Abstract
Purpose/Objectives
Conventionally fractionated whole breast irradiation (WBI) with a boost takes approximately 6–7 weeks. We evaluated a short course of hypofractionated, accelerated whole breast irradiation (HF) in which therapy is completed in 3 weeks inclusive of a sequential boost.
Materials/Methods
We delivered a whole breast dose of 36.63 Gy in 11 fractions of 3.33 Gy over 11 days followed by a lumpectomy bed boost in 4 fractions of 3.33 Gy delivered once daily for a total of 15 treatment days. Acute toxicities were scored using CTCAE v 4. Late toxicities were scored using the RTOG/EORTC scale. Cosmesis was scored using the Harvard Cosmesis Scale. Our primary endpoint was freedom from locoregional failure; we incorporated early stopping criteria based on predefined toxicity thresholds. Cosmesis was examined as a secondary endpoint.
Results
We enrolled 83 women with Stage 0-IIIa breast cancer. After a median follow up of 40 months, 2 cases of isolated ipsilateral breast tumor recurrence occurred (2/83, crude rate 2.4%). Three year estimated local recurrence-free survival is 95.9% (95% CI: 87.8% - 98.7%). The 3-year estimated distant recurrence-free survival is 97.3% (95% CI: 89.8% - 99.3%). Three year secondary malignancy free survival is 94.3% (95% CI: 85.3% - 97.8%). Twenty-nine patients (34%) had Grade 2 acute skin toxicity and 1 patient had a late grade 2 toxicity (fibrosis). One patient had acute grade 3 dermatitis while 2 patients experienced a grade 3 late skin toxicity. Ninety-four percent of evaluable patients had good/excellent cosmesis.
Conclusions
Our phase II institutional study offers one of the shortest courses of HF--delivered in 15 fractions inclusive of a sequential boost. We demonstrated expected low toxicity and high local control rates with good-excellent cosmetic outcomes. This fractionation scheme is feasible and and well-tolerated and offers women WBI in a highly convenient schedule.
Keywords: hypofractionated, whole breast radiation, sequential boost, breast cancer
Introduction
Early-stage breast cancer treatment options include breast-conservation with lumpectomy and adjuvant whole breast irradiation (WBI). Given excellent local control rates and low morbidity with current adjuvant radiation therapy technique and fractionation, it is natural that subsequent improvements in the field take patient convenience into account while decreasing healthcare costs (1). In the United States, a standard course of WBI with a sequential boost has consisted of 6–7 weeks of treatment, 5 days per week. This lengthy schedule is not always convenient and/or accessible for all patients; over the last several years there has been increasing interest and investigation into hypofractionated (HF) WBI.
The largest randomized trials to study HF WBI were the UK START A and B trials. The UK START A trial randomized women with pT1-3a, pN0-1 breast cancer after either lumpectomy or mastectomy to one of three radiation treatment arms with treatment time constant spanning 5 weeks. The control arm was standard fractionation (50 Gy in 25 fractions) vs. 39 Gy in 13 fractions vs. 41.6 Gy in 13 fractions, all over 5 weeks. Simultaneously, the UK START B trial randomized women with pT1-3a, pN0-1 breast cancer to standard fractionation vs. 40 Gy in 15 fractions over 3 weeks. In START A 71% of the lumpectomy patients received a boost while 51% of patients underwent a boost after lumpectomy in the START B; at the discretion of the treating physician in both trials. The primary endpoints for both trials were local regional relapse (LRR), normal tissue effect, and quality of life. There was no difference in LRR in either the UK START A or B trials (2,3,4), and late breast changes appeared to be better with HF.
Similarly, the Ontario Cooperative Oncology Group conducted a non-inferiority, randomized trial comparing standard WBI to HF WBI. Women who underwent lumpectomy and axillary dissection were randomized to 50 Gy in 25 fractions over 35 days or 42.5 Gy in 16 fractions over 22 days. Patients had to be pN0, with T1–T2 disease; neoadjuvant chemotherapy was disallowed, but adjuvant systemic treatment was allowed. Lumpectomy bed boost was not allowed. The primary endpoint was local recurrence. Long-term results revealed equivalent cumulative incidence of local recurrence in the standard arm (6.7%) vs. the HF WBI (6.2%) arm. There was no difference in overall survival, disease free survival, or cosmetic outcome (5,6).
The aforementioned randomized trials have proven equivalent local control and cosmetic outcomes with HF WBI schemes as compared to standard fractionation. These trials of HF primarily used 2-dimensional (2D) planning techniques with dose homogeneity measured on the central axis and without the benefits of currently available techniques to improve homogeneity such as field-in-field and electronic compensation methods. Furthermore, the trial designs did not include a standardized surgical bed boost, which is associated with demonstrably improved local control (7,8). While the START trials did include a variety of patients, Whelan et al limited their trial to smaller, node negative tumors. We conducted a Phase II prospective institutional trial evaluating accelerated HF WBI in which the goal was to complete therapy in 3 weeks inclusive of a sequential boost in a heterogeneous cohort of women in the era of modern 3D planning.
Methods and Materials
Trial Design/Patient Eligibility
We conducted a prospective, non-randomized, single arm phase II trial using a novel fractionation schedule in women with DCIS or invasive ductal, lobular, medullary, papillary, colloidal (mucinous), or tubular carcinoma (NCT00909909). To account for what we viewed as shortcomings in the existing data, we wanted our schema to be inclusive of a mandated sequential boost and still be complete in a total of 15 fractions. Table 1 details eligibility and exclusion criteria. In particular we didn’t exclude patients if they had high-risk features such as: lymphovascular invasion, close margins, young age, hormone-receptor negativity, or extensive intraductal component.
Table 1.
Eligibility/Exclusion Criteria
| Eligibility | Exclusion |
|---|---|
| Age ≥18 years old | Breast micro-calcifications prior to starting radiation treatment (RT) |
| ECOG performance status of 0–1 | > 9 positive axillary or sentinel lymph node |
| Stage 0–IIIA (pTis-T2, pN0-pN2a, M0) breast cancer | Lobular carcinoma in situ alone or non breast epithelial histology |
| Systemic treatment (chemotherapy or hormonal therapy) was allowed. | Multicentric disease |
| Neoadjuvant chemotherapy allowed. | Clinically or radiographically suspicious contralateral regional lymph nodes unless histologically confirmed negative for tumor |
| Negative margins (no tumor on ink). | Prior treatment for contralateral or synchronous breast cancer or if they had prior RT to the current breast. |
Co-morbid conditions:
| |
| No documented negative pregnancy test or unwilling to maintain safe and effective birth control |
Acute toxicities were scored using CTCAE v 4. Late toxicities were scored using the RTOG/EORTC scale. Cosmesis was scored by the treating physicians using the Harvard Cosmesis Scale. The study and the informed consent was reviewed by the XXX Institutional Review Board and approved. The Office of Human Research oversaw patient accrual and safety monitoring and the study was supported by the XX Institute of XX’s Core Center Support Grant (NCT00909909).
Radiation Treatment Planning and Technique
We delivered a whole breast dose of 36.63 Gy in 11 fractions of 3.33 Gy delivered 5 days per week, 1 fraction per day (EQD2=45 Gy, using linear quadratic formalism and an α/β ratio of 4, α value of 0.4, Tpot value of 13 days and an initial time lag of 14 days). Patients received a mandatory lumpectomy bed boost delivered in 4 fractions of 3.33 Gy delivered once daily (EQD2=15Gy). We chose 4 fractions for the boost since our trial did not exclude patients with high-risk features. CT based treatment planning was mandatory (14–63 days from last surgery or last cycle of chemotherapy) with the treatment planning scan including the entire breast. Radiation treatment began within 21–63 days from last surgery or last cycle of chemotherapy. Supine or prone positioning was allowed. Standard whole breast tangential fields were used while limiting dose to non-breast structures as per standard-of-care. On beams-eye views, the tangent beams could not include more than 3 cm of lung at any level. The heart was required to be excluded from the primary beam using blocks or other techniques (breath hold). The dose within the clinical treatment volume was required to be within 90–115% of prescription dose. A planning target volume (PTV) for the whole breast was not required at the time this study was initiated, and a graphical review of the plan at the discretion of the treating physician was considered acceptable. We allowed treatment of regional lymph nodes if indicated with a supraclavicular field and/or posterior axillary boost using the same treatment schedule. Nodal volumes were contoured and evaluated for coverage; internal mammary coverage was left to the discretion of the treating physician. A max dose not exceeding 107% was allowed in the supraclavicular volume, to constrain brachial plexus dose. Lumpectomy boost planning included contouring and visualization of the surgical cavity with a 1–2 cm margin to create a PTV. If the cavity could not be visualized the patient underwent an electron beam boost to a volume which encompassed a 2 cm margin on the lumpectomy scar.
Any combination of photon beams of energy 6 MV or higher, with or without the addition of electrons of any energy, were allowed for treatment provided the dosimetric requirements of adequately treating the whole breast and dose homogeneity were met. The tangents were prescribed to a point 1.5 cm anterior to the posterior edge of the fields at mid-separation, or to a point 1/3rd the distance from this point to the skin, or to an isodose line encompassing the PTV. Boost prescription point was to an isodose line that completely covered the volume/PTV. Wedges, field in field, and electronic compensation were allowed to achieve dose homogeneity.
Statistical Analysis
Our primary endpoint was freedom from local regional failure; we incorporated early stopping criteria based on predefined toxicity thresholds. Cosmesis was examined as a secondary endpoint. We used a fixed sample size of 83 patients with a primary end point of local-regional relapse rate. Assuming a true local-regional recurrence rate of 5%, the upper limit of a 95% confidence interval (calculated using the Wilson method) will exclude a local-regional failure rate of 12% with 83 patients (9). We also evaluated toxicities in these patients. In the first 16 patients enrolled, we closely monitored for pre-defined toxicities. A preset composite endpoint of toxicity was defined as greater than 25% rate of in breast toxicities (> grade 2 acute skin toxicity, moderate/severe fibrosis, retraction, edema, symptomatic fat necrosis, breast pain requiring narcotics) or a greater than 5% risk of non-breast toxicities (i.e. acute symptomatic pneumonitis > grade 2). We used probability calculations (posterior probabilities assuming uniform prior complication rate distribution) and estimated that we could exclude a 25% or higher rate of in-breast complications, if we observed 5 or fewer in-breast toxicities in the first 16 patients. Similarly, we could exclude a 5% or higher rate of non-breast complications if we observed no greater than 1 such toxicity.
All time intervals were calculated from the date of diagnosis. Nonparametric estimates of the survival or recurrence-free distributions or recurrence (failure) distribution were obtained by life table methods. Tests were declared statistically significant if the calculated P-value was ≤ 0.05. All tests used 2-sided P-values.
Results
We enrolled 83 women between 2009–2012 with Stage 0-IIIa breast cancer. Table 2 details the clinical characteristics of the patient population. Median follow up for our cohort of patients is 40 months with a range of 10.1–73 months. Median age at HF WBI is 53.4 years with a range of 33.1–79.1 years. Thirty percent of patients were less than 50 years old at HF WBI. Our patients had a mean separation of 22 cm (range 15.6–30.2 cm). Of note, 9.6% of our patients underwent regional radiation treatment with additional third or fourth field. Most of our cohort was treated supine, with 2 women undergoing prone treatment. Maximum point doses in our whole breast +/− regional RT plans ranged from 104–113% of prescription dose. Nine patients (10.8%) underwent neoadjuvant chemotherapy of which one patient had a pathological complete response after neoadjuvant chemotherapy.
Table 2.
Patient Clinical Characteristics
| Number of subjects | 83 |
| Follow-up (months) | |
| Median (Range) | 40 (10.1–73) |
| Age (years) | |
| Median (Range) | 53.4 (33.9–79.1) |
| ≥50 years N (%) | 58 (69.9) |
| < 50 years N (%) | 25 (30.1) |
| Breast Laterality N (%) | |
| Left | 40 (48.2) |
| Right | 43 (51.8) |
| Histology N (%) | |
| DCIS | 16 (19.2) |
| Invasive Ductal | 58 (69.9) |
| Invasive Lobular | 8 (9.6) |
| Other | 1 (1.2) |
| Tumor Size (mm) | |
| Median (Range) | 10.0 (0.0–40.0) |
| AJCC T Tumor Status N (%) | |
| T0 | 1 (1.2) |
| Tis | 17 (20.5) |
| T1 | 57 (68.7) |
| T2 | 8 (9.6) |
| AJCC N Nodal Status N (%) | |
| Nx | 8 (9.6) |
| N0 | 59 (70.1) |
| N1 | 15 (18.1) |
| N2 | 1 (1.2) |
| Estrogen Receptor N (%) | |
| Positive | 70 (84.3) |
| Negative | 13 (15.7) |
| Progesterone Receptor N (%) | |
| Positive | 67 (80.7) |
| Negative | 14 (16.9) |
| Unknown | 2 (2.4) |
| Her2Neu N (%) | |
| Positive | 4 (4.8) |
| Negative | 68 (81.9) |
| Unknown | 11 (13.3) |
| Radiation Treatment N (%) | |
| Whole Breast Alone | 75 (90.4) |
| Regional Radiation | 8 (9.6) |
| Systemic Therapy N (%) | |
| Hormone Thearpy | 64 (77.1) |
| Chemotherapy | 26 (31.3) |
| Neoadjuvant Chemotherapy | 9 (10.8) |
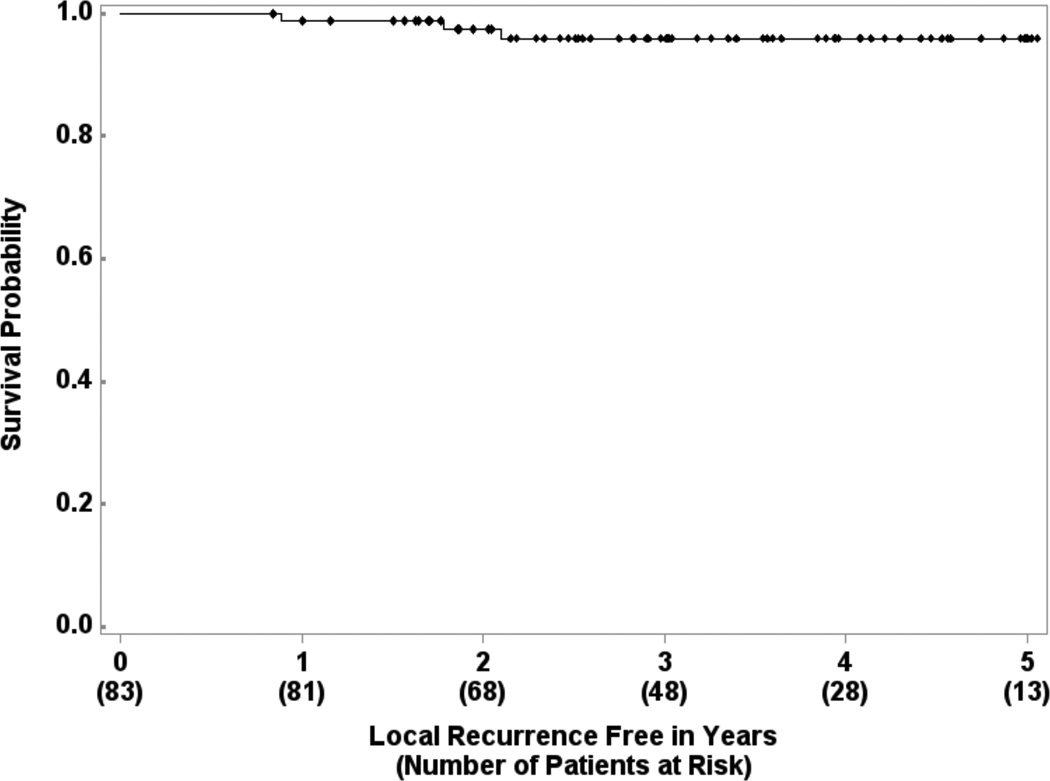
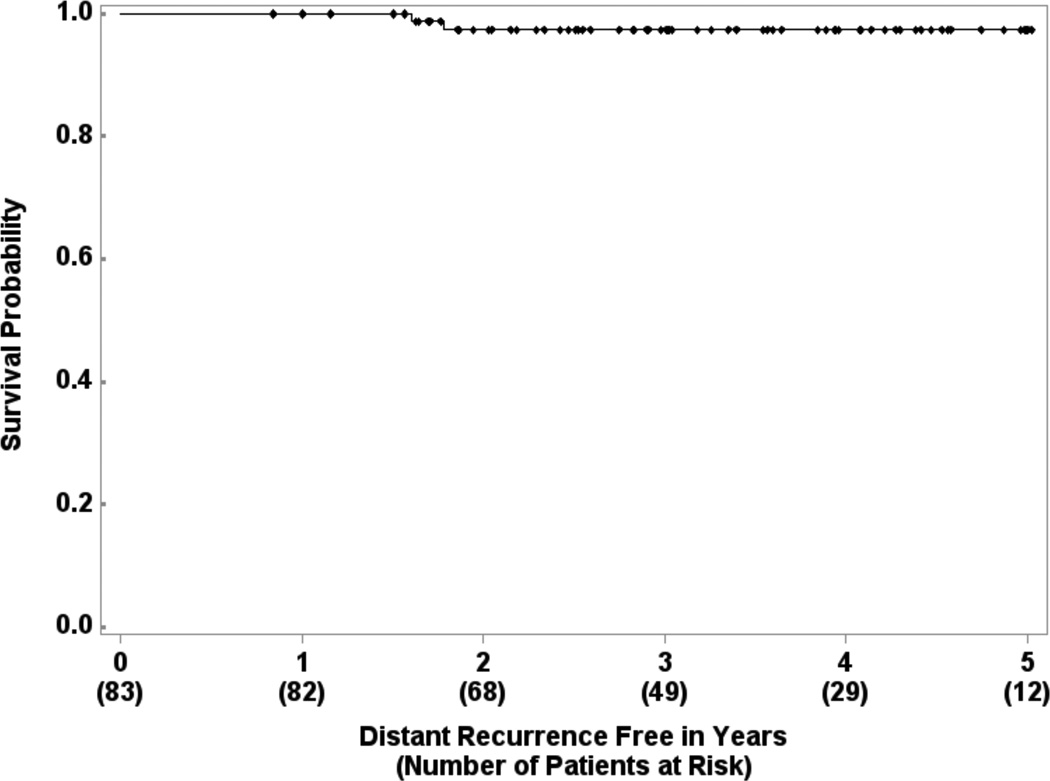
There were 2 cases of isolated ipsilateral breast tumor recurrence (IBTR) (2/83, crude rate 2.4%). Three year estimated local recurrence-free survival is 95.9% (95% CI: 87.8% – 98.7%) (Figure 1). Four patients experienced contralateral breast tumor recurrence (CBTR). The 3-year estimated distant recurrence-free survival is 97.3% (95% CI: 89.8% – 99.3%) (Figure 2). One patient had simultaneous IBTR and distant metastases. Another patient had an in-breast secondary malignancy (angiosarcoma) after a disease-free interval of 46.4 months. Three-year second malignancy-free survival is 94.3% (95% CI: 85.3% – 97.8%).
Figure 1.
Local recurrence-free survival in 83 patients treated with experimental schedule.
Figure 2.
Distant recurrence-free survival in 83 patients treated with experimental schedule.
Our initial cohort of 16 patients did not meet complication thresholds. In our entire cohort, grade 2 acute toxicity was recorded in 29 out of 83 patients (34%). One patient developed an acute grade 3 radiation dermatitis. One patient had a late grade 2 toxicity (fibrosis) approximately 4 months after completing treatment. Two patients experienced grade 3 late toxicity. Out of the two women with grade 3 late toxicity, 1 had developed an IBTR (after 22.8 months) and opted for repeat breast conservation with partial breast re-irradiation receiving 45 Gy in 1.5 Gy BID. Five months after re-irradiation, the patient developed a non-healing wound requiring hyperbaric oxygen and surgical intervention with incision and drainage. The other woman had a persistent skin reaction. There were no grade 4 or 5 toxicities in our cohort. Breast cosmesis evaluation revealed that 94% of 48 evaluable patients (those with physician evaluations at least 2 years post treatment) had a good or excellent cosmesis.
Discussion
American Society for Radiation Oncology (ASTRO) consensus guidelines outline HF WBI as suitable for patients > 50 years old, stage pT1-2 pN0, who do not undergo chemotherapy, and have radiation plan homogeneity +/– 7% along the central axis of the breast (10). Furthermore, ASTRO’s Choosing Wisely campaign suggests considering HF WBI in women >50 years old with early stage breast cancer as a part of breast conserving therapy (11). These recommendations exclude patients who were felt to require further investigation to determine safety and efficacy. We conducted a phase II institutional trial with one of the shortest courses of HF WBI reported from North America-- our whole breast schedule was completed in 11 fractions. We were comfortable testing our experimental schedule because we felt the radiobiological parameters of tumor control and toxicity in breast radiotherapy have been definitively established by the invaluable work of the UK trialists. We chose to sequence our boost for simplicity and familiarity and to allow for flexibility in boost technique. The entire treatment was delivered in 15 fractions with a boost; we tested our schedule in a more diversified cohort than some of the earlier trials, spanning DCIS through N2a disease, and including patients outside the ASTRO HF consensus guidelines.. Our data appears to 1) confirm that known radiobiological parameters of breast cancer continue to hold robustly in the 2–5 Gy fraction range, 2) the isoeffective doses can be expected to behave isoeffectively across heterogeneous risk groups.
The UK HF trials have demonstrated excellent local control and cosmetic outcomes with HF WBI treatment compared standard treatment (2,3,4). Whelan et al also conducted a Phase III randomized trial comparing standard WBI to HF WBI with long term results showing equivalent outcomes (5,6). In contrast to our HF WBI regimen in which we mandated a lumpectomy boost, the Canadian and UK trials did not uniformly use or disallowed a boost. Most recently, Shaitelman et. al reported the results of their trial which randomized women to conventional fractionation WBI vs. HF WBI with a tumor bed boost in both arms (12). Early toxicity results show that, even with tumor bed boost, HF WBI had significantly lower rates of toxicity. Nandi et al reported their experience of using START B fractionation with included boost in BCS patients. The authors showed low toxicity rates in a fairly heterogeneous population (13).
We felt it was acceptable to broaden our patient population in the context of a single-institution phase II. Interestingly, the LRR rates among heterogeneous groups of breast cancer patients is remarkably and uniformly low when adjuvant radiotherapy is delivered. In the Early Breast Cancer Trialists' Collaborative Group (EBCTCG) meta-analyses, the 5-year the local recurrence rates was 7.6% in women with DCIS, 7% in BCS patients, and 6% in post-mastectomy patients in the respective RT arms, regardless of nodal status. The respective, 10-year local recurrence rates after radiation were 12.9% with DCIS, 10% pN0 after BCS, 13% pN+ after BCS, 3% pN0 post mastectomy, and 7.5% pN+ after mastectomy (14,15,16). With large meta-analyses showing similar disease control rates after radiotherapy across the risk spectrum, we felt we could use a common statistical endpoint of local-regional control for all patients in our study.
Since the early HF WBI trials, there have been many advances in radiation planning and treatment. Several authors have evaluated HF WBI with intensity modulated radiation treatment (IMRT) delivered and a simultaneous integrated boost (SIB) and shown low acute skin toxicity rates (17,18,19,20). Mulliez et al examined HF in women with larger breasts randomized to prone and supine treatment position and showed 50% decrease in acute grade 2–3 dermatitis with prone positioning (21). Cante et al conducted a cohort study, offering women with early stage breast cancer HF with a concomitant boost and after a median follow up of 60 months, there was excellent local control with low late toxicity (22). Decreasing boost time, Pinnaro et al treated women who refused standard fractionation after BCS with HF WBI followed by a single 8 Gy boost with low toxicity observed (23). Furthermore, several authors have examined weekly fractionation schedules with low toxicity and good local control (24, 25). The wide variability in reported skin toxicities is likely due to differences in the toxicity scales used as well as the inherent subjectivity in assigning these grades. We tried to mitigate this in our series by using the CTCAE and RTOG criteria. Reported differences may also be due to differences in treatment technique, patient setup, and positioning. In our cohort, 1 of the 2 women with a grade 3 late skin toxicity occurred after re-irradiation to the ipsilateral breast, which was not related to the experimental fractionation. Ninety-four percent of our cohort had a good to excellent cosmetic outcome comparable to other HF WBI studies. We have outlined the details of the above-mentioned HF trials in Table 3.
Table 3.
Summary of Recent HF WBI Studies
| Study Name |
Inclusion Criteria |
# of Patients |
WBI Dose |
Boost Dose |
Regional Nodal RT |
Local Recurrence |
Toxicity |
|---|---|---|---|---|---|---|---|
| Manuscript Study | pTis-T2, pN2a, M0 | 83 | 36.63 Gy in 11 fractions | 13.32 Gy in 4 fractions | Allowed | 2.4% IBTR (crude) | Acute Skin: -Grade 2: 34% Late Skin: -Grade 2: 1.2% -Grade 3: 1.2% |
| Shaitelman et al (12) | Stage 0–II | 287 | 50 Gy in 25 fractions or 42.56 Gy in 16 fractions | 10–12.5 Gy in 4–5 fractions | -- | -- | Acute skin toxicity in 50 Gy arm: 77.9% Acute skin toxicity in 42.56 Gy arm: 46.4% Significant decrease in pruritis, breast pain, hyperpigmentation, and fatigue with HF-WBI. |
| Nandi et al (13) | pTis-T4, pN0-3, M0 after BCS or mastectomy | 135 | 40 Gy in 16 fractions | Only in BCS patients: 16 Gy total | Allowed | No local failure, but distant metastases observed | Acute Skin (BCS): -Grade 2: 24% -Grade 3: 4.8% Acute Skin (Mastectomy): -Grade 2: 6% |
| ARO 2010-10 (17) | pT0-T4, pN0-N1, M0 | 141 | 40 Gy in 16 fractions | 0.5 Gy SIB for 16 fractions | No | -- | Acute Skin: -Grade 2: 22% (3D) -Grade 2: 9% (IMRT) |
| Freedman et al (18, 19) | pTis-T2, pN0-N1, M0 | 75 | 45 Gy in 20 fractions | 2.8 Gy SIB for 20 fractions | No | 5 year rate −2.7% | Acute Skin: -Grade 2: 23% |
| Formenti et al (20) | Stage I–II | 91 | 40.5 Gy in 15 fractions | 0.5 Gy SIB 15 fractions | No | 1.1% local regional recurrence rate (crude) | Acute Skin: -Grade 1–2: 67% |
| Mulliez et al (21) | pTis-T2, pN0, M0. Bra cup >C | 100 | 40.05 in 15 fractions | 10 Gy in 4 fractions (not mandatory) | No | -- | Prone position decreased Grade 2–3 dermatitis 50% (p<0.001) and 3 fold decrease in Grade 2 edema (p=0.005) |
| Pinnaro et al (23) | pTis-T2, pN0-1, M0 | 39 | 34 Gy in 10 fractions | 8 Gy in a single fraction | No | -- | Acute Skin: -Grade 2: 10% Late Skin: -Grade 1: 28% |
| Cante et al (22) | pT1-T2, pNx/N0-1, M0 | 375 | 45 Gy in 20 fractions | 0.25 Gy SIB for 20 fractions | No | No local failure at 5 years. 1.1% regional failure | Late Skin: -Grade 2: 4.5% |
| Dragun et al (24) | pTis-T2, pNx/N0-N1, M0 | 42 | 30 Gy in 5 fractions once weekly | Optional used either 10 Gy in 5 fractions or 6 Gy once | No | Acute >Grade 2: -Dermatitis: 19% -Pain: 11.9% -Fatigue: 9.5% -Breast edema: 2.4% |
The UK FAST Trial randomized women to standard fractionation vs. 28.5 Gy in 5.7 Gy delivered once a week vs. 30 Gy in 6 Gy delivered once a week. Eligible patients were node negative with tumor size 3 cm or less and age > 50 years old after BCS. The primary endpoint was 2-year change in breast appearance. Although the trial is currently in follow up the early results show that 30 Gy over 5 weeks had worse breast appearance outcomes when compared to 28.5 Gy and 50 Gy. With a median follow up 37.3 months, there are 2 local relapses and 23 deaths (26).
In addition, there are several ongoing or completed large randomized trials investigating HF-WBI. UK FAST-Forward aims to assess shortening this fractionation schedule even further, building on the UK FAST trial. The control arm of the trial is 40 Gy in 15 fractions. The experimental arms include 27 Gy in 5 daily fractions of 5.4 Gy and 26 Gy in 5 daily fractions of 5.2 Gy. A 10 or 16 Gy boost maybe added to the surgical scar or lumpectomy site. The definitive US trial of HF WBI in early stage breast cancer is the RTOG 1005. In the standard arm, investigators prescribe 50 Gy in 25 fractions or 42.7 Gy in 16 fractions followed by a sequential boost of 12 Gy in 6 fractions or 14 Gy in 7 fractions. The experimental arm is HF WBI of 40 Gy in 15 fractions of 2.67 Gy daily with a concurrent boost for a total of 48 Gy at 3.2 Gy per fraction to lumpectomy site. This trial has completed accrual and is currently maturing.
Our fractionation scheme, 36.63 Gy in 11 fractions of 3.33 Gy followed by a boost, appears to be safe and effective with low toxicity and excellent cosmetic outcomes. The local recurrence free survival and local relapse rates are comparable to several randomized trials. Our study employs the shortest North American schedule tested, and does so in patients with heterogeneous risk profiles, including those receiving neoadjuvant chemotherapy, patients with positive axillary nodes, and patients with higher risk features such as young age and negative hormone receptors. Ten percent of our patients received regional nodal irradiation with this experimental HF, thus being the first prospective evaluation in the United States of an altered fractionation schedule for regional treatment. With a median follow up of 40 months we haven’t seen any brachial plexopathies, although some reports have shown potentially long latency periods before manifestation of plexopathy (27). A companion trial at our institution has completed enrollment with this fractionation in a cohort of post-mastectomy patients. While we will follow these patients carefully over the next several years, we believe there is little reason to doubt the radiobiological parameters of efficacy and toxicity now established by large in-human studies. However, until larger experiences are reported, HF regional nodal irradiation should be offered in the setting of a clinical trial.
Summary.
Conventionally fractionated whole breast irradiation with a boost takes 6–7 weeks. We report on a phase II institutional, single arm prospective trial of women with DCIS and invasive cancer undergoing short course hypofractionated whole breast radiation (HF-WBI) receiving 49.95 Gy in 3.33 Gy per fraction over 15 fractions inclusive of a boost. We found excellent local control rates and low toxicity allowing women to complete breast-conserving treatment in 3 weeks.
Acknowledgments
Support: Rutgers Cancer Institute of New Jersey’s Core Center Support Grant (NCT00909909) and Breast Cancer Research Foundation (BH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
Atif Khan: Advisory Board, Vertex Pharmaceuticals
This paper was presented at the 56th Annual Meeting of the American Society of Therapeutic Radiology & Oncology in San Francisco, CA, on September 15, 2014.
References
- 1.Bekelman JE, Sylwestrzak G, Barron J, et al. Uptake and costs of hypofractionated vs conventional whole breast irradiation after breast conserving surgery in the united states, 2008–2013. JAMA. 2014;312:2542–2550. doi: 10.1001/jama.2014.16616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bentzen SM, Agrawal RK, Aird EG, et al. The uk standardisation of breast radiotherapy (start) trial a of radiotherapy hypofractionation for treatment of early breast cancer: A randomised trial. Lancet Oncol. 2008;9:331–341. doi: 10.1016/S1470-2045(08)70077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bentzen SM, Agrawal RK, Aird EG, et al. The uk standardisation of breast radiotherapy (start) trial b of radiotherapy hypofractionation for treatment of early breast cancer: A randomised trial. Lancet. 2008;371:1098–1107. doi: 10.1016/S0140-6736(08)60348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haviland JS, Owen JR, Dewar JA, et al. The uk standardisation of breast radiotherapy (start) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013;14:1086–1094. doi: 10.1016/S1470-2045(13)70386-3. [DOI] [PubMed] [Google Scholar]
- 5.Whelan T, MacKenzie R, Julian J, et al. Randomized trial of breast irradiation schedules after lumpectomy for women with lymph node-negative breast cancer. J Natl Cancer Inst. 2002;94:1143–1150. doi: 10.1093/jnci/94.15.1143. [DOI] [PubMed] [Google Scholar]
- 6.Whelan TJ, Pignol JP, Levine MN, et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362:513–520. doi: 10.1056/NEJMoa0906260. [DOI] [PubMed] [Google Scholar]
- 7.Romestaing P, Lehingue Y, Carrie C, et al. Role of a 10-gy boost in the conservative treatment of early breast cancer: Results of a randomized clinical trial in lyon, france. J Clin Oncol. 1997;15:963–968. doi: 10.1200/JCO.1997.15.3.963. [DOI] [PubMed] [Google Scholar]
- 8.Bartelink H, Maingon P, Poortmans P, et al. Whole-breast irradiation with or without a boost for patients treated with breast-conserving surgery for early breast cancer: 20-year follow-up of a randomised phase 3 trial. Lancet Oncol. 2015;16:47–56. doi: 10.1016/S1470-2045(14)71156-8. [DOI] [PubMed] [Google Scholar]
- 9.Agresti A, Coull B. Approximate is better than "exact" for interval estimation of binomial proportions. The American Statistician. 1998;52:119–126. [Google Scholar]
- 10.Smith BD, Bentzen SM, Correa CR, et al. Fractionation for whole breast irradiation: An american society for radiation oncology (astro) evidence-based guideline. Int J Radiat Oncol Biol Phys. 2011;81:59–68. doi: 10.1016/j.ijrobp.2010.04.042. [DOI] [PubMed] [Google Scholar]
- 11.Hahn C, Kavanagh B, Bhatnagar A, et al. Choosing wisely: The american society for radiation oncology's top 5 list. Pract Radiat Oncol. 2014;4:349–355. doi: 10.1016/j.prro.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Shaitelman SF, Buchholz TA, Hunt KK, et al. Hypofractionated whole breast irradiation results in less acute toxicity and improved quality of life at six months compared to conventionally fractionated whole breast irradiation: Results of a randomized trial. International Journal of Radiation Oncology*Biology*Physics. 2014;90:1264. [Google Scholar]
- 13.Nandi M, Mahata A, Mallick I, et al. Hypofractionated radiotherapy for breast cancers--preliminary results from a tertiary care center in eastern india. Asian Pac J Cancer Prev. 2014;15:2505–2510. doi: 10.7314/apjcp.2014.15.6.2505. [DOI] [PubMed] [Google Scholar]
- 14.Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 15.Correa C, McGale P, Taylor C, et al. Overview of the randomized trials of radiotherapy in ductal carcinoma in situ of the breast. J Natl Cancer Inst Monogr. 2010;2010:162–177. doi: 10.1093/jncimonographs/lgq039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Darby S, McGale P, Correa C, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dellas K, Vonthein R, Zimmer J, et al. Hypofractionation with simultaneous integrated boost for early breast cancer: Results of the german multicenter phase ii trial (aro-2010-01) Strahlenther Onkol. 2014;190:646–653. doi: 10.1007/s00066-014-0658-5. [DOI] [PubMed] [Google Scholar]
- 18.Freedman GM, Anderson PR, Goldstein LJ, et al. Four-week course of radiation for breast cancer using hypofractionated intensity modulated radiation therapy with an incorporated boost. Int J Radiat Oncol Biol Phys. 2007;68:347–353. doi: 10.1016/j.ijrobp.2006.12.035. [DOI] [PubMed] [Google Scholar]
- 19.Freedman GM, Anderson PR, Bleicher RJ, et al. Five-year local control in a phase ii study of hypofractionated intensity modulated radiation therapy with an incorporated boost for early stage breast cancer. Int J Radiat Oncol Biol Phys. 2012;84:888–893. doi: 10.1016/j.ijrobp.2012.01.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Formenti SC, Gidea-Addeo D, Goldberg JD, et al. Phase i–ii trial of prone accelerated intensity modulated radiation therapy to the breast to optimally spare normal tissue. J Clin Oncol. 2007;25:2236–2242. doi: 10.1200/JCO.2006.09.1041. [DOI] [PubMed] [Google Scholar]
- 21.Mulliez T, Veldeman L, van Greveling A, et al. Hypofractionated whole breast irradiation for patients with large breasts: A randomized trial comparing prone and supine positions. Radiother Oncol. 2013;108:203–208. doi: 10.1016/j.radonc.2013.08.040. [DOI] [PubMed] [Google Scholar]
- 22.Cante D, Franco P, Sciacero P, et al. Five-year results of a prospective case series of accelerated hypofractionated whole breast radiation with concomitant boost to the surgical bed after conserving surgery for early breast cancer. Med Oncol. 2013;30:518. doi: 10.1007/s12032-013-0518-7. [DOI] [PubMed] [Google Scholar]
- 23.Pinnaro P, Soriani A, Landoni V, et al. Accelerated hypofractionated radiotherapy as adjuvant regimen after conserving surgery for early breast cancer: Interim report of toxicity after a minimum follow up of 3 years. J Exp Clin Cancer Res. 2010;29:9. doi: 10.1186/1756-9966-29-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dragun AE, Quillo AR, Riley EC, et al. A phase 2 trial of once-weekly hypofractionated breast irradiation: First report of acute toxicity, feasibility, and patient satisfaction. Int J Radiat Oncol Biol Phys. 2013;85:e123–e128. doi: 10.1016/j.ijrobp.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 25.Rovea P, Fozza A, Franco P, et al. Once-weekly hypofractionated whole-breast radiotherapy after breast-conserving surgery in older patients: A potential alternative treatment schedule to daily 3-week hypofractionation. Clin Breast Cancer. 2015 doi: 10.1016/j.clbc.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 26.Agrawal RK, Alhasso A, Barrett-Lee PJ, et al. First results of the randomised uk fast trial of radiotherapy hypofractionation for treatment of early breast cancer (cruke/04/015) Radiother Oncol. 2011;100:93–100. doi: 10.1016/j.radonc.2011.06.026. [DOI] [PubMed] [Google Scholar]
- 27.Johansson S, Svensson H Denekamp J. Dose response and latency for radiation-induced fibrosis, edema, and neuropathy in breast cancer patients. Int J Radiat Oncol Biol Phys. 2002;52:1207–1219. doi: 10.1016/s0360-3016(01)02743-2. [DOI] [PubMed] [Google Scholar]