Abstract
Context
Implantation is a reproductive bottleneck in women, regulated by fluctuations in ovarian steroid hormone concentrations. However, other nuclear receptor ligands are modifiers of endometrial differentiation leading to successful pregnancy. In the present study we analyzed the effects of peroxisome-proliferator-activated receptor β/δ (PPARβ/δ) activation on established cellular biomarkers of human endometrial differentiation (decidualization).
Objective
The objective of this work is to test the effects of PPARβ/δ ligation on human endometrial cell differentiation.
Design
Isolated primary human endometrial stromal cells (ESCs) were treated with synthetic (GW0742) or natural (all trans-retinoic acid, RA) ligands of PPARβ/δ, and also with receptor antagonists (GSK0660, PT-S58, and ST247) in the absence or presence of decidualizing hormones (10 nM estradiol, 100 nM progesterone, and 0.5 mM dibutyryl cAMP [3′,5′-cyclic adenosine 5′-monophosphate]). In some cases interleukin (IL)-1β was used as an inflammatory stimulus. Time course and dose-response relationships were evaluated to determine effects on panels of well characterized in vitro biomarkers of decidualization.
Results
PPARβ/δ, along with estrogen receptor α (ERα) and PR-A and PR-B, were expressed in human endometrial tissue and isolated ESCs. GW0742 treatment enhanced hormone-mediated ESC decidualization in vitro as manifested by upregulation of prolactin, insulin-like growth factor-binding protein 1, IL-11, and vascular endothelial growth factor (VEGF) secretion and also increased expression of ERα, PR-A and PR-B, and connexin 43 (Cx43). RA treatment also increased VEGF, ERα, PR-A, and PR-B and an active, nonphosphorylated isoform of Cx43. IL-1β and PPARβ/δ antagonists inhibited biomarkers of endometrial differentiation.
Conclusion
Ligands that activate PPARβ/δ augment the in vitro expression of biomarkers of ESC decidualization. By contrast, PPARβ/δ antagonists impaired decidualization markers. Drugs activating these receptors may have therapeutic benefits for embryonic implantation.
Keywords: uterus, decidualization, nuclear receptors, fatty acids, retinoic acid
Implantation is critical to the reproductive success of eutherian species, but its molecular mechanisms remain incompletely understood. In women and old-world primates that support spontaneous decidualization and menstruation (1), the endometrium proliferates and differentiates phasically in response to circadian (daily) and circatrigintan (monthly) fluctuations in gonadotropin and ovarian steroid hormone concentrations, respectively. 17β-estradiol (E2) and progesterone (P4) alone can transform the uterus to establish a vascularized, nutritive, and immune-privileged secretory endometrial platform that permits nidation of the blastocyst (2, 3). Although variations among hormone preparations, doses, and delivery routes exist in clinical practice, even menopausal endometrium can be rendered receptive by exclusive administration of these 2 ovarian steroids (4). Thus, we have come to understand that the myriad regulatory actions of E2 and P4 on the endometrium are mediated by elaboration of local growth factors (5), eicosanoids (6), and vitamins (7) synthesized or critically metabolized in situ. By contrast, proinflammatory cytokines, released in response to infection (eg, chronic endometritis) (8) or sterile inflammation (eg, endometriosis [9] and miscarriage [10]) can impair endometrial decidualization and reduce fecundity.
Studies over nearly 3 decades indicate that the cytotoxic cytokine interleukin (IL)-1β is a dominant product of activated monocytes (11). IL-1β secreted from resident endometrial macrophages can induce intrauterine inflammation and perturb decidualization. Inhibitory effects of IL-1β on decidual differentiation in vitro have been reported since the 1990s (12-14) and inverse correlations between in vitro fertilization (IVF) implantation rates and free serum IL-1β levels are reported (15). Further, multivariable logistical regression of cytokines in endometrial fluid collected immediately prior to IVF embryo transfer showed a negative correlation between IL-1β concentrations and clinical pregnancies (16). Our own reports have documented that IL-1β can disrupt several markers of secretory differentiation in human endometrial stromal cells (ESCs), induced in response to an endocrine stimulus composed of E2 (10 nM), P4 (100 nM), and dibutyryl cAMP (3′,5′-cyclic adenosine 5′-monophosphate; 0.5 mM) (17). Classical secreted factors (prolactin, insulin-like growth factor-binding protein 1 [IGFBP-1], and IL-11) and intracellular regulatory proteins (estrogen receptor α [ERα], PR-A and -B, and connexin 43 [Cx43]) were all downregulated by IL-1β exposure (18).
To advance our understanding of paracrinology during implantation, we used a human cell culture model that reliably represents the molecular and cellular features of secretory endometrial transformation, testing compounds that ligate the peroxisome proliferator-activated receptor β/δ (PPARβ/δ). PPARs are a subfamily of nuclear transcription factors that broadly regulate cell differentiation and metabolism. All 3 related receptor isoforms (PPARα, -β/δ, and –γ [NR1C1, -2 and -3 according to the Nuclear Receptors Nomenclature Committee (19)]) are found in human reproductive tissues (20-22). PPARβ/δ is the most widely expressed, but least investigated, of the PPARs and is the isoform that we have evaluated in the present project. PPARβ/δ is involved in triglyceride oxidation, which parallels glycogen storage in rodent (23) and human (24) decidua. PPARβ/δ ligands are increasingly recognized as anti-inflammatory mediators, suppressing chemokines and IL-1β secretion from macrophages (25) and are reported to have antiapoptotic effects in keratinocytes (26). PPARβ/δ can be activated by synthetic ligands (eg, GW0742) and also by naturally occurring ligands, including the polyunsaturated fatty acids, arachidonic and linoleic acid, and some of their metabolites (eg, prostacyclin [PGI2], 13S-hydroxyoctadecadienoic acid [HODE], and 15S-hydroxyeicosatetraenoic acid [HETE]). All-trans retinoic acid (RA), a vitamin A derivative actively metabolized within the uterus (27), has been proposed to exert endometrial decidualizing and antiapoptotic effects in that tissue via ligation of PPARβ/δ (28). Compounds that inhibit PPARβ/δ include antagonists (eg, PT-S58) and inverse agonists (eg, ST247) (29).
Materials and Methods
Source of human tissues and cells
Healthy, parous women with regular menstrual cycles, who had not received hormonal therapy for at least 3 months prior to surgery, were recruited. Endometrial tissue specimens were obtained from 5 participants (ranging in age from 30 to 37 years) without evidence of endometriosis or other pelvic pathology at the time of laparoscopy for tubal sterilization (n = 3) or for evaluation of suspected leiomyomata (n = 2) after providing written informed consent under institutional review board–approved study protocols at Wake Forest School of Medicine (No. 00 019 887) and the University of Utah School of Medicine (No. 00 124 276). Endometrial Pipelle biopsies collected under sterile conditions were transported to the laboratory on ice in DMEM/Ham’s F-12 (catalog No. 10-092cv, CellGro) containing 10% fetal bovine serum. The specimens were collected in the midproliferative phase to avoid effects of endogenous P4 and used to prepare primary cell cultures as described as follows. Histological staging (30) confirmed the clinical determination of the proliferative menstrual cycle phase. Paraffin-embedded endometrial biopsy tissues were sectioned 5-µm thick and subjected to hematoxylin-eosin staining and immunohistochemistry (IHC) as described previously (31) and summarized as follows.
Immunohistochemistry
Briefly, after mounting, the sections were deparaffinized in xylene and rehydrated in graded concentrations of ethanol. Antigen retrieval was performed by heating the slides in 10 mM sodium citrate (pH 6.0) at 100°C for 4 minutes. They were then exposed for 10 minutes to 3% hydrogen peroxide in methanol to quench endogenous hydrogen peroxidase activity. Each sample was then rinsed in water. Nonspecific binding was blocked with Super Block (catalog No. AAA-500, ScyTek Laboratories) overnight at 4°C. Sections were incubated overnight at 4°C with primary anti-PPARβ/δ antibodies (catalog No. NBP2-22 468, Novus Biologicals), anti-IL-1β antibodies (catalog No. 12 703, Cell Signaling Technology) or anti-ERα antibodies (catalog No. 13 258, Cell Signaling Technology) as a positive control, diluted 1:500, 1:400, and 1:400, respectively, in Tris buffer, pH 7.4, containing 0.5% casein as a blocking reagent. Staining was performed using EnVision Plus Systems (Dako) reagents, according to the manufacturer’s protocol, with mild counterstaining with Mayer’s hematoxylin. A negative staining control was performed by substituting the primary antibody with isotype-specific nonimmune immunoglobulin G controls (IgG XP, Biogenex, Cell Signaling) at concentrations matched to those of the primary antibodies.
Human endometrial stromal cells cultures, hormone treatment, and assessment of in vitro decidualization
Human ESC cultures were prepared from 5 proliferative phase biopsies, all subcultured at least twice to eliminate contamination by macrophages or other leukocytes, as described previously (31). Each culture was studied before the sixth passage to avoid cellular dedifferentiation (32). ESC cultures prepared by this protocol were more than 93% pure and retain phenotypic endometrial stromal markers in vitro, including functional estrogen and progesterone receptors (33). Cells were initially plated at a density of 25 000 cells/cm2 directly onto 10-cm polystyrene dishes without exogenous extracellular matrix and grown to approximately 80% confluence in phenol red-free DMEM/Ham’s F-12, supplemented with 5% charcoal-stripped fetal calf serum.
Peroxisome-proliferator-activated receptor ligand, retinoid, and kinase inhibitor treatment of endometrial stromal cell cultures
ESCs were stimulated with the PPARβ/δ agonist GW0742 (GW, catalog No. G3295, Sigma-Aldrich) as described elsewhere (34). In dose-response experiments, hormone and GW0742 effects were found to be additive for several decidual biomarkers, with an half maximal effective concentration (EC50) of the PPARβ/δ agonist of approximately 2.5 μM, so we selected 10 μM for the studies reported here. In some experiments RA was used as the ligand, also at a concentration of 10 μM (35). The PPARβ/δ antagonists GSK0660 (GSK, catalog No. G5795, Sigma-Aldrich), PT-S58 (catalog No. SML0410, Sigma-Aldrich) and the inverse agonist ST247 (catalog No. SML0424, Sigma-Aldrich) (29) also were evaluated. The half maximal inhibitory concentration (IC50) of GSK0660 is reported to be 1 µM for tube formation interference in human microvascular endothelial cells (36), so we studied this ligand at 1 and 10 μM concentrations. To induce decidualization, ESCs were subjected to our established differentiation protocol by incubation with 10 nM E2, 100 nM P4, and 0.5 mM dibutyryl cAMP (“hormones,” “H”) for up to 3 days (72 hours). To mimic inflammation, ESCs were exposed to 0.2 ng/mL (12 pM) recombinant IL-1β under conditions previously optimized (18). In some experiments, the MEK1/2 inhibitor, PD98059 (PD, catalog No. P215, Sigma-Aldrich) was used in ESC cultures at a concentration of 15 μM to assess relationships between extracellularly regulated kinase (ERK)1/2 inhibition and PPARβ/δ activation on decidualization pathways. At the end of the desired incubation period, cells were washed and scraped into Eppendorf tubes in phosphate-buffered saline, centrifuged, and then solubilized in lysis buffer before freezing at –80°C.
Western blot analyses
Western blots were performed on whole-cell extracts obtained by vortexing ESCs in extraction buffer (catalog No. FNN0011, Life Technologies). Total proteins (30 μg/lane, determined using the Thermo Scientific-Pierce BCA Protein Assay kit, catalog No. PI-23 227, Thermo Fisher Scientific) were run on NuPAGE Novex 4% to 12% Bis-Tris Protein Gels, transferred to polyvinylidene fluoride membranes and blocked with 5% skim milk in phosphate-buffered saline. Specific ERK1/2 cascade and Cx43 phosphoproteins were detected using validated monoclonal antibodies at optimized concentrations; these are described in detail in our prior reports (18, 35). After overnight labeling with the primary antiserum, blots were then incubated with a secondary goat antirabbit or antimouse antibody (1:300 ,000; catalog No. 31 460 and 31 430, Pierce Biotechnology Inc) linked to horseradish peroxidase and immunoreactive bands were visualized by the Enhanced Chemiluminescence (ECL) System (Amersham Pharmacia Biotech). The size of each band is indicated as its corresponding molecular mass (kDa) in the figures. In each case, quantification of Western blot ECL bands captured by laser densitometry were normalized to the intensity of corresponding, constitutive β-actin band densities for statistical analyses.
Immunofluorescence cytochemistry
To corroborate and localize the expression of Cx43, ESCs were cultured on Lab-Tek chamber slides before exposure to control media or media with hormones for 72 hours. The cells were fixed in 4% paraformaldehyde, and immunofluorescence cytochemistry was performed on an EVOS cellular imaging microscope after staining with anti-Cx43 or anti–β-actin antibodies as described (37). Counterstaining with DAPI (4’,6-diamidino-2-phenylindole) (blue fluorescence) was performed to identify ESC nuclei.
Enzyme-linked immunosorbent assay methods
Sensitive and specific commercial sandwich ELISA kits that we previously validated with ESC supernatants (37, 38) were used to quantify prolactin (Alpha Diagnostic International), and IGFBP-1, IL-11, and VEGF (R&D Biosystems) before and after coincubation with combinations of hormones and PPARβ/δ agonists or antagonists. As we and others have established, the first 3 are established markers of ESC decidualization (2, 39) and our prior findings indicate that VEGF secretion, an angiogeneic response, also correlates directly with decidualization (37). Supernatants (10 mL per plate) were aspirated and aliquoted for freezing and subsequent ELISA. For IL-11 and VEGF the conditioned media were diluted in sample buffer 1:5 prior to analysis.
Protein quantification and statistical analyses
The Western blot images are representative of experiments from a minimum of 3 cell preparations from independent participants. Nuclear receptor proteins (eg, ERα and PR-A and -B) were detected by Western blots using methods that we recently validated (18) and ERK1/2 pathway proteins and Cx43 levels in ESC lysates, along with their phospho-specific metabolites, were detected using antibodies validated in prior studies (37). All the normalized Western results were reported as means ± SEM of digitized band densities, relative to β-actin. Prolactin, IGFBP-1, IL-11, and VEGF were quantified in ESC conditioned media by ELISA as noted earlier. Expression levels of the cellular or secreted proteins were found to be normally distributed by Kolmogorov-Smirnov tests (17). Comparisons were determined using paired t tests for 2-group analyses with Bonferroni corrections. Aggregate data and their statistical analysis are indicated in the “Results” section. Statistical significance for all the analyses was accepted when 2-tailed tests yielded P less than .05 between the treatment groups.
Graphic model of study hypothesis
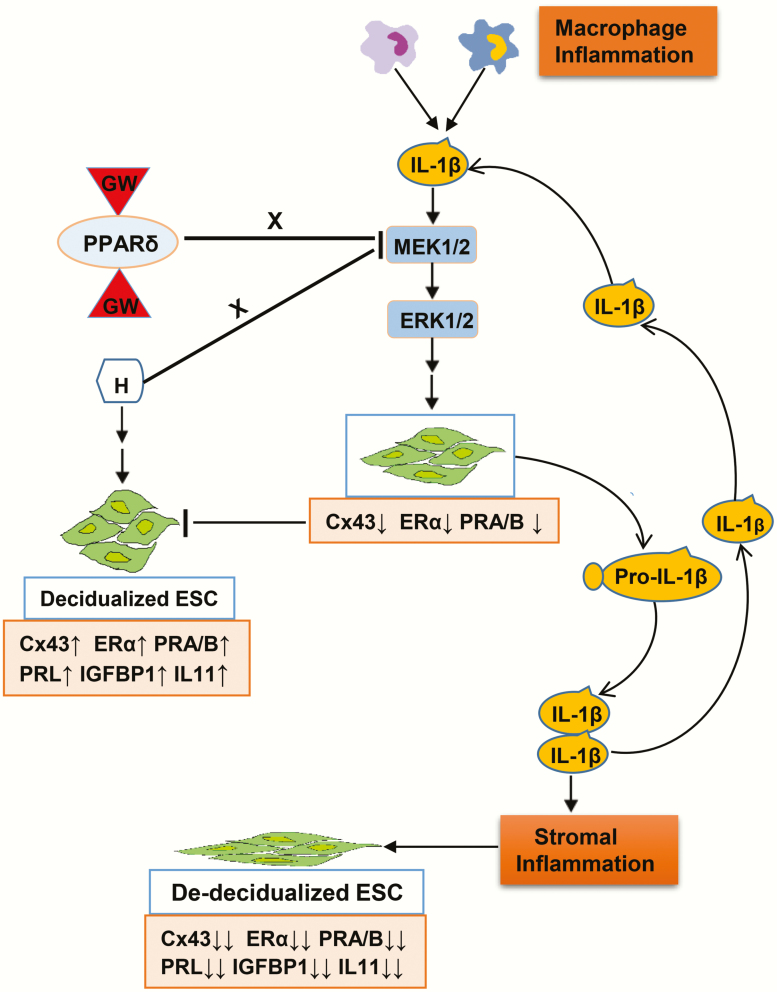
A graphic model summarizing multiple biomarkers of decidualization and the effects of cytokines, hormones, and PPARβ/δ ligands is provided in Fig. 1 to illustrate the hypothesis, findings, and presentation of the data. Macrophages and other inflammatory cells in the endometrial microenvironment are thought to be a primary source of IL-1β and other proinflammatory cytokines, but autocrine and paracrine sources also are activated. The major biomarkers evaluated in this report are represented in the pink boxes, with arrows indicating direction and magnitude of production and secretion. Corresponding alterations in ESC shape (green cell figures) also are represented in the illustration.
Figure 1.
Graphic model of study hypothesis. Macrophages and other inflammatory cells in the endometrial microenvironment are a primary source of interleukin 1β (IL-1β) and other proinflammatory cytokines, but endometrial stromal cell (ESC) autocrine and paracrine sources also contribute. The major biomarkers evaluated in this report are represented in the pink boxes, with arrows indicating direction and magnitude of production or secretion. Corresponding alterations in ESC shape are represented in the cartoon.
Results
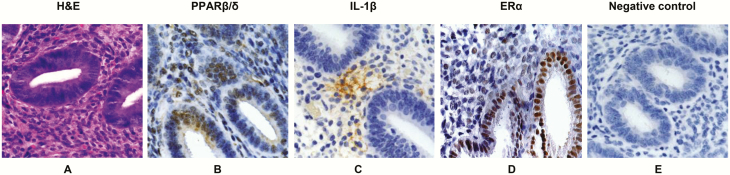
To establish that in vivo endometrial tissue expresses the end point decidualization factors that we are investigating in our in vitro model, we undertook immunohistochemical studies with validated antibodies against PPARβ/δ, IL-1β, and ERα. This allows us to identify different endometrial cell types and cellular localization of these decidual markers, which could be essential to the in vivo decidualization process. The histomorphology of proliferative endometrium with simple tubular glands is highlighted in hematoxylin-eosin–stained tissue (Fig. 2A). IHC was performed on adjacent sections with anti-human PPARβ/δ (Fig. 2B) and anti–IL-1β (Fig. 2C) antiserum. Anti-human ERα (Fig. 2D) and nonimmune serum (Fig. 2E) on serial sections provided positive and negative IHC controls, respectively. Similar to our prior findings with PPARγ in isolated human ESCs (20), the PPARβ/δ receptor isoform was found to be concentrated in endometrial epithelial and stromal cell nuclei, indicating that their component cells are all potential targets of PPARβ/δ ligands (Fig. 2B). By contrast, IL-1β immunoreactivity showed focal, lacy cytoplasmic staining of the cytokine, similar to the pattern of distribution in a prior immunofluorescence staining study (17) (Fig. 2C). ERα-labeled epithelial and stromal nuclei provided positive controls (Fig. 2D), and there was no brown immunoperoxidase staining in the negative controls (Fig. 2E).
Figure 2.
Immunohistochemistry of peroxisome-proliferator-activated receptor β/δ (PPARβ/δ), interleukin1β (IL-1β), and estrogen receptor α (ERα) in endometrium. A, A hematoxylin-eosin (H&E)-stained section of proliferative endometrium is provided to illustrate relevant tissue morphology. B, Immunoperoxidase histochemistry (brown precipitate) shows nuclear localization of PPARβ/δ in endometrial glands and stroma. C, Immunoperoxidase histochemistry (brown precipitate) shows focal, lacy stromal cytoplasmic localization of IL-1β in an adjacent section. D, As a positive control, anti-ERα antibodies stained epithelial and stromal nuclei. E, A negative control, in which the primary antibodies were substituted with nonimmune serum. All sections were counterstained with Mayer’s hematoxylin. Similar findings were observed in n = 3 tissue sections. Magnification × 200.
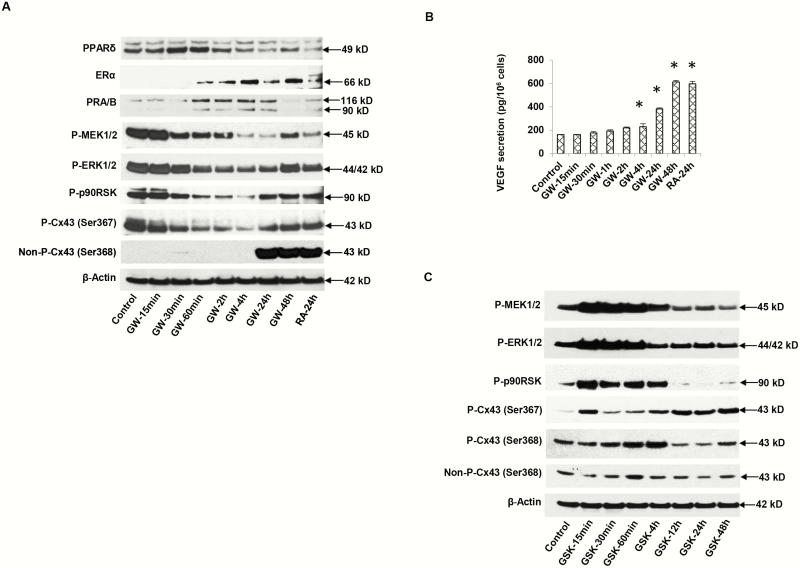
The IHC findings were corroborated by Western blot analyses of primary human ESC lysates, verifying that the anticipated 49 kDa PPARβ/δ protein band was present in the blotted gels (Fig. 3A, panel 1). The Western data indicate that PPARβ/δ receptors are quite prominent in ESCs under basal conditions and undergo modest upregulation 30 to 60 minutes after exposure to the agonist ligand, GW0742 (GW), followed by downregulation 4 to 48 hours later (Fig. 3A, panel 1, lanes 6-8). Similarly, RA for 24 hours also caused a downregulation of PPARβ/δ (Fig. 3A, panel 1, lane 9). The effects of GW on ESC ERα (66 kDa) and PR (-A and -B isoforms, 90 and 116 kDa, respectively) kinetics were different, revealing a gradual increase in the former, and a plateau of the latter receptor isoforms from 1 to 24 hours after initiation of GW treatment (Fig. 3A, panels 2 and 3, lanes 4-8). RA treatment showed modest increases in ERα and PR after 24 hours (Fig. 3A, panels 2 and 3, lane 9).
Figure 3.
A, Time-course of peroxisome-proliferator-activated receptor β/δ (PPARβ/δ) agonist GW0742 (GW) effects on endometrial stromal cell (ESC) protein lysates by Western blotting. GW treatment for 15 minutes to 48 hours showed changes in nuclear receptors: PPARβ/δ (49 kDa, panel 1), estrogen receptor α (ERα) (66 kDa, panel 2), PR-A and -B (90 and 116 kDa, respectively, panel 3). Phosphoproteins of the extracellularly regulated kinase (ERK)1/2 cascade included phospho-MEK1/2 (45 kDa, panel 4), phospho-ERK1/2 (42 kDa, panel 5), and phospho-p90 RSK (90 kDa, panel 6). Connexin 43 (Cx43) isoforms: phospho-Cx43 Ser367 (43 kDa, panel 7), nonphospho-Cx43 Ser368 (43 kDa, panel 8). β-Actin levels (42 kDa, panel 9) are shown. Lane 1 represents untreated control cells and lane 9, ESCs treated for 24 hours with all-trans retinoic acid (RA) as a positive control. B, Under the same conditions, ESC supernatant concentrations of vascular endothelial growth factor (VEGF) were determined by enzyme-linked immunosorbent assay. Means ± SEM of triplicate samples are shown. GW at 24 and 48 hours and RA at 24 hours each significantly stimulated VEGF secretion over control conditions (P < .05, t tests with Bonferroni corrections, n = 3). C, Time-course effects of PPARβ/δ antagonist, GSK0660 (GSK) on ESC protein markers by Western blotting. Phosphoproteins of the extracellularly regulated kinase (ERK)1/2 cascade included phospho-MEK1/2 (45 kDa, panel 1), phospho-ERK1/2 (42 kDa, panel 2), phospho-p90 RSK (90 kDa, panel 3), Cx43 isoforms: Phospho-Cx43 Ser367 (43 kDa, panel 4), phospho-Cx43 Ser368 (43 kDa, panel 5), and nonphospho-Cx43 Ser368 (43 kDa, panel 6). β-Actin levels (42 kDa, panel 7) are shown. Lane 1 represents untreated control cells.
Culturing ESCs with GW or RA also showed similar direct effects on the pattern of ERK1/2 cascade phosphoproteins associated with ESC differentiation (17). Suppression of ERK1/2 pathway phosphorylation by GW was demonstrated in time-course experiments, showing that phospho-MEK1/2 (Fig. 3A, panel 4), phospho-ERK1/2 (Fig. 3A, panel 5), and phospho-p90 RSK (Fig. 3A, panel 6) were all progressively downregulated across the GW treatment course, with a gradual return toward baseline after 48 hours. These findings were accompanied by parallel reductions in phospho-Cx43 (Fig. 3A, panel 7) and a dramatic increase in nonphospho-Cx43 species (Fig. 3A, panel 8) 24 to 48 hours following GW treatment. RA also caused a large increase in nonphospho-Cx43 (Fig. 3A, panel 8, lane 9). By contrast, no changes in β-actin were noted during the time course (Fig. 3A, panel 9). Upregulation of nonphospho-Cx43 has been reported by Laird (40) and ourselves (35) to contribute to maximal intercellular coupling via Cx43 gap junctions. Moreover, we observed a time-dependent increase in VEGF secretion in response to GW or RA treatment (Fig. 3B). That ERα, PR, nonphospho-Cx43, and VEGF were all increased 24 to 48 hours after GW or RA treatment, is consistent with conditions that accelerate ESC differentiation (18).
To corroborate these observations, we tested the effects of a PPARβ/δ antagonist, GSK0660 (GSK), on several of the same ESC ERK1/2 pathway components. Treatment with GSK resulted in essentially mirror-image effects with respect to those of GW. A prompt upregulation of ERK1/2 pathway phosphorylation, with increased phospho-MEK1/2, -ERK1/2, and -p90 RSK, occurred within 15 minutes of GSK exposure (Fig. 3C, panels 1-3, lanes 1 and 2). Overall, concentrations of those same phosphoproteins fell to or below baseline values by 12 hours after GSK exposure (Fig. 3C, panels 1-3, lanes 6-9). In general, GSK stimulated phosphorylation of Cx43 (Fig. 3C, panels 4 and 5). Unlike GW and RA, which caused a large increase in nonphospho-Cx43 by 24 hours (Fig. 3A, panel 8, lanes 7-9), GSK had little effect on the expression of active, nonphospho-Cx43 isoforms or β-actin (Fig. 3C, panels 6 and 7, respectively).
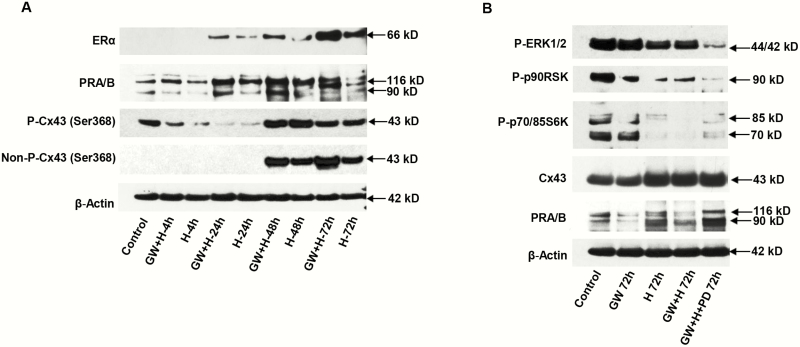
The results of these experiments supported the hypothesis that PPARβ/δ agonists are upstream regulators that suppress the ERK1/2-MAPK pathway and subsequently promote ESC decidualization, so we next inquired as to whether GW could augment signaling associated with hormone-induced ESC differentiation. Using the E2 + P4 + cAMP hormones (“H”) that we (18, 37, 38) and others (2) demonstrated can induce ESC decidualization, additive effects of GW were noted. In Fig. 4A, time- and hormone-dependent increases in ERα (panel 1) and PR-A and PR-B (panel 2) were noted after the addition of GW (“GW + H”) for 48 or 72 hours (2.1 ± 0.6-fold and 2.5 ± 0.5-fold higher, respectively, in GW + H-treated cells [Fig. 4A, panels 1 and 2] relative to H alone [P < .05, t test, n = 3]). Expression of the active, nonphospho-Cx43 species was not detected before 48 hours, but was 1.7 ± 0.5-fold and 7.0 ± 2.0-fold higher, respectively, in GW + H-treated cells (Fig. 4A, panel 4) at the 48-hour and 72-hour time points, relative to H alone (P < .05, t test, n = 3). GW did not have an appreciatively additive effect to hormones on the inactive phospho-Cx43 isoform, nor were differential treatment effects seen on β-actin levels (Fig. 4A, panels 3 and 5, respectively).
Figure 4.
Effects of peroxisome-proliferator-activated receptor β/δ (PPARβ/δ) agonist (GW), hormones (H), and MEK inhibitor (PD) were assessed. A, Time-course and additive effects of GW + H for up to 72 hours were observed for endometrial stromal cell (ESC) expression of estrogen receptor α (ERα) (panel 1), PR-A and -B (panel 2), nonphospho-connexin 43 (Cx43) Ser368 (panel 4), and phospho-Cx43 Ser368 (panel 3). β-Actin levels (panel 5) are shown. Lane 1 represents untreated control cells. B, Relative to controls, a combination of GW, H, and PD for 72 hours led to the lowest phospho-extracellularly regulated kinase (ERK)1/2 (panel 1), phospho-p90 RSK (panel 2), phospho-p70/85 S6Kinase (panel 3) and was associated with the highest levels of total Cx43 (panel 4) and PR-A and -B (panel 5) (P < .05, t-test, n = 3). β-Actin levels (panel 6) are shown as loading controls.
In contrast to their stimulatory effects on markers of ESC differentiation, GW and H repressed the inflammatory ERK1/2 phosphoprotein cascade. Inhibitory effects on phospho-ERK1/2 (Fig. 4B, panel 1), phospho-p90RSK (panel 2), and phosho-p70/85 S6Kinase (panel 3) were noted by GW, H, and their combination. Cx43 and PR-A and PR-B isoforms, biomarkers of ESC decidualization, all tended to be stimulated (panels 4 and 5). Our previous studies demonstrated that treatment with the MEK1/2-specific inhibitor, PD98059 (PD), also can contribute to an enhanced decidual phenotype in ESCs, in part by blocking the inhibitory effects of endogenous IL-1β action (17, 18). As shown in Fig. 4B (lane 5), incubation with a combination of GW + H + PD resulted in superimposed effects, with the lowest levels of phospho-ERK1/2, phospho-p90 RSK, and phospho-p70/85 S6Kinase compared to control (0.3 ± 0.1-fold, P < .05, n = 3, for all 3 kinases). Those effects were accompanied by high levels of total Cx43 (2.9 ± 0.6-fold) and PR (2.0 ± 0.2-fold compared to control, P < .05, n = 3). GW + H or H alone had more subtle actions on ERK pathway phosphorylation (Fig. 4B, lanes 2 and 3). β-Actin levels did not change in response to the treatments (panel 6).
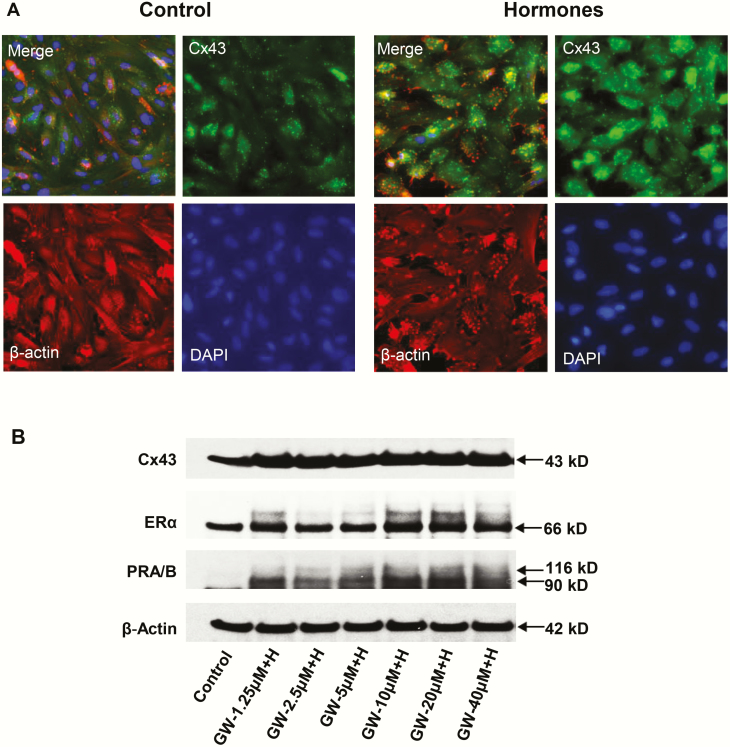
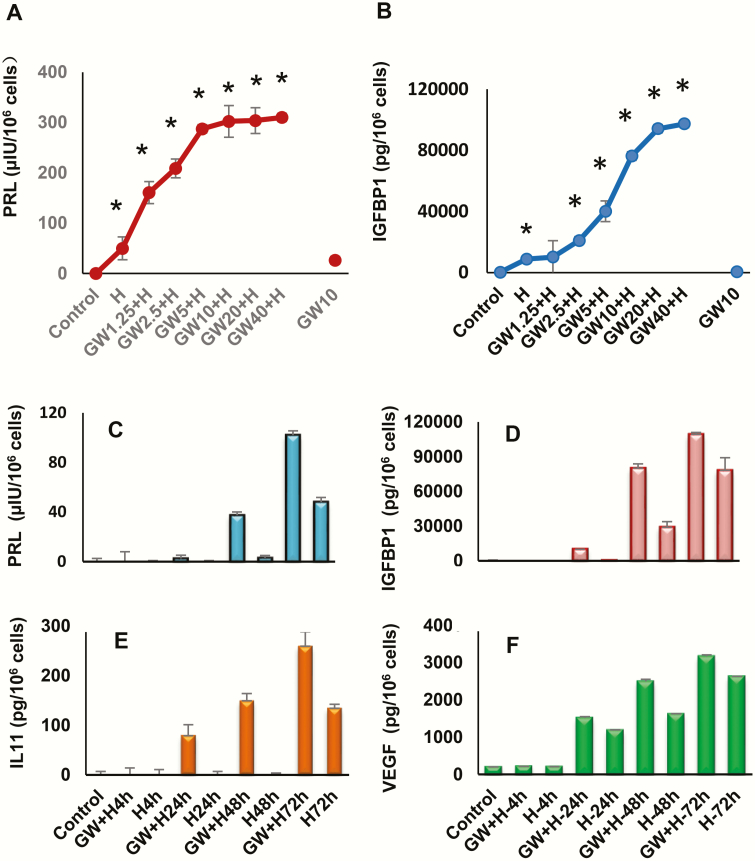
Fig. 5A shows the results of H (“hormone”) treatment on immunofluorescent Cx43 (green) staining (upper right panels) in ESC cultures. DAPI nuclear staining (lower right panels), β-actin (lower left panels), and a merged frame (upper left panels) are included for comparison. Punctate immunostaining of Cx43 gap junctions was significantly upregulated (2.6 ± 0.1-fold compared to control, P < .05, n = 4) following 72 hours of H exposure. The nonuniform distribution of fluorescent signal in the cultures may be due to differences in cell cycle. Dose-response experiments in Western blots indicated that GW + H stimulation of decidualization markers Cx43, ERα, PR-A and PR-B had an EC50 of approximately 2.5 μM GW (Fig. 5B). GW alone (at concentrations as high as 10 μM) had little independent effect on secreted biomarkers (Fig. 6A and 6B), but in the presence of H for 72 hours, GW stimulated prolactin and IGFBP-1 secretion beyond H alone and with EC50s ranging from 1 to 5 μM. Time-course investigations showed that prolactin, IGFBP-1, IL-11, and VEGF secretion were all higher under GW + H stimulation than H treatment alone, within 24 to 72 hours (Fig. 6C-6F).
Figure 5.
A, The effects of hormone (“H”) treatment on immunofluorescent connexin 43 (Cx43) (green) staining in endometrial stromal cell (ESC) cultures for 72 hours are shown in the upper right panels. A 2.6 ± 0.5-fold increase in Cx43 pixel count (P < .05, t test, n = 4) was noted. DAPI (4’,6-diamidino-2-phenylindole) nuclear staining (lower right panels), β-actin (lower left panels), and a merged frame (upper left panels) also are presented. B, Western blot results in ESCs treated with different concentrations of peroxisome-proliferator-activated receptor β/δ (PPARβ/δ) agonist (GW) combined with decidualizing hormones (“H”) for Cx43, estrogen receptor α (ERα) and PR-A and PR-B are shown. Standard E2 + P4 + 3′,5′–cyclic adenosine 5′-monophosphate (cAMP) hormone concentrations were kept constant while GW levels were increased. ERα (panel 2) and PR-A and -B (panel 3) both revealed dose-dependent increases by the addition of GW (“GW + H”).
Figure 6.
Dose-responsive effects of peroxisome-proliferator-activated receptor β/δ (PPARβ/δ) agonist (GW) and hormones (“H”) alone and in combination and time-course effects (GW + H) on A, prolactin (PRL) and B, insulin-like growth factor-binding protein 1 (IGFBP-1) secretion were evaluated by enzyme-linked immunosorbent assay (ELISA). In the presence of H, GW had apparent EC50s ranging from 1 to 5 μM. The incubation time was 72 hours. Asterisks signify dose-response effects different from control conditions (P < .05, n = 3).Time-course effects of GW on secretory behavior of differentiated endometrial stromal cells. ELISAs were used to quantify C, PRL, D, IGFBP-1, E, interleukin 11 (IL-11), and F, vascular endothelial growth factor (VEGF) secretion by these cells. The data reflect the effects of up to 72 hours of H alone, and also the additive responses to a combination of 10 μM GW + H.
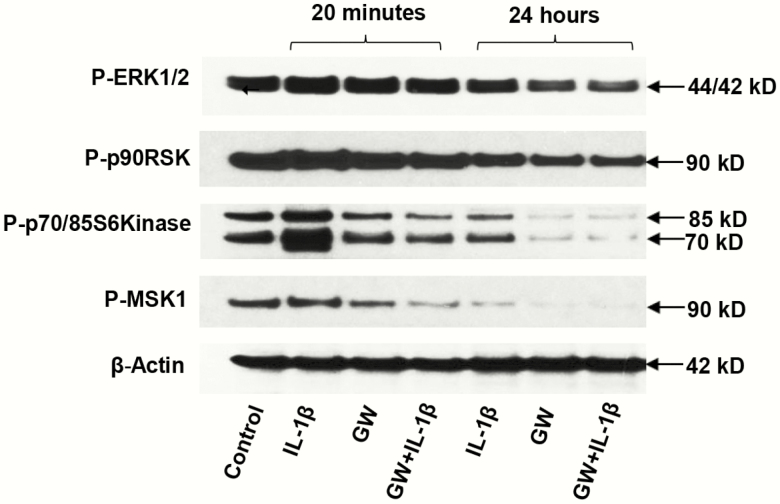
To assess possible mechanisms of GW on hormone-induced ESC differentiation, we evaluated its action under conditions of IL-1β–mediated inhibition of decidualization biomarkers. As reported previously, IL-1β impedes ESC decidualization (13), predominantly via activation of the ERK1/2 signal transduction pathway (17, 18). When ESCs were exposed briefly (20 minutes) or more chronically (24 hours) to 12 pM IL-1β, we observed that phospho-ERK1/2, phospho-p90 RSK, phospho-p70/85 S6K, and phospho-MSK1 were all transiently upregulated after 20 minutes (Fig. 7, panels 1-4, respectively). In ESCs coincubated with GW + IL-1β, ERK1/2 cascade phosphoproteins were reduced relative to controls (Fig. 7, lanes 4 and 7). After 24 hours of GW + IL-1β, phospho-ERK1/2 was 0.4 ± 0.1-fold (P < .05, n = 3) control levels; phospho-p70/85 S6K was reduced to 0.3 ± 0.1-fold (P < .05, n = 3), and phospho-MSK1 decreased to 0.3 ± 0.1-fold (P < .05, n = 3). These results indicated that the PPARβ/δ agonist can block IL-1β-mediated ERK1/2 activation.
Figure 7.
Effects of peroxisome-proliferator-activated receptor β/δ (PPARβ/δ) agonist (GW) on interleukin 1β (IL-1β)-mediated inhibition of decidualization biomarkers in endometrial stromal cells (ESCs). ESCs were exposed briefly (20 minutes) or chronically (24 hours) to IL-1β, GW, or a combination of the compounds. Phospho (P)-extracellularly regulated kinase (ERK)1/2, P-p90 RSK, P-p70/85 S6K, and P-MSK1 were all significantly reduced by the combination after 24 hours (panels 1-4, respectively) (≤ 0.4 ± 0.1-fold, P < .05, t test, n = 3). β-Actin levels did not change in response to the treatments (panel 5).
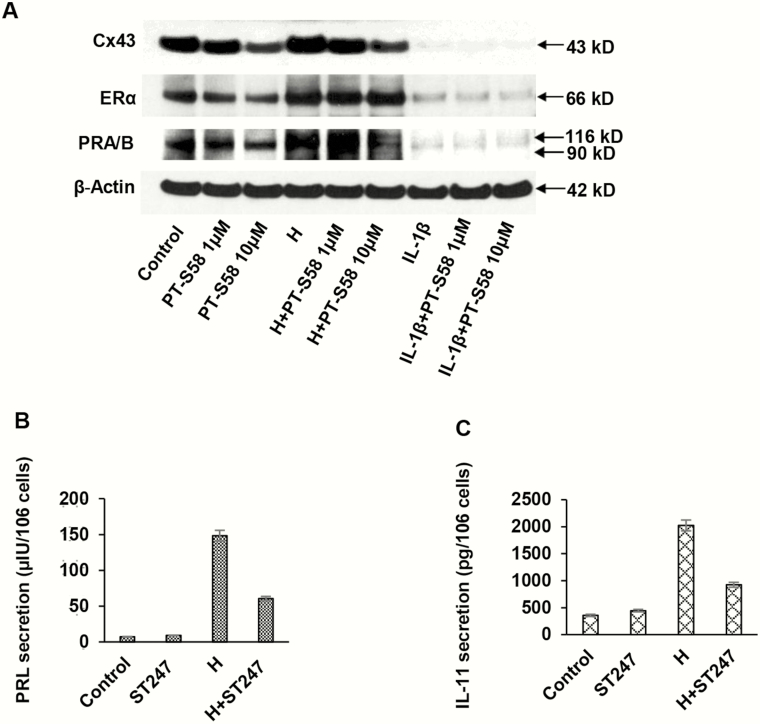
The effects of PPARβ/δ signaling in ESCs were further tested using high-affinity PPARβ/δ inhibitors derived from GSK0660. The antagonist PT-S58 (29) was shown to dose-dependently reduce levels of Cx43 and basal and hormone-induced ERα, but it had little additional inhibitory effects in the presence of IL-1β (Fig. 8A). At the 10-μM concentration, PT-S58 suppressed basal Cx43 to 0.5 ± 0.1-fold and hormone-stimulated Cx43 to 0.3 ± 0.1-fold control levels (P < .05, n = 3). It is well known that ESCs under basal conditions secrete little prolactin or IL-11, but the inverse PPARβ/δ agonist ST247 suppressed hormone-induced secretion of both proteins by more than 50% (Fig. 8B and 8C, respectively).
Figure 8.
Alternative peroxisome-proliferator-activated receptor β/δ (PPARβ/δ) inhibitors (PT-S58 and ST247) were used to assess effects on endometrial stromal cell (ESC) biomarkers. A, After a 72-hour incubation period, PT-S58 appeared to dose-dependently suppress basal connexin 43 (Cx43) (panel 1) and basal and hormone-induced estrogen receptor α (ERα) and PR (panels 2 and 3). Interleukin 1β (IL-1β) strongly inhibited Cx43, ERα, and PR to such an extent that it is difficult to ascertain whether the PPARβ/δ antagonist caused further inhibition. At the 10-μM concentration, PT-S58 suppressed basal Cx43 to 0.5 ± 0.1-fold and hormone-stimulated Cx43 to 0.3 ± 0.1-fold control levels (P < .05, t test, n = 3). β-Actin levels were not affected (panel 4). The inverse PPARβ/δ agonist, ST247 (10 μM), was incubated with ESCs for 72 hours in the absence or presence of H. Hormone-induced B, prolactin and C, IL-11 secretion were both inhibited more than 50% by ST247, although the inverse agonist had no effect under control conditions (P < .05, t test, n = 3).
Discussion
In the present series of experiments, we evaluated the role of synthetic and natural PPARβ/δ ligands using human endometrial biopsies and a well-characterized model of human ESC decidualization (2, 37, 41). The overall pathways affected are represented in Fig. 1. Our collective findings indicate that PPARβ/δ are expressed in endometrial epithelial and stromal cell nuclei in situ and can be autoregulated by agonist ligands (GW and RA) in ESCs in vitro. PPARβ/δ ligation accelerates the hormone-induced decidualization program in ESCs, with upregulation of characteristic biomarkers, including ERα, PR-A and PR-B and Cx43, as a result of ERK1/2 inhibition (18). In addition, classical and emerging secretory products of decidualized endometrium (eg, prolactin, IGFBP-1, IL-11, and VEGF) are stimulated by PPARβ/δ agonists. By contrast, ligands that antagonize PPARβ/δ have opposing effects on ESC differentiation. The precise cellular functions of these biomarker proteins secreted at the embryo-maternal interface are not fully elucidated, but all 4 are intimately associated with angiogenesis (42-44), a process that is crucial to successful implantation (45).
We (37) and others (46) have reported that biochemical evidence of ESC decidualization is optimal after 7 to 14 days of hormone treatment in vitro, but reproducibly detectable differences in prolactin, IGFBP-1, IL-11, and VEGF can be discerned within as few as 72 hours of hormone exposure (Fig. 6A-6F). We suspect that heterogeneity of Cx43 expression (see Fig. 5A) may be due to differences in cell cycles. We chose this earlier time point because it allowed accurate assessment of signaling protein phosphorylation that is difficult to monitor over weeks of hormone exposure. In Fig. 4B, treatment with a combination of GW + H + PD yields a composite biochemical pattern of low phospho-ERK1/2, low phospho-p90 RSK, and low phospho-p70/85 S6K, with high Cx43 and PR. These markers correspond to a robust decidualized ESC phenotype. The superimposed effects appear to reflect converging influences of indirect inhibition of ERK1/2 by GW-PPARβ/δ complexes, along with classical differentiating effects of H on ESC.
Prior studies in rodent species indicated that PPARβ/δ pathways are regulated locally at embryonic implantation sites (47, 48). Our study used a commercially available, synthetic PPARβ/δ agonist as well as RA, a natural, endogenous ligand of this class of receptors. However, RA signaling is complex and mediated via either PPARβ/δ or retinoic acid receptors (RARs) depending on the relative cellular concentrations of these 2 nuclear receptors and their respective binding proteins, fatty acid binding protein 5 (FABP5) and cellular RA binding protein 2 (CRABP2). When RA is transported to the nucleus via FABP5-PPARβ/δ, it promotes endometrial stromal cell survival, decidualization, and embryo development (49-52). By contrast, RA signaling via CRABP2-RAR in ESCs has been associated with ESC growth arrest and apoptosis (28). In vitro studies with ESCs indicate that during decidualization, initial dominance of the CRABP2-RAR pathway results in an inhibition of differentiation (53, 54), whereas decidualization is stimulated when the pathway is shifted to FABP5-PPARβ/δ (49). Alterations in signaling pathways may explain why the RA effects are dependent on timing of exposure. We found that in situ RA biosynthesis was significantly increased (1.4-fold, P < .03) during ESC decidualization and that RA augmented local endometrial VEGF production (52) and Cx43 dephosphorylation, a protein modification that results in functional Cx43 gap junction intercellular communication (35). The latter effect was noted to be RAR independent, because it was not mitigated by the pan-RAR inverse agonist BMS493. Our findings add support to the concept that RA promotes ESC decidualization via the alternative, nonclassical FABP5-PPARβ/δ pathway (28, 35).
Synthetic agonist ligands of PPARβ/δ are currently under pharmaceutical development, and among their therapeutic activities in preclinical models are reduction in triglycerides and suppression of IL-1β action (25). In human keratinocytes, PPARβ/δ signaling attenuates IL-1α and IL-1β action by upregulation of endogenous IL-1 receptor antagonist (IL-1ra) (55). IL-1β positively autoregulates its own production in ESCs (17) and in other cell systems (11), but this proinflammatory, autocrine loop was disrupted by GW in ESCs. Moreover, PPARβ/δ signaling can reverse untoward metabolic effects associated with poor pregnancy outcomes (21). We calculated the EC50 of GW to be approximately 2.5 μM, very similar to that deduced (2.6 μM) by Wu and colleagues for the related PPARβ/δ agonist GW501516 (56). Although we did not include every end point permutation, we did show that PPARβ/δ antagonists and inverse agonists impede the expression of many of the same differentiation markers (18). For example, Cx43, ERα and PR-A/PR-B expression were all reduced by PT-S58 (Fig. 8A). Because poor reproductive outcomes in endometriosis (57), polycystic ovary syndrome (58), idiopathic primary infertility (59), IVF (15), recurrent miscarriage (60), and preterm birth (61) have all been attributed in part to detrimental effects by IL-1β, we postulate that PPARβ/δ agonists might mitigate some of these clinical settings and should be considered for future clinical trials. To our knowledge, no such interventions have yet been attempted using the extant PPARβ/δ pharmacopeia. In vivo correlations, and ultimately, human clinical trials, will be needed to definitively prove the hypothesis that PPARβ/δ action is necessary for implantation and early pregnancy survival. Identifying the species that best represents a model for human implantation (nonhuman primates vs rodents) is an ongoing challenge but an important future goal. Obviously, the safety profiles of such new therapeutics will need to be assiduously vetted in animal models before application in human conception cycles could be approved, but they may provide novel treatments for the unmet needs of couples with implantation defects.
Acknowledgments
The authors thank the operating room and nursing staffs of Wake Forest Baptist Hospital, who contributed to the protocol and the generous participation of the study participants.
Financial Support: This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (Grants R01 HD55379 and U01 HD66439 to N.S. and R.N.T.).
Glossary
Abbreviations
- CRABP2
cellular RA binding protein 2
- Cx43
connexin 43
- E2
17β-estradiol
- ELISA
enzyme-linked immunosorbent assay
- ERα
estrogen receptor α
- ERK
extracellularly regulated kinase
- ESCs
endometrial stromal cells
- FABP5
fatty acid binding protein 5
- IGFBP-1
insulin-like growth factor-binding protein 1
- IHC
immunohistochemistry
- IL-1β
interleukin 1β
- P4
progesterone
- PD
PD98059
- PPARβ/δ
peroxisome-proliferator-activated receptor β/δ
- RA
all trans-retinoic acid
- RARs
retinoic acid receptors
- VEGF
vascular endothelial growth factor
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: The data sets generated and analyzed during the present study are not publicly available but will be provided by the corresponding author on reasonable request.
References
- 1. Bellofiore N, Ellery SJ, Mamrot J, Walker DW, Temple-Smith P, Dickinson H. First evidence of a menstruating rodent: the spiny mouse (Acomys cahirinus). Am J Obstet Gynecol. 2017;216(1):40.e1-40.e11. [DOI] [PubMed] [Google Scholar]
- 2. Gellersen B, Brosens JJ. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr Rev. 2014;35(6):851-905. [DOI] [PubMed] [Google Scholar]
- 3. Lessey BA, Young SL. What exactly is endometrial receptivity? Fertil Steril. 2019;111(4):611-617. [DOI] [PubMed] [Google Scholar]
- 4. Devroey P, Pados G. Preparation of endometrium for egg donation. Hum Reprod Update. 1998;4(6):856-861. [DOI] [PubMed] [Google Scholar]
- 5. Shifren JL, Tseng JF, Zaloudek CJ, et al. Ovarian steroid regulation of vascular endothelial growth factor in the human endometrium: implications for angiogenesis during the menstrual cycle and in the pathogenesis of endometriosis. J Clin Endocrinol Metab. 1996;81(8):3112-3118. [DOI] [PubMed] [Google Scholar]
- 6. Smith SK. Prostaglandins and growth factors in the endometrium. Baillieres Clin Obstet Gynaecol. 1989;3(2):249-270. [DOI] [PubMed] [Google Scholar]
- 7. Taylor RN, Kane MA, Sidell N. Pathogenesis of endometriosis: roles of retinoids and inflammatory pathways. Semin Reprod Med. 2015;33(4):246-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu D, Kimura F, Zheng L, et al. Chronic endometritis modifies decidualization in human endometrial stromal cells. Reprod Biol Endocrinol. 2017;15(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zondervan KT, Becker CM, Koga K, Missmer SA, Taylor RN, Viganò P. Endometriosis. Nat Rev Dis Primers. 2018;4(1):9. [DOI] [PubMed] [Google Scholar]
- 10. Christiansen OB, Nielsen HS, Kolte AM. Inflammation and miscarriage. Semin Fetal Neonatal Med. 2006;11(5):302-308. [DOI] [PubMed] [Google Scholar]
- 11. Dinarello CA. A clinical perspective of IL-1β as the gatekeeper of inflammation. Eur J Immunol. 2011;41(5):1203-1217. [DOI] [PubMed] [Google Scholar]
- 12. Kariya M, Kanzaki H, Takakura K, et al. Interleukin-1 inhibits in vitro decidualization of human endometrial stromal cells. J Clin Endocrinol Metab. 1991;73(6):1170-1174. [DOI] [PubMed] [Google Scholar]
- 13. Frank GR, Brar AK, Jikihara H, Cedars MI, Handwerger S. Interleukin-1 beta and the endometrium: an inhibitor of stromal cell differentiation and possible autoregulator of decidualization in humans. Biol Reprod. 1995;52(1):184-191. [DOI] [PubMed] [Google Scholar]
- 14. Vićovac LM, Starkey PM, Aplin JD. Comment: effect of cytokines on prolactin production by human decidual stromal cells in culture: studies using cells freed of bone marrow-derived contaminants. J Clin Endocrinol Metab. 1994;79(6):1877-1882. [DOI] [PubMed] [Google Scholar]
- 15. Spandorfer SD, Neuer A, Liu HC, Rosenwaks Z, Witkin SS. Involvement of interleukin-1 and the interleukin-1 receptor antagonist in in vitro embryo development among women undergoing in vitro fertilization-embryo transfer. J Assist Reprod Genet. 2003;20(12):502-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boomsma CM, Kavelaars A, Eijkemans MJ, et al. Endometrial secretion analysis identifies a cytokine profile predictive of pregnancy in IVF. Hum Reprod. 2009;24(6):1427-1435. [DOI] [PubMed] [Google Scholar]
- 17. Yu J, Berga SL, Zou W, et al. IL-1β inhibits connexin 43 and disrupts decidualization of human endometrial stromal cells through ERK1/2 and p38 MAP kinase. Endocrinology. 2017;158(12):4270-4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yu J, Berga SL, Zou W, Taylor RN. Interleukin-1β inhibits estrogen receptor-α, progesterone receptors A and B and biomarkers of human endometrial stromal cell differentiation: implications for endometriosis. Mol Hum Reprod. 2019;25(10):625-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nuclear Receptors Nomenclature Committee. A unified nomenclature system for the nuclear receptor superfamily. Cell. 1999;97(2):161-163. [DOI] [PubMed] [Google Scholar]
- 20. Pritts EA, Zhao D, Ricke E, Waite L, Taylor RN. PPAR-gamma decreases endometrial stromal cell transcription and translation of RANTES in vitro. J Clin Endocrinol Metab. 2002;87(4):1841-1844. [DOI] [PubMed] [Google Scholar]
- 21. Wieser F, Waite L, Depoix C, Taylor RN. PPAR action in human placental development and pregnancy and its complications. PPAR Res. 2008;2008:527048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hutter S, Knabl J, Andergassen U, Jeschke U. The role of PPARs in placental immunology: a systematic review of the literature. PPAR Res. 2013;2013:970276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hall K. Lipids in the mouse uterus during early pregnancy. J Endocrinol. 1975;65(2):233-243. [DOI] [PubMed] [Google Scholar]
- 24. Staff AC, Ranheim T, Khoury J, Henriksen T. Increased contents of phospholipids, cholesterol, and lipid peroxides in decidua basalis in women with preeclampsia. Am J Obstet Gynecol. 1999;180(3 Pt 1):587-592. [DOI] [PubMed] [Google Scholar]
- 25. Lee CH, Chawla A, Urbiztondo N, et al. Transcriptional repression of atherogenic inflammation: modulation by PPARdelta. Science. 2003;302(5644):453-457. [DOI] [PubMed] [Google Scholar]
- 26. Di-Poï N, Tan NS, Michalik L, Wahli W, Desvergne B. Antiapoptotic role of PPARbeta in keratinocytes via transcriptional control of the Akt1 signaling pathway. Mol Cell. 2002;10(4):721-733. [DOI] [PubMed] [Google Scholar]
- 27. Pierzchalski K, Taylor RN, Nezhat C, et al. Retinoic acid biosynthesis is impaired in human and murine endometriosis. Biol Reprod. 2014;91(4):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pavone ME, Reierstad S, Sun H, Milad M, Bulun SE, Cheng YH. Altered retinoid uptake and action contributes to cell survival in endometriosis. J Clin Endocrinol Metab. 2010;95(11):E300-E309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Naruhn S, Toth PM, Adhikary T, et al. High-affinity peroxisome proliferator-activated receptor β/δ-specific ligands with pure antagonistic or inverse agonistic properties. Mol Pharmacol. 2011;80(5):828-838. [DOI] [PubMed] [Google Scholar]
- 30. Noyes RW, Hertig A, Rock J. Dating the endometrial biopsy. Fertil Steril. 1950;1:3-25. [DOI] [PubMed] [Google Scholar]
- 31. Yu J, Boicea A, Barrett KL, et al. Reduced connexin 43 in eutopic endometrium and cultured endometrial stromal cells from subjects with endometriosis. Mol Hum Reprod. 2014;20(3):260-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chowdhury I, Banerjee S, Driss A, et al. Curcumin attenuates proangiogenic and proinflammatory factors in human eutopic endometrial stromal cells through the NF-κB signaling pathway. J Cell Physiol. 2019;234(5):6298-6312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tseng JF, Ryan IP, Milam TD, et al. Interleukin-6 secretion in vitro is up-regulated in ectopic and eutopic endometrial stromal cells from women with endometriosis. J Clin Endocrinol Metab. 1996;81(3):1118-1122. [DOI] [PubMed] [Google Scholar]
- 34. Bishop-Bailey D, Bystrom J. Emerging roles of peroxisome proliferator-activated receptor-beta/delta in inflammation. Pharmacol Ther. 2009;124(2):141-150. [DOI] [PubMed] [Google Scholar]
- 35. Wu J, Taylor RN, Sidell N. Retinoic acid regulates gap junction intercellular communication in human endometrial stromal cells through modulation of the phosphorylation status of connexin 43. J Cell Physiol. 2013;228(4):903-910. [DOI] [PubMed] [Google Scholar]
- 36. Capozzi ME, McCollum GW, Savage SR, Penn JS. Peroxisome proliferator-activated receptor-β/δ regulates angiogenic cell behaviors and oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 2013;54(6):4197-4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yu J, Berga SL, Johnston-MacAnanny EB, et al. Endometrial stromal decidualization responds reversibly to hormone stimulation and withdrawal. Endocrinology. 2016;157(6):2432-2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yu J, Wu J, Bagchi IC, Bagchi MK, Sidell N, Taylor RN. Disruption of gap junctions reduces biomarkers of decidualization and angiogenesis and increases inflammatory mediators in human endometrial stromal cell cultures. Mol Cell Endocrinol. 2011;344(1-2):25-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dimitriadis E, Salamonsen LA, Robb L. Expression of interleukin-11 during the human menstrual cycle: coincidence with stromal cell decidualization and relationship to leukaemia inhibitory factor and prolactin. Mol Hum Reprod. 2000;6(10):907-914. [DOI] [PubMed] [Google Scholar]
- 40. Laird DW. Connexin phosphorylation as a regulatory event linked to gap junction internalization and degradation. Biochim Biophys Acta. 2005;1711(2):172-182. [DOI] [PubMed] [Google Scholar]
- 41. Irwin JC, de las Fuentes L, Giudice LC. Growth factors and decidualization in vitro. Ann N Y Acad Sci. 1994;734:7-18. [DOI] [PubMed] [Google Scholar]
- 42. Giudice LC, Mark SP, Irwin JC. Paracrine actions of insulin-like growth factors and IGF binding protein-1 in non-pregnant human endometrium and at the decidual-trophoblast interface. J Reprod Immunol. 1998;39(1-2):133-148. [DOI] [PubMed] [Google Scholar]
- 43. Clapp C, Thebault S, Jeziorski MC, Martínez De La Escalera G. Peptide hormone regulation of angiogenesis. Physiol Rev. 2009;89(4):1177-1215. [DOI] [PubMed] [Google Scholar]
- 44. Ramathal CY, Bagchi IC, Taylor RN, Bagchi MK. Endometrial decidualization: of mice and men. Semin Reprod Med. 2010;28(1):17-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Taylor RN, Lebovic DI, Hornung D, Mueller MD. Endocrine and paracrine regulation of endometrial angiogenesis. Ann N Y Acad Sci. 2001;943:109-121. [DOI] [PubMed] [Google Scholar]
- 46. Gellersen B, Brosens IA, Brosens JJ. Decidualization of the human endometrium: mechanisms, functions, and clinical perspectives. Semin Reprod Med. 2007;25(6):445-453. [DOI] [PubMed] [Google Scholar]
- 47. Ding NZ, Ma XH, Diao HL, Xu LB, Yang ZM. Differential expression of peroxisome proliferator-activated receptor delta at implantation sites and in decidual cells of rat uterus. Reproduction. 2003;125(6):817-825. [DOI] [PubMed] [Google Scholar]
- 48. Pakrasi PL, Jain AK. Cyclooxygenase-2 derived PGE2 and PGI2 play an important role via EP2 and PPARdelta receptors in early steps of oil induced decidualization in mice. Placenta. 2008;29(6):523-530. [DOI] [PubMed] [Google Scholar]
- 49. Zheng WL, Sierra-Rivera E, Luan J, Osteen KG, Ong DE. Retinoic acid synthesis and expression of cellular retinol-binding protein and cellular retinoic acid-binding protein type II are concurrent with decidualization of rat uterine stromal cells. Endocrinology. 2000;141(2):802-808. [DOI] [PubMed] [Google Scholar]
- 50. Niederreither K, Subbarayan V, Dollé P, Chambon P. Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat Genet. 1999;21(4):444-448. [DOI] [PubMed] [Google Scholar]
- 51. Ulven SM, Gundersen TE, Weedon MS, et al. Identification of endogenous retinoids, enzymes, binding proteins, and receptors during early postimplantation development in mouse: important role of retinal dehydrogenase type 2 in synthesis of all-trans-retinoic acid. Dev Biol. 2000;220(2):379-391. [DOI] [PubMed] [Google Scholar]
- 52. Sidell N, Feng Y, Hao L, et al. Retinoic acid is a cofactor for translational regulation of vascular endothelial growth factor in human endometrial stromal cells. Mol Endocrinol. 2010;24(1):148-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Brar AK, Kessler CA, Meyer AJ, Cedars MI, Jikihara H. Retinoic acid suppresses in-vitro decidualization of human endometrial stromal cells. Mol Hum Reprod. 1996;2(3):185-193. [DOI] [PubMed] [Google Scholar]
- 54. Ozaki R, Kuroda K, Ikemoto Y, et al. Reprogramming of the retinoic acid pathway in decidualizing human endometrial stromal cells. PloS One. 2017;12(3):e0173035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chong HC, Tan MJ, Philippe V, et al. Regulation of epithelial-mesenchymal IL-1 signaling by PPARbeta/delta is essential for skin homeostasis and wound healing. J Cell Biol. 2009;184(6):817-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wu CC, Baiga TJ, Downes M, et al. Structural basis for specific ligation of the peroxisome proliferator-activated receptor δ. Proc Natl Acad Sci U S A. 2017;114(13):E2563-E2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lebovic DI, Chao VA, Martini JF, Taylor RN. IL-1beta induction of RANTES (regulated upon activation, normal T cell expressed and secreted) chemokine gene expression in endometriotic stromal cells depends on a nuclear factor-kappaB site in the proximal promoter. J Clin Endocrinol Metab. 2001;86(10):4759-4764. [DOI] [PubMed] [Google Scholar]
- 58. González F. Inflammation in polycystic ovary syndrome: underpinning of insulin resistance and ovarian dysfunction. Steroids. 2012;77(4):300-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Fitzgerald HC, Salamonsen LA, Rombauts LJ, Vollenhoven BJ, Edgell TA. The proliferative phase underpins endometrial development: altered cytokine profiles in uterine lavage fluid of women with idiopathic infertility. Cytokine. 2016;88:12-19. [DOI] [PubMed] [Google Scholar]
- 60. Tang AW, Quenby S. Recent thoughts on management and prevention of recurrent early pregnancy loss. Curr Opin Obstet Gynecol. 2010;22(6):446-451. [DOI] [PubMed] [Google Scholar]
- 61. Romero R, Espinoza J, Gonçalves LF, Kusanovic JP, Friel LA, Nien JK. Inflammation in preterm and term labour and delivery. Semin Fetal Neonatal Med. 2006;11(5):317-326. [DOI] [PMC free article] [PubMed] [Google Scholar]