beyond 6 months after completion of radiation therapy. Only 3 women (4.6%) experienced a chest wall or nodal recurrence as a first site of relapse. Freedom from local failure, including local failure after distant relapse, was 92% at 5 years, and the 5-year overall survival was 90%.
Conclusions:
This is the first prospective trial conducted in the United States to demonstrate the safe and effective use of hypofractionated PMRT. We have demonstrated a low complication rate while achieving excellent local control. Toxicity was better than anticipated based on previously published series of PMRT toxicities. Although our fractionation was novel, the radiobiological equivalent dose is similar to other hypofractionation schedules. This trial was the basis for the creation of Alliance A221505 (RT CHARM), which is currently accruing patients in a phase 3 randomized design.
Introduction
Postmastectomy radiation therapy (PMRT) is an important component of the multidisciplinary management of patients with locally advanced breast cancer. PMRT has been shown in randomized trials to improve breast cancer survival in patients with lymph node–positive disease.1-3 Hypofractionation has become the new standard of care in many types of breast cancer radiation, with the publication of randomized trials demonstrating its safety and efficacy.4,5 Cosmetic results from the UK Start B trial demonstrated an improvement with hypofractionation in breast shrinkage, telangiectasias, and breast edema.4 Radiobiology research has given us a linear quadratic formula to describe the relationship among dose per fraction, time, and total dose of radiation.6,7
The UK Start A and Start B trials used a total of 4 different dose fractionation schedules, thus enabling investigators to model the radiobiological effects for normal breast tissue and breast cancer based on daily and total dose. Those results have demonstrated that breast cancer and normal breast cells share a similar radiation dose sensitivity, theoretically negating the benefit of significant fractionation.8 This has laid the groundwork for exploration into additional settings for breast cancer hypofractionation, such as postmastectomy and regional nodal patients. A 2019 published randomized trial from Beijing, China, treated 820 patients with either a standard fractionation of 50 Gy in 25 fractions or 43.5 Gy of in 16 fractions after mastectomy. Results from this trial support the safety and efficacy of hypofractionated PMRT, at least in the setting of the nonreconstructed chest wall (CW).9
In 2009, with institutional review board approval, we conducted a prospective phase 2 trial (NCT01417286) using hypofractionated PMRT with a unique dose schedule. Patients enrolled at the Rutgers Cancer Institute of New Jersey and at the Huntsman Cancer Institute at the University of Utah. Early toxicity results of our study were published in 2017 with a median follow-up of 32 months. That analysis demonstrated a 3% local failure rate and no grade 3 toxicities.10 Here we present the 5-year update of our prospective phase 2 trial.
Methods and Materials
Patients
Between 2010 and 2014, 69 women over 18 years of age, with American Joint Committee on Cancer version 7, stage IIA-IIIC invasive epithelial breast cancer and negative surgical margins (no tumor on ink) were enrolled to receive a hypofractionated PMRT regimen. Table 1 demonstrates the patient characteristics. Patients were allowed (not required) to undergo breast reconstruction and receive neoadjuvant/adjuvant chemotherapy and antihormone therapy (before, during, or after radiation therapy [RT]). Staging was the higher of clinical or pathologic staging if neoadjuvant chemotherapy was administered; otherwise, pathologic staging was used. Treatment characteristics are shown in Table 2. Exclusion criteria included metastatic disease, prior chest radiation, coexisting medical conditions with life expectancy <2 years, collagen vascular disease, and prior malignancy within 5 years of enrollment. High-risk features such as inflammatory breast cancer (T4d), lymphovascular invasion, close margins, young age, and negative hormone receptors were allowed. One patient had inflammatory breast cancer (T4d) and 1 had skin ulceration (T4b). Two patients had N3 disease, one of whom also had T4b.
Table 1.
Patient characteristics
| Total (%) | |
|---|---|
| Age, y | Median: 54 |
| ≤40 | 11 (16) |
| 41-50 | 20 (30) |
| 51-60 | 8 (12) |
| 61-70 | 22 (33) |
| >70 | 6 (9) |
| Laterality | |
| Right | 38 (57) |
| Left | 28 (42) |
| Bilateral | 1 (2) |
| Final Stage* | |
| IIA | 14 (21) |
| IIB | 25 (37) |
| IIIA | 22 (33) |
| IIIB | 4 (6) |
| IIIC | 2 (3) |
| Histology | |
| IDC | 62 (93) |
| ILC | 5 (7) |
| Grade | |
| I | 10 (15) |
| II | 18 (27) |
| III | 38 (57) |
| ECE | |
| No | 35 (52) |
| Yes | 18 (27) |
| Unknown | 14 (21) |
| LVSI | |
| No | 33 (49) |
| Yes | 27 (40) |
| Unknown | 7 (10) |
| ER+ | 51 (76) |
| PR+ | 45 (67) |
| Her2+† | 15 (22) |
| ER–/PR–/Her2– | 9 (13) |
Abbreviations: ECE = extracapsular extension; ER = estrogen receptor; LVSI = lymphovascular space invasion; PR = progesterone receptor.
Higher of pathologic or prechemotherapy clinical stage.
100% of Her2 patients received Her2-directed therapy.
Table 2.
Treatment characteristics
| n (%) | |
|---|---|
| Neoadjuvant chemotherapy | 33 (49) |
| Anthracycline-based | 21 (57) |
| Taxane-based (no anthracyclines) | 9 (24) |
| Other | 3 (8) |
| Adjuvant Chemotherapy | 37 (55) |
| Anthracycline regimen | 24 (65) |
| Taxane-based | 12 (33) |
| No chemotherapy received | 5 (7) |
| Both neoadjuvant and adjuvant chemotherapy | 8 (12) |
| Adjuvant tamoxifen (ER/PR+) | 22/53 (42) |
| Adjuvant AI (ER/PR+) | 23/52 (43) |
| Both | 1 (1) |
| No hormone therapy (ER/PR+) | 8/53 (15) |
| Pretherapy nodal bx (neoadjuvant) | |
| Negative | 2 |
| Positive | 19 |
| Convert to pCR nodes from + nodes | 5/27 (19) |
| pCR, breast only | 10/33 (30) |
| pCR, both | 5/33 (15) |
| No nodal evaluation (T3N0) | 1 (1) |
| Sentinel node only | 10 (15) |
| Axillary dissection | 56 (84) |
| Range of nodes dissected | 3-29 |
| Median no. of nodes | 12.5 |
| Range positive | 0-16 |
| Median positive | 2 |
| Breast reconstruction | |
| Yes | 41 (59) |
| No | 28 (41) |
Abbreviations: AI = aromatase inhibitor; ER = estrogen receptor; pCR = pathologic complete response; PR = progesterone receptor.
Radiation therapy
Using 3-dimensional planning techniques, patients received radiation to the CW or reconstructed breast and regional lymph nodes to 36.63 Gy in 11 fractions of 3.33 Gy per day, delivered 5 days a week. Bolus was used on all patients per institutional standard; however, bolus thickness and frequency were at the discretion of the treating provider. Coverage of the internal mammary (IM) lymph nodes was not required but occurred in 28% of patients. Among patients with IM node coverage, planning techniques used included partially wide tangents (74%), matched photon/electron (16%), and intensity modulated RT (10%). The use of a boost was optional and was prescribed as 3.33 Gy × 4 fractions using en-face 6 to 9 MeV electrons to the mastectomy scar with a 2-cm block edge margin. Volume-based planning was not required because this protocol was written before the creation of the Radiation Therapy Oncology Group breast cancer atlas.11 Ninety-seven percent of patients received the optional boost because was the practice pattern at the time for both enrolling institutions. Without consideration of the scar boost, the CW was to be encompassed by the 90% to 115% prescription isodose line. If electrons were used in a matching technique, the max dose allowed was 120%, not to exceed 2 cm3 of tissue. The heart was ideally excluded from the primary tangent field with a maximum daily dose of no more than 2 Gy/d. The ipsilateral lung width visible at any level on the tangent beam’s eye view had to be <3 cm. A 3-dimensional lung constraint was not predetermined but retrospectively collected as ipsilateral lung V15, calculated to be an approximate biological equivalent dose to evaluating V20 in standard fractionation, where Vx = volume (V) receiving (x) dose of radiation. The brachial plexus was routinely contoured with the brachial/axillary vessels as a surrogate landmark and treated to a max dose of 39.2 Gy, 107% of the prescription dose. Further details of radiation treatment planning were thoroughly described in our previous report.10
Statistical analysis
The primary hypothesis was grade ≥3 non–reconstruction-related toxicities being noninferior to historical control in patients receiving standard-fractionation PMRT. These nonreconstruction toxicities were compiled as our primary endpoint and constituted late dermatitis, CW pain, painful/symptomatic brachial plexopathy, and acute symptomatic pneumonitis grade ≥3 on the Common Terminology Criteria for Adverse Events (CTCAE; version 4.0) scale. Early stopping rules were put in place during enrollment but were not met. Early toxicity was defined as occurring during or within 6 months of the completion of radiation. Late toxicity was defined as occurring greater than 6 months after the last day of radiation.
Secondary endpoints were local and locoregional recurrence rates (LR; LRR). LR was defined as a CW or skin failure, and LRR was defined as local or regional nodal recurrence (axillary, supraclavicular, or IM). Using a fixed sample size of 67 patients, for up to 5 recurrences, a 90% confidence interval would detect a true recurrence rate between 3.7% and 14.5%. If this was met, we believed it was appropriate and would be deemed noninferior to previously reported recurrence rates.1-3,9 All intervals were calculated from the date of the end of RT.
Results
Disease control
There were 2 CW recurrences as the first site of relapse (3%) at 22 and 54 months post-RT. One patient was successfully salvaged and remains free of disease. One patient had CW and nodal recurrence concomitantly. The combined local regional relapse rate is 5%. Two additional patients experienced a local relapse after a distant relapse. Including a local failure after failing distantly, our combined 5-year locoregional-free survival rate is 92% (Fig. 1). In multivariable analysis, an increasing number of positive lymph nodes was predictive for LRR (P = .039); estrogen receptor status predicted for any recurrence and for overall survival (P = .0014 and P = .0234, respectively). Twelve patients developed distant disease for a 5-year distant-disease-free survival of 77% and 5-year overall survival of 90% (Figs. 2 and 3).
Fig. 1.
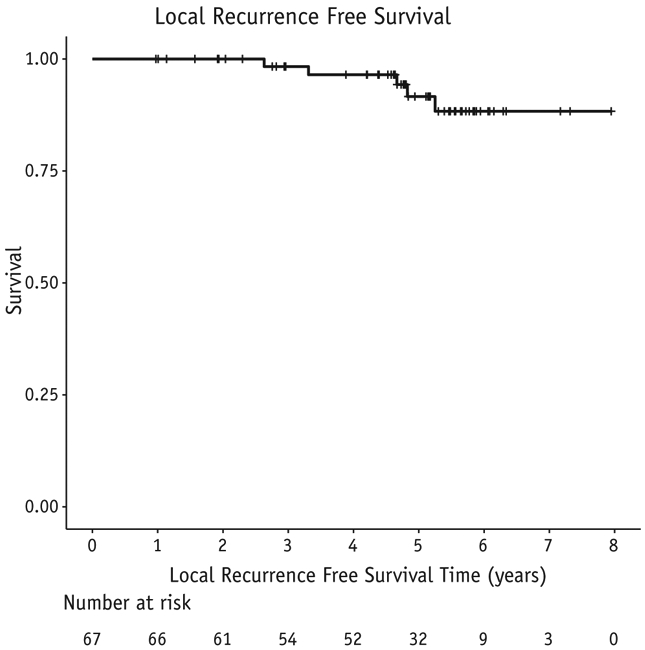
Local recurrence-free survival in 67 patients treated with experimental schedule.
Fig. 2.
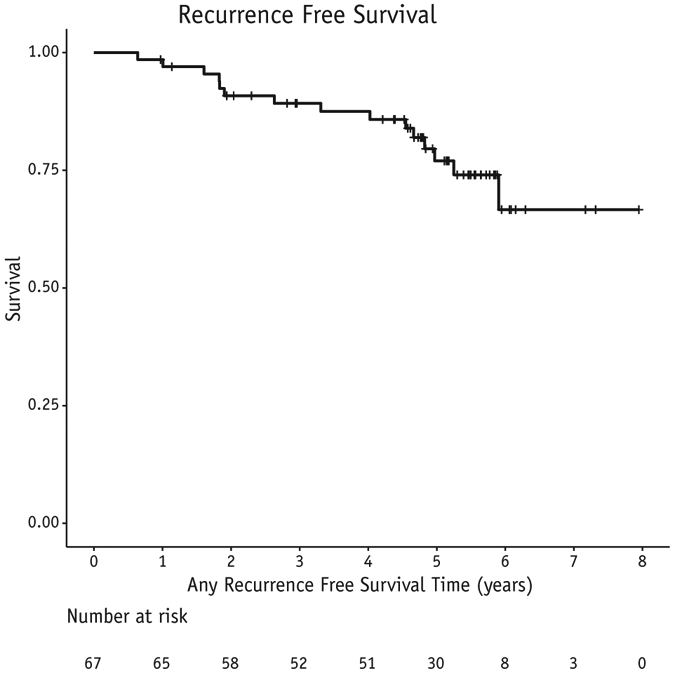
Distant or local recurrence-free survival in 67 patients treated with experimental schedule.
Fig. 3.
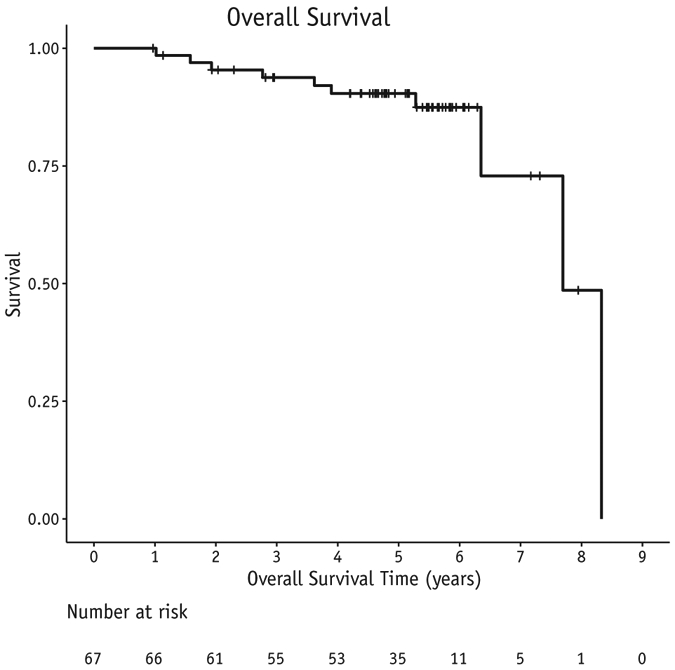
Overall survival in 67 patients treated with experimental schedule.
Dose-volume analysis
All patients had normal tissue dose volume histogram analysis performed retrospectively using axial computed tomography simulation information (Table 2). Dosimetry data is availble in Table 3. Average mean heart dose was 1.3 Gy (range, 0.26-3.81) and the average ipsilateral lung V15 Gy was 25% (range, 3.3%-41.8%). Maximum dose to the brachial plexus, contoured as axillary vessels, averaged 37.0 Gy (range, 34.0-39.2).
Table 3.
Dosimetry data
| Average | Range | |
|---|---|---|
| Mean heart, Gy | 1.3 | (0.3-3.8) |
| Heart V20,* % | 0.3 | (0-4.3) |
| Ipsilateral lung V15,* % | 24.8 | (3.3-41.8) |
| Ipsilateral lung V18,* % | 23.5 | (2.8-39.4) |
| Max brachial plexus (>0.2 cm3), Gy | 37.4 | (34-39.2) |
Vx = volume (V) receiving x dose or greater, expressed as percentage of total volume.
Planning target volumes (PTVs) for the CW and nodal volume were evaluated randomly for 30 of the patients. The average CW PTV receiving 95% of the prescribed dose was 97%. The average axillary PTV receiving 95% of the prescribed dose was 92%.
Toxicities
Toxicity results are listed in Table 4. The main toxicity was radiation dermatitis. There were no grade 3 acute or late toxicities in our hypofractionation patients. One patient developed an infected postmastectomy seroma that spontaneously started draining after 1 fraction of RT. The patient was taken off study and was treated with conventionally fractionated PMRT. The event was not attributable to radiation. Twenty-eight percent of patients developed acute grade 2 dermatitis, all cases of which resolved within 6 months from the end of RT. Grade 1 acute skin toxicity occurred in 54% of patients; grade 1 late toxicity occurred in 30%, usually as hyperpigmentation. Additional late grade 2 toxicities included 8% CW pain and 3% fatigue. Only 1 patient (1.5%) experienced late grade 2 lymphedema; this patient had axillary lymph node dissection with 26 lymph nodes removed.
Table 4.
Treatment-related toxicities
| Grade 1 toxicities |
Grade 2 toxicities |
|||
|---|---|---|---|---|
| Acute, n (%) |
Late, n (%) |
Acute, n (%) |
Late, n (%) |
|
| Skin | 37 (55) | 20 (30) | 19 (28) | 0 |
| Fatigue | 15 (22) | 12 (18) | 8 (12) | 2 (3) |
| Pain | 13 (19) | 9 (13) | 2 (3) | 5 (8) |
| Lymphedema | 1 (2) | 1 (2) | 2 (3) | 1 (2) |
| Subcutaneous | 0 | 11 (17) | 1 (2) | 0 |
| Telangiectasia | 0 | 11 (17) | 0 | 0 |
| Other* | 2 (3) | 0 | 1 (2) | 0 |
Bronchospasm (wheezing), shoulder stiffness.
Five patients reported late grade 2 pain that persisted 6 months after treatment. Of these 5 patients, 1 reported CW pain that responded to non-narcotics and persisted for around a year but had resolved by last follow-up. The other patient reported pain that seemed to be related to expander contracture; this improved significantly at last follow-up, but she continued to have CW tightness. Two patients reported late grade 2 fatigue, which was scored as multifactorial and not attributed solely to radiation treatment.
Reconstruction
Forty-three patients had plans for breast reconstruction. Of the 43, 40 patients (93%) had pre-RT reconstruction, of which 88% were temporary expanders, 7% were immediate implants, and 5% were prior augmentation implants. Three patients (7%) had post-RT reconstruction. Of the 43 patients, 35% had grade 3 or 4 reconstruction complications attributable to RT.
Discussion
The Early Breast Cancer Trialists’ Cooperative Group (EBCTCG) evaluated 22 randomized trials and concluded that adding radiation to node-positive breast cancer after mastectomy decreased local recurrence at 10 years from 26% to 8% and decreased 20-year breast cancer mortality from 66% to 58%.12 These benefits were found to be independent of the effect of cytotoxic systemic therapy. A similar benefit was not found for node-negative women. Fourteen of the 22 randomized trials analyzed by the EBCTCG used a standard fractionation of 50 Gy in 25 fractions of 2.0 Gy to the CW and regional nodes. Regional lymph nodes include levels 1 to 3 of the axilla, the supraclavicular fossa, and usually the first 3 ipsilateral intercostal IM nodes. Our 5-year locoregional recurrence rate of 5% is in line with the results of the these prospective PMRT trials despite our unique and convenient fractionation.
Targeting the IM nodes is an area of controversy, considering they were targeted in 20 of the 22 included trials in the EBCTCG meta-analysis. In addition, both the National Cancer Institute of Canada MA.20 and European Organization for Research and Treatment of Cancer 22922 trials demonstrate a small but significant improvement in disease-free survival by adding IM node radiation.12-14 Targeting the IM nodes when performing regional nodal irradiation appears to be the accepted current standard, given their inclusion in recent trial design (Alliance A11202 and A221505, NRG B51, and National Cancer Institute of Canada MA.39). Our trial was run before the results of MA.20 and European Organization for Research and Treatment of Cancer 22922 were known; therefore, IM nodes were treated “optionally” in only 28% of patients. Adding IM coverage to the remaining 72% of patients would likely increase the average heart and lung dosimetry to those reported here.
Hypofractionation is traditionally accelerated, thus delivering the total dose of radiation in a shorter period of time and requiring a lower total dose for similar effectiveness. This lower total dose of radiation may explain the improved cosmetic effects found in the hypofractionation arms of UK Start trials. Toxicity rates from PMRT trials vary based on reporting scale used, but our schedule appears to be similar or better, with no grade 3 events reported and only 12% late grade 2 events. The hypofractionation prospective PMRT trial from China reported that 1% of patients in both treatment arms experienced a grade 3 or worse toxicity, and 21% experienced late grade 1 to 2 skin toxicity.9
Hypofractionation is inherently more cost-effective for both the patient and society. In an economic statistical Markov chain analysis, Khan et al demonstrated that for a low/middle-income country, hypofractionated PMRT likely would result in an improvement in breast cancer survival by improving access to care.15 In an era of medical cost reduction, the Centers for Medicare and Medicaid Services have proposed an innovative payment model for radiation oncology with the goal of improving quality and value-based care (Centers for Medicare and Medicaid Services rule 5527-P).16 Short-course radiation trials such as this may help radiation oncology practices survive the changing financial reimbursement of the future while improving patients’ quality of life. Trials using modern hypofractionated radiation with an appropriate biological effective dose (BED) and modern chemotherapy were lacking but are now being pursued. Fear of hypofractionation is likely based on faulty assumptions. Several single-institution hypofractionated PMRT trials dating back to the 1950s and 1960s reported excessive toxicity. At that time, there was little understanding of the radiobiology of breast cancer, and as a result these trials gave too high a BED, resulting in unacceptable toxicity.17-19 The Vancouver BC trial by Ragaz et al used 37.5 Gy in 15 fractions (35 Gy to the mid axilla) in 16 fractions and demonstrated acceptable toxicity.3 The BED of this fractionation is only 80% the BED of 50 Gy in 2-Gy fractionations. This reduced BED, although potentially less toxic, may explain the higher-than-expected 20-year local recurrence rate of 13%.20
Modern trials with conventional chemotherapy and radiation doses that use our understanding of linear-quadratic equivalent doses are starting to be published. Small trials from Pakistan, Greece, and Thailand appear to show that hypofractionated PMRT with an appropriate BED can be safely performed, at least in nonreconstructed breast patients.21-23 The UK Start A and B trials, although designed for early-stage postlumpectomy patients, ended up treating a few mastectomy (531; 9%) and regional nodal (451; 7%) patients.8,24,25 There was no description of breast reconstruction in these patients, but no excess CW or nodal toxicity was reported. Of the regional nodal irradiation patients, there was only 1 case of brachial plexopathy in the UK Start A trial (3.2 Gy × 13 arm), without description of symptoms or severity. The recently published trial from Beijing, China, is the first modern randomized PMRT fractionation trial, and it demonstrates very promising results.9 An important detail about this reported trial is that all patients had flat, unreconstructed CWs that were treated with electrons. Our trial adds to the momentum of hypofractionation in this patient population yet adds a novel dose/fractionation that is perhaps the most convenient published to date. The outcomes of our dose schedule are perhaps best interpreted with the addition of our mastectomy scar boost, considering 97% of our patients received the boost. The 2-Gy BED to the regional nodes and CW is estimated to be 45 Gy with 60 Gy to the mastectomy scar. Only 1.5% of our patients experienced CTCAE grade 2 lymphedema. This lower-than-expected lymphedema rate for a group in which 84% underwent axillary dissection may partially be explained by the lower BED delivered to the regional nodes (45 Gy) but is more likely reflective of the limitations of CTCAE grading, which relies on severity of arm symptoms and an assessment of completing instrumental activities of daily living. Routine arm measurements were not collected. This trial did not mandate consistent contouring of targets and organs; going forward, we would advise using volume-based planning per the Radiation Therapy Oncology Group Breast Contouring atlas with hypofractionated dose constraints, such as those that appear in Alliance A221505.11
Conclusions
An unexplored population yet to be adequately studied for safe hypofractionation is women with a reconstructed CW. Although reconstructed patients (41%) were included on our trial with acceptable reconstruction toxicities, this was our motivation behind the creation of the phase 3 randomized trial, Alliance A221505 (RT CHARM), which started enrolling patients in March 2018 and has enrolled 400 of 880 planned patients as of December 2019.10,26 A detailed update of our reconstruction outcomes with comparison to a matched conventionally fractionated cohort will be reported in a separate publication.
Acknowledgments
Funding was provided by a Rutgers Cancer Institute of New Jersey’s Core Center Support Grant (P30CA072720), the Breast Cancer Research Foundation (B.H.), Huntsman Cancer Hospital, Huntsman Cancer Institute, and the University of Utah School of Medicine, Department of Radiation Oncology.
Footnotes
Disclosures: none.
References
- 1.Overgaard M, Hansen P, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. N Engl J Med 1997;337:949–955. [DOI] [PubMed] [Google Scholar]
- 2.Overgaard M, Jensen M, Overgaard J, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82C randomized trial. Lancet 1999;353:1641–1648. [DOI] [PubMed] [Google Scholar]
- 3.Ragaz J, Jackson SM, Le N, et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med 1997;337:956–962. [DOI] [PubMed] [Google Scholar]
- 4.Haviland JS, Owen JR, Dewar JA, et al. The UK standardisation of breast radiotherapy (Start) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol 2013;14:1086–1094. [DOI] [PubMed] [Google Scholar]
- 5.Whelan TJ, Pignol JP, Levine MN, et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med 2010; 362:513–520. [DOI] [PubMed] [Google Scholar]
- 6.Bentzen S, Baumann M. The linear-quadratic model in clinical practice In: Steel G, editor. Basic Clinical Radiobiology. London: Arnold; 2002. p. 134–146. [Google Scholar]
- 7.Fowler J The linear-quadratic formula and progress in fractionated radiotherapy. Br J Radiol 1989;62:679–694. [DOI] [PubMed] [Google Scholar]
- 8.Yarnold J, Ashton A, Bliss J, et al. Fractionation sensitivity and dose response of late adverse effects in the breast after radiotherapy for early breast cancer: Long-term results of a randomised trial. Radiother Oncol 2005;75:9–17. [DOI] [PubMed] [Google Scholar]
- 9.Wang SL, Fang H, Song YW, et al. Hypofractionated versus conventional fractionated postmastectomy radiotherapy for patients with high-risk breast cancer: A randomised, non-inferiority, open-label, phase 3 trial. Lancet Oncol 2019;20:352–360. [DOI] [PubMed] [Google Scholar]
- 10.Khan AJ, Poppe MM, Goyal S, et al. Hypofractionated postmastectomy radiation therapy is safe and effective: First results from a prospective phase ii trial. Journal of clinical oncology: official journal of the American Society of Clinical. Oncology 2017;35:2037–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White JR, Tai A, Arthur DW, et al. Breast cancer atlas for radiation therapy planning: Consensus definitions In: Breast Cancer Atlas for Radiation Therapy Planning: Consensus Definitions. Radiation Therapy Oncology Group; 2011. Available at: https://www.rtog.org/LinkClick.aspx?fileticket==vzJFhPaBipE%3d&tabid==236. Accessed May 1, 2020. [Google Scholar]
- 12.Early Breast Cancer Trialists’ Collaborative Group, McGale P, Taylor C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: Meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 2014;383:2127–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poortmans P, Struikmans H, Collette S, et al. OC-0523: Lymph node RT improves survival in breast cancer: 10 years results of the EORTC ROG and BCG phase III trial 22922/10925. Radiother Oncol 2014;11: S206. [Google Scholar]
- 14.Whelan TJ, Olivotto IA, Ackerman I, et al. NCIC-CTG MA.20: An intergroup trial of regional nodal irradiation in early breast cancer. J Clin Oncol 2011;29:LBA1003. [Google Scholar]
- 15.Khan AJ, Rafique R, Zafar W, et al. Nation-scale adoption of shorter breast radiation therapy schedules can increase survival in resource constrained economies: Results from a Markov chain analysis. Int J Radiat Oncol Biol Phys 2017;97:287–295. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Medicare and Medicaid Services. Proposed radiation oncology (RO) model, CMS-5527-P. Available at: https://www.hhs.gov/sites/default/files/CMS-5527-P.pdf. Accessed April 12, 2020.
- 17.Stoll BA, Andrews JT. Radiation-induced peripheral neuropathy. Br Med J 1966;1:834–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galecki J, Hicer-Grzenkowicz J, Grudzien-Kowalska M, et al. Radiation-induced brachial plexopathy and hypofractionated regimens in adjuvant irradiation of patients with breast cancer—a review. Acta Oncol 2006;45:280–284. [DOI] [PubMed] [Google Scholar]
- 19.Johansson S, Svensson H, Larsson LG, et al. Brachial plexopathy after postoperative radiotherapy of breast cancer patients—a long-term follow-up. Acta Oncol 2000;39:373–382. [DOI] [PubMed] [Google Scholar]
- 20.Ragaz J, Olivotto I, Spinelli J, et al. Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy: 20 year results of the British Columbia randomized trial. J Natl Cancer Inst 2005;97:116–126. [DOI] [PubMed] [Google Scholar]
- 21.Koukourakis MI, Panteliadou M, Abatzoglou IM, et al. Postmastectomy hypofractionated and accelerated radiation therapy with (and without) subcutaneous amifostine cytoprotection. Int J Radiat Oncol Biol Phys 2013;85:e7–e13. [DOI] [PubMed] [Google Scholar]
- 22.Pinitpatcharalert A, Chitapanarux I, Euathrongchit J, Tharavichitkul E, Sukthomya V, Lorvidhaya V. A retrospective study comparing hypofractionated radiotherapy and conventional radiotherapy in postmastectomy breast cancer. J Med Assoc Thai 2011;94:S94–S102. [PubMed] [Google Scholar]
- 23.Shahid A, Athar MA, Asghar S, Zubairi T, Murad S, Yunas N. Post mastectomy adjuvant radiotherapy in breast cancer: A comparision of three hypofractionated protocols. J Pak Med Assoc 2009;59:282–287. [PubMed] [Google Scholar]
- 24.Group ST, Bentzen SM, Agrawal RK, et al. The UK standardisation ofbreast radiotherapy (Start) trial B of radiotherapy hypofractionation for treatment of early breast cancer: A randomised trial. Lancet 2008;371:1098–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Owen JR, Ashton A, Bliss JM, et al. Effect of radiotherapy fraction size on tumour control in patients with early-stage breast cancer after local tumour excision: Long-term results of a randomised trial. Lancet Oncol 2006;7:467–471. [DOI] [PubMed] [Google Scholar]
- 26.ClinicalTrials.gov [Internet]. Bethesda, MD: National Library of Medicine (US) Identifier NCT03414970, Hypofractionated radiation therapy after mastectomy in preventing recurrence in patients with stage iia-iiia breast cancer; 2018; [about 4 screens]. Available at: https://clinicaltrials.gov/ct2/show/NCT03414970. Accessed May 1, 2020. [Google Scholar]


