Abstract
Background
The tryptophan-catabolizing enzyme indoleamine 2,3-dioxygenase 1 (IDO1), which subverts T-cell immunity at multiple levels, is itself subject to inherent T-cell reactivity. This intriguing deviation from central tolerance has been interpreted as counterbalancing IDO1-mediated immunosuppression. Based on this hypothesis, clinical studies employing an IDO1 peptide-based vaccine approach for cancer treatment have been initiated, but there remains a pressing need to further investigate the immunological ramifications of stimulating the anti-IDO1 T-cell response in this manner.
Methods
CT26 colon carcinoma tumors were evaluated for expression of IDO1 protein by western blot analysis, immunofluorescence microscopy and flow cytometry. Mouse IDO1-derived peptides, predicted to bind either major histocompatibility complex (MHC) class I or II of the H2d BALB/c strain, were emulsified in 50% Montanide for prophylactic or therapeutic vaccine treatment of CT26 tumor-bearing mice initiated either 7 days prior to or following tumor cell injection, respectively. In some therapeutic treatment experiments, administration of programmed cell death protein 1-binding antibody (anti-PD1 antibody) or epacadostat was concurrently initiated. Tumor size was determined by caliper measurements and comparative tumor growth suppression was assessed by longitudinal analyses of tumor growth data. For adoptive transfer, T cells from complete responder animals were isolated using paramagnetic beads and fluorescence-activated cell sorting.
Results
This study identifies mouse MHC class I-directed and II-directed, IDO1-derived peptides capable of eliciting antitumor responses, despite finding IDO1 expressed exclusively in tumor-infiltrating immune cells. Treatment of established tumors with anti-PD1 antibody and class I-directed but not class II-directed IDO1 peptide vaccines produced an enhanced antitumor response. Likewise, class I-directed and II-directed IDO1 peptides elicited an enhanced combinatorial response, suggesting distinct mechanisms of action. Consistent with this interpretation, adoptive transfer of isolated CD8+ T cells from class I and CD4+ T cells from class II peptide-vaccinated responder mice delayed tumor growth. The class II-directed response was completely IDO1-dependent while the class I-directed response included an IDO1-independent component consistent with antigen spread.
Conclusions
The in vivo antitumor effects demonstrated with IDO1-based vaccines via targeting of the tumor microenvironment highlight the utility of mouse models for further exploration and refinement of this novel vaccine-based approach to IDO1-directed cancer therapy and its potential to improve patient response rates to anti-PD1 therapy.
Keywords: indoleamine-pyrrole 2,3,-dioxygenase; vaccination; adaptive immunity; programmed cell death 1 receptor; immunotherapy
Introduction
Attempts to deploy the immune system to fight cancer date back over a century,1 but it has not been until the recent advent of ‘immune checkpoint inhibitors’2 that widespread realization of the potential of immunotherapy for treating patients with solid tumors has begun to be realized. These therapeutic antibodies, initially directed against cytotoxic T-lymphocyte-associated antigen 4 followed by programmed cell death protein 1 (PD1) and programmed death-ligand 1 (PDL1), remove regulatory checks that enable tumors to escape immune surveillance. The antitumor efficacy observed with these agents is attributed, in particular, to their ability to unleash T-cell-mediated immunity. While immune checkpoint inhibitor treatment can result in durable responses in the segment of responsive patients, the majority of patients still progress and do not show a long-term benefit. Responsiveness also varies widely between different tumor types. Identifying complementary interventions that overcome the observed limitations of current immune checkpoint inhibitors to unleash latent immune responsiveness against tumors is clearly of the utmost importance for extending the benefits of immunotherapy to the large contingent of patients who remain poorly responsive.
A number of approaches to enhance immune checkpoint inhibitor efficacy are currently being considered, such as targeting other immune regulators or engineering T cells through chimeric antigen receptor T technology. Therapeutic vaccines are another obvious possibility despite the disappointing historical record.3 4 The success of checkpoint inhibitors suggests that a fundamental flaw in the therapeutic vaccine approach has been the inability to overcome a dominantly suppressive tumor microenvironment solely with a positive immune stimulus. Breaching the immune checkpoint barrier should provide an opportunity for directing and amplifying the tumor-directed response through vaccination. One of the key challenges for developing a vaccine is, of course, epitope selection. A great deal of attention has been directed towards identifying tumor antigens, with the most recent focus on targeting neoantigens based on individualized tumor mutation profiles.5 Possible downsides to this strategy are that the vaccines have to be specifically tailored to individual tumors and there is the possibility of mutational drift leading to tumoral immune escape.
Vaccine-based targeting of tumoral immune escape mechanisms offers the possibility of a more generalizable approach. This strategy was initially formulated around the finding, first in patients with cancer but then extended to healthy individuals, of naturally occurring, cytotoxic T cells specifically directed against the enzyme indoleamine 2,3-dioxygenase 1 (IDO1).6 7 IDO1 is a tryptophan catabolizing enzyme. However, unlike the functionally related hepatic enzyme TDO2 which exerts homeostatic control over the available tryptophan, IDO1 is implicated in moderating immune activity particularly in the context of chronic inflammation.8 IDO1 affects T-cell functionality at multiple levels, both through direct inhibition of effector T-cell function as well as induction and activation of regulatory T cells.9 The presence of a resident pool of autoreactive effector T cells directed against IDO1 prompted the hypothesis that these cells might represent a counterregulatory mechanism to dampen immunosuppression.10 This in turn suggested that an IDO1-directed therapeutic vaccine strategy might be self-reinforcing by combining a tumor targeted approach that also reduces the level of immunological protection. Based on this hypothesis, early phase clinical trials with IDO1-derived peptides have been carried out in patients with lung cancer and melanoma,11–14 and a trial combining IDO1 peptide vaccination with the anti-PD1 therapy Keytruda (KEYNOTE-764; NCT03562871) in non-small cell lung cancer (NSCLC) is currently recruiting.
An obvious challenge facing the clinical development of IDO1-derived peptide vaccines is the lack of an established precedent for effectively treating cancer with either therapeutic peptide vaccines or IDO1-targeted strategies. In particular, the failure of the ECHO-301/KEYNOTE-252 trial (NCT02752074) to demonstrate a benefit from combining the IDO1 small molecule inhibitor epacadostat with the anti-PD1 antibody pembrolizumab over pembrolizumab alone in patients with advanced melanoma argues that a deeper mechanistic understanding is vital to developing an effective treatment strategy.15 In this study, we report the identification of major histocompatibility complex (MHC) class I-directed and class II-directed, mouse IDO1-derived peptides capable of eliciting CD8+ and CD4+ T-cell-mediated antitumor responses, respectively, despite IDO1 expression being localized to infiltrating immune cells rather than tumor cells. These responses were cooperative and could be further enhanced by combining with anti-PD1 antibody administration. Analysis of complete responder mice indicated that the CD4 response remains fully IDO1-dependent while the CD8 response is mixed between IDO1-dependent and IDO1-independent components consistent with antigen spread. These findings provide important validation for the ongoing clinical development of IDO1-derived peptides as therapeutic vaccine agents, either alone or in combination with checkpoint inhibitors, and reveal important insights regarding how this approach can elicit an effective immunological antitumor response.
Materials and methods
Cell culture
Tumor-derived mouse cell lines CT26 (colorectal adenocarcinoma), 4T1 (mammary carcinoma), Pan02 (pancreatic adenocarcinoma), B16F10 (melanoma), LLC (Lewis lung carcinoma) were cultured in Dulbecco’s Modified Eagle Medium, and RENCA (renal adenocarcinoma) was cultured in RPMI 1640. Media were supplemented with penicillin, streptomycin and 10% fetal bovine serum. All cell lines were obtained from American Type Culture Collection.
Transgenic mouse strains
Female BALB/c mice were purchased from the Charles River Laboratory, Congenic BALB/c strain transgenic Rag–/– mice were obtained from the Jackson Laboratory and Ido1-/- mice were previously provided by A. Mellor.16
Tumor engraftment
CT26 cells (1×105) and RENCA cells (1×106) were suspended in 100 µL of serum free media and were injected subcutaneously in the flank of female BALB/c mice. Female C57BL/6 mice were similarly injected with B16F10, Pan02 and LLC cells (1×105). Orthotopic 4T1 mouse mammary carcinoma tumors were established by injecting 1×104 cells in the mammary fat pad of female BALB/c mice aged 5–6 week. Tumor volumes were measured by Vernier calipers.
Immunoblot analysis
Tumors were excised at 400 mm3 and immunoblot analysis on whole tumor lysates was performed as previously described.17 Epididymis lysates from wild-type (WT) and Ido1-/- male BALB/c strain mice were included as positive and negative controls for IDO1. Primary antibodies for IDO1 (clone D7Z7U) and β-tubulin (clone AA2) were purchased from Cell Signaling Technologies (Cat# 68572) and EMD Millipore (Cat# 05–661), respectively.
Peptide vaccination
Mouse IDO1-derived peptides synthesized by GenScript that were predicted to bind MHC class I or II of the H2d BALB/c mouse strain (online supplementary additional files 1-3) were selected for in vivo validation using the epitope prediction database syfpeithi (http://www.syfpeithi.de)18 to identify the highest scoring peptides. Independent confirmatory ranking of the MHC class I-directed peptides was performed using a second algorithm RANKPEP (http://imed.med.ucm.es/Tools/rankpep.html).19 Peptides were dissolved in either ultrapure water or dimethyl sulfoxide at 5 or 10 mM, depending on solvent and solubility guidance. Dissolved peptides were subsequently emulsified 1:1 v/v with the clinical grade adjuvant Montanide ISA 51 VG (36 362Z, Seppic) for a dose of 100 µg total peptide in a total volume of 100 µL. For the EP2+EP6 peptide combination, 50 µg of each peptide was used. The emulsified peptide vaccine solution was injected subcutaneously at the base of the tail of female BALB/c mice aged 7–8 weeks with a 27-gauge needle. Control mice were given water and Montanide emulsification. For prophylactic studies, a single dose of peptide vaccine was administered to mice 7 days prior to CT26 tumor cell implantation. For established tumor treatment studies, peptide vaccination was administered 1 week following tumor cell injection and once again every 2 weeks thereafter. The end point was a tumor volume of ≥1000 mm3.
jitc-2020-000605supp001.pdf (29MB, pdf)
Immunofluorescence microscopy
Sections cut from CT26 tumors frozen in optimal cutting temperature (OCT) compound were fixed with acetone and stored at −20°C. For immunofluorescence microscopy, slides were blocked with 40 µg/mL goat antimouse IgG-Fab (H+L) (Jackson ImmunoResearch) and subsequently with 10% normal goat serum (Jackson ImmunoResearch). Slides were incubated overnight at 4°C with the following primary antibodies: antimouse IDO1 (clone 4B7; Millipore), biotin antimouse PDL1 (clone 10F.9G2; BioLegend), antimouse CD45.2-FITC (clone 104; BioLegend), biotin antimouse CD11b (M1/70; BioLegend), antimouse Gr1 (clone RB6-8C5; BioLegend), antimouse CD11c-Alexafluor 488 (clone N418; BioLegend) and antimouse CD11c-FITC (N418; BioLegend).
Cell sorting
CT26 tumors were excised at 500 mm3 volume and dissociated using a gentleMACS Tissue Dissociator (Miltenyi Biotec) as per the manufacturer’s guidelines. Total CD45+ cells from the CT26 tumors were enriched by a MACS separation system with paramagnetic anti-CD45 beads (Miltenyi Biotec). Cells were stained with cell viability dye (APC-Cy7), antimouse CD45-APC (clone 30-F11; BioLegend); antimouse CD11b-PE/Cy7 (clone M1/70; BioLegend) and antimouse CD11c-FITC (N418; BioLegend). CD45+CD11b+CD11c+ and CD45+CD11b+CD11c- populations were isolated by fluorescence-activated cell sorting (BD FACSAria III), affixed to slides using a Shandon Cytospin3 and air dried. For immunofluorescence microscopy, slides were blocked with 40 µg/mL goat antimouse IgG-Fab (H+L) (Jackson ImmunoResearch) and subsequently with 10% normal goat serum (Jackson ImmunoResearch). Slides were incubated overnight at 4°C with the following primary antibodies: antimouse IDO1 (clone 4B7; Millipore) and antimouse CD19-Cy5 (eBio1D3; eBioscience).
Flow cytometry
Flow cytometric analysis of IDO1-expressing cells harvested from digested CT26 tumor samples was conducted on a BD FACSCanto (BD Biosciences) with the following antibodies as indicated: cell viability dye (APC-Cy7), antimouse CD45-APC (clone 30-F11; BioLegend); antimouse CD11b-PE/Cy7 (clone M1/70; BioLegend), antimouse Gr1-PerCP (clone RB6-8C5; BioLegend) and/or antimouse CD11c-PE (cloneN418; BioLegend). Percentage of IDO1-expressing cells (CD45+Gr1-CD11b+CD11c+) from CT26 tumors from mice treated with Montanide, peptide vaccines (EP2, EP6, EP2+EP6) or α-PD1 antibody were calculated. Analysis of T-cell populations for comparison between naïve and complete responder mice was performed by isolating total splenocytes and staining with the following antibodies: antimouse CD4-APC/Cy7 (clone GK1.5; BioLegend), antimouse CD8a-APC (clone 53–6.7; BioLegend), antimouse CD44-FITC (clone IM7; BioLegend), antimouse CD62L-PE/Cy7 (clone MEL-14; BioLegend). Analysis of markers was performed using FlowJo software.
In vivo antibody and drug treatment
InVivoPlus antimouse PD1 from BioXCell (clone: RMP1-14 Cat# BP0146) was administered by intraperitoneal injection at 10 mg/kg in 100 µL total volume twice a week. The IDO1 enzyme inhibitor epacadostat (ChemieTek Cat# CT-EPAC) was administered by oral gavage, twice a day, at 30 mg/kg in 100 µL of vehicle (3% N, N-diethylacetamide, 10% 1-hydroxypropyl-β-cyclodextrin).
Adoptive transfer
Mice that completely responded to the various treatment combinations (EP2+αPD1, EP6+αPD1 and EP2 +EP6+αPD1) were challenged twice with CT26 tumor cells (online supplementary additional file 9). Mice which rejected multiple tumor cell injections were used for adoptive transfer experiments. Spleen and inguinal lymph nodes from tumor-resistant mice were harvested. Total T cells were isolated using a MACS separation system with paramagnetic anti-CD90.2 beads (Miltenyi Biotec). CD8+ and CD4+ populations were isolated by first incubating with antimouse CD8-FITC antibody (clone 53–6.7; BioLegend) followed by antifluorescein isothiocyanate (FITC) magnetic beads (Miltenyi Biotec) to isolate CD8+ cells with the MACS system. CD4+ T cells from the flow-through were isolated similarly using antimouse CD4-FITC antibody (clone RM4-5; BioLegend) with the MACS system. Isolated total T cells were injected intravenously into female BALB/c mice (5×106/mouse) harboring CT26 tumors—injected 10 days prior to adoptive transfer. Similarly, 5×106 isolated CD4+, CD8+ cells or the combination (2.5×106 CD4+ and CD8+ cells each) were similarly injected into CT26 tumor-bearing mice. The end point was a tumor volume of ≥1000 mm3.
Statistical analysis
All reported tests are two-tailed and were considered significant at p values <0.05. Longitudinal analyses of tumor growth data were carried out over entire segments of the tumor growth curves using the online TumGrowth software program.20 Pairwise determinations across groups were adjusted according to the Holm method. All graphs were generated using Prism 7 software (GraphPad). Graphical depictions of tumor volume changes over time are presented as means±SEM computed at each time point with censoring of individual animals removed from the study after reaching the experimental end point. For experiments in which treatment resulted in complete responses in some animals, individual tumor growth curves are included for clarification of the observed changes in group means.
Results
Prophylactic IDO1-derived peptide vaccination suppresses growth of IDO1+ CT26 tumors through an adaptive immune response against host IDO1
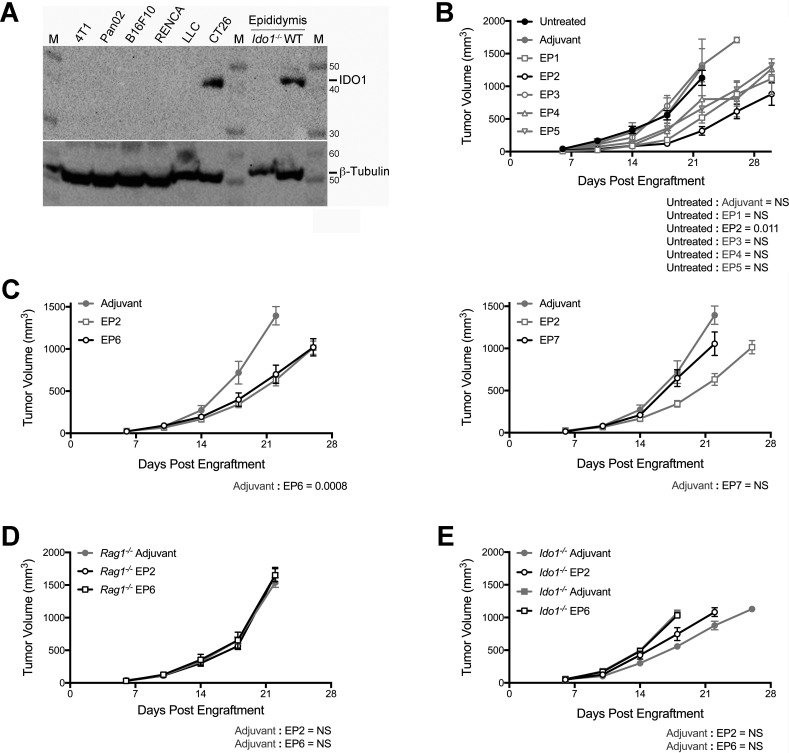
If, as identified in humans, mice harbor a subset of IDO1-reactive effector T cells, then vaccination with IDO1-derived peptides could potentially activate these T cells to mount a response against an IDO1-expressing tumor. To identify a tumor model in which to address this hypothesis, IDO1 expression was evaluated in a panel of different engrafted tumors. The highest level of IDO1 expression was observed in tumors formed by CT26 colonic carcinoma cells (figure 1A). To identify potential vaccine candidates, computer-based predictive ranking of immunogenic peptide sequences within the mouse IDO1 protein was performed. Since the CT26 cell line is of BALB/c origin, peptides optimized for binding the H2d subset of MHC class I molecules were specifically selected. Five 9-mer peptides (EP1–5) were selected for evaluation (online supplementary additional files 1-3). Prophylactic vaccination of mice with each of these five different class I-directed peptides and subsequent challenge with CT26 tumor cells 7 days later elicited a range of responses from peptide EP2 which caused the most pronounced delay in tumor outgrowth to EP3 which had no discernible impact (figure 1B).
Figure 1.
Growth delay of indoleamine 2,3-dioxygenase 1 (IDO1)-positive CT26 tumors by prophylactic vaccination with major histocompatibility complex (MHC) class I-directed or II-directed IDO1 peptides requires intact adaptive immunity and host IDO1. (A) Western blot analysis of tumors formed by six different mouse tumor-derived cell lines for comparison of IDO1 protein levels (top panel) with β-tubulin as a loading control (bottom panel). Epididymis lysates from wild-type (WT) and Ido1-/- male BALB/c strain mice were included as positive and negative controls. M designates the molecular weight marker. (B) Growth curves of CT26 tumors in mice administered single doses of five different MHC class I-directed, IDO1 peptide vaccines (EP1–5) 7 days prior to tumor engraftment. Overall responses are plotted as means±SEM together with concurrent results from both untreated and vehicle-treated animals. Growth curves for all groups are represented with gray lines except for the treatment cohort exhibiting the strongest response (EP2) and the untreated cohort which are distinguished with black lines. (n=6 tumors/cohort). (C) Growth curves of CT26 tumors in mice treated with two different MHC class II-directed, IDO1 peptide vaccines (EP6 left, EP7 right) 7 days prior to tumor engraftment. Overall responses (black lines) are plotted as means±SEM. Concurrent results from both vehicle and EP2 peptide-treated animals (gray lines) are included on each graph for comparison (n=10 tumors/cohort). (D) Growth curves of CT26 tumors in Rag1–/– mice vaccinated with the MHC class I-directed and II-directed IDO1 peptides EP2 and EP6 7 days prior to tumor engraftment. Overall responses (black lines) are plotted as means±SEM. Concurrent results from vehicle-treated animals (gray lines) are included on each graph for comparison (n≥6 tumors/cohort). (E) Growth curves of CT26 tumors in Ido1-/- mice vaccinated with EP2 and EP6 7 days prior to tumor engraftment. Overall responses (black lines) are plotted as means±SEM. Concurrent results from vehicle-treated animals (gray lines) are included on each graph for comparison (n=10 tumors/cohort). P values from longitudinal analysis of tumor growth for each peptide vaccine-treated group compared with untreated or vehicle-treated animals are included on each graph.
Two additional 15-mer peptides (EP6–7), predicted to be MHC class II-restricted (online supplementary additional files 1-3), were likewise evaluated for their impact on CT26 tumor growth following prophylactic vaccination. The responses to these two peptides were quite dissimilar, with EP6 eliciting a degree of tumor growth delay comparable to EP2, the most effective class I-directed peptide (concurrently evaluated in this experiment), while EP7 produced no discernible effect on tumor growth (figure 1C). To confirm that the observed tumor growth suppression elicited by both class I-directed and class II-directed peptides was immune-mediated, the same prophylactic vaccination protocol with the EP2 or EP6 peptides followed by CT26 tumor challenge was carried out in Rag1-nullizygous (Rag1–/–) mice. As anticipated, there was no observable effect of these peptides on tumor growth in the absence of adaptive immunity (figure 1D). To directly demonstrate that these two peptides could elicit IDO1-directed T-cell responses, ELISPOT analysis of recall responses from vaccinated animals was carried out (online supplementary additional file 4). To further confirm that the immune response was directed against IDO1 expression in the CT26 tumor cells, prophylactic vaccinations followed by CT26 tumor challenge were carried out in Ido1-nullizygous (Ido1-/-) mice. Unexpectedly, the absence of IDO1 expression in the host was sufficient to completely negate the ability of these peptides to elicit tumor growth suppression (figure 1E). Overall, these results present confirmatory evidence for the presence in mice of a latent capacity for IDO1-directed, T-cell immune responsiveness that can be stimulated by peptide vaccination directed towards either class I or class II presentation. However, further genetic characterization revealed that the relevant response is directed primarily against expression of IDO1 in the host rather than in the engrafted tumor cells.
Immunofluorescence localization of IDO1 expression predominantly within a subset of B lymphoid cells with dendritic cell characteristics infiltrating CT26 tumors
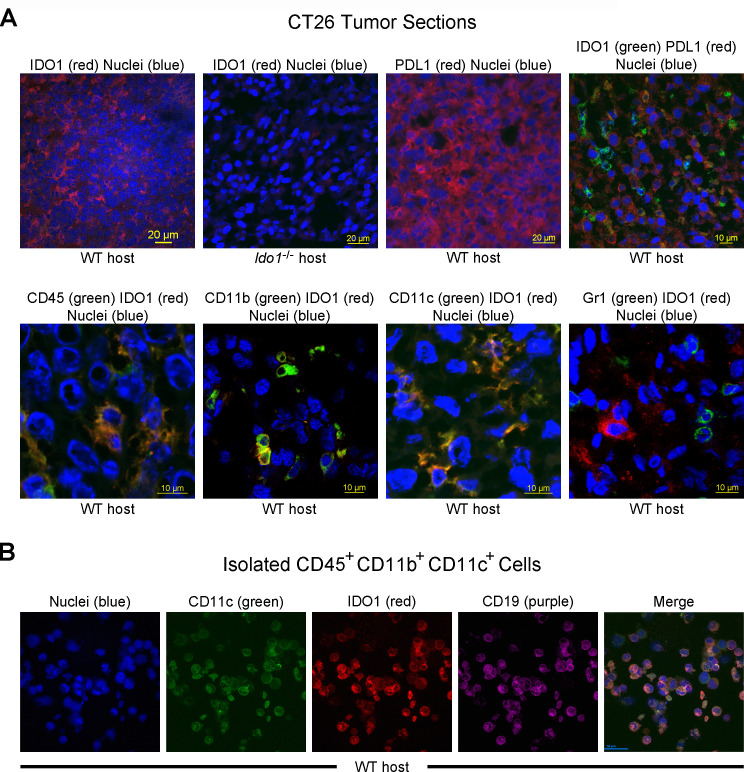
To explore why the IDO1 peptide-based vaccination response against CT26 tumors required host IDO1 expression, the cellular localization of IDO1 within these tumors was examined by immunofluorescence microscopy. Staining for IDO1 was not distributed across the tumor, as would be expected if it was expressed in the engrafted CT26 tumor cells, but rather was restricted to cell clusters and was not observed at all when the host was Ido1-nullizygous (figure 2A). The observed expression in CT26 tumor cells of another interferon (IFN)γ-regulated gene product, PDL1, provided a clear contrast. Cultured CT26 cells upregulated PDL1, but not IDO1, expression in response to IFNγ induction (online supplementary additional file 5), while in vivo CT26 tumors exhibited widespread PDL1 staining which did not appear to overlap with IDO1 (figure 2A). Instead, IDO1 staining overlapped with CD45 (figure 2A), revealing that infiltrating host immune cells are the source of the high level of IDO1 expression found in CT26 tumors. Only a subset of the total CD45+ cells in tumors were also IDO1+ and additional staining also showed apparent overlap of IDO1 expression with the cell surface markers CD11b and CD11c but not Gr1 (figure 2A).
Figure 2.
Indoleamine 2,3-dioxygenase 1 (IDO1) expression is localized to infiltrating immune cells within CT26 tumors. (A) Confocal images of CT26 tumor sections. (top row, left to right) Immunofluoresence staining of sections from wild-type (WT) and Ido1-/- mice for IDO1 (Cy3, red), and from WT mice for programmed death-ligand 1 (PDL1) (Cy3, red) and the combination of IDO1 (fluorescein isothiocyanate (FITC), green) and PDL1 (Cy3, red). Nuclei were stained on all sections (DAPI, blue). (bottom row, left to right) Staining of CT26 tumor sections from WT mice for combinations of IDO1 (Cy3, red) with CD45, CD11b, Gr1 and CD11c (FITC, green). Nuclei were stained on all sections (DAPI, blue). (B) Confocal images of a field of FACS-isolated CD45+, CD11b+, CD11c+ cells from a CT26 tumor in a WT host. (left to right) Immunofluoresence staining for nuclei (DAPI, blue), CD11c (FITC, green), IDO1 (Cy3, red), CD19 (Cy5, purple) and the composite image.
To refine the characterization of the IDO1-expressing immune cell population, dissociated CT26 tumor preparations were evaluated by flow cytometry. Because IDO1+ staining could not be adequately discriminated directly on the flow cytometer, enriched CD45+ cells, separated from IDO1– tumor cells with magnetic beads (online supplementary additional file 6A), were FACS sorted into CD11b+ CD11c+ and CD11b+ CD11c- populations (online supplementary additional file 6B) for immunofluorescence microscopy analysis based on our whole tumor staining data. As anticipated from the staining of whole tumor sections, IDO1+ staining was only observed in the subpopulation of CD11b+ cells that were also CD11c+ and not in the cells that were CD11c- (online supplementary additional file 6C). Additional staining demonstrated that within the CD45+ CD11b+ CD11c+ population, IDO1 staining coincided with staining for CD19 (figure 2B). A similar distribution of surface markers has previously been associated with an IDO1-expressing subpopulation of B lymphoid cells with dendritic cell characteristics.21 We conclude that it is infiltrating immune cells, apparently corresponding to the previously described population of IDO1+ DC-like B cells, and not the tumor cells themselves that account for the high level of IDO1 expression associated with CT26 tumors.
The class I-directed and II-directed IDO1 peptides show enhanced combinatorial antitumor activity but not with IDO1 enzyme inhibition
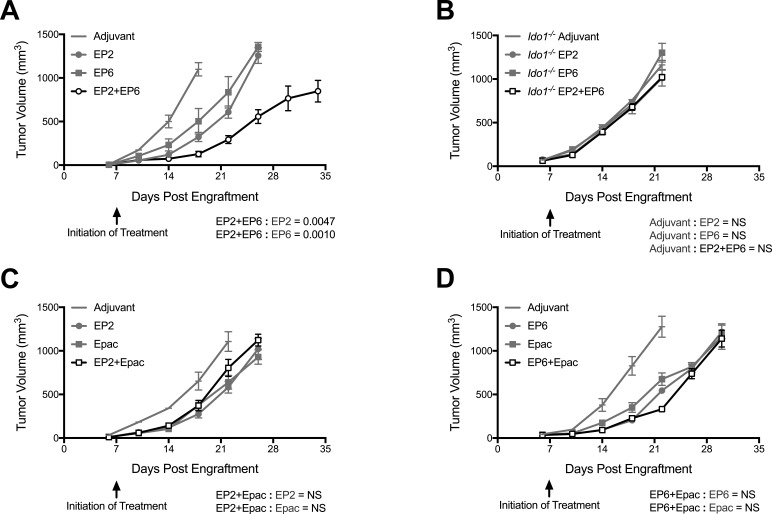
Responsiveness of established tumors to therapeutic IDO1 peptide vaccine treatment was investigated using the most effective peptides from the initial prophylactic vaccination screen. Both the class I-directed peptide (EP2) and the class II-directed peptide (EP6) elicited similar delays in the outgrowth of CT26 tumors established 1 week prior to initiating treatment, while animals treated with both peptides in combination exhibited an enhanced degree of tumor growth suppression beyond that elicited by either peptide alone (figure 3A). As in the prophylactic setting, no antitumor response was observed against tumors established in Ido1-/- mice (figure 3B). Treatment of established tumors with the small molecule IDO1 inhibitor epacadostat also resulted in tumor growth suppression, however, in contrast to the effect of combining the two peptides, combining epacadostat with either of the peptides did not result in a discernible enhancement of tumor growth suppression (figure 3C, D). These vaccination studies in tumor-bearing mice establish the important precedent that effective use of such peptide vaccines might be possible in the therapeutic setting and suggest that class I-directed and II-directed peptides might act through complementary mechanisms.
Figure 3.
Major histocompatibility complex (MHC) class I-directed and II-directed indoleamine 2,3-dioxygenase 1 (IDO1) peptides cooperate together but not with IDO1 enzyme inhibition. (A) Growth curves of CT26 tumors in wild-type (WT) mice immunized with the MHC class I-directed and II-directed peptides EP2 and EP6 either separately or together beginning 7 days after tumor engraftment. Responses to adjuvant alone, individual peptides (gray lines) and combined peptides (black lines) are plotted as means±SEM (n=8 tumors/cohort). (B) Evaluation of EP2 and EP6 both separately and in combination in Ido1-/- mice as described in A (n=8 tumors/cohort). (C) Growth curves of CT26 tumors in WT mice treated with EP2 and epacadostat either separately or together beginning 7 days after tumor engraftment. Responses to adjuvant alone, EP2 and epacadostat individually (gray lines), and combined treatment (black lines) are plotted as means±SEM (n=10 tumors/cohort). (D) Evaluation of EP6 and epacadostat both separately and in combination as described in B (n≥9 tumors/cohort). P values for longitudinal tumor growth comparisons between combined and individual peptide vaccine-treated groups are included on each graph.
The class I-directed but not class II-directed peptide shows cooperative antitumor activity with anti-PD1 antibody
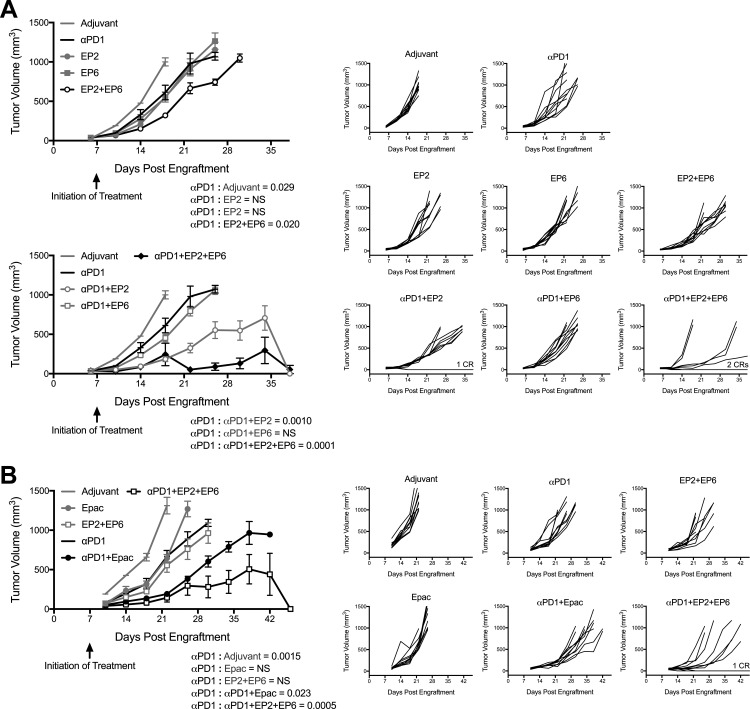
Anti-PD1 antibodies are currently at the forefront of cancer immunotherapy, with a demonstrated benefit in multiple tumor types. Our microscopy analysis of CT26 tumors showing compartmentalization of IDO1 within infiltrating immune cells and the PD1 ligand PDL1 within tumor cells, suggested that the targeting of both might produce complementary effects. To test this hypothesis, we carried out IDO1 peptide-based vaccination in combination with anti-PD1 antibody administration. Combining the class I-directed peptide EP2 with anti-PD1 produced a more pronounced suppression of CT26 tumor growth than either agent alone (figure 4A and online supplementary additional file 7A). In contrast, combining the class II-directed peptide EP6 with anti-PD1 had no additional impact on tumor growth over that of either agent alone (figure 4A and online supplementary additional file 7A). Since the impact of treatment with the combination of class I-directed and II-directed peptides on tumor growth was similarly cooperative, we also evaluated the response to treatment with both peptides together with anti-PD1. Combining all three agents (EP2+EP6+anti-PD1) elicited the most pronounced effect on tumor growth suppression among the different treatment groups (figure 4A and online supplementary additional file 7A, C). Notably, it has only been by combining anti-PD1 antibody with IDO1 peptide vaccination that we have observed complete responders, that is mice for which treatment resulted in the complete elimination of both tumors. In this experiment, complete responders were observed in the anti-PD1+EP2-treated (one of five mice) and anti-PD1+EP2+EP6-treated (two of five mice) cohorts (figure 4A).
Figure 4.
Programmed cell death protein 1-binding antibody (anti-PD1 antibody) cooperativity with major histocompatibility complex (MHC) class I-directed and II-directed indoleamine 2,3-dioxygenase 1 (IDO1) peptides compared with epacadostat. (A) Growth curves of CT26 tumors in wild-type (WT) mice receiving the MHC class I-directed and II-directed peptides EP2 and EP6 either separately or together with or without the anti-PD1 antibody beginning 7 days after tumor engraftment. The experiment is divided between two graphs for clarity. (top left) Responses to adjuvant alone, individual peptides or anti-PD1 alone (gray lines), and the combined peptides (black lines), are plotted as means±SEM (n=10 tumors/cohort). (bottom left) Responses to adjuvant alone, anti-PD1 alone or with the individual peptides (gray lines), and anti-PD1 with the combined peptides (black lines) are plotted as means±SEM (n=10 tumors/cohort). (B) Growth curves of CT26 tumors in WT mice treated with the combination of EP2+EP6 or the IDO1 inhibitor epacadostat either without or with anti-PD1 antibody beginning 7 days after tumor engraftment. (left side) Responses to adjuvant alone, epacadostat, anti-PD1 or EP2+EP6 individually (gray lines), and combinations of epacadostat or EP2+EP6 with anti-PD1 (black lines) are plotted as means±SEM (n=10 tumors/cohort). P values for longitudinal tumor growth comparisons between the anti-PD1 and other treatment groups are included on each graph. P values from additional pairwise determinations are shown in online supplementary additional file 4. (right sides (all)) Individual growth curves for each treatment condition (X-axis is set at −100 on the Y-axis). In groups with complete responders (CRs), the number of animals represented is indicated on the graph.
To determine how the biological response to immunizing against IDO1 compared with inhibiting its enzymatic activity, we compared EP2+EP6 vaccination to epacadostat administration either without or in combination with anti-PD1. Epacadostat treatment produced a tumor growth suppressive effect comparable to that of anti-PD1 treatment (figure 4B and online supplementary additional file 7B). When combined, epacadostat+anti-PD1 did show an enhanced degree of tumor growth suppression over either agent alone but the combination of EP2+EP6+anti-PD1 was even more pronounced in this effect (figure 4B and online supplementary additional file 7B, C). Furthermore, unlike the EP2+EP6+anti-PD1-treated group, no complete responses were observed in the epacadostat+anti-PD1-treated cohort, although the number of mice evaluated was too small to confidently rule out the possibility that complete responses might occur. These data confirm that cooperative antitumor effects can be produced by targeting both IDO1 and PD1 in this model, with the combination of vaccination against IDO1 apparently producing an even more robust antitumor response than blocking the activity of the enzyme itself.
IDO1 levels are reduced in the tumor infiltrating immune cells of mice administered IDO1 peptides
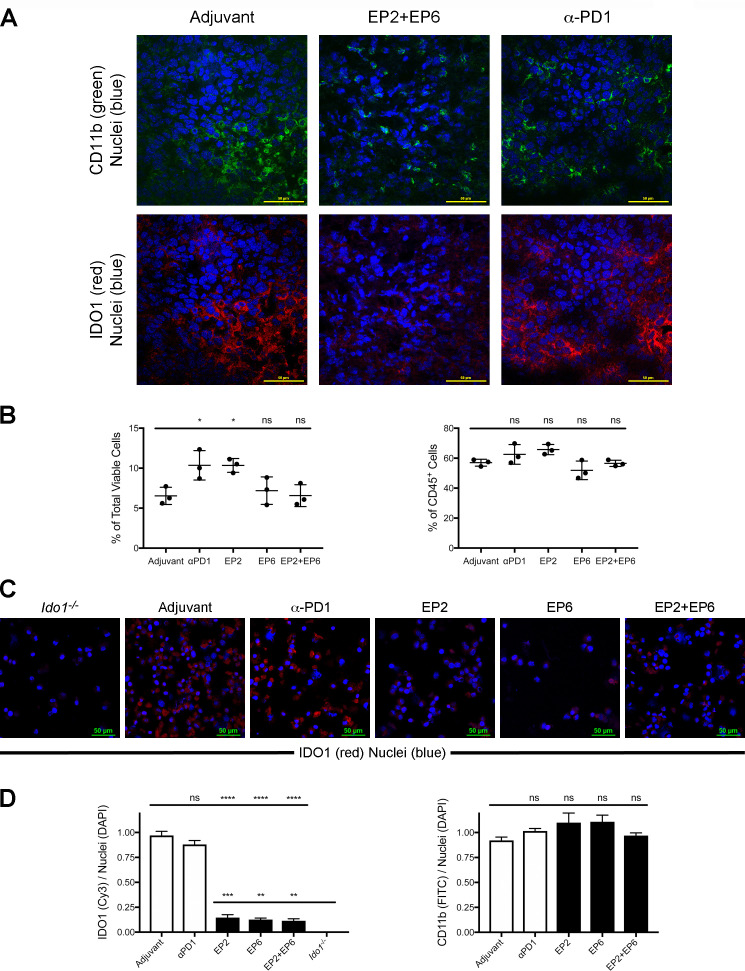
Based on our determination that IDO1 expression is localized to a specific subset of infiltrating immune cells within the CT26 tumors, we evaluated the impact of IDO1 peptide vaccine treatment on these cells. In response to EP2+EP6 treatment, IDO1 staining within tumors was markedly reduced while CD11b, one of the markers of the IDO1-expressing infiltrating immune cells, was not significantly affected (figure 5A). This contrasts with anti-PD1 antibody treatment, which caused a similar reduction in tumor growth rate as EP2+EP6 treatment without a noticeable effect on IDO1 staining (figure 5A). Flow cytometry analysis of the IDO1-expessing CD45+ CD11b+ CD11c+ population within dissociated tumors revealed no evidence that vaccine treatment resulted in a significant decrease in the proportion of the these cells relative to the overall number of cells or the number of infiltrating immune cells within tumors (figure 5B and online supplementary additional file 8). Rather, when these cells were isolated and evaluated for IDO1 expression, it was apparent that the level of IDO1 expression in the cells obtained from vaccine-treated animals was significantly lower than in cells from either vehicle or anti-PD1-treated animals, although still significantly higher than in cells from Ido1-/- animals (figure 5C, D). These results indicate that the effect of administering either the class I-directed or II-directed IDO1 peptides is to reduce IDO1 expression within the tumor without demonstrably reducing the population of infiltrating DC-like B cells that express IDO1.
Figure 5.
Major histocompatibility complex (MHC) class I-directed and II-directed indoleamine 2,3-dioxygenase 1 (IDO1) peptide administration does not reduce the proportional representation of IDO1-expressing tumor infiltrating immune cells but does reduce their expression of IDO1. (A) Confocal images of CT26 tumor sections from wild-type (WT) mice administered either adjuvant alone, EP2+EP6 IDO1 peptides or antiprogrammed cell death protein 1 (anti-PD1) antibody as noted. Immunofluoresence staining for CD11b (fluorescein isothiocyanate (FITC), green), IDO1 (Cy3, red) and nuclei (DAPI, blue) was performed on all sections. (B) Quantitative comparison of the proportional representation of the CD11bhi CD11chi (IDO1-expressing) subset following administration of adjuvant alone, anti-PD1 and the individual EP2 or EP6 peptides alone or combined identified by flow cytometry as shown in online supplementary additional file 8 from (left) within the total population of viable cells from dissociated tumors and (right) within the CD45+ population of tumor-infiltrating immune cells. Graphed as means±SEM with significance determined by one-way analysis of variance (ANOVA) with Tukey’s multiple comparison test. (C) Confocal images of a field of FACS-isolated CD45+, CD11b+, CD11c+ cells from CT26 tumors obtained from an Ido1-/- host or from WT hosts administered either adjuvant alone, EP2, EP6 or EP2+EP6 IDO1 peptides, or anti-PD1 antibody as noted. Immunofluoresence staining for IDO1 (Cy3, red) and nuclei (DAPI, blue) was evaluated on all sections. (D) Quantitative comparison of IDO1 (Cy3)/nuclei (DAPI) (left) and CD11b (FITC)/nuclei (DAPI) (right) staining per field from FACS-isolated CD45+, CD11b+, CD11c+ cells from treatment groups described in (D). Graphed as means±SEM with significance determined by one-way ANOVA with Tukey’s multiple comparison test. ns, not significant. *P<0.05;**p<0.01; ***p<0.001, ****p<0.0001.
Class I-directed and II-directed IDO1 peptides elicit durable CD8-mediated and CD4-mediated antitumor responses
Complete responses resulting from IDO1 peptide+anti-PD1 antibody treatments were found to be durable and subsequent challenges with CT26 tumor cells failed to grow, consistent with these animals having developed immunological resistance (online supplementary additional file 9). Complete responder animals treated with EP2+EP6+anti-PD1 exhibited significant twofold to fourfold increases in splenic CD44hi CD62Lhi (activated) and CD44hi CD62Llo (memory) T cells compared with naïve mice in both the CD4+ and CD8+ compartments (figure 6A–C and online supplementary additional file 10). This observed expansion, especially of the memory T-cell populations, is consistent with the heightened responsiveness of the complete responders to tumor rechallenge. Cytokine profiling of isolated splenic T cells showed evidence of mixed inflammatory cytokine polarization for the complete responder animals compared with naïve controls. CD4+ cells, in particular, exhibited increased induction of interleukin (IL)17a (significant), IL6 and IFNγ (trending) and decreased induction of IL4, tumor necrosis factor (TNF)-α (significant), IL10 and IL2 (trending) while CD8+ cells from both naïve and complete responder animals exhibited markedly lower levels of overall cytokine induction with the exception of TNFα (online supplementary additional file 11). Together, these data suggest that the durable antitumor response elicited in some EP2+EP6+anti-PD1-treated animals is due to its ability to induce T-cell memory.
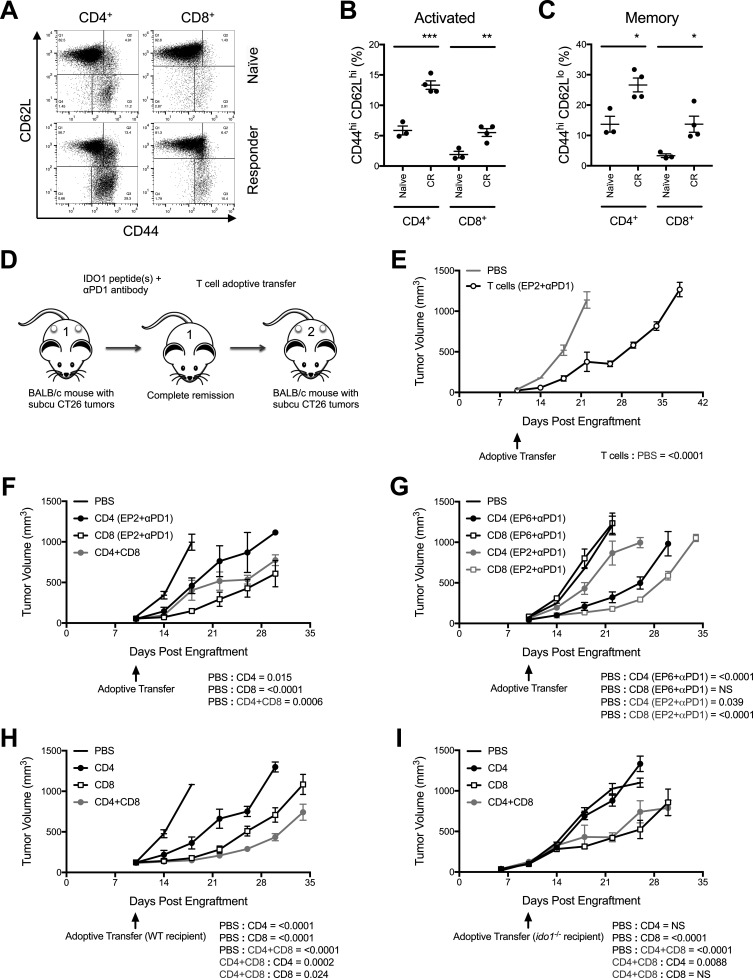
Figure 6.
Major histocompatibility complex (MHC) class I-directed and II-directed responses are predominantly mediated by CD8+ and CD4+ T cells, respectively. (A) Flow cytometry characterization of splenic T-cell populations from a complete responder (CR) mouse (bottom panels) compared with a naïve mouse (top panels). CD4+ CD8- (left panels) and CD8+ CD4- (right panels) gated populations assessed for levels of CD44 (X-axis) and CD62L (Y-axis). (B) Quantitative comparison of the proportional representation of CD44hi CD62Lhi (activated) populations of CD4+ and CD8+ T cells in the spleens of CR mice relative to naïve mice identified by flow cytometry analysis as shown in (E). Graphed as means±SEM with significance determined by two-tailed Student’s t-test (n≥3 mice/group). (C) Quantitative comparison of the proportional representation of CD44hi CD62Llo (memory) populations as described for B. (D) Schematic illustration of the basic strategy for adoptive transfer of T cells from IDO1 peptide(s)+programmed cell death protein 1-binding antibody (anti-PD1 antibody)-treated, CR mice to treatment naïve, tumor-bearing mice. (E) Growth curves of CT26 tumors in WT mice that received T cells isolated 10 days following tumor rechallenge from a CR mouse previously treated with anti-PD1 antibody and the MHC class I-directed IDO1 peptide EP2. Responses to PBS (gray line) and T cells (black line) are plotted as means±SEM (n=8 tumors/cohort). (F) Growth curves of CT26 tumors in WT mice that received flow cytometry sorted T-cell subsets isolated 10 days following tumor rechallenge from a CR mouse treated with anti-PD1 and EP2. Responses to PBS, CD4+, CD8+ (black lines), and combined CD4+ and CD8+ T cells (gray line) are plotted as means±SEM (n=4 tumors/cohort). (G) Growth curves of CT26 tumors in WT mice that received flow cytometry sorted T-cell subsets isolated 10 days following tumor rechallenge from CR mice treated with either anti-PD1 and the MHC class II-directed IDO1 peptide EP6 or anti-PD1 and EP2. Responses to PBS, CD4+ and CD8+ T cells from EP6+anti-PD1 (black lines) or EP2+anti-PD1 (gray lines) treated donors are plotted as means±SEM (n=6 tumors/cohort). (H) Growth curves of CT26 tumors in WT mice that received flow cytometry sorted T-cell subsets isolated 10 days following tumor rechallenge from a CR mouse treated with anti-PD1 and EP2+EP6. Responses to PBS, CD4+, CD8+ (black lines) and combined CD4+ and CD8+ T cells (gray line) are plotted as means±SEM (n=6 tumors/cohort). (I) As described above in G except the recipient mice were Ido1-/- (n=6 tumors/cohort). P values for longitudinal tumor growth comparisons between the PBS (no cells) and different T-cell adoptive transfer cohorts are included on graphs E–I. P values for comparisons between the combined and individual CD4+ and CD8+ adoptive transfer cohorts are also included for graphs H and I. *P<0.05;**p<0.01; ***p<0.001.
To directly test if the acquisition of CT26 tumor resistance was due to the induction of T-cell memory, a series of adoptive transfer experiments were performed (figure 6D). Transferring T cells isolated from spleens of complete responders previously treated with EP2+anti-PD1 to treatment-naïve, CT26 tumor-bearing mice resulted in tumor growth suppression (figure 6E) consistent with the expectation that the antitumor activity observed in response to IDO1 peptide-based vaccination is T-cell-mediated. To further refine the subset of T cells responsible for the antitumor response, isolated CD4+ and CD8+ T-cell populations from EP2+anti-PD1-treated complete responders were transferred. Most of the capacity to suppress tumor growth was associated with the CD8+ T-cell population (figure 6F). A less pronounced effect was observed with CD4+ cells and combining CD4+ cells with CD8+ cells did not demonstrably improve the response relative to that achieved with CD8+ cells alone (figure 6F). These findings are consistent with the predicted class I-restricted binding of the EP2 peptide to which the responding cells would be expected to be CD8+ CTLs. Conversely, the class II-directed EP6 peptide should signal to MHC class II-responsive CD4+ helper T cells. Although occurring less frequently than with anti-PD1+EP2, we were able to identify an anti-PD1+EP6-treated complete responder with which to address this hypothesis. When transferred to treatment-naïve, CT26 tumor-bearing mice, it was the CD4+ cells in this instance that suppressed tumor growth while the CD8+ cells were ineffectual (figure 6G). These data are consistent with the specific MHC binding restriction predicted for both of these peptides, and indicate that eliciting CD4+ as well as CD8+ T-cell responses with IDO1 peptide vaccines may provide potential treatment benefits.
Responder mice develop both IDO1-directed as well as IDO1-independent antitumor T-cell responses
The greatest degree of tumor growth suppression was observed by combining both the EP2 and EP6 peptides together with the anti-PD1 antibody, suggesting complementary mechanisms. To examine the immunological basis for this complementarity, isolated CD4+ and CD8+ T cells from EP2+EP6+anti-PD1 complete responders were adoptively transferred to treatment-naïve, CT26 tumor-bearing mice. Under these conditions, both T-cell subsets elicited tumor growth suppression, although the CD8+ cells were more effective (figure 6H). Furthermore, combining CD4+ and CD8+ cells further enhanced the degree of tumor growth suppression (figure 6H), consistent with the CD4+-mediated and CD8+-mediated effects being non-redundant. To assess how important the IDO1-directed response is to antitumor activity, isolated CD4+ and CD8+ T cells from EP2+EP6+anti-PD1-treated, WT complete responders were adoptively transferred to treatment-naïve Ido1-/- mice with established CT26 tumors. In this context, where IDO1 was absent in the host, the transfer of CD4+ cells resulted in no evidence of tumor growth suppression when compared with mice that did not receive any T cells (figure 6I). CD8+ cells, on the other hand, retained the ability to elicit a clear tumor growth suppressive effect, although this did not become apparent until approximately 1 week later than was observed in WT mice (figure 6I). Transferring CD4+ together with CD8+ cells into Ido1-/- animals did not produce an enhanced antitumor response (figure 6I), unlike what was observed in WT recipient animals. These data indicate that the CD4-elicited response against the class II-directed peptide EP6 is entirely dependent on host expression of IDO1 in this model while the CD8-elicited response directed against the class I-directed peptide is more complex, being initially directed entirely against host IDO1 but subsequently developing an IDO1-independent component in the complete responders that contributes to the overall antitumor effect.
Discussion
Enthusiasm for cancer immunotherapy has been stoked by the successes of immune checkpoint inhibitors. This has included a renewed interest in meeting the challenge of developing effective therapeutic cancer vaccines. Immunization against infections is the oldest form of immune-based intervention, far predating Edward Jenner’s cowpox vaccination experiments in the 1700s.22 Infection by a wide variety of foreign pathogens can now be controlled through the use of vaccines, but cancer, which arises from random mutational events as a disease of altered self, has been much less amenable to this approach. Furthermore, it is clear that while cancers possess abnormal neoantigens, they also foster a state of dominant immune tolerance, likely an important factor in the widespread failure of the many vaccines designed to activate an antitumor immune response. It has been surmised that this hurdle to effective cancer vaccine development might be surmounted by vaccinating against key immunoregulatory components of the tumor microenvironment such as IDO1.23 In this study, we describe the development of a mouse model to explore this concept. Using this model, we have demonstrated that resident IDO1-reactive T cells are indeed present in the mouse, as was found in humans, and that in an established tumor setting these T cells can be stimulated by peptide vaccination to promote an effective, IDO1-directed immune response.
With regard to human tumors, it remains to be fully resolved where IDO1 expression is most relevant, directly in the tumor cells or in associated stromal cells,24 and the answer may involve a complex mix of both compartments that may also vary between tumors. While IDO1 is readily inducible in a variety of human tumor cell lines, mouse cell lines have proven to be much more refractory to IDO1 expression. To ensure the presence of the vaccine target, our initial choice of a model system was one in which the engrafted CT26 tumors were known to express high levels of IDO1, reportedly within the tumor cells.25 Unexpectedly, however, our initial findings from vaccinating mice with various peptides were indicative of the antitumor response being directed against IDO1 expressed not in the tumor cells but in the host. Further examination of the CT26 tumors by immunofluorescence microscopy confirmed that IDO1 was expressed in a specific subset of infiltrating immune cells. These IDO1+ cells exhibited a surface marker profile corresponding to a splenic population of B lymphoid cells with dendritic cell attributes previously identified as expressing IDO1.2121 While a different model will be required to study vaccine treatment in the context of intratumoral IDO1, the current studies establish the fundamental precedent that the expression of IDO1 directly within the cells of a tumor is not a prerequisite for mounting an effective IDO1-directed therapeutic antitumor vaccine response. This finding has important ramifications for the clinical development of IDO1-based vaccines as it expands the range of tumors for which this therapeutic approach may be considered. Furthermore, it mitigates concern of that target antigen presentation will be circumvented as a consequence of low initial levels of class I expression or subsequent selective pressure to reduce antigen presentation on the tumor cells.
The lack of IDO1 expression directly in the tumor cells provides a rationale as to why no enhanced cooperativity between IDO1 peptide vaccination and IDO1 enzyme inhibition was observed, since T-cell responses directed against the IDO1-expressing, infiltrating immune cells and inhibition of their immunosuppressive IDO1 activity may act redundantly. In contrast, administration of an antibody against PD1 demonstrated a clear enhanced combinatorial effect with class I-directed IDO1 peptide vaccination, an outcome that is consistent with the distinct compartmentalization of the corresponding PD1 ligand, PDL1, in the tumor cells and IDO1 in the infiltrating immune cells. The observed cooperativity between anti-PD1 and the class I-directed but not the class II-directed IDO1 peptide suggests that there may be more redundancy between the impact of targeting the PD1/PDL1 interaction and the CD4+ T helper cell-mediated aspect of the IDO1 peptide vaccine response than with the CD8+ T effector cell aspect. However, the further efficacy enhancement achieved with the triple combination suggests that the benefits of combining the class I and class II peptides and the class I peptide with anti-PD1 are not entirely redundant.
A general concern in considering the use of anti-PD1 antibody in combination with any IDO1-directed therapy is the failure of the phase 3 ECHO-301/KEYNOTE-252 trial (NCT02752074) in which treatment of patients with advanced stage melanoma with a combination of epacodostat+pembrolizumab produced no additional survival benefit when compared with pembrolizumab treatment alone.15 Similar to previous reports in B16 mouse melanoma models,26 27 treatment with the combination of epacadostat+anti-PD1 antibody produced cooperative suppression of tumor growth in the CT26 model, indicative of a higher degree of responsiveness in this experimental model than in the clinical setting. Compared with the inhibitor, the IDO1 peptide vaccine+anti-PD1 antibody combination treatment response appeared more pronounced and produced complete responses not observed in the epacadostat+anti-PD1 treatment arm, indicative of IDO1 peptide vaccination eliciting a more robust immunological response than IDO1 inhibition. Detailed comparative analysis of the immunological underpinnings of the antitumor responses elicited by IDO1 peptide vaccination versus IDO1 inhibition will be required to mechanistically clarify the differences between these two types of IDO1-directed intervention.
Complete elimination of pre-established tumors was most effectively achieved when IDO1-derived, MHC class II as well class I peptide vaccination was combined with anti-PD1 antibody administration. These represented durable responses as the original tumors failed to re-emerge. Furthermore, the complete responder animals were fully protected against subsequent re-challenge with CT26 tumor cells. This outcome bolsters the expectation that IDO1-based vaccines directed at stimulating CD8+ and CD4+ T-cell responses can improve patient responses to immune checkpoint blocking antibody therapy, and these elements are incorporated in the ongoing clinical trials in patients with NSCLC of IDO1 peptide vaccines in combination with the anti-PD1 antibody pembrolizumab with or without chemotherapy (NCT03562871) and in patients with metastatic melanoma in combination with the anti-PD1 antibody nivolumab and a PD-L1-based vaccine (NCT03047928).
Unlike current vaccine approaches that direct CD8+ cytotoxic T cells against tumor-specific antigens (eg, neoantigens), the IDO1-targeted vaccine approach was conceived of as a means to undermine the tumor-promoting immune suppression associated with chronic inflammation,23 28 where IDO1 is an integral player.29 30 This study provides direct evidence in support of the fundamental re-envisaging of tumor vaccines that targeting IDO1 entails, beyond conventional neoantigens given that T-cell-mediated antitumor responses were generated against WT IDO1 epitopes not expressed by the tumor cells, and beyond just CD8+ T cells given that a distinct CD4+ T-cell-mediated antitumor response, which complemented the CD8+ T-cell response, could be generated. Expression of IDO1 is highly regulated and immunosuppressive IDO1+ cells may be reverted into immune competent cells by shifting the inflammatory stimulus.31 In this regard, it is intriguing that neither the MHC class I-directed nor II-directed IDO1 peptides caused apparent reductions in the number of IDO1-expressing immune cells within the tumors of treated animals, but instead significantly decreased IDO1 expression levels within these cells. This outcome was particularly unexpected for the class I-directed peptide, as the CD8+ T cells it elicits had been expected to act through IDO1-targeted cell depletion. Instead, both IDO1-directed CD4+ and CD8+ T cells may contribute to IDO1-targeted cell reprogramming, although the cooperativity consistently observed by combining the two peptides indicates that there are complementary differences in their mechanisms of action that remain to be uncovered.
Ironically, this unconventional cancer vaccine strategy may ultimately segue into the realm of conventional tumor vaccines given that a component of CD8+ T-cell response present in the complete responder animals was shown in adoptive transfer experiments to be IDO1-independent. This indication of antigen spread32 suggests that the class I-directed IDO1 peptide vaccination may be fostering the development of CD8+ T-cell responses against tumor neoantigens. Although this interpretation of the data remains speculative, it suggests an avenue for investigating the dynamics of developing a broader antitumor immune response than is directly elicited by the IDO1 peptide vaccine and how this might contribute to the overall efficacy of the response. With proof of concept for IDO1 peptide vaccines now established, this preclinical model system should provide a valuable resource for gaining further insight into the immunological basis of the antitumor response in order to rationally optimize treatment design and analysis.
Footnotes
Contributors: Conceptualization: AJM, AWP, MHA, M-BZ. Methodology: SD, AJM, AWP, JBDH, ES-W, KLK, AM, LMFM, LM-N. Investigation: SD, JBDH, ES-W, KLK, AM, DG, IL, LMFM. Writing—original draft: AJM, SD. Writing—review and editing: AJM, AWP, MHA, SD, M-BZ, KLK, JBDH, LM-N. Funding acquisition: AJM, M-BZ, MHA. Supervision: AJM, AWP, M-BZ, MHA.
Funding: The work was supported through a sponsored research agreement with IO Biotech (AJM), with additional laboratory support through NIH grant R01 CA191191 and the Lankenau Medical Center Foundation and Main Line Health (AJM).
Competing interests: IO Biotech, which provided financial support for this project, is conducting IDO1 peptide vaccine-based clinical trials. KLK, IL and AWP are employed by IO Biotech, M-BZ is a Founder, Chief Executive Officer and a shareholder in IO Biotech, MHA is a Founder, Chief Scientific Officer and shareholder in IO Biotech and AJM is a Scientific Advisory Board member and shareholder in IO Biotech.
Patient consent for publication: Not required.
Ethics approval: All studies involving mice were approved by the Lankenau Institute for Medical Research IACUC and conform with AALAC guidelines.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1.Coley WB. The treatment of malignant tumors by repeated inoculations of erysipelas. with a report of ten original cases. 1893. Clin Orthop Relat Res 1991;105:487–511. [PubMed] [Google Scholar]
- 2.Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov 2018;8:1069–86. 10.1158/2159-8290.CD-18-0367 [DOI] [PubMed] [Google Scholar]
- 3.Eggermont AMM. Therapeutic vaccines in solid tumours: can they be harmful? Eur J Cancer 2009;45:2087–90. 10.1016/j.ejca.2009.05.004 [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med 2004;10:909–15. 10.1038/nm1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yarchoan M, Johnson BA, Lutz ER, et al. Targeting neoantigens to augment antitumour immunity. Nat Rev Cancer 2017;17:209–22. 10.1038/nrc.2016.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sørensen RB, Berge-Hansen L, Junker N, et al. The immune system strikes back: cellular immune responses against indoleamine 2,3-dioxygenase. PLoS One 2009;4:e6910. 10.1371/journal.pone.0006910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sørensen RB, Hadrup SR, Svane IM, et al. Indoleamine 2,3-dioxygenase specific, cytotoxic T cells as immune regulators. Blood 2011;117:2200–10. 10.1182/blood-2010-06-288498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prendergast GC, Smith C, Thomas S, et al. Indoleamine 2,3-dioxygenase pathways of pathogenic inflammation and immune escape in cancer. Cancer Immunol Immunother 2014;63:721–35. 10.1007/s00262-014-1549-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munn DH, Mellor AL. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol 2013;34:137–43. 10.1016/j.it.2012.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andersen MH. Immune regulation by self-recognition: novel possibilities for anticancer immunotherapy. J Natl Cancer Inst 2015;107:djv154. 10.1093/jnci/djv154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bjoern J, Iversen TZ, Nitschke NJ, et al. Safety, immune and clinical responses in metastatic melanoma patients vaccinated with a long peptide derived from indoleamine 2,3-dioxygenase in combination with ipilimumab. Cytotherapy 2016;18:1043–55. 10.1016/j.jcyt.2016.05.010 [DOI] [PubMed] [Google Scholar]
- 12.Iversen TZ, Engell-Noerregaard L, Ellebaek E, et al. Long-lasting disease stabilization in the absence of toxicity in metastatic lung cancer patients vaccinated with an epitope derived from indoleamine 2,3 dioxygenase. Clin Cancer Res 2014;20:221–32. 10.1158/1078-0432.CCR-13-1560 [DOI] [PubMed] [Google Scholar]
- 13.Kjeldsen JW, Iversen TZ, Engell-Noerregaard L, et al. Durable clinical responses and long-term follow-up of stage III-IV non-small-cell lung cancer (NSCLC) patients treated with IDO peptide vaccine in a phase I Study-A brief research report. Front Immunol 2018;9:2145. 10.3389/fimmu.2018.02145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nitschke NJ, Bjoern J, Iversen TZ, et al. Indoleamine 2,3-dioxygenase and survivin peptide vaccine combined with temozolomide in metastatic melanoma. Stem Cell Investig 2017;4:77. 10.21037/sci.2017.08.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muller AJ, Manfredi MG, Zakharia Y, et al. Inhibiting IDO pathways to treat cancer: lessons from the ECHO-301 trial and beyond. Semin Immunopathol 2019;41:41–8. 10.1007/s00281-018-0702-0 [DOI] [PubMed] [Google Scholar]
- 16.Smith C, Chang MY, Parker KH, et al. Ido is a nodal pathogenic driver of lung cancer and metastasis development. Cancer Discov 2012;2:722–35. 10.1158/2159-8290.CD-12-0014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muller AJ, DuHadaway JB, Jaller D, et al. Immunotherapeutic suppression of indoleamine 2,3-dioxygenase and tumor growth with ethyl pyruvate. Cancer Res 2010;70:1845–53. 10.1158/0008-5472.CAN-09-3613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rammensee HG, Friede T, Stevanoviíc S. MHC ligands and peptide motifs: first listing. Immunogenetics 1995;41:178–228. 10.1007/BF00172063 [DOI] [PubMed] [Google Scholar]
- 19.Reche PA, Glutting J-P, Reinherz EL. Prediction of MHC class I binding peptides using profile motifs. Hum Immunol 2002;63:701–9. 10.1016/S0198-8859(02)00432-9 [DOI] [PubMed] [Google Scholar]
- 20.Enot DP, Vacchelli E, Jacquelot N, et al. TumGrowth: an open-access web tool for the statistical analysis of tumor growth curves. Oncoimmunology 2018;7:e1462431. 10.1080/2162402X.2018.1462431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson BA, Kahler DJ, Baban B, et al. B-lymphoid cells with attributes of dendritic cells regulate T cells via indoleamine 2,3-dioxygenase. Proc Natl Acad Sci U S A 2010;107:10644–8. 10.1073/pnas.0914347107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riedel S. Edward Jenner and the history of smallpox and vaccination. Proc 2005;18:21–5. 10.1080/08998280.2005.11928028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pedersen AW, Kopp KL, Andersen MH, et al. Immunoregulatory antigens-novel targets for cancer immunotherapy. Chin Clin Oncol 2018;7:19. 10.21037/cco.2018.01.03 [DOI] [PubMed] [Google Scholar]
- 24.Munn DH, Mellor AL. Ido in the tumor microenvironment: inflammation, counter-regulation, and tolerance. Trends Immunol 2016;37:193–207. 10.1016/j.it.2016.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koblish HK, Hansbury MJ, Bowman KJ, et al. Hydroxyamidine inhibitors of indoleamine-2,3-dioxygenase potently suppress systemic tryptophan catabolism and the growth of IDO-expressing tumors. Mol Cancer Ther 2010;9:489–98. 10.1158/1535-7163.MCT-09-0628 [DOI] [PubMed] [Google Scholar]
- 26.Holmgaard RB, Zamarin D, Munn DH, et al. Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. J Exp Med 2013;210:1389–402. 10.1084/jem.20130066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spranger S, Koblish HK, Horton B, et al. Mechanism of tumor rejection with doublets of CTLA-4, PD-1/PD-L1, or IDO blockade involves restored IL-2 production and proliferation of CD8+ T cells directly within the tumor microenvironment. J Immunother Cancer 2014;2:3–14. 10.1186/2051-1426-2-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andersen MH. The T-win® technology: immune-modulating vaccines. Semin Immunopathol 2019;41:87–95. 10.1007/s00281-018-0695-8 [DOI] [PubMed] [Google Scholar]
- 29.Muller AJ, Sharma MD, Chandler PR, et al. Chronic inflammation that facilitates tumor progression creates local immune suppression by inducing indoleamine 2,3 dioxygenase. Proc Natl Acad Sci U S A 2008;105:17073–8. 10.1073/pnas.0806173105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prendergast GC, Mondal A, Dey S, et al. Inflammatory Reprogramming with IDO1 Inhibitors: Turning Immunologically Unresponsive 'Cold' Tumors 'Hot'. Trends Cancer 2018;4:38–58. 10.1016/j.trecan.2017.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grohmann U, Bianchi R, Orabona C, et al. Functional plasticity of dendritic cell subsets as mediated by CD40 versus B7 activation. J Immunol 2003;171:2581–7. 10.4049/jimmunol.171.5.2581 [DOI] [PubMed] [Google Scholar]
- 32.Gulley JL, Madan RA, Pachynski R, et al. Role of antigen spread and distinctive characteristics of immunotherapy in cancer treatment. J Natl Cancer Inst 2017;109:djw261. 10.1093/jnci/djw261 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2020-000605supp001.pdf (29MB, pdf)