Abstract
This protocol describes a method based on iodine and a base as mild coupling reagents to synthetize deoxyribonucleic guanidines (DNGs)—oligodeoxynucleotide analogues with a guanidine backbone. DNGs display unique properties, such as high cellular uptake with low toxicity and increased stability against nuclease degradation but have been impeded in their development by the requirement for toxic and iterative manual synthesis protocols. The novel synthesis method reported here eliminates the need for toxic mercuric chloride and pungent thiophenol critical to previous DNG synthesis methods and translates their synthesis to a MerMade™ 12 automated oligonucleotide synthesizer. This method can be used to synthesize DNG strands up to 20 bases in length, along with 5’-DNG-DNA-3’ chimeras, at 1–5 μmol scales in a fully automated manner. We also present detailed and accessible instructions to adapt a MerMade™ 12 oligonucleotide synthesizer to enable the parallel synthesis of DNG and DNA/RNA oligonucleotides. Since DNG linkages alter the overall charge of the oligonucleotides, we also describe purification strategies to generate oligonucleotides with varying lengths and numbers of DNGs, based on extraction or preparative scale gel electrophoresis, along with methods to characterize the final products. Overall, this article provides an overview of the synthesis, purification, and handling of DNGs and mixed charge DNG-DNA oligonucleotides.
Basic Protocol 1: Preparation of a MerMade™ synthesizer for guanidine couplings
Basic Protocol 2: Synthesis of DNG strands on a MerMade™ synthesizer
Basic Protocol 3: Purification of DNG strands using preparative acetic acid urea (AU) PAGE
Basic Protocol 4: Characterization of DNG strands using MALDI-MS
Basic Protocol 5: Characterization of DNG strands using AU PAGE
Support Protocol 1: Synthesis of initiator-functionalized CPG
Support Protocol 2: Synthesis of thiourea monomer
Keywords: Deoxynucleic guanidines (DNG), oligonucleotide backbone modifications, positively charged oligonucleotides, automated oligonucleotide synthesis
INTRODUCTION
DNG oligonucleotides are attractive synthetic targets for biological applications due to their internucleoside guanidinium linkages, which have been shown to increase their nuclease stability, melting temperature, and cellular internalization compared to unmodified oligonucleotides (Jain et al., 2012; Skakuj et al., 2019). In this protocol, we describe an automated DNG synthesis method performed on a MerMade™ 12 oligonucleotide synthesizer, with minimal modifications to the synthesizer, which allows the user to synthesize both DNG and standard oligonucleotides routinely (see Basic Protocol 1). This automated DNG synthesis relies on an iodine-base complex for the activation of thiourea monomers during coupling, foregoing the toxic mercury couplings usually associated with this oligonucleotide modification (see Basic Protocol 2, Skakuj et al., 2019, and utilizes accessible building blocks (see Supporting Protocols 1 and 2), enabling a more widespread use of this oligonucleotide modification.
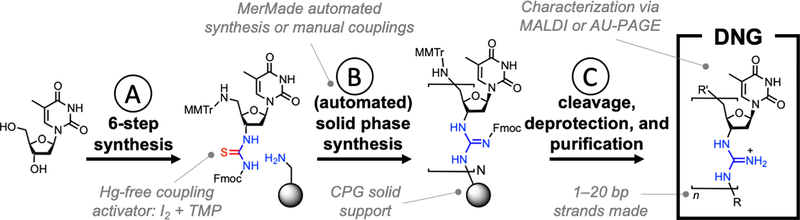
In addition to DNG oligonucleotides, 5’-DNG modifications can be incorporated into DNA and RNA oligonucleotides using this strategy. Interfacing DNGs with DNA produces mixed charge oligonucleotides (polyampholytes) whose properties will be determined by the dominant species in the chimera. As a result, DNG-DNA chimeric oligonucleotides require purification strategies that can be adapted as the overall charge of the oligonucleotide is varied. We find that oligonucleotides with 30% or less DNG character can be purified using reverse-phase HPLC or PAGE techniques as described previously (Albright & Slatko, 1994; Sinha & Jung, 2015). However, for oligonucleotides with more DNG character, we developed new purification strategies based on acetic acid-urea (AU) PAGE (traditionally used for histone purification) and aqueous extractions, which could be reliably used to purify these classes of nucleic acids (see Basic Protocols 2 and 3). We also provide methods for obtaining matrix assisted laser desorption/ionization (MALDI) mass spectra of DNG strands (see Basic Protocol 4) and to assess their purity using analytical AU PAGE (see Basic Protocol 5). Together, the methods described in this protocol seek to facilitate access to DNG oligonucleotides as well as mixed charge 5’-DNG-DNA-3’ chimeras in view of the unique materials and biological properties (e.g., rapid cell uptake, charge mediated assembly, etc.) of these structures that stem from their altered backbone. An outline of the synthesis procedure is shown on Figure 1.
Figure 1.
(A) Beginning with thymidine (left), a six-step synthesis procedure leads to a thiourea monomer that can be used to make DNG strands. (B) An automated (or optionally manual) synthesis that uses a mercury-free coupling chemistry creates a DNG strand atop a CPG support (which may or may not already bear standard DNA strands) analogous to standard oligonucleotide synthesis. (C) Following cleavage and deprotection using standard conditions, the strand can be purified and analyzed using AU PAGE and MALDI. This sequence can result in DNG-only or DNG-DNA chimeric strands of various lengths.
BASIC PROTOCOL 1: PREPARATION OF A MERMADE™ SYNTHESIZER FOR GUANIDINE COUPLINGS
A MerMade™ 12 oligonucleotide synthesizer can be adapted to synthesize DNA and DNG oligonucleotides simultaneously (Figure 2ab). This protocol will guide users on how to accomplish this MerMade™ 12 adaptation in four steps: (1) an additional activator must be added to the plumbing, (2) an alternative wash must be programmed (3) the delivery amounts of the new reagents must be calibrated, and (4) a new synthesis cycle must be written. The authors have carried out this procedure on the LabView software version 2.7.14 from BioAutomation; if certain options outlined here are not found in older versions of the software, we recommend contacting the vendor to upgrade to newer software.
Figure 2.
Simplified plumbing diagram of a MM12. (A) before any alterations and (B) after changes to allow for guanidine-backbone couplings. Green indicates gas lines, blue indicates standard phosphoramidite activator lines, black indicates phosphoramidite or DNG monomer lines, and red indicates alternative DNG activator lines. Dashed lines indicate old connections that have been removed in the altered plumbing system. (C) Splitting of the argon line on a MerMade 12™ to accommodate two types of activators. (D) Re-routing of an activator line from the activator manifold to the dedicated DNG activator on a MerMade 12™.
Materials
Dichloromethane (DCM, Sigma-Aldrich, 270997)
N,N-Dimethylformamide (DMF, Sigma-Aldrich, 227056)
1:1 DCM:acetonitrile (ACN, Sigma-Aldrich, 217004) solution
Controlled pore glass (CPG) beads (e.g. Unylinker support 1000 Å, ChemGenes) packed in 1 or 5 μmol oligonucleotide synthesis columns (Glen Research, 20–0050-02 or BioAutomation M4–1000)
MerMade™ 12 oligonucleotide synthesizer (BioAutomation) running LabView software version 2.7.14, configured for standard oligonucleotide synthesis at the 1 or 5 μmol scale with spare reagent bottle attachment points (usually a few spares are supplied with the instrument at installation)
Extra plastic screw fittings and ferrules
Closed plastic screw plug
Empty reagent bottles for alternative activator, alternative wash and thiourea monomer
Tee junction connector
Plumb alternative activator
-
1
Drain all activator solution from the synthesizer and shut off argon supply.
-
2
Choose a monomer position to be dedicated solely to guanidine-backbone couplings.
-
3
Disconnect the activator line corresponding to that position from the activator manifold (blue rectangle, Figure 2bc) and reconnect it to a spare bottle attachment port that will hold the alternative activator. Plug the open position left behind on the activator manifold with a closed plastic screw.
-
4
Disconnect the activator argon line from the argon manifold (green circle, Figure 2bd) and connect it to a Tee junction.
-
5
Connect one of the two remaining ports of the Tee junction back into the argon gas manifold and connect the other to the gas intake of the spare bottle attachment port designated for the alternative activator (Figure 2bd).
-
6
Refill standard and alternative activator bottles with acetonitrile and open argon flow. Ensure no gas or reagents are leaking from the newly connected lines and test the flow of reagents to the reagent ports.
Add alternative wash option
-
7
Open the configuration file named “config.ini”, located in the folder Documents > Bioautomation > Mermade12, using a simple text editor. Change the line that states “DEA Wash=0” to read “DEA Wash=1”. Save the file and restart the software.
NOTE: In the MerMade™ software, under “Service” > “Editors” > “Edit Script File”, in the “Base Script Editor” there will now appear an “Alt Wash 1” option.
Calibrate instrument
-
8
Add 10 mL of 1:1 ACN:DCM solution to the thiourea monomer reagent bottle and 10 mL DCM to the alternative activator reagent bottle. Add 100 mL DMF to the alternative wash bottle.
-
9
On the main screen, click “Service” > “Valves” > “Calibrate valves” to reach the “Injection Head Calibration” screen. This menu will display the current valve settings for dispensing liquids from each port. Adjust injection times (ms) under the “Liquid Cal” tab according to the manufacturer’s instructions such that the dispensed volumes for the DNG monomer, alternative activator, and alternative wash positions match the volume input entered in the software.
-
10
Click “Save” when the injection head calibration is completed and exit from the software.
Program a DNG synthesis cycle
-
11
Click on “Service” > “Editors” > “Edit Script File” and load a base script file for standard DNA synthesis at the 1 or 5 μmole scale.
NOTE: Base script files are found in C: ˃ MM ˃ Script and come with the instrument during installation.
-
12
Save this script file under a new name and reserve it for DNG synthesis.
-
13
Click on “Alt Wash 1” to reach the “Alt Wash Settings” screen. Designate a reagent port that will be used as the alternative wash (DMF) and copy the settings from acetonitrile washes, except for the parameters below. The parameters below are defined for 1 μmole syntheses, with the information for 5 μmole syntheses contained in parentheses.
ALT WASH 1
1 umol (5 umol) scales
Injection Parameters
Injection volume: 275 (800) μL
No. of injections: 1
No. of sub injections: 1
Regular valves
Wait to vacuum pulse: 3.0 (2.0) s
No of vacuum pulses: 1 (2)
Vacuum pulse lag: 0.0 s
Wait time: 6.0 s
-
14
Click “Calibrate valve” to reach the “Injection head calibration” screen. Under the “Vacuum cal” tab, adjust the length of the vacuum pulse such that the injected volume of DMF matches the volume input. Click “Save”, then “Done” once the vacuum calibration is completed and exit back to the “Base Script Editor”.
-
15
In the “Base Script Editor”, select the amidite ports that have alternative activator routing based on the number labels found on the instrument. These will be dedicated for DNG monomers.
-
16
Click on “Edit settings” for one chosen amidite port, and under “Reagent and cycle settings”, select “Copy from base A, a” (or other standard phosphoramidite base coupling parameters), then click “Next”.
-
17
In the “Reagent Editor” screen, change the following values under the “Amidite” tab.
NOTE: No significant DNG-specific modifications are required in the “Deblock”, “Capping”, “Oxi/Sul”, “Acetonitrile” and “Other Options” tabs if modifying from a standard phosphoramidite protocol.
AMIDITE
1 umol (5 umol) scales
Injection parameters
Injection volume: 180 (540) μL
No of injections: 1
No of sub injections: 4 (10)
Regular valves
Wait to vacuum pulse: 300.0 (250) s
No of vacuum pulses: 2 (3)
Vacuum pulse lag : 10.0 s
Wait time: 600.0 (900) s
ACTIVATOR
Injection parameters
Injection volume: 60 (180) μL
-
18
Click on “Vacuum calibration” and add a CPG-loaded oligonucleotide synthesis column to the synthesis ports that will be used for DNG synthesis. Under the “Vacuum cal” tab, adjust the length of the vacuum pulses (ms) for the chosen amidite/activator ports such that the CPG beads are in contact with the injected solutions and remain covered until the final drain step. Click “Save”, then “Done” when the calibration is complete.
-
19
Click “Next” to proceed to the “Cycle Editor” screen. Enter the cycle steps for DNG synthesis as outlined in Table 1.
-
20
Click “Finish” to return to the “Base Script Editor”. Save changes by overwriting the DNG synthesis script file.
-
21
Repeat steps 6–10 for other amidite ports that are to be used for DNG synthesis. Alternatively, use the “Copy from base” option under the “Reagent and cycle settings” menu to copy the settings of another DNG port.
-
22
Once all port reassignments are completed, save changes by overwriting the DNG synthesis script file and exit from the MerMade™ software.
Table 1.
Steps of a DNG synthesis cycle on the MerMade™ 12 synthesizer.
| 1) START TRITYL | 11) ACN WASH |
| 2) DEBLOCK | 12) ACN WASH |
| 3) DEBLOCK | 13) ACN WASH |
| 4) DEBLOCK | 14) COUPLING |
| 5) DEBLOCK | 15) COUPLING |
| 6) ACN WASH | 16) ACN WASH |
| 7) ACN WASH | 17) CAPPING |
| 8) ACN WASH | 18) ACN WASH |
| 9) END TRITYL | 19) ACN WASH |
| 10) ALT WASH 1 | 20) ACN WASH |
Program a DNG sequence file
-
23
Open a simple text editor (e.g. Notepad) and define a short name for the sequence to be synthesized. Add a comma and a space, then enter the desired oligonucleotide sequence (5’ to 3’ direction) using the letter codes associated to amidite ports with alternative DNG activator routing to specify the coupling of DNG monomers.
-
24
Save the sequence file as “.txt” under the exact same name as the sequence.
BASIC PROTOCOL 2: SYNTHESIS OF DNG STRANDS ON A MERMADE™ SYNTHESIZER
This section guides readers through the automated synthesis of DNG or 5’-DNG-DNA-3’ oligonucleotides using a DNG-enabled MerMade™ 12 oligonucleotide synthesizer that has been modified as described in Basic Protocol 1. This protocol was validated using the initiator-functionalized CPG beads and the thiourea monomers whose syntheses are reported in Support Protocols 1 and 2 (Challa & Bruice, 2004). The initiator-functionalized CPG will provide the primary amine functional group necessary to couple with the thiourea monomer upon activation with an iodine-base complex and produce a protected guanidine internucleoside linkage. This coupling chemistry can proceed in an automated manner and has enabled the synthesis of DNG oligonucleotides up to 20 bases in length and 5’-DNG-DNA-3’ chimeras of various ratios. Guanidine-backbone enabled MerMade™ 12 instruments can routinely achieve 95% coupling yield per step (Skakuj et al., 2019).
Materials
Dichloromethane (Sigma-Aldrich, 270997)
Acetonitrile (Sigma-Aldrich, 217004)
N,N-Dimethylformamide (Sigma-Aldrich, 227056)
Iodine
2,2,6,6-tetramethylpiperidine (TMP)
Thiourea monomer (see Supporting Protocol 2)
Cap A solution (80% Tetrahydrofuran (THF)/10% pyridine/10% acetic anhydride)
Cap B solution (16% 1-Methylimidazole in THF)
Deblock solution (3% Dichloroacetic acid in DCM)
40% Ammonium hydroxide solution in water
40% Methylamine solution in water
Tris(hydroxymethyl)aminomethane (Tris) buffer (1 M, pH 8)
20% Acetic acid solution
CPG solid phase support functionalized with initiator at the 5’ end (see Support Protocol 1)
Guanidine-backbone enabled MerMade™ 12 oligonucleotide synthesizer (see Basic Protocol 1)
Small scintillation vials (6 mL, ensure vial fits within existing amidite bottles on the synthesizer)
Large scintillation vials (26 mL)
Aluminum foil
Oven (55 °C)
Nitrogen gas
House vacuum
Pliers
Blunt-ended needle
Lyophilizer or high vacuum pump with cold trap
Syringe filters (PES or other hydrophilic material, 0.2 μm)
Syringes
Make activator solution
-
1
Weight out 126.9 mg (0.50 mmol) of solid iodine into a clear-glass 26 mL scintillation vial with a cap.
-
2
Add 9.75 mL of anhydrous DCM into the vial to make a 50 mM iodine solution.
NOTE: Scintillation vials made of clear glass allow you to see whether any iodine crystals remain undissolved on the bottom. Ensure the iodine is completely dissolved; sonication helps.
-
3
Add 253 μL of TMP to the iodine solution. The solution should change color from very dark burgundy to orange.
NOTE: This solution is light sensitive and will decompose over time, store wrapped in aluminum foil and use within 24 h.
Make thiourea monomer solution
-
4
Weigh out 79.4 mg (0.10 mmol) of thiourea monomer (see Supporting Protocol 2) into a small scintillation vial, which will be inserted into an amidite container on the synthesizer.
NOTE: Using a small container to hold the monomer solution, instead of pouring it into the large bottles that are mounted on the synthesizer, minimizes loss of material on the edges of the glass and evaporation of solvent. If the monomer stock was stored in the freezer, wait for it to warm to room temperature before opening the container.
-
5
Add 2 mL of DCM followed by 2 mL ACN, for a final solution of 25 mM in 1:1 ACN/DCM.
Perform automated synthesis
-
6
Pour the activator solution (see Basic Protocol 2, steps 1–3) into the alternative activator bottle and attach it to the synthesizer.
NOTE: Cover this bottle and the line leading from it to the column with aluminum foil to prevent degradation of the alternative activator solution.
-
7
Place the vial containing the thiourea monomer solution (see Basic Protocol 2, steps 4–5) into a larger bottle and attach it to the synthesizer at the amidite position defined by the DNG synthesis script file.
-
8
Prime the lines leading from the activator and thiourea monomer to the column by going to “Service” > “Valves” > “Injection Head Test” to reach the “Injection Line Test” function of the synthesizer. Allow reagent to flow through the entire length of the tubing with a small amount dispensing into a waste container held below the respective ports. Exit to the main screen when completed.
-
9
Set up the synthesis using the command prompts on the MerMade™ software. Input the desired sequence file into the synthesizer, making sure it uses the letter or number code defined for the amidite port in which the thiourea monomer is found. Select the DNG synthesis script file, load a column with initiator-functionalized CPG (see Support Protocol 1), and begin the synthesis.
NOTE: DNG coupling efficiency can be assessed by UV-Vis spectroscopy using the MMTr cleavage method (Eckstein, 1991).
Cleave and deprotect oligonucleotides
-
10
When the synthesis has finished (20 min per DNG coupling at the 1 μmol scale or 30 min per DNG coupling at the 5 μmol scale), dry the solid support beads under vacuum for a few seconds to remove solvent leftovers from the synthesis.
-
11
Remove the top frit from the column by using a pair of pliers to squeeze the plastic housing (5 μmol scale) or a blunt needle (0.2–1 μmol scale) to disrupt the frit; then transfer the solid support to a 26 mL scintillation vial.
-
12
Add a 1:1 solution of 40% aqueous ammonia and 40% aqueous methylamine (AMA) to the solid support (approximately 1 mL for a 1 μmol or 3 mL for a 5 μmol scale synthesis).
NOTE: Concentration of ammonia solutions decreases over time as ammonia gas evaporates. Store this solution for up to a week at 4°C after opening to ensure a reliable ammonia concentration.
-
13
Place the capped vial in a 55 °C oven for 30 min.
NOTE: It is important to cap the vial tightly as the ammonia and methylamine can evaporate at elevated temperatures.
-
14
Remove the vial from the oven and let cool for a few minutes prior to opening.
CAUTION: Use caution when opening the vial as the contents are under slight pressure and filled with ammonia and methylamine gas. Open the vial in the hood behind a glass shield.
-
15
Add Tris buffer into the vial to a concentration of approximately 0.1 M and then use a gentle stream of nitrogen to evaporate the ammonia and methylamine.
NOTE: The Tris buffer will prevent the removal of the MMTr group if the contents of the vial are accidentally dried completely during subsequent steps.
a) Purification by PAGE
If the oligonucleotide contains more than 30% DNG character, they tend to adsorb onto CPG beads and must be extracted using acid. To prepare these samples, follow the steps below. Figure 3a shows expected PAGE purification outcomes for T10 and T20 DNG.
Figure 3.
Purification and characterization of DNG-containing oligonucleotides. (A) Analytical AU PAGE gel (24%) showing T10 DNG before (lane 1) and after AU PAGE purification (lane 2), as well as the crude for T20 DNG (lane 3) and the purified product by AU PAGE (lane 4). Gel was stained with SimplyBlue SafeStain. (B) MALDI-TOF-MS trace measured in negative mode for a 5’-(Tg)6(Tp)14-3’ chimera, where g represents guanidine and p represents phosphate linkages, purified using HPLC. (C) MALDI-TOF MS trace measured in positive mode for T10 DNG (without a thiol modifier at the 3’ end) purified using the acid extraction approach.
-
16a.
Add 1 mL 20% acetic acid solution to the solid support to reconstitute the oligonucleotide.
-
17a.
Filter the solution through a 0.2 μm syringe filter.
-
18a.
At this point, the oligonucleotide can be purified by PAGE, frozen, or lyophilized for storage. Purify the oligonucleotide using standard denaturing PAGE (Albright & Slatko, 1994) if negatively charged or using AU PAGE (see Basic Protocol 3 and Figure 3a) if cationic.
b) Purification by HPLC
Strands that are <30% DNG in length can be purified using trityl-ON reverse-phase HPLC protocols as described previously (Sinha & Jung, 2015); to prepare these samples, follow the protocol below. Figure 3b shows the expected outcome of HPLC purification for DNG-DNA chimeras with 30% DNG linkages.
-
16b.
Add distilled water to the solid support to reconstitute the oligonucleotides.
-
17b.
Filter through a 0.2 μm syringe filter.
-
18b.
At this point, the oligonucleotide can be purified by HPLC according to previously reported procedures (Singa & Jung 2015), frozen, or lyophilized for storage.
c) Purification by simple extraction
Certain lengths of DNG-only oligonucleotides can be purified by a simple extraction protocol that relies on the strong adsorption of long DNG strands to glass surfaces compared to shorter failure strands. We have found this method to be effective for DNG oligonucleotides around 10 bases in length since their increased affinity for CPG beads allows selective removal of shorter oligonucleotides. Figure 3c shows the expected outcome for the purification of T10 DNG using simple extraction.
-
16c.
Wash solid support 2x with deionized water (1 mL for a 1 μmol scale synthesis)
-
17c.
Add 20% acetic acid to the solid support beads (0.5 mL for a 1 μmol scale synthesis) to solubilize the full length DNG product strands.
-
18c.
Filter the acetic acid solution through a 0.2 μm syringe filter.
-
19c.
Lyophilize the resulting solution to remove acetic acid and reconstitute in 1 mL deionized water.
BASIC PROTOCOL 3: PURIFICATION OF DNG STRANDS USING PREPARATIVE ACETIC ACID UREA (AU) PAGE
AU PAGE was initially developed to separate small, highly-positively charged histone proteins (Ryan & Annunziato, 2001; Shechter et al., 2007). Due to the similarity of this analyte to DNG oligomers, we adapted these protocols to enable DNG purification. With the exception of the gel and buffer compositions, this protocol proceeds very similarly to standard denaturing PAGE. Like DNA, DNG oligonucleotides have a constant mass-to-charge ratio, although they are cationic instead of negatively charged, enabling their separation by length upon denaturation; DNGs also retain their absorbance at 260 nm, which facilitates their detection and extraction from the gel. AU PAGE can be used to achieve single nucleotide separation of DNG strands for characterization and purification (Figure 3a). In this protocol readers will purify oligonucleotides using preparative AU PAGE.
Materials
Urea
Glacial acetic acid
Deionized water
40% acrylamide in water (19:1 acrylamide:bisacrylamide)
Ammonium persulfate
Tetramethylethylenediamine (TEMED)
5% acetic acid solution
8 M urea, 5% acetic acid solution
0.6% methyl green, 50% glycerol solution
Hoefer 600 gel electrophoresis supplies (16 cm gels, 0.15 cm spacers)
UV lamp
Silica plate
Plastic wrap
15 and 50 mL conical tubes
NAP-10 columns (17085402, GE Life Sciences)
1.5 mL plastic tubes
Heat block
Casting the gel
-
1
Prepare 50 mL of a 24% acetic acid-urea acrylamide solution by mixing 18 g of urea, 30 mL of 40% acrylamide (19:1 acrylamide:bisacrylamide) solution and 2.5 mL glacial acetic acid.
-
2
Sonicate until fully dissolved (40 kHz, 3 min), then adjust volume to 50 mL with deionized water.
-
3
Add 150 mg ammonium persulfate and 300 μL TEMED in succession, mixing thoroughly between each addition to initiate polymerization.
-
4
Sonicate briefly to remove bubbles (40 kHz, 10 s), then cast between gel plates, installing a 10-well comb.
-
5
Allow gel to polymerize for 10 min, then remove comb, wash wells with water, and install in gel box using 5% acetic acid as running buffer.
Running the gel
-
6
Resuspend DNG strand (0.5 μmol synthesis) in 100 μL 5% acetic acid. Add 100 μL of 8 M urea, 5% acetic acid solution, mix, heat to 95 °C for 5 min, and then load over 3 wells while hot.
-
7
In a separate well, load 20 μL 0.6% methyl green, 50% glycerol solution as loading dye.
NOTE: The loading dye usually leaves a trace across the gel during the run and will cloud oligonucleotide detection if loaded in the same well.
-
8
Run at 250 V for 3.5 h with reversed polarization. The loading dye should have reached the bottom of the gel.
NOTE: Reversed polarization should be used for oligonucleotides and DNG-DNA chimeras that are expected to carry an overall positive charge (i.e. the number of DNG linkages should be superior to the number of phosphate linkages).
Extracting the DNG strands from the gel
-
9
Separate the gel from the plates and image on a plastic wrap over a silica plate with fluorescent indicator using a UV lamp.
-
10
Excise the desired bands using a razor blade into a 15 mL conical tube and crush the gel to fine pieces using a 200 μL pipette tip or a glass rod.
-
11
Add 6 mL 5% acetic acid solution and incubate at 55 °C with mixing for 16 h.
-
12
Pellet gel by centrifugation (2000 rcf, 4 min) and collect supernatant into a separate conical tube. Add 2 mL 5% acetic acid to the gel fragments and centrifuge again, collecting the supernatant in the same tube.
-
13
Lyophilize the supernatant, then resuspend in 1 mL 5% acetic acid.
-
14
Desalt the oligonucleotide using a NAP-10 column pre-equilibrated with 5% acetic acid, according to the manufacturer’s protocol.
-
15
Lyophilize and resuspend in 1 mL deionized water. Measure concentration using absorbance at 260 nm and extinction coefficients calculated from DNA strands of equivalent lengths using the OligoAnalyzer tool (Integrated DNA Technologies, https://www.idtdna.com/calc/analyzer). Store at 4 °C at concentrations in the 10–100 μM range.
BASIC PROTOCOL 4: CHARACTERIZATION OF DNG STRANDS USING MALDI-MS
Successful synthesis of DNG strands can be routinely verified using MALDI-MS. Generally, DNG-rich oligonucleotides need to be deposited in higher amounts on the plate and produce stronger signal in positive mode, as opposed to DNA samples. The presence of acetic acid during deposition also results in superior signal. This procedure describes MALDI-MS characterization of DNG-containing oligonucleotides and produces results analogous to those reported in Figure 3bc and previous publication by the authors (Skakuj et al., 2019).
Materials
2’,6’-Dihydroxyacetophenone (DHAP)
Methanol
Saturated ammonium citrate solution
Glacial acetic acid
1.5 mL plastic tubes
MALDI plate
Preparation of the DHAP matrix for MALDI
Weigh 25 mg of 2’,6’-dihydroxyacetophenone in a 1.5 mL plastic tube.
Add 300 μL methanol to the tube and vortex to dissolve the matrix.
Add 111 μL saturated ammonium citrate to this solution and vortex. This mixture should turn bright yellow, and a precipitate will appear. Use the supernatant in subsequent steps. Note. This solution should be made fresh for best results.
For anionic oligonucleotides containing DNG linkages
-
4a.
Spot 0.3 μL of DNG-modified oligonucleotide onto a MALDI plate.
-
5a.
Add 0.3 μL DHAP matrix onto the sample and allow to dry completely (5 min).
NOTE: Appearance of the sample should be light yellow and opaque.
-
6a.
Assess purity of the oligonucleotide using a MALDI source in negative mode. Figure 3b shows an example MALDI-TOF MS trace for an HPLC-purified DNG-DNA chimera with 30% DNG linkages.
For cationic oligonucleotides containing DNG linkages
-
4b.
Reconstitute an aliquot of the DNG-containing oligonucleotide to be analyzed at 100 μM in 20% acetic acid. Drop 2 μL onto a MALDI plate.
NOTE: DNG oligonucleotides do not fly as well as DNA and RNA by MALDI. Increasing the concentration of the oligonucleotide or adding subsequent layers of sample prior to adding the matrix can improve results.
-
5b.
Add 0.2 μL DHAP matrix onto the sample and allow to dry completely (5 min).
NOTE: A thin white film should appear with occasional crystallized regions.
-
6b.
Assess purity of the oligonucleotide using a MALDI source in positive mode. Figure 3c shows an example MALDI-TOF MS trace for a T10 DNG strand purified using the simple extraction procedure.
BASIC PROTOCOL 5: CHARACTERIZATION OF DNG STRANDS USING AU PAGE
An assessment of the purity of DNG oligonucleotides post-synthesis can be obtained using analytical AU PAGE. This procedure was adapted from previous protocols aimed at separating and characterizing histone proteins, which are arginine-rich and also have small molecular weights (Ryan & Annunziato, 2001; Shechter et al., 2007). Moreover, since DNGs contain guanidinium groups, they can be detected reliably with anionic protein stains. It is worth noting that these dyes are less sensitive than DNA intercalators and thus, the amount of DNG loaded onto the gel should be scaled up accordingly. An example of expected results for crude and purified 10-mer and 20-mer DNG syntheses is shown in Figure 3a. This procedure allows the reader to characterize DNG-containing strands using analytical AU PAGE gels stained imaged using standard anionic protein stains.
Materials
Urea
Glacial acetic acid
Deionized water
40% acrylamide in water (19:1 acrylamide:bisacrylamide)
Ammonium persulfate
Tetramethylethylenediamine (TEMED)
5% acetic acid solution
8 M urea, 5% acetic acid solution
0.6% methyl green, 50% glycerol solution
Bio-Rad mini-PROTEAN gel electrophoresis supplies (0.15 cm spacers)
15 and 50 mL conical tubes
SimplyBlue™ SafeStain (LC6065, Thermo Fisher)
Heat block
Gel scanner (e.g. ChemiDoc MP, Bio-Rad)
Casting the gel
-
1
Prepare 15 mL of a 24% acetic acid-urea acrylamide solution by mixing 5.4 g of urea, 9 ml of 40% acrylamide (19:1 acrylamide:bisacrylamide) solution, and 750 μL glacial acetic acid.
-
2
Sonicate (40 kHz, 3 min) until fully dissolved, then adjust volume to 15 mL with deionized water.
-
3
Add 45 mg ammonium persulfate and 90 μL TEMED in succession, mixing thoroughly in between each addition to initiate polymerization.
-
4
Sonicate (40 kHz, 10 s) briefly to remove bubbles, then cast between gel plates, installing a 10-well comb.
-
5
Allow gel to polymerize for 10 min, then remove comb, wash wells with water, and install in gel box using 5% acetic acid as buffer.
Running the gel
-
6
Resuspend DNG strand (300–700 pmol) in 10 μL of 5% acetic acid. Add 10 μL of 8 M urea, 5% acetic acid solution, mix, heat to 95 °C for 5 min, and then load onto gel while hot.
NOTE: Colorimetric protein stains are typically not as sensitive as fluorescent DNA intercalators for detection. Loading higher amounts of samples is preferred (500–700 pmol) if analyzing shorter DNG strands (10-mers).
-
7
Load 10 μL of 0.6% methyl green, 50% glycerol solution as loading dye on a separate lane.
-
8
Run at 200 V for 1.5 h with reversed polarization.
-
9
Stain in SimplyBlue™ SafeStain for at least 1 h, microwaving at full intensity for 30 sec, two times, allowing the gel to cool down in between.
-
10
Destain in deionized water overnight and image on gel scanner.
NOTE: A representative analytical AU PAGE gel for T10 and T20 DNG before and after AU PAGE purification is shown in Figure 3a.
SUPPORT PROTOCOL 1: SYNTHESIS OF INITIATOR-FUNCTIONALIZED CPG
The initiator monomer (I5) couples through a phosphate linkage to an existing deprotected hydroxyl on solid support and thus bears a phosphoramidite on its 3′ hydroxyl group. However, its 5′ hydroxyl group is replaced by an amine, which allows for subsequent DNG couplings to form guanidine linkages. A five step synthesis results in I5 with an approximate final yield of 9% (Figure 4). The authors routinely start this synthetic sequence with 5 g of thymidine. This synthetic sequence is based upon published literature with modifications by the authors (Challa & Bruice, 2004). I5 is then coupled to a CPG solid support bearing a 5’ hydroxyl or O-DMT group and then acts as the initiator for DNG synthesis.
Figure 4.
Synthetic scheme for initiator I5. Synthesis proceeds via 5 steps: (a) Ts-Cl, Py; (b) NaN3, NaI, DMF; (c) Pd/C, H2; (d) MMTr-Cl, Py; (e) 2-cyanoethyl-N,N-diisopropylchlorophosphoramidite, DIPEA, DCM.
Materials
Thymidine (dT)
p-Toluenesulfonyl chloride (TsCl)
Sodium azide (NaN3)
Sodium iodide (NaI)
10% Palladium on carbon (Pd/C)
Hydrogen gas (H2)
Monomethoxytrityl chloride (MMTr-Cl)
Triethylamine (TEA)
2-cyanoethyl-N,N-diisopropylchlorophosphoramidite
Diisopropylethylamine (DIPEA)
Pyridine, anhydrous (Sigma-Aldrich, 270970)
N,N-Dimethylformamide, anhydrous (Sigma-Aldrich, 227056)
Dichloromethane, anhydrous (Sigma-Aldrich, 270997)
Methanol
Hexanes
O-DMT- or hydroxyl-terminated CPG beads (e.g. Unylinker support 1000 Å, ChemGenes)
Celite
Nitrogen or argon
Whatman filter paper No. 1
Vacuum filtration apparatus
Round bottom flasks
Stir bars
Hot plate with stirring
Blast shield
Condenser
Ice bath
Hot oil bath
Silica gel chromatography supplies
Scintillation vials (6 and 25 mL)
Reflux condenser
MerMade™ 12 oligonucleotide synthesizer equipped for phosphoramidite synthesis with at least one spare port
RediSep Rf Gold Silica Flash Columns 69–2203 series, Teledyne Isco
Synthesis of I1
-
1
Weigh out thymidine (5 g, 20.7 mmol, 1 equiv.) into a 250 mL round bottom flask and add a stir bar.
-
2
Add pyridine (35 mL) to the flask and ensure the thymidine is dissolved. Then cool to 0 °C by stirring in an ice bath.
-
3
Dissolve p-toluenesulfonyl chloride (4.54 g, 23.7 mmol, 1.15 equiv.) in pyridine (5 mL) and add to the flask dropwise over 10 min.
-
4
Remove from ice and allow the solution to warm to room temperature while stirring.
-
5
Pour the solution into ca. 200 mL of cold distilled water; a white precipitate should form instantly.
-
6
Vacuum filter the precipitate using Whatman No. 1 filter paper and wash with water and diethyl ether.
NOTE: The product I1 is a white powder usually obtained in ~80% yield. 1H NMR (500 MHz, DMSO-d6) δ 11.31 (s, 1H), 7.79 (d, J = 8.3 Hz, 2H), 7.47 (d, J = 8.1 Hz, 2H), 7.38 (d, J = 1.4 Hz, 1H), 6.14 (t, J = 6.9 Hz, 1H), 4.26 (dd, J = 10.9, 3.3 Hz, 1H), 4.21 – 4.13 (m, 2H), 3.86 (dt, J = 5.8, 3.5 Hz, 1H), 2.41 (s, 3H), 2.15 (dt, J = 13.8, 6.9 Hz, 1H), 2.06 (ddd, J = 13.6, 6.6, 3.9 Hz, 1H), 1.77 (d, J = 1.2 Hz, 3H).
Synthesis of I2
-
7
Add I1 (6.4 g, 16.1 mmol, 1 equiv.), sodium azide (1.17 g, 17.9 mmol, 1.1 equiv.), and sodium iodide (0.24 g, 1.61 mmol, 0.1 equiv.) to a 250 mL round bottom flask charged with a stir bar and equipped with a reflux condenser.
CAUTION: Sodium azide is highly toxic and explosive, weigh out using a plastic spatula in a hood. Ensure no contact with acid or heavy metals. Store waste in a clearly labeled separate container for disposal.
NOTE: Sodium azide will not fully dissolve in DMF, only ensure that I1 is fully dissolved.
-
8
Add anhydrous DMF (30 mL) to the flask and stir to ensure I1 is fully dissolved.
-
9
Heat the reaction to 110 °C under nitrogen for 1 h while stirring.
CAUTION: Use a blast shield to cover the reaction for extra safety.
-
10
Let the reaction cool to room temperature while stirring, then filter off remaining solids using a pad of Celite and DMF as wash.
-
11
Remove the solvent using a rotary evaporator under reduced pressure.
-
12
Purify the product by silica gel chromatography with a DCM to 30% methanol gradient (RediSep Rf Gold Silica Flash Columns 69–2203 series, Teledyne Isco).
NOTE: The product I2 is a white solid usually obtained in ~60% yield.
1H NMR (500 MHz, DMSO-d6) δ 11.32 (s, 1H), 7.49 (d, J = 1.3 Hz, 1H), 6.20 (dd, J = 7.6, 6.4 Hz, 1H), 5.40 (d, J = 4.4 Hz, 1H), 4.19 (dq, J = 7.5, 3.9 Hz, 1H), 3.84 (td, J = 5.2, 3.6 Hz, 1H), 3.56 (d, J = 5.3 Hz, 2H), 2.25 (dt, J = 13.9, 6.8 Hz, 1H), 2.08 (ddd, J = 13.5, 6.5, 3.7 Hz, 1H), 1.79 (d, J = 1.2 Hz, 3H).
Synthesis of I3
-
13
Weight out I2 (0.5 g, 1.87 mmol) into a 250 mL round bottom flask with a stir bar.
-
14
Add 20 mL of methanol to the flask and dissolve the contents.
-
15
Degas the contents of the flask by bubbling argon or nitrogen through the solution while stirring vigorously for approx. 15 min.
-
16
Weight out Pd/C (100 mg) into a scintillation vial.
-
17
CAUTION: Pd/C can ignite spontaneously in air or upon addition of solvent. Keep separated from the starting material until fully wetted.
-
18
Add 2 mL of water to the Pd/C to create a slurry and add quickly to the round bottom flask, washing with an additional 1 mL of water to transfer the entire contents of the vial.
-
19
Bubble hydrogen gas slowly (approx. 1 bubble per second) through the solution until all starting material is consumed.
-
20
NOTE: TLC with 15/85 methanol/DCM solvent system and UV illumination to monitor starting material consumption.
-
21
Once the reaction is complete, stop hydrogen gas flow and once again bubble argon or nitrogen through the solution for 15 min.
-
22
Filter the reaction mixture through a plug of Celite, washing with copious amounts of methanol.
CAUTION: Ensure the Pd/C never becomes dry during filtration. When done, add water and transfer to waste container.
-
23
Remove the solvent under vacuum with a rotary evaporator.
NOTE: The product I3 is a white foam usually obtained in ~90% yield and can be used without further purification. 1H NMR (500 MHz, DMSO-d6) δ 7.65 (d, J = 1.4 Hz, 1H), 6.14 (dd, J = 7.7, 6.2 Hz, 1H), 5.17 (s, 1H), 4.19 (dt, J = 6.5, 3.4 Hz, 1H), 3.64 (td, J = 5.2, 3.3 Hz, 1H), 2.72 (dd, J = 5.2, 1.4 Hz, 2H), 2.12 (ddd, J = 13.9, 7.7, 6.4 Hz, 1H), 2.03 (ddd, J = 13.3, 6.3, 3.4 Hz, 1H), 1.78 (d, J = 1.2 Hz, 3H).
Synthesis of I4
-
24
Weight out I3 (1.35 g, 5.6 mmol, 1 equiv.) into a 250 mL round bottom flask with a stir bar.
-
25
Dissolve I3 in 35 mL of pyridine.
-
26
Add triethylamine to the solution (0.86 mL, 6.16 mmol, 1.1 equiv.).
-
27
Cool the solution to 0 ºC in an ice bath while stirring.
-
28
Weigh out MMTr-Cl into a scintillation vial and dissolve in 8 mL of pyridine.
-
29
Add the MMTr-Cl solution to the round bottom flask dropwise over 30 min.
-
30
Let the solution stir at 0 ºC for 2 h, then warm to room temperature.
-
31
Remove the solvent from the reaction under reduced pressure using a rotary evaporator.
-
32
Purify the product using silica chromatography with a DCM to methanol gradient and a constant 5% TEA.
NOTE: The MMTr protecting group is acid sensitive and will be removed upon contact with silica if 5% TEA is not present in the solvent to neutralize it. If performing a dry loading on the silica column, adsorb the crude reaction mixture to Celite instead of silica.
NOTE: The product I4 is a white foam that is usually obtained in ~70% yield. 1H NMR (500 MHz, DMSO-d6) δ 11.30 (s, 1H), 7.44 – 7.38 (m, 5H), 7.33 – 7.23 (m, 6H), 7.21 – 7.15 (m, 2H), 6.84 (d, J = 8.9 Hz, 2H), 6.14 (t, J = 6.7 Hz, 1H), 5.22 (d, J = 4.7 Hz, 1H), 4.18 (dt, J = 6.8, 4.3 Hz, 1H), 3.81 (dt, J = 6.4, 4.3 Hz, 1H), 3.72 (s, 3H), 2.64 (t, J = 8.1 Hz, 1H), 2.33 – 2.25 (m, 1H), 2.22 – 2.10 (m, 2H), 2.09 – 2.01 (m, 1H), 1.69 (d, J = 1.2 Hz, 3H).
Synthesis of I5
NOTE: Perform this synthesis under an inert atmosphere (glove box or air-free Schlenk technique)
-
33
Weight out I4 (0.80 mg, 1.6 mmol, 1 equiv.) into a scintillation vial charged with a stir bar.
-
34
Add DCM (16 mL) to dissolve I4.
-
35
Add DIPEA (1.0 g, 1.4 mL, 7.8 mmol, 5 equiv.).
-
36
Add 2-cyanoethyl N,N,-diisopropylchlorophoshoramidite (1.1 g, 1.0 mL, 4.7 mmol, 3 equiv.) and let stir at room temperature for 2 h.
-
37
Remove the solvent using a rotary evaporator under reduced pressure.
-
38
Purify the product with flash silica chromatography with a 60/40 hexanes/DCM solvent with 5% TEA under inert atmosphere.
NOTE: Two closely traveling spots will be visible by UV illumination on the TLC plate; these correspond to the two diastereomers (differing in the chirality at the phosphorus center). Both should be collected and used, as the chiral center is removed during oxidation step.
NOTE: Phosphorus 31P NMR is an excellent tool for identifying and assessing purity of this material. Product should produce two signals (originating from the two diastereomers), at 147.5 and 147.1 ppm. Extensive decomposition of the product can be seen in the phosphorus NMR, at 11.9 and 15.8 ppm, which is most likely due to hydrolysis to the H-phosphonate.
NOTE: The product I5 is an oil that can be obtained in ~30% yield after accounting for phosphorus degradation impurities. 1H NMR (400 MHz, DMSO-d6) δ 11.31 (s, 1H), 7.65 (s, 1H), 7.45 – 7.36 (m, 5H), 7.28 (ddd, J = 14.2, 8.2, 6.1 Hz, 6H), 7.22 – 7.13 (m, 2H), 6.88 – 6.79 (m, 2H), 6.13 (s, 1H), 6.06 (s, 1H), 4.50 (ddd, J = 20.2, 10.7, 6.3 Hz, 1H), 4.04 (dddt, J = 30.2, 10.4, 7.6, 5.8 Hz, 3H), 3.72 (d, J = 1.9 Hz, 3H), 3.53 – 3.40 (m, 3H), 2.71 (dt, J = 42.5, 5.9 Hz, 2H), 2.39 – 2.13 (m, 2H), 1.70 (dd, J = 5.4, 1.1 Hz, 3H), 1.23 – 1.00 (m, 12H). 31P NMR (162 MHz, DMSO-d6) δ 147.52 (h, J = 9.6, 9.6, 9.6, 9.1, 9.1 Hz), 147.12 (h, J = 9.8, 9.8, 9.8, 9.5, 9.5 Hz), 16.25 – 15.38 (m), 12.18 – 11.51 (m). HRMS–ESI (m/z): [M + Na]+ calcd for C39H48N5NaO6P, 736.3234; found 7736.3239
Coupling of I5 to CPG beads
-
39
Resuspend I5 in anhydrous DCM at a concentration of 0.1 M in a 6 mL scintillation vial.
-
40
Load vial in a spare reagent bottle on a MerMade™ 12 oligonucleotide synthesizer.
-
41
In a simple text editor (e.g. Notepad), define a short name for the coupling to be performed. Add a comma and a space, then enter the letter or number code attributed to the chosen spare bottle in a standard phosphoramidite script file.
-
42
Save the sequence file with a “.txt” extension under the exact same name as the sequence.
-
43
Set up a synthesis on a MerMade™ 12 oligonucleotide synthesizer equipped with standard phosphoramidite chemistry reagents, by following the manufacturer’s instructions and the command prompts provided by the software. Load the newly defined sequence file and a standard phosphoramidite synthesis script file. Load a hydroxyl or O-DMT terminated CPG-filled column in the instrument’s main chamber. Verify reagent levels and flow, then start the synthesis.
-
44
Once synthesis has completed, proceed to Basic Protocol 2 or store the I5-initiated CPG at −20 °C.
SUPPORT PROTOCOL 2: SYNTHESIS OF THIOUREA MONOMER
The propagating thiourea monomer (P6) bears 3′ and 5′ amines in place of hydroxyls, so it can be added to a growing DNG strand via a guanidine linkage and accept further DNG couplings. The six-step synthesis can yield P6 in approximately 9% final yield (Figure 5). The authors routinely begin this synthesis with 20 g of thymidine to prepare 6 g (~ 7 mmol) of P6 monomer. This synthetic sequence is based upon published literature with modifications by the authors (Challa & Bruice, 2004; Eleuteri et al., 1996; Lavandera et al., 2001; Ruda et al., 2011). Similar synthetic protocols have been reported for the other bases; however, they have not been verified by the authors (Linkletter et al., 2001; Szabo & Bruice, 2004).
Figure 5.
Synthetic scheme for P6. Synthesis proceed via 6 steps: (a) MsCl, Py; (b) TEA, EtOH; (c) NaN3, DMF; (d) Pd/C, H2; (e) MMTr-Cl, Py; (f) Fmoc-isothiocyanate, DCM.
Materials
Thymidine
Methanesulfonyl chloride (MsCl)
Triethylamine (TEA)
Sodium azide (NaN3)
10% Palladium on carbon (Pd/C)
Hydrogen gas
Monomethoxytrityl chloride (MMTr-Cl)
Fluorenylmethoxycarbonyl isothiocyanate (Fmoc-NCS)
Deionized water
Ethanol
Pyridine, anhydrous (Sigma-Aldrich, 270970)
N,N-Dimethylformamide, anhydrous (Sigma-Aldrich, 227056)
Dichloromethane, anhydrous (Sigma-Aldrich, 270997)
Methanol
Ethyl acetate
Blast shield
Hot plate with stirring
TLC plates
UV lamp
Silica gel chromatography supplies
Celite
Nitrogen or argon
Vacuum filtration apparatus with filter papers
Rotary evaporator
Ice bath
Heating mantle
Round bottom flasks
Stir bars
Erlenmeyer flask (2 L)
Scintillation vials (25 mL)
Reflux condenser
Lyophilizer
Synthesis of P1
-
1
Dissolve thymidine (20 g, 83 mmol, 1 equiv.) with anhydrous pyridine (120 mL) in a round bottom flask equipped with a stir bar. Cool to 0 °C in an ice bath.
-
2
Add MsCl (19.2 mL, 248 mmol, 3 equiv.) dropwise over the course of approx. 30 min using an addition funnel.
-
3
Let warm to room temperature once the addition is complete and stir for 2 h.
-
4
Use a rotary evaporator to concentrate the solution to approx. 50 mL, then pour this crude reaction into an Erlenmeyer flask filled with 1.5 L of cold water. A white precipitate should form right away. Let stir for a few minutes.
-
5
Vacuum filter the precipitate with Whatman No. 1 filter paper. Wash the solids with approx. 100 mL of water. Dry the wet precipitate using a lyophilizer or under high vacuum.
NOTE: The product P1 is a white powder that is usually obtained in ~90% yield. 1H NMR (500 MHz, DMSO-d6) δ 11.41 (s, 1H), 7.51 (d, J = 1.4 Hz, 1H), 6.22 (t, J = 7.1 Hz, 1H), 5.30 (td, J = 4.8, 3.1 Hz, 1H), 4.46 (t, J = 4.1 Hz, 2H), 4.37 (dt, J = 5.1, 3.6 Hz, 1H), 3.33 (s, 3H), 3.25 (s, 3H), 2.54 – 2.51 (m, 2H), 1.79 (d, J = 1.2 Hz, 3H).
Synthesis of P2
-
6
Suspend P1 (26.7 g, 67 mmol) in 700 mL of anhydrous ethanol and TEA (70 mL, 10% by volume) in a round bottom flask equipped with a large stir bar and a reflux condenser.
NOTE: The solids will not dissolve but ensure the solution is stirring.
-
7
Heat the solution and maintain at reflux overnight.
NOTE: The solids will become viscous initially but eventually return to a free-flowing suspension when the reaction is complete.
-
8
Cool the reaction mixture to room temperature, then vacuum filter with Whatman 1 paper washing with 100 mL of ethanol.
-
9
Dry the resulting solid using a lyophilizer or high vacuum.
NOTE: The product P2 is a white powder that can usually be obtained in ~90% yield. 1H NMR (500 MHz, DMSO-d6) δ 7.59 (d, J = 1.4 Hz, 1H), 5.90 (d, J = 3.8 Hz, 1H), 5.35 (dd, J = 2.9, 1.6 Hz, 1H), 4.54 – 4.44 (m, 2H), 4.24 – 4.17 (m, 1H), 3.20 (s, 3H), 2.61 (dd, J = 13.1, 1.5 Hz, 1H), 2.52 (dd, J = 4.1, 2.8 Hz, 1H), 1.76 (d, J = 1.2 Hz, 3H).
Synthesis of P3
-
10
Dissolve P2 (9.1 g, 30.1 mmol, 1 equiv.) in 100 mL DMF then add sodium azide (4.3 g, 66.2 mmol, 2.2 equiv.) in a round bottom flask equipped with a stir bar and a reflux condenser.
CAUTION: Sodium azide is highly toxic and explosive. Weigh out with a plastic spatula in a hood. Avoid contact with acids and heavy metals. Store waste in a clearly labeled separate container for disposal.
-
11
Heat the reaction to 130 °C for 6 h under nitrogen. Monitor the reaction progress by TLC using neat ethyl acetate as the solvent.
CAUTION: Use a blast shield as an extra layer of protection.
-
12
Cool the reaction to room temperature and filter through a pad of Celite, washing with approx. 100 mL of DMF.
-
13
Remove solvent from the resulting crude mixture with a rotary evaporator.
-
14
Purify the crude product on silica gel column with a gradient from DCM to 30% MeOH in DCM.
NOTE: The product P3 will be a viscous amber oil that is usually obtained in ~60% yield. 1H NMR (500 MHz, DMSO-d6) δ 11.36 (s, 1H), 7.52 (m, 1H), 6.14 (t, J = 6.6 Hz, 1H), 4.45 (dt, J = 7.7, 5.5 Hz, 1H), 3.92 (td, J = 5.6, 4.0 Hz, 1H), 3.67 (dd, J = 13.3, 4.0 Hz, 1H), 3.59 (dd, J = 13.3, 5.8 Hz, 1H), 2.50 (m, 1H), 2.33 (ddd, J = 14.0, 7.1, 5.5 Hz, 1H), 1.80 (d, J = 1.3 Hz, 3H).
Synthesis of P4
-
15
Dissolve P3 (7.86 g, 26.9 mmol) in approx. 90 mL of methanol in a round bottom flask with a stir bar. Degas the solution by bubbling argon or nitrogen through it for approx. 15 min while stirring.
-
16
Weigh out palladium on carbon (1.18 g) into a glass scintillation vial. Add approx. 10 mL of water to the vial and create a slurry.
CAUTION: Palladium on carbon is flammable especially when mixed with organic solvents. Keep the palladium on carbon separate from the starting material until fully wetted.
-
17
Quickly transfer the palladium slurry into the reaction flask, washing with a small amount of additional water.
-
18
Begin to bubble hydrogen gas through the solution at a rate of approx. one bubble per second while stirring vigorously. Monitor reaction progress using TLC (60/40 ethyl acetate/hexanes) checking for complete consumption of starting material. Reaction is usually complete within 6 h.
-
19
Stop hydrogen flow and bubble argon or nitrogen through the solution vigorously for approx. 15 min.
-
20
Filter solution through a pad of Celite, washing with copious amounts of methanol until the filtrate no longer gives a strong signal on a TLC plate upon UV illumination.
CAUTION: Do not allow the Celite to dry completely while performing the filtration. Once done, add water to the Celite and dispose into a designated container.
-
21
Remove solvent using a rotary evaporator followed by high vacuum.
NOTE: Product P4 is a white foam that can usually be obtained in 90% yield. 1H NMR (400 MHz, Methanol-d4) δ 7.51 (d, J = 1.2 Hz, 1H), 6.14 (t, J = 6.6 Hz, 1H), 4.35 (dt, J = 7.7, 5.5 Hz, 1H), 3.95 (ddd, J = 5.5, 4.7, 3.7 Hz, 1H), 3.68 (dd, J = 13.3, 3.8 Hz, 1H), 3.57 (dd, J = 13.4, 4.7 Hz, 1H), 2.49 (ddd, J = 14.0, 7.8, 6.2 Hz, 1H), 2.39 (ddd, J = 14.0, 7.0, 5.4 Hz, 1H), 1.88 (d, J = 1.3 Hz, 3H).
Synthesis of P5
-
22
Dissolve P4 (4.21 g, 17.5 mmol, 1 equiv.) in 350 mL of anhydrous pyridine and 5% triethylamine by volume (approx. 17 mL) in a round bottom flask with a stir bar and an addition funnel. Cool the solution to 0 °C.
Make sure the thymidine is fully dissolved before proceeding to the next step to minimize double additions of the monomethoxytrityl chloride on the same nucleoside.
-
23
Weigh out MMTr–Cl (5.42 g, 16.5 mmol, 1 equiv.) in a 26 mL scintillation vial followed by 25 mL of anhydrous pyridine. Transfer this solution into the addition funnel, washing the vial with additional pyridine to aid in complete transfer.
-
24
Add the MMTr-Cl solution dropwise over approx. 30 min while maintaining reaction at 0 °C.
-
25
Once the addition is complete allow the reaction to warm to room temperature for 30 min and stir for a further 2 h.
-
26
Remove the solvent using a rotary evaporator under reduced pressure. Load the crude reaction mixture onto Celite using methanol.
CAUTION: Avoid exposing the product to acidic conditions, including silica gel, as degradation of the product through removal of MMTr group will occur. This can be observed by the appearance of an orange color due to the release of the trityl cation.
-
27
Purify the product using 0.5/5/94.5 MeOH/TEA/DCM solvent system.
NOTE: Product P5 is a white foam that can usually be obtained in 25% yield. 1H NMR (500 MHz, Methanol-d4) δ 7.49 – 7.44 (m, 4H), 7.40 – 7.33 (m, 3H), 7.30 – 7.23 (m, 4H), 7.20 – 7.14 (m, 2H), 6.86 – 6.80 (m, 2H), 6.16 (dd, J = 7.4, 4.1 Hz, 1H), 3.82 – 3.76 (m, 1H), 3.76 (s, 3H), 3.39 (q, J = 7.8 Hz, 1H), 2.62 (dd, J = 12.6, 3.8 Hz, 1H), 2.37 (dd, J = 12.6, 6.6 Hz, 1H), 2.29 (ddd, J = 13.8, 7.9, 4.1 Hz, 1H), 2.18 (dt, J = 13.8, 7.7 Hz, 1H), 1.78 (d, J = 1.2 Hz, 3H).
Synthesis of P6
-
28
Dissolve P5 (1.58 g, 2.0 mmol, 1 equiv) in 40 mL of anhydrous DCM at room temperature in a round bottom flask with a stir bar.
-
29
Weigh out Fmoc–NCS (0.67 g, 2.4 mmol, 1.2 equiv) into a 6 mL scintillation vial.
-
30
Add Fmoc–NCS to the reaction in one batch and let stir at room temperature for 16 h.
-
31
Remove solvent under reduced pressure using a rotary evaporator and purify the product using a DCM to ethyl acetate gradient.
NOTE: The column chromatography should be performed rapidly, as the acidic silica will degrade the product on the column via removal of the MMTr group.
NOTE: The product P6 is a slightly yellow foam which can usually be obtained in 70% yield. 1H NMR (400 MHz, Methanol-d4) δ 7.86 – 7.73 (m, 3H), 7.67 (d, J = 7.5 Hz, 2H), 7.49 – 7.20 (m, 15H), 7.14 (t, J = 7.3 Hz, 2H), 6.85 – 6.73 (m, 2H), 6.20 (dd, J = 7.7, 3.7 Hz, 1H), 5.27 (q, J = 8.5 Hz, 1H), 4.50 (d, J = 6.9 Hz, 2H), 4.29 (t, J = 6.8 Hz, 1H), 3.98 (dt, J = 8.1, 4.0 Hz, 1H), 3.73 (s, 3H), 2.61 (ddd, J = 17.3, 13.5, 6.3 Hz, 2H), 2.50 – 2.34 (m, 2H), 1.80 (d, J = 1.1 Hz, 3H). 13C NMR (126 MHz, DMSO-d6) δ 179.54, 163.80, 157.39, 153.28, 150.39, 146.24 (d, J = 6.6 Hz), 143.31 (d, J = 13.5 Hz), 140.75, 137.70, 136.45, 129.63, 128.27, 127.92, 127.75, 127.21, 126.10, 125.63 (d, J = 6.5 Hz), 120.24, 113.04, 109.66, 83.19, 81.98, 69.73, 67.41, 59.83, 54.95, 46.04, 20.84, 14.15, 12.25. HRMS–ESI (m/z): [M + H]+ calcd for C46H44N5O6S, 794.3007; found 794.3016.
COMMENTARY
Background Information
Positively charged oligonucleotide analogues have been an attractive synthetic target for the past three decades, due their expected increased hybridization strength with complementary DNA or RNA strands and their altered recognition by enzymes and cells (Jain et al., 2012; Skakuj et al., 2019). These properties are anticipated to increase both their stability and extent of uptake when placed in a physiological setting. This has resulted in the development of three distinct classes of positively charged oligonucleotides where the negatively charged phosphate backbone is entirely replaced by a cationic moiety, as opposed to appended onto the nucleotide. To date, successful positively charged oligonucleotide analogues have been joined through either: guanidinium, S-methylthiourea, or nucleosyl-amino acid internucleoside linkages (Meng & Ducho, 2018). Additionally, phosphoramidate linkages that bear positively charged side groups can be used to preserve the phosphorus center and eliminate its negative charge (Letsinger et al., 1988).
The first phosphate backbone replacements with substituted guanidines were reported by Herdewijn in 1992–1993 and further investigated with unsubstituted guanidines, starting in 1994, by Bruice who coined the term deoxyribonucleic guanidine (DNG) for its guanidinium internucleoside linkages (Dempcy et al., 1994; Pannecouque et al., 1992; Vandendriessche, Van Aerschot, et al., 1993; Vandendriessche, Voortmans, et al., 1993). We became interested by this oligonucleotide analogue due to its advantageous structural characteristics: DNGs are achiral, maintain a positive charge across a broad range of pH (due to the high pKa of the guanidinium groups), show high stability towards nuclease degradation, and can be synthesized reliably from accessible building blocks (Jain et al., 2012).
A major limitation of DNGs was the synthetic method for coupling nucleoside monomers together, which relied on a mercury-mediated abstraction of sulfur to activate a thiourea monomer towards nucleophilic attack from a 5′-amine on the growing strand. In the 30 years of development of these strands, mercury-mediated coupling was the sole reported method for the synthesis of DNGs, which mirrors its prominence in the synthesis of guanidines reported in the organic chemistry literature (Jain et al., 2012). While efficient at carrying out this reaction, mercury presents a number of challenges limiting its applications, including exposure of researchers to this toxic metal, the generation of toxic waste streams with expensive disposal, contamination of instruments, and the possible contamination of final products with mercury. As a result, while a solid phase synthesis of DNGs was developed using mercuric chloride as the coupling agent, it was never translated to an automated system, and the DNGs synthesized using this approach were not characterized in a biological context. To increase access to this structure and facilitate further investigations with DNGs, we sought to develop an alternative coupling methodology that did not rely on toxic reagents and could be automated using standard oligonucleotide synthesizers.
The methods for accessing DNG oligonucleotides described here consist of three components: a novel coupling chemistry that avoids the use of mercury, a translation of the synthesis to an automated oligonucleotide synthesizer, and accessible methods to purify and characterize these oligonucleotides. (Figure 1) The coupling chemistry developed here sought to utilize the thiourea monomer whose synthesis is well established and built upon reports that describe abstraction of sulfur from thioureas by iodine (Ali et al., 2010; Gaffney & Jones, 2014). During the development of this chemistry, we found that a balance of acidity and basicity of the reagents had to be achieved in order to prevent the removal of backbone protecting groups from the growing strand —acidic conditions could cause the removal of MMTr while bases could remove the Fmoc protecting groups from the guanidine backbone. The stability of protecting groups can be assayed by including a disulfide linkage at the 3’ end of the growing strand: this allows for cleavage from the solid support through reduction of the disulfide linkage under mild conditions, such that the nucleobase and backbone protecting groups are retained. MALDI-MS analysis of strands cleaved this way showed that Fmoc backbone protecting groups were stable through multiple rounds of DNG synthesis (Skakuj et al., 2019). While the balance of base strength and amount was critical, we found that the stability of the base in solution with iodine was paramount for the automation of the process since both reagents must be stored as solutions on the instrument. Triethylamine was by far the most commonly used base for iodine-mediated thiourea activation, however, we found that it quickly decomposed and precipitated upon storage in solution with iodine. A screen of bases revealed that 2,2,6,6-tetramethylpiperidine provided sufficient basicity and stability in solution with iodine to enable automated synthesis (Skakuj et al., 2019).
In our hands, the synthesis method described here could be used to make DNG oligonucleotides up to 20 bases in length (Figure 3a). This oligonucleotide length shows the power of automation, as it would be prohibitively time consuming without an oligonucleotide synthesizer. Automated syntheses were carried out on a range of scales from approximately 0.5–5.0 μmol. Average yields of individual couplings were found to be about 95% over eight additions measured on a synthesizer at the 1 μmol scale (Skakuj et al., 2019). While longer strands of oligonucleotides were only made using the automated protocol, we have successfully coupled the DNGs manually over numerous cycles. Thus, we do not believe automation is necessary to carry out this chemistry.
A challenge with DNGs, as with many polyampholytes, is their solution properties. Chimeric strands of DNA and DNG behave much like DNA when their ratio on a single strand is approximately ≤30% DNG to DNA. Therefore, many chimeric strands can be purified by reverse phase (RP)-HPLC, using trityl groups to separate success strand from failures (Figure 3b). As the number of DNG bases increases, in addition to the expected failure and success peaks, additional slow-eluting peaks appear in the RP-HPLC chromatogram (data not shown). We hypothesize that these late-eluting peaks are due to secondary structures or assemblies of these strands. Once the ~30% DNG to DNA threshold is reached, injections into the HPLC may no longer produce any product peaks, suggesting the material precipitated or permanently adhered to the column. We found that standard protocols for denaturing urea polyacrylamide gel electrophoresis worked well to characterize and purify DNG-DNA conjugates with up to 40% DNG character (data not shown).
DNG oligonucleotides required a different purification strategy. Inspired by the gel electrophoresis techniques developed for histones (small cationic proteins bearing many arginine side chains), we adapted the acetic acid-urea PAGE for use with DNG strands (Ryan & Annunziato, 2001; Shechter et al., 2007). By using higher acrylamide concentrations and increasing the amount of crosslinker compared to previously reported histone separation gels, we were able to achieve single base resolution on this separation. (Figure 3c). This technique can now be used to purify and/or analyze DNG oligonucleotides with good resolution.
While working on DNG purification protocols, we serendipitously discovered an extraction procedure that can quickly yield 10-mer DNGs with high purity (Figure 3c). Unfortunately, we suspect it will likely not work for DNGs of shorter lengths and we have not applied it to DNGs of longer lengths. In this extraction procedure, the DNGs are cleaved and deprotected using AMA and the solution is dried with a stream of nitrogen. At this stage, the oligonucleotides are adsorbed to the glass surface, likely through electrostatic association with deprotonated hydroxyls of the solid support. By washing the beads with pure water, the shorter failures sequences are selectively desorbed from the surface, leaving behind only the longer full-length strands. These strands can be liberated with a solution of 20% acetic acid in water. 10-mer DNGs of reasonable purity can be quickly obtained in this way, but more importantly, this procedure illustrates the types of secondary interactions not found with standard oligonucleotides but that must be kept in mind while working with DNGs. We suspect that these strands can be purified using a cation exchange resin, however, this has been elusive in our hands.
Taken together, the methods presented in this protocol provide a reliable synthetic route to access DNG and 5’-DNG-DNA-3’ chimeric oligonucleotides using mild reagents and automated procedures, further facilitating the access to mixed charge oligonucleotides with programmed patterns. The synthetic approaches and purification strategies presented here are also amenable to scale up, which enables their study in a biological context and will likely facilitate applications in other fields.
Critical Parameters
Yields of DNGs suffer when the two solutions that make up the coupling reaction (activator and thiourea monomer) are allowed to come into contact with the solid support prior to reacting fully. The thiourea activation reaction can be easily followed by monitoring the disappearance of the orange color. The rate of thiourea activation by the iodine solution is highly dependent on the mixing of these two reagents when they are dispensed onto the column and their concentrations. Ensure that the coupling solution is fully mixed and clear when it is pulled onto the beads by the first vacuum pulse. This can be controlled by changing the concentrations of reagents and relative volumes added to the column, the number of pulses the synthesizer uses to dispense the reagent, and the time prior to the first vacuum pulse that pulls the mixture onto the beads. These variables should be optimized to ensure rapid and complete reaction prior to contact with the solid support.
Troubleshooting
Knowledge of basic operating and maintenance procedures for the MerMade™ 12 is recommended prior to implementing Basic Protocol 1 and 2. If the user is not familiar with procedures such as editing a script file, as well as performing injection volume and vacuum pulse calibrations on the instrument, we recommend referring to the user manual and/or contacting the manufacturer for support.
During long synthesis (over 10 bases), the alternative activator solution may begin to form a precipitate at the end of the dispensing port; this can block the flow of alternative activator solution. To prevent the synthesis from failing, we recommend stopping the synthesis at a safe step, after approximately 10 bases, and briefly washing off the dispensing port with dichloromethane to remove any accumulated solids.
Coupling yields can be assessed during synthesis by UV-Vis spectroscopy using the MMTr cleavage method (Eckstein, 1991). By analyzing coupling yields at every step, it is possible to assess the operation of the instrument and the expected overall yield for the synthesis. We recommend testing yields at each coupling step during the implementation of this protocol and then every time coupling conditions or monomers are varied to ensure their compatibility with the methods described in this protocol.
Understanding/Expected Results
The synthesis protocol described here can be used to make pure DNG or 5’-DNG-DNA-3’ chimeras of various lengths (1–20 bases reported) on a range of scales (0.5–5 μmol reported). Successful synthesis of final products can be confirmed by MALDI-TOF mass spectrometry and PAGE (Figure 3).
Time Considerations
When the building blocks (see Support Protocols 1 and 2) and reagents needed are at hand and the synthesizer has been converted to be compatible with DNG synthesis (see Basic Protocol 1), the synthesizer can be prepared and started (including the making of activator and monomer solutions) within 1 h. The cleavage and deprotection of the oligonucleotides using AMA solution can be completed within a day. Standard RP-HPLC purification can be completed within an hour of drying the crude oligonucleotide product. Subsequent lyophilization steps take approximately one day, followed by another day after removal of the trityl group. PAGE purification can be completed within one day, however, extraction of the oligonucleotide from the crushed gel fragments takes one to two days. The simple extraction protocol can yield pure 10-mer DNG within one day.
The major investment of time is the synthesis of monomers. Once familiar with the protocols, researchers can complete most individual reactions within two to three days, including reaction time, work-up, purification, drying, and characterization. This means that a single batch of P6 monomer can be made in about three weeks.
As an alternative to synthesizing an initiator unit that switches the strand from a 5′ hydroxyl to an amine, a commercially available terminal amino-modifier (such as 10–1905 from Glen Research) with a MMTr-protected amine can be coupled using phosphoramidite chemistry, then deprotected with standard deblock solution and coupled using the guanidinium chemistry. This saves much time and synthetic effort but will result in a short, abasic spacer region at the interface of the phosphate- and guanidine-backbones.
ACKNOWLEDGEMENTS
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award U54CA199091. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The project was also supported by the Prostate Cancer Foundation and the Movember Foundation under award 17CHAL08, the Vannevar Bush Faculty Fellowship program sponsored by the Basic Research Office of the Assistant Secretary of Defense for Research and Engineering and funded by the Office of Naval Research through grant N00014-15-1-0043, and the NTU-NU Institute for NanoMedicine located at the International Institute for Nanotechnology, Northwestern University, USA and the Nanyang Technological University, Singapore. K. E. B. gratefully acknowledges support from a Banting Fellowship from the Government of Canada. The authors would like to thank Robert Stawicki and Jennifer Delgado for their help in setting up the MerMade™ 12 for DNG synthesis.
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
LITERATURE CITED
- Albright LM, & Slatko BE (1994). Denaturing Polyacrylamide Gel Electrophoresis. Current Protocols in Human Genetics, 00(1), A.3F.1–A.3F.4. doi: 10.1002/0471142905.hga03fs00 [DOI] [PubMed] [Google Scholar]
- Ali AR, Ghosh H, & Patel BK (2010). A greener synthetic protocol for the preparation of carbodiimide. Tetrahedron Letters, 51(7), 1019–1021. doi: 10.1016/j.tetlet.2009.12.017 [DOI] [Google Scholar]
- Challa H, & Bruice TC (2004). Deoxynucleic guanidine: synthesis and incorporation of purine nucleosides into positively charged DNG oligonucleotides. Bioorganic & Medicinal Chemistry, 12(6), 1475–1481. doi: 10.1016/j.bmc.2003.12.043 [DOI] [PubMed] [Google Scholar]
- Dempcy RO, Almarsson O, & Bruice TC (1994). Design and synthesis of deoxynucleic guanidine: a polycation analogue of DNA. Proceedings of the National Academy of Sciences of the United States of America, 91, 7864–7868. doi: 10.1073/pnas.91.17.7864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein F (1991). Oligonucleotides and analogues : a practical approach. Oxford; New York: IRL Press. [Google Scholar]
- Eleuteri A, Reese CB, & Song QL (1996). Synthesis of 3’,5’-dithiothymidine and related compounds. Journal of the Chemical Society, Perkin Transactions 1(18), 2237–2240. doi: 10.1039/p19960002237 [DOI] [Google Scholar]
- Gaffney BL, & Jones RA (2014). Synthesis of c-di-GMP analogs with thiourea, urea, carbodiimide, and guanidinium linkages. Organic Letters, 16(1), 158–161. doi: 10.1021/ol403154w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain ML, Bruice PY, Szabo IE, & Bruice TC (2012). Incorporation of Positively Charged Linkages into DNA and RNA Backbones: A Novel Strategy for Antigene and Antisense Agents. Chemical Reviews, 112(3), 1284–1309. doi: 10.1021/cr1004265 [DOI] [PubMed] [Google Scholar]
- Lavandera I, Fernández S, Ferrero M, & Gotor V (2001). First regioselective enzymatic acylation of amino groups applied to pyrimidine 3’,5’-diaminonucleoside derivatives. Improved synthesis of pyrimidine 3’,5’-diamino-2’,3’,5’-trideoxynucleosides. Journal of Organic Chemistry, 66(11), 4079–4082. doi: 10.1021/jo010048a [DOI] [PubMed] [Google Scholar]
- Letsinger RL, Singman CN, Histand G, & Salunkhe M (1988). Cationic oligonucleotides. Journal of the American Chemical Society, 110(13), 4470–4471. doi: 10.1021/ja00221a089 [DOI] [Google Scholar]
- Linkletter BA, Szabo IE, & Bruice TC (2001). Solid-phase synthesis of oligopurine deoxynucleic guanidine ( DNG ) and analysis of binding with DNA oligomers. Nucleic Acids Research, 29(11), 2370–2376. doi: 10.1093/nar/29.11.2370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng M, & Ducho C (2018). Oligonucleotide analogues with cationic backbone linkages. Beilstein Journal of Organic Chemistry, 14, 1293–1308. doi: 10.3762/bjoc.14.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannecouque C, Wigerinck P, Van Aerschot A, & Herdewijn P (1992). Dimeric Building-Blocks with N-Cyanoguanidine Linkage for Oligonucleotide Synthesis. Tetrahedron Letters, 33(49), 7609–7612. doi: 10.1016/S0040-4039(00)60837-7 [DOI] [Google Scholar]
- Ruda GF, Nguyen C, Ziemkowski P, Felczak K, Kasinathan G, Musso-Buendia A, … Gilbert IH (2011). Modified 5’-trityl nucleosides as inhibitors of Plasmodium falciparum dUTPase. ChemMedChem, 6(2), 309–320. doi: 10.1002/cmdc.201000445 [DOI] [PubMed] [Google Scholar]
- Ryan CA, & Annunziato AT (2001). Separation of histone variants and post-translationally modified isoforms by triton/acetic acid/urea polyacrylamide gel electrophoresis. Current Protocols in Molecular Biology, Chapter 21, Unit 21 22. doi: 10.1002/0471142727.mb2102s45 [DOI] [PubMed] [Google Scholar]
- Shechter D, Dormann HL, Allis CD, & Hake SB (2007). Extraction, purification and analysis of histones. Nature Protocols, 2(6), 1445–1457. doi: 10.1038/nprot.2007.202 [DOI] [PubMed] [Google Scholar]
- Sinha ND, & Jung KE (2015). Analysis and Purification of Synthetic Nucleic Acids Using HPLC. Current Protocols in Nucleic Acid Chemistry, 61(1), 10.15.11–10.15.39. doi: 10.1002/0471142700.nc1005s61 [DOI] [PubMed] [Google Scholar]
- Skakuj K, Bujold KE, & Mirkin CA (2019). Mercury-Free Automated Synthesis of Guanidinium Backbone Oligonucleotides. Journal of the American Chemical Society, 141(51), 20171–20176. doi: 10.1021/jacs.9b09937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo IE, & Bruice TC (2004). DNG cytidine: Synthesis and binding properties of octameric guanidinium-linked deoxycytidine oligomer. Bioorganic and Medicinal Chemistry, 12(15), 4233–4244. doi: 10.1016/j.bmc.2004.05.010 [DOI] [PubMed] [Google Scholar]
- Vandendriessche F, Van Aerschot A, Voortmans M, Janssema G, Busson R, Van Overbeke A, … Herdewijn (1993). Synthesis, Enzymatic Stability and Base-pairing Properties of OIigothymidylates Containing Thymidine dimers with different N-substituted Guanidine linkages. Journal of the Chemical Society, Perkin Transactions 1, 1567–1575. doi: 10.1039/P19930001567 [DOI] [Google Scholar]
- Vandendriessche F, Voortmans M, Hoogmartens J, Van Aerschot A, & Herdewijn P (1993). Synthesis of Novel N-Substituted Guanidine Linked Nucleoside Dimers and Their Incorporation into Oligonucleotides. Bioorganic & Medicinal Chemistry Letters, 3(2), 193–198. doi: 10.1016/S0960-894x(01)80875-1 [DOI] [Google Scholar]
KEY REFERENCES
- Skakuj K, Bujold KE, & Mirkin CA (2019). Mercury-Free Automated Synthesis of Guanidinium Backbone Oligonucleotides. Journal of the American Chemical Society, 141(51), 20171–20176. doi: 10.1021/jacs.9b09937Original publication describing the coupling of DNGs using iodine-base activator.
- Linkletter BA; Szabo IE; Bruice TC (1999). Solid-Phase Synthesis of Deoxynucleic Guanidine (DNG) Oligomers and Melting Point and Circular Dichroism Analysis of Binding Fidelity of Octameric Thymidyl Oligomers with DNA Oligomers. Journal of the American Chemical Society, 121(16), 3888–3896. doi: 10.1021/ja984212wExtensive synthesis data and description of melting experiments of 8-mer DNGs (gT)8 with complementary strands bearing increasing numbers of mismatches.
- Jain ML, Bruice PY, Szabo IE, & Bruice TC (2012). Incorporation of Positively Charged Linkages into DNA and RNA Backbones: A Novel Strategy for Antigene and Antisense Agents. Chemical Reviews, 112(3), 1284–1309. doi: 10.1021/cr1004265Review article written by Bruice, et al. that summarizes their laboratory’s investigations into positively charged oligonucleotide analogues, including DNGs, from 1996 to 2010.
- Meng M, & Ducho C (2018). Oligonucleotide analogues with cationic backbone linkages. Beilstein Journal of Organic Chemistry, 14, 1293–1308. doi: 10.3762/bjoc.14.111Review article that summarizes the recent advancements in positively charged oligonucleotides.
- Shechter D, Dormann HL, Allis CD, & Hake SB (2007). Extraction, purification and analysis of histones. Nature Protocols, 2(6), 1445–1457. doi: 10.1038/nprot.2007.202Protocol article describing the AU PAGE technique in the context of histone purification and analysis, which served as inspiration for DNG purification.