Abstract
OBJECTIVES
The aim of this study was to compare the incidence of permanent pacemaker (PPM) implantation after aortic valve replacement by rapid-deployment bioprosthesis (RDB) and standard valve (Standard).
METHODS
All patients undergoing aortic valve replacement between 2015 and 2018, in 1 centre, were included. A multivariate analysis on the whole cohort and then a propensity score matching were used to compare the 2 groups. The primary end point was PPM implantation.
RESULTS
We studied 924 patients (256 RDBs and 668 Standards). Overall, 67 PPM were implanted, 37 (14.5%) in the RDB group and 26 (3.9%) in the Standard group (P < 0.0001, univariate analysis). The multivariate analysis in the unmatched population found 4 independent factors associated with PPM implantation: right bundle branch block with odds ratios (ORs 3.7, 95% CI 2.9–6.7; P < 0.0001), RDB (OR 3.6, 95% CI 2.0–6.2; P < 0.0001), age (OR 1.1, 95% CI 1.0–1.1; P < 0.006) and endocarditis (OR 3.4, 95% CI 1.0–11.0; P < 0.04). In the propensity score-matched RDB group (203 patients per group), 25 patients required PPM implantation versus 3 in the Standard group (12.3% vs 1.5%, P < 0.0001). RDBs also had more postoperative left bundle branch block and new onset of atrial fibrillation (30.2% vs 5.1%, P < 0.0001 and 34.0% vs 24.1%, P = 0.029). RDBs had lower operating times (in min): aortic cross-clamping = 62 (44–76.5) vs 72 (57.5–91.5) and cardiopulmonary bypass = 81 (63–98.5) vs 91 (75–112), P < 0.0001. There was no significant difference in other outcomes.
CONCLUSIONS
RDBs were associated with reduced operating times, increased risk of atrial fibrillation and PPM implantation as compared with standard aortic valves.
Keywords: Cardiac surgery, Aortic valve replacement, Rapid-deployment bioprosthesis, Pacemaker implantation
INTRODUCTION
Aortic stenosis is still the most common valvular heart disease [1]. Surgical aortic valve replacement (AVR) remains the standard treatment for severe aortic stenosis in patients with low and intermediate surgical risk, based on the European System for Cardiac Operative Risk Evaluation (EuroSCORE) II [2]. Since 2017, bioprostheses are recommended for patients older than 60 years in Europe and older than 50 years in the USA. Both American and European cardiology societies also clearly emphasize the importance of including patient preference (class I) in choosing valve type [1, 3]. In patients at high surgical risk, transcatheter AVR (TAVR) has become the procedure of choice [4]. Recent studies have shown encouraging results in patients at intermediate surgical risk [5], and even in low-risk patients [6], however, with an increased incidence of atrioventricular (AV) blocks and pacemaker implantation [7, 8]. When surgery is indicated, AVR can be combined with other interventions, such as coronary bypass and mitral or tricuspid valve replacements, thus increasing the duration of both cardiopulmonary bypass and aortic cross-clamping. Long operative time increases the risk of postoperative organ dysfunction, such as acute kidney injury [9], and infectious complications, such as pneumonia or deep wound infection [10–12].
Based on the model of TAVR, rapid-deployment bioprostheses (RDBs) have been developed for surgical implantation. RDBs are built on the design platform of the standard bioprostheses with the addition of a balloon-expandable stainless steel cloth-covered frame. After aortic opening, standard bioprostheses implantation requires 12–15 sutures whereas RDBs are anchored by the frame expansion and utilize only 3 guiding sutures to secure annular placement, thereby reducing the aortic cross-clamp and bypass time [13–15]. In addition, RDBs allow for more protective procedures in patients with fragile annulus (redo AVR, massive calcifications, endocarditis). Compared to TAVR, RDBs have 3 advantages: valvular resection with decalcification of the aortic annulus, a valve implantation under direct vision to avoid paravalvular leaks by proper fitting of the prosthesis into the annulus; and the possibility of performing concomitant procedures, such as coronary artery bypass grafting.
Conduction disorders, including third-degree AV block, are well-known complications of AVRs and sometimes require permanent pacemaker (PPM) implantation [16]. With conventional aortic valves, the rate of PPM implantation is ∼5% [17, 18]. Several studies showed an increased risk of conduction abnormalities and PPM implantation (between 8.5% and 17%) with RDBs [19–23]. However, only a few studies compared RDBs to standard bioprostheses and only included small numbers of patients.
The aim of our study was to compare the incidence of conductive disorders and PPM implantation after AVR with RDB and standard bioprostheses [22, 23].
MATERIALS AND METHODS
This is an observational study based on the analysis of a prospectively collected database. All adult patients who underwent an AVR between January 2015 and September 2018 in 1 teaching hospital were included (Clinic Ambroise Paré, Neuilly-sur-Seine, France). Operative indications for AVR agreed with the current European guidelines during the study [2]. The choice of RDB versus a standard bioprosthesis was left to the surgeon's judgement, based on the potential advantages of RDB summarized in the ‘Introduction’ section: fragile annulus, combined surgery and long procedure for any reason. The standard bioprostheses used were Avalus®, Crown®, Magna®, Mitroflow®, Perimount® and Trifecta®. Among RDBs, only the Intuity® valve (Edwards Lifesciences, Irvine, CA, USA) was used. This valve is built from the structure of a Carpentier-Edwards Perimount valve and was implanted for the first time in 2005.
AVRs could be associated with bypass surgery. Patients with other valvular diseases requiring a repair or a replacement were excluded from the study, as were patients undergoing a replacement of the ascending aorta. Mechanical valves were also excluded. Regarding the observational and retrospective nature of the study, specific consent was not required, but patients signed a no opposition to the computerization of the essential data of their intervention at entrance in our institution. The study design and the analysis of the database were approved by our local ethics committee in October 2018.
From the patient’s medical records, we collected preoperative and postoperative clinical, biological, electrocardiogram, echocardiographic and surgical data, in particular, the medical history, type of aortic valvular disease, EuroSCORE II, operating data (including duration of cardiopulmonary bypass and aortic cross-clamping), type and size of the prosthesis, main complications after surgery, preoperative and postoperative echocardiography, length of stay and hospital mortality.
The primary end point was the rate of postoperative PPM implantation. Secondary outcomes included: operating times; conduction and rhythmic disorders [defined as the appearance first-degree AV block, second-degree AV block and third-degree AV block, left bundle branch block, left hemiblock, right bundle branch block (RBBB) and new-onset atrial fibrillation]; postoperative echocardiographic measurements (aorta-left ventricle mean pressure gradient and paravalvular regurgitation); and clinical postoperative complications, including infections, acute renal failure requiring dialysis, reintubation, stroke, myocardial infarction, duration of mechanical ventilation, length of stay in intensive care unit (ICU) and hospital mortality.
Statistical analysis
Data were expressed as mean ± standard deviation or median (interquartile range) for continuous variables, as appropriate according to their distribution. Categorical variables were expressed as a number (percentage). Comparison between RDBs versus conventional valves was made on clinical, biological, electrocardiogram, echocardiographic and surgical characteristics. Categorical variables were compared in 2 groups from the χ2 test. The normality of distribution of the quantitative variables was verified by Shapiro’s test. Comparison of normally distributed variables used Student’s t-test. Other variables were compared by a Mann–Whitney non-parametric test. The software used was Prism Graph 8.0.
A multivariate analysis was conducted in the unmatched population by stepwise logistic regression to determine the independent preoperative and intraoperative factors associated with PPM implantation. We initially performed a univariate analysis, and only those variables that achieved a P-value of ≤ 0.1 were included in the multivariate analysis. A constant was added into the model, and the factors were unforced. The significance threshold adopted was P-value <0.05. Linearity was checked. The software used was SPSS 25.0.
Clinical end points were secondarily compared between the RDB and Standard groups using a propensity score framework. The aim of the propensity score was to balance patient baseline characteristics. The propensity score was generated with all of the available preoperative items: diabetes, renal insufficiency requiring dialysis, history of PPM or implantable cardioverter defibrillator, preoperative atrial fibrillation, preoperative conductive disorders, chronic obstructive pulmonary disease, prior cardiac surgery, preoperative left ventricular ejection fraction, size of valve, endocarditis and dyspnoea. The propensity score matching algorithm used the method of nearest neighbour, with a calliper of 0.2, without replacement. There were no missing data in the matched populations. An inter-group comparison was secondarily performed for quality control, using McNemar tests for binary end points and paired t-tests for continuous end points.
RESULTS
Between January 2015 and September 2018, 924 patients were included (flow chart in Fig. 1). Among these patients, 256 received an RDB and 668 received a standard biological valve. Overall, 67 PPM were implanted, 37 (14.5%) in the RDB group and 26 (3.9%) in the Standard group (P < 0.0001). In the whole cohort (unmatched population), patients were older in the RDB group with a majority of men. They also had a higher EuroSCORE II and more comorbidities (e.g. arterial hypertension, chronic renal failure and left ventricular dysfunction).
Figure 1:
Study CONSORT flow chart. AVR: aortic valve replacement; RDB: rapid-deployment bioprosthesis.
Propensity score matching
The propensity score allowed the selection of 203 unique pairs of patients with similar characteristics. Standardized mean differences of each variable of the matched population are presented in Table 1 and Fig. 2. Preoperative characteristics of patients (unmatched and matched populations) are presented in Table 1. Aortic stenosis was the most common indication for the surgery. A combined surgery with bypass was performed in 60.6% of patients.
Table 1:
Comparison of preoperative patient characteristics between the RDB and Standard in the unmatched and matched cohorts
| Unmatched cohort |
Matched cohort |
||||||
|---|---|---|---|---|---|---|---|
| RDB | Standard | P-value | RDB | Standard | P-value | SMD | |
| (n = 256) | (n = 668) | (n = 203) | (n = 203) | ||||
| Age (years) | 75.4 ± 7.5 | 72.4 ± 10 | <0.0001 | 74.9 ± 7.3 | 74.6 ± 8.4 | 0.64 | 0.075 |
| Male gender, n (%) | 196 (76.6) | 436 (65.3) | 0.001 | 149 (73.4) | 143 (70.4) | 0.51 | 0.02 |
| BMI (kg/m2), mean ± SD | 27.2 ± 4.8 | 27.4 ± 4.9 | 0.74 | 27.3 ± 4.9 | 27.1 ± 4.3 | 0.56 | 0.03 |
| History | |||||||
| Arterial hypertension, n (%) | 197 (77.0) | 443 (66.3) | 0.02 | 153 (75.4) | 151 (74.4) | 0.82 | 0.07 |
| Diabetes mellitus, n (%) | 71 (27.7) | 181 (27.1) | 0.85 | 56 (27.6) | 56 (27.6) | 0.99 | 0.09 |
| Chronic renal failure, n (%) | 114 (44.5) | 238 (35.6) | 0.001 | 89 (43.8) | 83 (40.9) | 0.55 | 0.06 |
| Dialysis, n (%) | 2 (0.8) | 4 (0.6) | 0.76 | 1 (0.5) | 2 (1.0) | 0.56 | 0.0001 |
| AF, n (%) | 50 (19.5) | 116 (17.4) | 0.44 | 38 (18.7) | 36 (17.7) | 0.80 | 0.01 |
| COPD, n (%) | 21 (8.2) | 41 (6.1) | 0.26 | 17 (8.4) | 21 (10.3) | 0.50 | 0.02 |
| Preoperative LVEF > 50%, n (%) | 199 (77.7) | 577 (86.3) | 0.003 | 154 (75.9) | 168 (82.8) | 0.09 | 0.09 |
| Preoperative LVEF 31–50%, n (%) | 49 (19.1) | 78 (11.7) | 0.003 | 41 (20.2) | 31 (15.3) | 0.19 | 0.09 |
| Preoperative LVEF ≤ 30%, n (%) | 8 (3.1) | 13 (1.9) | 0.25 | 8 (3.9) | 4 (2.0) | 0.24 | 0.11 |
| NYHA III/IV, n (%) | 98 (38.3) | 207 (31.0) | 0.04 | 79 (38.9) | 72 (35.5) | 0.47 | 0.08 |
| Endocarditis, n (%) | 9 (3.5) | 36 (5.4) | 0.24 | 7 (3.4) | 8 (3.9) | 0.79 | 0.08 |
| EuroSCORE II, median (IQR) | 3.6 (2.1–5.6) | 2.1 (1.2–3.7) | <0.0001 | 3.5 (1.9–5.4) | 3.1 (1.8–5.0) | 0.75 | 0.10 |
| Redux, n (%) | 16 (6.3) | 36 (5.4) | 0.61 | 13 (6.4) | 15 (7.4) | 0.70 | 0.02 |
| Urgent surgery, n (%) | 5 (2.0) | 11 (1.6) | 0.75 | 4 (2.0) | 3 (1.5) | 0.70 | 0.08 |
| Aortic valve condition | |||||||
| Stenosis, n (%) | 240 (93.8) | 578 (86.5) | 0.002 | 190 (93.6) | 187 (92.1) | 0.56 | 0.0001 |
| Regurgitation, n (%) | 6 (2.3) | 66 (9.9) | <0.0001 | 6 (3.0) | 6 (3.0) | 0.99 | 0.03 |
| Mixed, n (%) | 8 (3.1) | 14 (2.1) | 0.36 | 5 (2.5) | 7 (3.4) | 0.56 | 0.03 |
| Others, n (%) | 2 (0.8) | 9 (1.3) | 0.48 | 2 (1.0) | 3 (1.5) | 0.65 | 0.11 |
| Preoperative ECG | |||||||
| History of PPM, n (%) | 12 (4.7) | 24 (3.6) | 0.44 | 13 (6.4) | 12 (5.9) | 0.84 | 0.07 |
| History of ICD, n (%) | 1 (0.4) | 2 (0.3) | 0.83 | 1 (0.5) | 1 (0.5) | 0.99 | 0.0001 |
| First-degree AVB, n (%) | 19 (7.5) | 36 (5.2) | 0.24 | 13 (6.4) | 12 (5.9) | 0.84 | 0.04 |
| Second-degree AVB, n (%) | 0 (0) | 1 (0.1) | 0.54 | 0 (0) | 0 (0) | ||
| Third-degree AVB, n (%) | 0 (0) | 1 (0.1) | 0.54 | 0 (0) | 0 (0) | ||
| LBBB, n (%) | 31 (12.3) | 59 (8.9) | 0.1 | 28 (13.8) | 22 (10.8) | 0.36 | 0.11 |
| RBBB, n (%) | 29 (11.5) | 76 (11.5) | 0.98 | 24 (11.8) | 20 (9.9) | 0.52 | 0.03 |
Intergroup comparison between the matched groups.
AF: atrial fibrillation; AVB: atrioventricular block; AVR: aortic valve replacement; BMI: body mass index; COPD: chronic obstructive pulmonary disease; ECG: electrocardiogram; EuroSCORE: European System for Cardiac Operative Risk Evaluation; ICD: implantable cardioverter defibrillator; IQR: interquartile range; LBBB: left bundle branch block; LVEF: left ventricular ejection fraction; NYHA: Dyspnoea classification from New York Heart Association; PPM: permanent pacemaker, RBBB: right bundle; RDB: rapid-deployment bioprosthesis; Redux: prior cardiac surgery; SD: standard deviation; SMD: standardized mean difference; Standard: Standard bioprosthesis.
Figure 2:
Density of standardized mean differences before (red curve and frequency distribution) and after matching (blue curve and frequency distribution).
Intraoperative main characteristics (matched and unmatched populations) are given in Table 2. Both the aortic cross-clamping and cardiopulmonary bypass times were shorter in the RDB group. The prostheses were larger in the RDB group, with more prostheses with a diameter of 25 mm, as compared with the standard group.
Table 2:
Comparison of intraoperative patient characteristics between the RDB and Standard in the unmatched and matched cohorts
| Unmatched cohort |
Matched cohort |
|||||
|---|---|---|---|---|---|---|
| RDB | Standard | P-value | RDB | Standard | P-value | |
| (n = 256) | (n = 668) | (n = 203) | (n = 203) | |||
| Full sternotomy, n (%) | 256 (100) | 572 (85.5) | <0.0001 | 203 (100) | 203 (100) | |
| ACC time (min) | 66 (48–80) | 62 (53–73) | 0.96 | 62 (44–77) | 72 (58–92) | <0.0001 |
| CPB time (min) | 84 (67–100) | 79.5 (69–92) | 0.01 | 81 (63–99) | 91 (75–112) | <0.0001 |
| Prosthesis size (mm), n (%) | ||||||
| 19 | 17 (6.6) | 29 (4.3) | 0.15 | 16 (7.9) | 13 (6.4) | 0.56 |
| 21 | 60 (23.4) | 193 (28.8) | 0.09 | 46 (22.7) | 60 (29.6) | 0.11 |
| 23 | 91 (35.5) | 232 (34.7) | 0.73 | 72 (35.5) | 77 (37.9) | 0.61 |
| 25 | 71 (27.7) | 150 (22.4) | 0.09 | 59 (29.1) | 39 (19.2) | 0.02 |
| 27 | 17 (6.6) | 59 (8.8) | 0.28 | 10 (4.9) | 14 (7.0) | 0.40 |
| 29 | 0 (0) | 5 (0.7) | 0.17 | 0 (0) | 0 (0) | 0.99 |
| Prothesis size (mm), mean ± SD | 23.1 ± 2.1 | 23.1 ± 2.0 | 0.95 | 23.1 ± 2.0 | 22.8 ± 2.0 | 0.06 |
| Isolated AVR, n (%) | 82 (32.0) | 525 (78.6) | <0.0001 | 79 (38.9) | 81 (39.9) | 0.06 |
| AVR with bypass, n (%) | 174 (68.0) | 143 (21.4) | <0.0001 | 124 (61.1) | 122 (60.1) | −0.03 |
| Epicardial PPM, n (%) | 1 (0.4) | 1 (0.1) | 0.48 | 1 (0.5) | 1 (0.5) | |
These data were included into the propensity score matching; therefore, the value indicates the standard mean difference.
ACC: aortic cross-clamp; AVR: aortic valve replacement; CPB: cardiopulmonary bypass; PPM: permanent pacemaker; RDB: rapid-deployment bioprosthesis; SD: standard deviation; Standard: Standard bioprosthesis.
Primary and secondary outcomes in unmatched and matched populations are summarized in Table 3. PPM implantations were also more frequent in the RDB group as compared to the Standard group in the matched cohort (12.3% vs 1.5%, P < 0.0001). Most often, the conduction disorder leading to PPM implantation was observed upon ICU admission (21/26, 81%). Persistent postoperative third-degree AV block was the main indication for PPM implantation, followed by sinus dysfunction. In the RDB group, there were also more third-degree AV blocks as compared to the Standard group (34.0% vs 24.1%, P = 0.03), more sinus dysfunction, left bundle branch blocks and cases of new onset of atrial fibrillation. In contrast, there was no difference in the postoperative RBBBs, first-degree AV blocks and left anterior hemiblocks. No significant differences in the rates of other postoperative complications were noted between the 2 groups, especially in infectious complications and renal failure requiring dialysis.
Table 3:
Comparison of postoperative patient characteristics between the RDB and Standard in the unmatched and matched cohorts, including the primary and secondary outcomes
| Unmatched cohort |
Matched cohort |
|||||
|---|---|---|---|---|---|---|
| RDB | Standard | P-value | RDB | Standard | P-value | |
| (n = 256) | (n = 668) | (n = 203) | (n = 203) | |||
| Primary outcome | ||||||
| Pacemaker implantation, n (%) | 37 (14.5) | 26 (3.9) | <0.0001 | 25 (12.3) | 3 (1.5) | <0.0001 |
| Other rhythmic outcomes | ||||||
| ICD implantation, n (%) | 2 (0.8) | 1 (0.1) | 0.13 | 1 (0) | 0 (0) | 0.79 |
| CRT implantation, n (%) | 1 (0.4) | 1 (0.1) | 0.48 | 0 (0) | 0 (0) | |
| Third-degree AV block, n (%) | 51 (20) | 49 (7.5) | <0.0001 | 37 (18.3) | 17 (8.7) | 0.004 |
| Second-degree AV block, n (%) | 1 (0.4) | 1 (0.2) | 0.48 | 1 (0.5) | 0 (0) | 0.32 |
| First-degree AV block, n (%) | 31 (12.2) | 67 (10.2) | 0.36 | 20 (9.9) | 22 (11.3) | 0.74 |
| New LBBB, n (%) | 79 (31.0) | 41 (6.3) | <0.0001 | 61 (30.2) | 10 (5.1) | <0.0001 |
| New RBBB, n (%) | 11 (4.3) | 45 (6.9) | 0.16 | 7 (3.5) | 14 (7.2) | 0.17 |
| Left anterior hemiblock, n (%) | 10 (3.9) | 34 (5.2) | 0.45 | 7 (3.5) | 8 (4.1) | 0.79 |
| Sinus dysfunction, n (%) | 2 (0.8) | 6 (0.9) | 0.86 | 1 (0.5) | 1 (0.5) | |
| New onset of AF, n (%) | 83 (32.4) | 139 (20.8) | 0.0002 | 69 (34.0) | 49 (24.1) | 0.03 |
| Clinical outcomes | ||||||
| Reoperation for bleeding, n (%) | 3 (1.2) | 8 (1.2) | 0.97 | 1 (0.5) | 5 (2.5) | 0.09 |
| Any transfusion, n (%) | 162 (63.3) | 336 (50.3) | 0.0004 | 127 (62.6) | 117 (57.3) | 0.31 |
| Reintubation, n (%) | 11 (4.3) | 25 (3.7) | 0.70 | 7 (3.4) | 11 (5.4) | 0.33 |
| Cerebral ischaemic events, n (%) | 6 (2.3) | 9 (1.3) | 0.28 | 6 (3.0) | 6 (3.0) | 0.99 |
| Myocardial infarction, n (%) | 9 (3.5) | 15 (2.2) | 0.27 | 8 (3.9) | 7 (3.4) | 0.79 |
| Pneumonia, n (%) | 41 (16) | 69 (10.3) | 0.02 | 31 (15.3) | 32 (15.8) | 0.89 |
| Dialysis, n (%) | 4 (1.6) | 20 (3.0) | 0.22 | 3 (1.5) | 8 (3.9) | 0.13 |
| Deep wound sternal infection, n (%) | 5 (2.0) | 10 (1.5) | 0.62 | 5 (2.5) | 5 (2.5) | 0.99 |
| Mechanical ventilation (h), median (IQR) | 5 (4–8) | 4 (4–7) | 0.10 | 5 (4–8) | 5 (4–10) | 0.25 |
| Length of stay in ICU (days), median (IQR) | 4 (4–5) | 4 (3–5) | 0.08 | 4 (4–5) | 4 (4–5) | 0.87 |
| Hospital mortality, n (%) | 9 (3.5) | 24 (3.6) | 0.95 | 8 (3.9) | 14 (6.9) | 0.19 |
| Echographic outcomes | ||||||
| Paravalvular leak > mild, n (%) | 6 (2.3) | 16 (2.4) | 0.96 | 5 (2.5) | 5 (2.5) | 0.99 |
| Mean pressure gradient (mmHg), median (IQR) | 11 (9–15) | 12 (9–16) | 0.09 | 12 (9–15) | 13 (9–16) | 0.42 |
AF: atrial fibrillation; AV: atrioventricular; CRT: cardiac resynchronization therapy; ICD: implantable cardioverter defibrillator; ICU: intensive care unit; IQR: interquartile range; LBBB: left bundle branch block; RBBB: right bundle branch block; RDB: rapid-deployment bioprosthesis; Standard: Standard biological bioprostheses.
Univariate and multivariate analysis
The perioperative factors associated with PPM implantation after AVR, in the unmatched cohort, are shown in Table 4. In this univariate analyses, age, EuroSCORE II, first-degree AV block, preoperative RBBB, endocarditis and RDB valve implementation were associated with PPM implantation. The size of the valve was not associated with PPM implantation. Among these variables, the multivariate logistic regression in the entire cohort selected 4 independent factors associated with PPM implantation (Table 5, Fig. 3): RBBB [OR 3.7, 95% confidence interval (CI) 2.9–6.7; P < 0.0001], RDB [odds ratio (OR) 3.6, 95% CI 2.0–6.2; P < 0.0001], age (OR 1.1 per year, 95% CI 1.0–1.1; P = 0.006) and endocarditis (OR 3.4, 95% CI 1.01–11.0; P = 0.04).
Table 4:
Factors associated with pacemaker implantation by univariate analyses on the unmatched whole cohort of patients
| OR | Confidence interval | P-value | |
|---|---|---|---|
| Age (years) | 1.07 | 1.03–1.11 | 0.0001 |
| BMI (kg/m2) | 1.01 | 0.96–1.06 | 0.75 |
| Male gender | 1.03 | 0.59–1.79 | 0.91 |
| Renal failure requiring dialysis | 2.81 | 0.32–24.4 | 0.35 |
| Diabetes mellitus | 1.29 | 0.74–2.25 | 0.37 |
| Preoperative atrial fibrillation | 1.65 | 0.91–2.99 | 0.1 |
| Endocarditis | 2.32 | 0.94–5.72 | 0.07 |
| Preoperative LVEF ≤30% | 0.69 | 0.09–5.22 | 0.72 |
| EuroSCORE II (per point) | 1.05 | 1.01–1.09 | 0.03 |
| Valve size | 1.03 | 0.91–1.16 | 0.60 |
| First-degree AV block | 3.03 | 1.40–6.51 | 0.005 |
| LBBB | 1.20 | 0.53–2.71 | 0.67 |
| RBBB | 3.67 | 2.03–6.64 | <0.0001 |
| Left anterior hemiblock | 1.74 | 0.59–5.06 | 0.31 |
| RDB | 4.03 | 2.38–6.84 | <0.0001 |
| Cardiopulmonary bypass time (per min) | 1.01 | 0.99–1.02 | 0.11 |
| Aortic cross-clamping time (per min) | 1.01 | 0.99–1.02 | 0.36 |
AV: atrioventricular; BMI: body mass index; EuroSCORE: EuroSCORE: European System for Cardiac Operative Risk Evaluation; LBBB: left bundle branch block; LVEF: left ventricle ejection fraction; OR: odds ratio; RDB: rapid-deployment bioprosthesis; RBBB: right bundle branch block.
Table 5:
Independent factors associated with pacemaker implantation using a multivariate analysis on the unmatched whole cohort of patients
| OR | Confidence interval | P-value | |
|---|---|---|---|
| Preoperative RBBB | 3.7 | 2.0–7.0 | <0.0001 |
| RDB | 3.6 | 2.0–6.0 | <0.0001 |
| Age (years) | 1.1 | 1.0–1.1 | 0.006 |
| Endocarditis | 3.4 | 1.0–11.0 | 0.04 |
| First-degree AV block | 2.2 | 0.9–5.0 | 0.07 |
| EuroSCORE II | 1.0 | 0.9–1.1 | 0.8 |
AV: atrioventricular; EuroSCORE: European System for Cardiac Operative Risk Evaluation; OR: odds ratio; RBBB: right bundle branch block; RDB: rapid-deployment bioprostheses.
Figure 3:
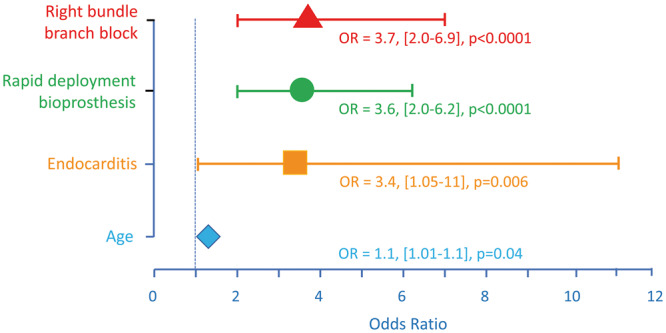
Independent factors associated with permanent pacemaker (PPM) implantation. The logistic regression identified 4 independent factors of PPM implantation, including 3 preoperative factors and only 1 intraoperative factor: the insertion of a rapid-deployment bioprosthesis. For age, the OR is given for 1-year change. The vertical dotted line shows the reference line (OR = 1). OR: odds ratio.
DISCUSSION
The major finding of this study was an increased risk of PPM implantation using RDB (Intuity valve) for AVR as compared to patients receiving standard bioprostheses (14.5% vs 3.9%). The rate of PPM implantation with standard bioprostheses was low [24] and may have been lowered by the fact that older patients and patients with fragile aortic annulus were more likely to receive an RDB. The rate of PPM implantation with RDBs confirmed the previously reported studies, supporting the hypothesis of a technology-inherent risk. However, except in 1 study, which found a PPM implantation rate at 17% with the Perceval valve [19], other studies reported lower incidences, ranging from 7.9% to 11.9% [25, 26]. In the TRANSFORM study [13], the largest American study on Intuity valve including 839 patients, the rate of PPM implantation was 11.9% and 73% of patients who required PPM had preoperative RBBB. The most recent European study found a rate of 8.6% of PPM on 500 consecutive Intuity valve implantations [21]; while the other conductive disorders were not described. In these studies, mortality was low (<3%) and the haemodynamic profile was excellent with postoperative mean transvalvular gradient <15 mmHg and a very low rate of paravalvular leakage (<2%) [13, 21]. These incidences of PPM implantation are nevertheless lower than those found after a procedure by TAVR, which is estimated between 12% and 36% [7, 8]. The underlying mechanism of these conductive disorders is probably related to direct trauma of the conduction pathways during the pneumatic expansion of the bioprosthesis with or without secondary inflammation [20, 22, 23, 25, 26]. In our study, the valve size was not associated with the pacemaker implantation, but conduction disturbances were most often observed immediately, arguing for a mechanical stress. The ratio between the valve size and the native aortic annular diameter is probably important to understand the role of valve oversizing in the occurrence of conductive disorders. Unfortunately, we did not collect these data in our database. The experience of surgeons with these valves can improve over time, possibly allowing a less traumatic insertion, better depth of implantation and optimal sizing. However, the date of surgery was not selected as an independent factor in our multivariate analysis.
We also found that preoperative conductive disorders, such as first-degree AV block and RBBB, were independently associated with postoperative PPM implantation. These observations are in line with the TRANSFORM study, also studying the Intuity valve [26]. In this study, other risk factors were found, such as female gender and larger valve size, that we did not confirm in our study. In contrast, we found that Intuity valves were associated with a higher risk of postoperative atrial fibrillation. This has not been reported until now in the existing literature. In a retrospective study [14], a comparatively high rate of new-onset atrial fibrillation (33%) was reported with the Perceval valve.
RDBs effectively decreased both cardiopulmonary bypass and aortic cross-clamping times [27]. Our results confirmed these findings with an average decrease of 10 min. This can be useful in patients undergoing a high-risk procedure, especially in combined surgery or in patients with a fragile aortic annulus making AVR with a standard biological valve difficult. These benefits on operative times were not associated with a significant reduction in clinical postoperative complications, especially infectious complications. In addition, we found no detectable change in the haemodynamic results as compared to standard bioprostheses with similar in-hospital aorta-left ventricle pressure gradients and a low rate of paravalvular regurgitation. These results did not confirm the meta-analysis [25] on the Perceval valve, reporting a reduction in the incidence of acute renal failure.
Limitations
Our study has several limitations. First, being a monocentric study, the results correspond to a specific experience. The retrospective nature of the analysis brings only a low risk of bias since the database was prospectively collected on a registry. However, we cannot eliminate the impact of an important cofactor that was not available such as the ratio between the native aortic annular diameter and the size of the prosthesis. In addition, the choice of the prosthesis was not randomized but left to the discretion of the surgeon. This prevents the assertion of the causal link of the observed associations. Furthermore, we only studied intra-hospital events without a long-term follow-up. Finally, although there was no difference in favour of the RDB on clinical postoperative complications (such as renal failure and infectious complication) despite a reduction in operative times, we could not eliminate a lack of power in our study for these analyses.
CONCLUSION
RDB implantation with Intuity valve is associated with a 3.6-fold increased risk of PPM implantation as compared to Standard valve bioprostheses in our single-institution study. The choice of this type of valve reduces operating times and yields good haemodynamic results, without significant decreases in the rates of clinical complications after surgery. To improve the benefit/risk ratio, the final decision whether or not to insert RDB should be made on a case-by-case basis, considering age, the existence of preoperative conduction disorders and the expected duration of the intervention, especially in case of combined surgery.
Conflict of interest: Pierre Squara receives patent maintenance fees from Medtronic. All other authors declared no conflict of interest.
Author contributions
Morgane Herry: Conceptualization; Data curation; Formal analysis; Writing—original draft. Driss Laghlam: Conceptualization; Data curation; Formal analysis; Validation; Writing—original draft. Olivier Touboul: Data curation; Formal analysis; Investigation; Validation; Writing—original draft. Lee S. Nguyen: Conceptualization; Methodology; Software; Supervision; Validation. Philippe Estagnasié: Conceptualization; Project administration; Supervision; Validation; Writing—review & editing. Alain Brusset: Conceptualization; Data curation; Formal analysis; Methodology; Software; data base creation and maintenance. Pierre Squara: Conceptualization; Project administration; Supervision; Validation; Writing—review & editing.
Abbreviations
- AV
Atrioventricular
- AVR
Aortic valve replacement
- CI
Confidence interval
- EuroSCORE
European System for Cardiac Operative Risk Evaluation
- ICU
Intensive care unit
- OR
Odds ratio
- PPM
Permanent pacemaker
- RBBB
Right bundle branch block
- RDB
Rapid-deployment bioprosthesis
- TAVR
Transcatheter aortic valve replacement
REFERENCES
- 1. Iung B, Baron G, Butchart EG, Delahaye F, Gohlke-Barwolf C, Levang OW. et al. A prospective survey of patients with valvular heart disease in Europe: the Euro Heart Survey on Valvular Heart Disease. Eur Heart J 2003;24:1231–43. [DOI] [PubMed] [Google Scholar]
- 2. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ. et al. ; ESC Scientific Document Group. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739–91. [DOI] [PubMed] [Google Scholar]
- 3. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Fleisher LA. et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol 2017;70:252–89. [DOI] [PubMed] [Google Scholar]
- 4. Deeb GM, Reardon MJ, Chetcuti S, Patel HJ, Grossman PM, Yakubov SJ. et al. 3-year outcomes in high-risk patients who underwent surgical or transcatheter aortic valve replacement. J Am Coll Cardiol 2016;67:2565–74. [DOI] [PubMed] [Google Scholar]
- 5. Reardon MJ, Van Mieghem NM, Popma JJ, Kleiman NS, Sondergaard L, Mumtaz M. et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med 2017;376:1321–31. [DOI] [PubMed] [Google Scholar]
- 6. Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O’Hair D. et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med 2019;380:1706–15. [DOI] [PubMed] [Google Scholar]
- 7. Schroeter T, Linke A, Haensig M, Merk DR, Borger MA, Mohr FW. et al. Predictors of permanent pacemaker implantation after Medtronic CoreValve bioprosthesis implantation. Europace 2012;14:1759–63. [DOI] [PubMed] [Google Scholar]
- 8. Gaede L, Blumenstein J, Liebetrau C, Dorr O, Kim WK, Nef H. et al. Outcome after transvascular transcatheter aortic valve implantation in 2016. Eur Heart J 2018;39:667–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sirvinskas E, Andrejaitiene J, Raliene L, Nasvytis L, Karbonskiene A, Pilvinis V. et al. Cardiopulmonary bypass management and acute renal failure: risk factors and prognosis. Perfusion 2008;23:323–7. [DOI] [PubMed] [Google Scholar]
- 10. Miholic J, Hudec M, Domanig E, Hiertz H, Klepetko W, Lackner F. et al. Risk factors for severe bacterial infections after valve replacement and aortocoronary bypass operations: analysis of 246 cases by logistic regression. Ann Thorac Surg 1985;40:224–8. [DOI] [PubMed] [Google Scholar]
- 11. Allou N, Bronchard R, Guglielminotti J, Dilly MP, Provenchere S, Lucet JC. et al. Risk factors for postoperative pneumonia after cardiac surgery and development of a preoperative risk score. Crit Care Med 2014;42:1150–6. [DOI] [PubMed] [Google Scholar]
- 12. He S, Chen B, Li W, Yan J, Chen L, Wang X. et al. Ventilator-associated pneumonia after cardiac surgery: a meta-analysis and systematic review. J Thorac Cardiovasc Surg 2014;148:3148–55.e1-5. [DOI] [PubMed] [Google Scholar]
- 13. Barnhart GR, Accola KD, Grossi EA, Woo YJ, Mumtaz MA, Sabik JF. et al. TRANSFORM (multicenter experience with Rapid Deployment Edwards INTUITY Valve system for aortic valve replacement) US clinical trial: performance of a rapid deployment aortic valve. J Thorac Cardiovasc Surg 2017;153:241–51.e2. [DOI] [PubMed] [Google Scholar]
- 14. Mujtaba SS, Ledingham SM, Shah AR, Pillay T, Schueler S, Clark S.. Aortic valve replacement with a conventional stented bioprosthesis versus sutureless bioprosthesis: a study of 763 patients. Braz J Cardiovasc Surg 2018;33:122–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wahlers TCW, Andreas M, Rahmanian P, Candolfi P, Zemanova B, Giot C. et al. Outcomes of a rapid deployment aortic valve versus its conventional counterpart: a propensity-matched analysis. Innovations (Phila) 2018;13:177–83. [DOI] [PubMed] [Google Scholar]
- 16. Schurr UP, Berli J, Berdajs D, Hausler A, Dzemali O, Emmert M. et al. Incidence and risk factors for pacemaker implantation following aortic valve replacement. Interact CardioVasc Thorac Surg 2010;11:556–60. [DOI] [PubMed] [Google Scholar]
- 17. Socie P, Nicot F, Baudinaud P, Estagnasie P, Brusset A, Squara P. et al. Frequency of recovery from complete atrioventricular block after cardiac surgery. Am J Cardiol 2017;120:1841–6. [DOI] [PubMed] [Google Scholar]
- 18. Robich MP, Schiltz NK, Johnston DR, Mick S, Krishnaswamy A, Iglesias RA. et al. Risk factors and outcomes of patients requiring a permanent pacemaker after aortic valve replacement in the United States. J Card Surg 2016;31:476–85. [DOI] [PubMed] [Google Scholar]
- 19. Mazine A, Teoh K, Bouhout I, Bhatnagar G, Pelletier M, Voisine P. et al. Sutureless aortic valve replacement: a Canadian multicentre study. Can J Cardiol 2015;31:63–8. [DOI] [PubMed] [Google Scholar]
- 20. Mogilansky C, Balan R, Deutsch C, Czesla M, Massoudy P.. New postoperative conduction abnormalities after the implantation of a rapid-deployment aortic valve prosthesis. Interact CardioVasc Thorac Surg 2019;28:581–6. [DOI] [PubMed] [Google Scholar]
- 21. Andreas M, Wallner S, Habertheuer A, Rath C, Schauperl M, Binder T. et al. Conventional versus rapid-deployment aortic valve replacement: a single-centre comparison between the Edwards Magna valve and its rapid-deployment successor. Interact CardioVasc Thorac Surg 2016;22:799–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. D’Onofrio A, Tessari C, Filippini C, Bagozzi L, Diena M, Alamanni F. et al. Early and mid-term results of rapid deployment valves: the Intuity Italian Registry (INTU-ITA). Ann Thorac Surg 2018;106:1742–9. [DOI] [PubMed] [Google Scholar]
- 23. Andreas M, Coti I, Rosenhek R, Shabanian S, Mahr S, Uyanik-Uenal K. et al. Intermediate-term outcome of 500 consecutive rapid-deployment surgical aortic valve procedures. Eur J Cardiothorac Surg 2019;55:527–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Alkhouli M, Alqahtani F, Ziada KM, Aljohani S, Holmes DR, Mathew V.. Contemporary trends in the management of aortic stenosis in the USA. Eur Heart J 2020;41:921–8. [DOI] [PubMed] [Google Scholar]
- 25. Meco M, Montisci A, Miceli A, Panisi P, Donatelli F, Cirri S. et al. Sutureless perceval aortic valve versus conventional stented bioprostheses: meta-analysis of postoperative and midterm results in isolated aortic valve replacement. J Am Heart Assoc 2018;7:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Romano MA, Koeckert M, Mumtaz MA, Slachman FN, Patel HJ, Chitwood WR Jr. et al. Permanent pacemaker implantation after rapid deployment aortic valve replacement. Ann Thorac Surg 2018;106:685–90. [DOI] [PubMed] [Google Scholar]
- 27. Borger MA, Moustafine V, Conradi L, Knosalla C, Richter M, Merk DR. et al. A randomized multicenter trial of minimally invasive rapid deployment versus conventional full sternotomy aortic valve replacement. Ann Thorac Surg 2015;99:17–25. [DOI] [PubMed] [Google Scholar]




