Abstract
Biomimetic pharmacophores deposited at the air/water interface self-assemble into distinct crystalline nanostructures. The shapes and dimensions of the nanostructures are significantly modulated by the lipid environments in mixed lipid/DAG-lactone monolayers.
Keywords: DAG-lactones, biomimetic chemistry, nanorods, nanofibers, Langmuir films, lipid monolayers, lipid-fibril interactions
Nanofibers and nanorods have been encountered in natural biological systems, primarily amyloid peptides and proteins[1–6], but have been also produced through diverse synthetic routes employing metals and semiconductors[7, 8], organic[9, 10] and inorganic substances[11]. In general, two fundamental aspects govern nanostructure formation and morphology. First, specific molecules exhibiting distinct structural features or organization properties need to be distinguished or synthesized. Second, nanostructures typically form in appropriate physico-chemical environments which facilitate assembly of the molecular building blocks into distinct configurations. Nanofibers comprising biomimetic molecules, in particular, have attracted growing interest due to the diverse structural features and design parameters that can be borrowed from the realm of biology[12]. We describe here the formation at the air/water interface of unique nanorods and nanofibers comprising sn-1,2-diacylglycerol-lactone (DAG-lactone), a biomimetic pharmacophore[13]. Remarkably, we observed that the morphology and organization of the DAG-lactone nanostructures were significantly modulated by lipid molecules co-deposited with the pharmacophore at the air/water interface. Besides of the interesting self-assembly properties of the DAG-lactone, the pronounced structural modulation induced by the lipids in the monolayer environments might be biologically-significant because DAG-lactones operate within the environment of the cell membrane[14].
Figure 1A depicts the di-phenyl DAG-lactone investigated here. Previous studies have shown that di-phenyl DAG-lactones can drive translocation of PKC to cellular membranes and closely interact with membrane bilayers through the hydrophobic rigid diphenyl “molecular rod”[15]. We initially investigated the self-assembly properties of pure 1 deposited and compressed at the air/water interface (i.e. Langmuir monolayers, Figure 1B–E). Indeed, the microscopy images in Figure 1 point to a remarkable self-assembly of 1 at the air/water interface.
Figure 1. Langmuir monolayers of DAG-lactone 1.
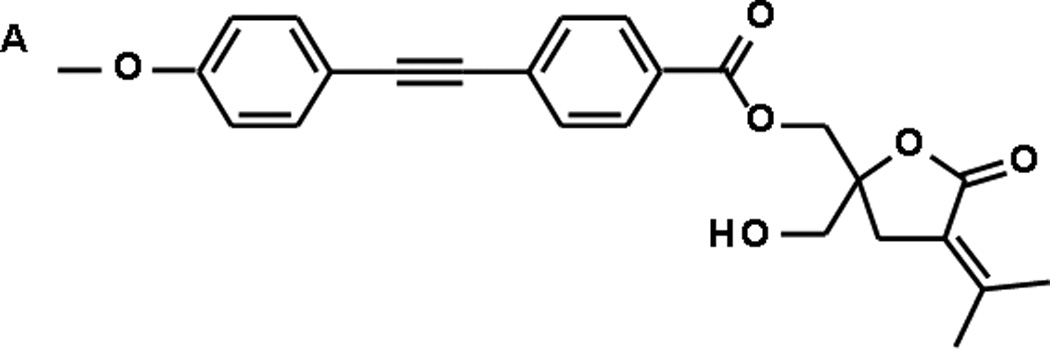
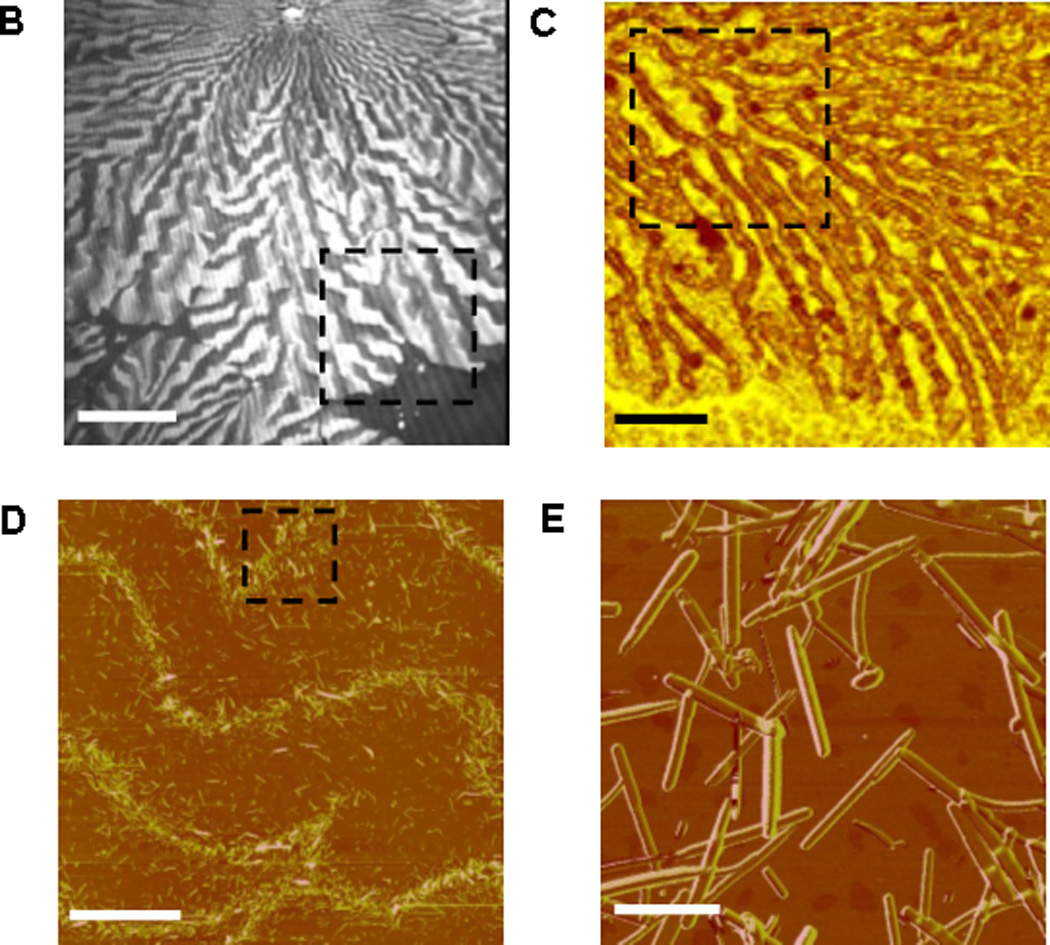
A. Molecular structure of 1; B. BAM image of 1 monolayer (pressure 24 mN/m), bar corresponds to 100 µm; C. SWLI image of approximate area highlighted in B, bar corresponds to 25 µm; D. AFM image of approximate area highlighted in C, bar corresponds to 10 µm; E. AFM image of approximate area highlighted in D, bar corresponds to 1 µm.
Figure 1B depicts the Brewster angle microscopy (BAM) image of 1 monolayer compressed to a pressure of 24mN/m. The bright features in Figure 1B correspond to condensed domains of 1 formed through compression of the monolayer[16]. Figure 1C provides a closer look at the 1 domains using Scanning White Light Interferometry (SWLI)[17, 18]. The SWLI image demonstrates that the edges of the condensed domains of 1 comprise a network of condensed stripes. The atomic force microscopy (AFM) images in Figure 1D–E reveal that within the elongated stripes, 1 assembled into small, sub-micron rods. Importantly, while previous studies have demonstrated that amphiphilic molecules produce organized monolayers at the air/water interface[19–21], the formation of nanorod assemblies is unique. Furthermore, when 1 was simply dissolved in an organic solvent and dried, the needle-like nanostructures in Figure 2 were not detected, indicating that the air/water interface constitutes an essential platform for 1 self-assembly.
Figure 2. AFM of lipid/1 films formed at the air/water interface.
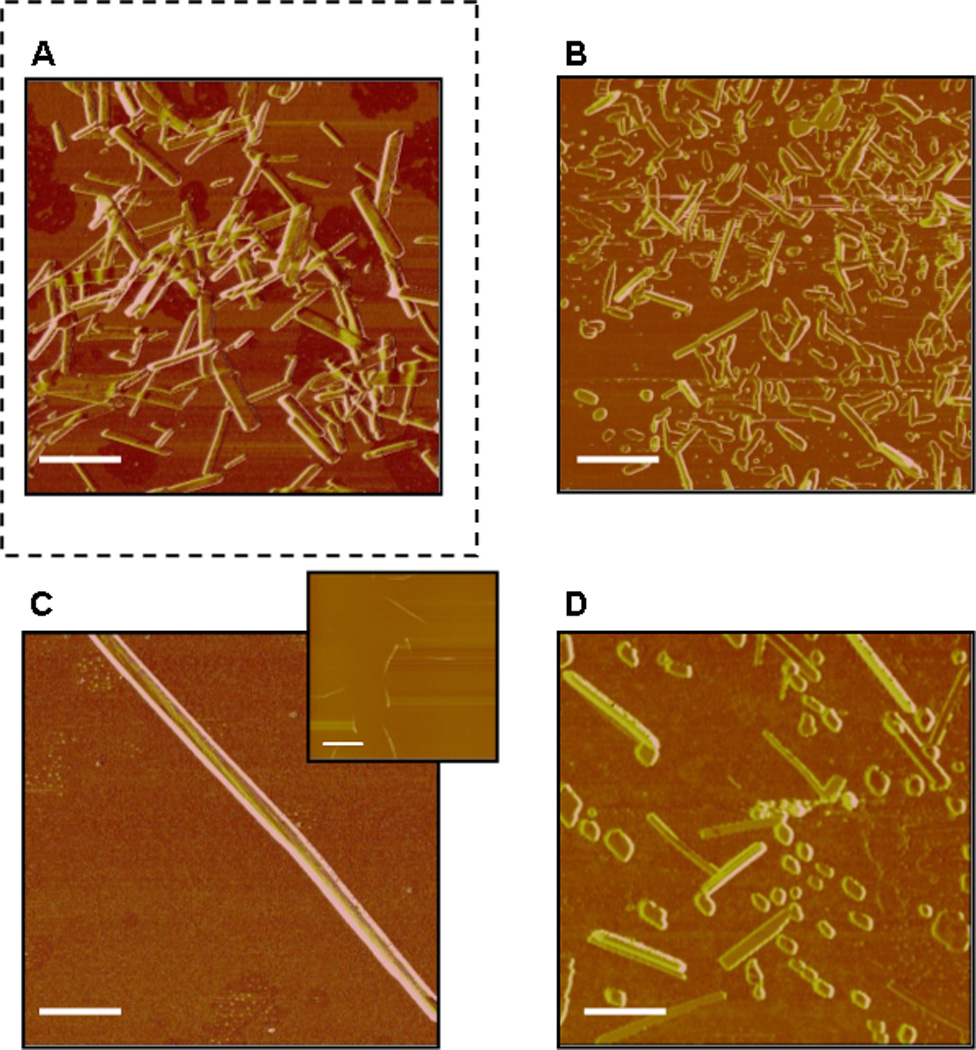

A. Pure 1; Equimolar mixtures of 1 and: B. cholesterol; C. DMPC; D. DMPS; E. DOPG; F. POPG. The scale bar in images corresponds to 1 µm (scale bar in insert of C corresponds to 15 µm).
Figure 2 presents AFM images of Langmuir monolayers of pure DAG-lactone 1 (Figure 2A) and 1 deposited in equimolar mixtures with different lipids (Figure 2B–F). All images in Figure 2 were acquired after deposition of the monolayers on water in a Langmuir trough, equilibration for a few minutes, isothermal compression to around 30 mN/m, and transfer onto solid substrates. Previous studies of Langmuir monolayers of several rigid-rod DAG-lactone derivatives demonstrated that lipid molecules strongly affect monolayer properties and organization at the air/water interface[16].
The AFM images (Figure 2B–F) and the corresponding statistical analysis (Figure 1, Supporting Information) demonstrate that even small variations in phospholipid headgroup, length, or degree of saturation of the acyl chains exert dramatic effects upon the structural features of the DAG-lactone nanostructures. For example, deposition of 1 with cholesterol resulted in shorter nanorods compared to 1 alone (Figure 2B). In comparison, mixing of 1 with dimyristoylphosphatidylcholine (DMPC) yielded extremely long and uniformly thick fibers (Figure 2C). The DMPC-modulated nanofibers of 1 were considerably longer than all the other needle-like structures observed. In contrast to the DMPC-induced fibers, when 1 was co-deposited with the negatively-charged dimyristoylphosphatidylserine (DMPS), which exhibits the same acyl backbone as DMPC albeit displaying a charged headgroup, the AFM experiment indicated the formation of a significant population of short fragments, as shown in Figure 2D.
Differences in the shapes and dimensions of 1 nanostructures were also apparent between films in which the DAG-lactone was mixed with dioleylphosphatidylglycerol (DOPG, Figure 2E) or palmitoyloleylphosphatidylglycerol (POPG, Figure 2F); these two lipids have identical head group and differences in lengths and saturation of the acyl chains. Specifically, abundant square-shaped “nano-islets” fused to elongated nanorods were detected in the mixed 1/DOPG monolayers (Figure 2E), while shorter thin nanorods were observed in the 1/POPG films (Figure 2F). Significant shape modulations of 1 structures were recorded in mixed films comprising other lipid molecules.
To further characterize the structural features of 1 nanostructures formed at air/water interface we carried out x-ray diffraction (XRD) experiments, comparing the as-synthesized powder of 1 (Figure 3A, bottom pattern) and the nanorods formed at the air/water interface that were transferred onto glass slides (Figure 3A, top pattern). The sharp reflections observed in the XRD pattern corresponding to the 1 nanorods self-assembled at the air/water interface (top spectrum, Figure 3A) indicate a high degree of crystallinity. The small reflections at around 6° and 11° in the film sample (Figure 3A, top) indicates that the film generated at the air-water interface constitutes also a small fraction of 1 organized in structures that are similar to those produced in the as-synthesized powder form.
Figure 3. XRD structure analysis of 1.


A. XRD patterns of 1 rods formed at the air/water interface showing a major Bragg peak at d = 19.1 Å and its second and third order reflections at 9.5 and 6.3 Å, respectively (top). The pre-dissolved powder (bottom) shows major peaks at different positions.B–C: Molecular models depicting possible organization of 1 at the air/water interface. B. Monolayer organization (note the large distance between the hydrophobic chains); C. Proposed bilayer structure.
Importantly, the distinctly different peak positions and intensities of 1 nanorod films compared to those of the powdered sample underscore the important role of the water interface in induction of the crystalline structures of 1. Furthermore, the fact that milligrams of the as-synthesized powder sample produced the XRD pattern in Figure 3A (bottom), while a much smaller sample quantity extracted from the film gave rise to the high reflections in Figure 3A (top), confirms the very high propensity for formation of crystalline structures of 1 at the air/water interface.
Based on the XRD and AFM data, and further employing molecular modelling, we aimed to illuminate the molecular organization of 1 at the air/water interface that leads to nanorod formation. Figure 3B depicts a monolayer of 1, an organization which seems relatively unfavourable due to the substantial distance between the di-phenyl side-chains. However, Figure 3C shows that a bilayer arrangement of 1 allows closer interactions of the acyl chains, which is promoted by π–π stacking interactions[22]. Importantly, the bilayer organization obtained by molecular modeling exhibits a thickness of ~19 Å which is very close in value to the spacing of the first order Bragg peak (19.1 Å) observed in the XRD pattern (Figure 3A).
The bilayer organization depicted in Figure 3C suggests that interactions between the condensed assemblies of 1 occur both with the polar lipid headgroups (expected to exhibit affinity to the lactone residues of 1), as well as with the lipid acyl chains, which are attracted to the hydrophobic di-phenyl moieties. This model implies that even slight structural differences among the lipid molecules would play a significant role in shaping the structural features of the 1 nanorods, as indeed observed in the AFM experiments in Figure 2
Representative surface-area/pressure isotherms of monolayers of 1 and several lipids, depicted in Figure 4, lend support to the proposed relationship between the lipids and 1 nanostructures formed at the air/water interface. The compression isotherm of 1 alone (Figure 4A) displays onset in surface pressure at a molecular area ~30 Å2 that is larger than the roughly estimated projected area of the hydrophobic chain of the molecule (~20 Å2). This initial liquid-expanded phase is followed by a transition to a condensed phase, approximately at 7 mN/m (Figure 4A)[16] at around half molecular area of the onset, as indeed depicted in the proposed model (Figure 3C).
Figure 4. Surface-pressure/area isotherms of 1/lipid monolayers.

A. 1 alone (no lipids added); B. 1/DMPC; C. 1/cholesterol; D. 1/DMPS. All 1/lipid monolayers were at a 1:1 mole ratio.
The presence of different lipids at equimolar concentrations within mixed monolayers (Figure 4B–D) gave rise to dramatic modifications of the isotherms, indicating pronounced interactions between 1 and the co-deposited lipids. The isotherm of 1/DMPC, for example, yields a single liquid-expanded phase (Figure 4B), similar to DMPC alone[23, 24]. 1/cholesterol, in contrast, yielded a highly condensed monolayer (Figure 4C), while 1/DMPS featured an initial liquid-expanded film that abruptly transformed into a condensed phase (Figure 4D).
The significant differences in the surface pressure/area isotherm profiles of the 1/lipid monolayers apparent in Figure 4, combined with the structural models depicted in Figure 3, point to the degree of monolayer rigidity as a likely prominent structural determinant of the nanostructures of 1 formed at the air/water interface. Specifically, in fluid monolayers, such as 1/DMPC (Figure 4B), 1 can assemble into highly elongated structures with minimal hindrance from the co-deposited phospholipid molecules, as apparent in the AFM image in Figure 2C. However, in the presence of lipids that give rise to highly condensed monolayers, such as cholesterol (Figure 4C), the π–π stacking interactions between the assembled DAG-lactones are much more constrained, leading to shorter and irregular assemblies, visible in the AFM analysis (Figure 2B).
In summary, we have discovered that a biomimetic DAG-lactone pharmacophore forms unique crystalline nanostructures at the air/water interface. Our results indicate that the water surface provided a platform for self-assembly of DAG-lactone 1 nanorods and consequent interactions with co-deposited lipid molecules. The structures and overall morphologies of the DAG-lactone assemblies were significantly modulated by the lipid environments in mixed lipid/DAG-lactone monolayers. Thermodynamic, microscopic, and spectroscopic analyses indicate that the structure and organization of 1 are determined both by the hydrophilic attraction between the lactone moieties and lipids’ headgroups, as well as hydrophobic interactions between the rigid side-residue of 1 and the lipids’ acyl chains. Furthermore, monolayer rigidity appears to be an important factor affecting the assembly properties of the DAG-lactone nanostructures. Other DAG-lactone derivatives examined in our laboratory exhibited similar aggregation properties, pointing to the generality of this self-assembled nanostructure phenomenon. The observation of lipid-modulated self-assembly of DAG-lactone nanostructures points to biomimetic chemistry as an intriguing frontier for nanotechnology research.
Experimental Section
Materials
Phospholipids, including 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC), 1,2-dimyristoyl-sn-glycero-3-[phospho-L-serine] (DMPS), 1,2-dioleoyl-sn-glycero-3-[phospho-rac-(1-glycerol)] (DOPG), 1-palmitoyl-2-oleoyl-sn-glycero-3-[phospho-rac-(1-glycerol)] (POPG) and cholesterol were obtained from Avanti Polar Lipids (Alabaster, AL) as a powder and used as provided. The DAG-lactone 1 was synthesized as described elsewhere[15]. Chloroform (CHCl3) and methanol (CH3OH) were HPLC grade (Frutarom Ltd., Haifa, Israel). Parent solutions (2 mM) of the phospholipids and the DAG lactone were prepared by co-dissolution in chloroform. Glass substrates for X-ray diffraction and AFM measurements were obtained from Menzel-Glaser (Romikal Ltd, Beer Sheva, Israel).
Surface-pressure/area isotherms
Surface-pressure/area isotherms were recorded using a computerized Langmuir trough (model 622/D1, Nima Technology Ltd., Coventry, U.K.). The water subphase used in the Langmuir trough was doubly purified with a Barnstead D7382 water purification system (Barnstead Thermolyne Corporation, Dubuque, IA), yielding 18.3 mΩ resistivity. The surface pressure was monitored using 1-cm-wide filter paper as a Wilhelmy plate. For each isotherm experiment, the desired amount of 1 or 1/lipid mixture in chloroform (total concentration of 2 mM) was spread on the water subphase and equilibrated for 15 min, allowing for solvent evaporation prior to compression. Compression was carried out at a constant barrier speed of 8 cm2 min−1. Each isotherm represents three experimental runs, which were reproducible within an error of 1.0 Å2 molecule−1.
Atomic Force Microscopy (AFM)
AFM images were obtained with a 100 µm scanner using a Dimensions 3100 instrument (Veeco, United States). Microfabricated Si-cantilevers - NSC15 (Mikromasch, United States) with integrated pyramidal tips were used. The 512 pixel × 512 pixel images were taken in a noncontact mode with a scan size of up to 80 µm, at a scan rate of 0.250 −1 Hz.
Scanning White Light Interferometry (SWLI)
SWLI images were obtained with a New View 200 (Zygo, United States) with 50× mirou objective NA 0.65. The images size are 240 pixel × 320 pixel with a pixel size of 0.9µm2 down to 0.22 µm2, depending on the zoom.
Brewster Angle Microscopy (BAM)
A Brewster angle microscope (NFT, Gottingen, Germany) mounted on a Langmuir film balance was used to observe the microscopic structures in situ. The light source of the BAM was a frequency-doubled Nd:YAG laser with a wavelength of 532 nm and 20–70 mW primary output power in a collimated beam. The BAM images were recorded with a CCD camera. The scanner objective was a Nikon superlong working distance objective with nominal 10× magnification and a diffraction limited lateral resolution of 2 µm. The images were corrected to eliminate side ratio distortion originating from a nonperpendicular line of vision of the microscope.
Molecular modeling
The proposed molecular models were generated with the aid of Cerius2 (Accelrys Inc., San-Diego, United States). The molecules were positioned manually in monolayer and bilayer forms envisaged to suit both the interfacial environment and the XRD pattern of the fibrilar assemblies.
Fluorescence confocal microscopy
Fluorescence images were acquired on an UltraView ERS confocal system (spinning disc), Perkin Elmer Co., equipped with an Axiovert-200 M microscope (Zeiss, Germany) with a Plan-Neofluar 63×/1.4 oil objective. Excitation was at 405 nm using a diode laser source. Images of DPH-doped films were acquired in fast sequential mode using an EM 445/60W (Blue) filter.
X-ray diffraction (XRD)
XRD measurements were carried out using a Bruker D8 Discover X-ray diffractometer in standard locked couple regime with an X-ray wavelength of 1.54 Å from a copper anode and parallel beam. The samples were represented by squares 2 cm on a side. The measurements were optimized for the signal/noise ratio by sample position, and 2′ increments with each measurement were made over a time period of about one hour. The data were processed by standard Eva program (Brukker) for better representation.
Acknowledgements
This work was supported in part by the intramural research program of the National Cancer Institute, Center of Cancer Research, the National Institutes of Health.
References
- 1.Reches M, Gazit E. Current Nanoscience. 2006;2:105–111. [Google Scholar]
- 2.Wang C, Huang L, Wang L, Hong Y, Sha Y. Biopolymers. 2007;86:23–31. doi: 10.1002/bip.20681. [DOI] [PubMed] [Google Scholar]
- 3.Hamada D, Yanagihara I, Tsumoto K. Trends in biotechnology. 2004;22:93–97. doi: 10.1016/j.tibtech.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Arvinte T, Cudd A, Drake AF. The Journal of biological chemistry. 1993;268:6415–6422. [PubMed] [Google Scholar]
- 5.Shi Y, Stouten PF, Pillalamarri N, Barile L, Rosal RV, Teichberg S, Bu Z, Callaway DJ. Biophysical chemistry. 2006;120:55–61. doi: 10.1016/j.bpc.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 6.Azriel R, Gazit E. The Journal of biological chemistry. 2001;276:34156–34161. doi: 10.1074/jbc.M102883200. [DOI] [PubMed] [Google Scholar]
- 7.Reches M, Gazit E. Science (New York, N.Y. 2003;300:625–627. doi: 10.1126/science.1082387. [DOI] [PubMed] [Google Scholar]
- 8.Barakat Nasser AM, Kim Bongsoo, Kim HY. J. Phys. Chem. C. 2009;113:531–536. [Google Scholar]
- 9.Zhang X, Goux WJ, Manohar SK. Journal of the American Chemical Society. 2004;126:4502–4503. doi: 10.1021/ja031867a. [DOI] [PubMed] [Google Scholar]
- 10.Di Benedetto Francesca, Mele Elisa, Camposeo Andrea, Athanassiou Athanassia, Cingolani Roberto, Pisignano D. Adv. Mater. 2008;20:314–318. [Google Scholar]
- 11.Wu Jianbo, Zhang Hui, Ma Xiangyang, Jun Li FS, Du Ning, Yang D. Materials Letters. 2006;60:3895–3898. [Google Scholar]
- 12.Lashuel Hilal A, LaBrenz Steven R, Woo Linda, Serpell Louise C, Kelly JW. J. Am. Chem. Soc. 2000;122:5262–5277. doi: 10.1021/ja9937831. [DOI] [PubMed] [Google Scholar]
- 13.Marquez VE, Blumberg PM. Accounts of Chemical Research. 2003;36:434–443. doi: 10.1021/ar020124b. [DOI] [PubMed] [Google Scholar]
- 14.Griner EM, Kazanietz MG. Nature reviews. 2007;7:281–294. doi: 10.1038/nrc2110. [DOI] [PubMed] [Google Scholar]
- 15.Malolanarasimhan K, Kedei N, Sigano DM, Kelley JA, Lai CC, Lewin NE, Surawski RJ, Pavlyukovets VA, Garfield SH, Wincovitch S, Blumberg PM, Marquez VE. J. Med. Chem. 2007;50:962–978. doi: 10.1021/jm061289j. [DOI] [PubMed] [Google Scholar]
- 16.Philosof-Mazor L, Volinsky R, Comin MJ, Lewin NE, Kedei N, Blumberg PM, Marquez VE, Jelinek R. Langmuir. 2008;24:11043–11052. doi: 10.1021/la802204n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adi S, Adi H, Chan HK, Young PM, Traini D, Yang R, Yu A. Langmuir. 2008;24:11307–11312. doi: 10.1021/la8016062. [DOI] [PubMed] [Google Scholar]
- 18.Madani-Grasset Frédéric, Phamb Nhan T, Glynosa Emmanouil, Koutsosa Vasileios. Materials Science and Engineering B. 2008;152:125–131. [Google Scholar]
- 19.Jin Y, Chen S, Xin R, Zhou Y. Colloids and surfaces. 2008;64:229–235. doi: 10.1016/j.colsurfb.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 20.Rapaport H, Kuzmenko I, Lafont S, Kjaer K, Howes PB, Als-Nielsen J, Lahav M, Leiserowitz L. Biophys J. 2001;81:2729–2736. doi: 10.1016/S0006-3495(01)75915-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Birdi KS. New York: Kluwer Academics/Plenum Publishers; 1999. [Google Scholar]
- 22.Gazit E. Chemical Society reviews. 2007;36:1263–1269. doi: 10.1039/b605536m. [DOI] [PubMed] [Google Scholar]
- 23.Kundu S, Chakraborty H, Sarkar M, Datta A. Colloids and surfaces. 2009;70:157–161. doi: 10.1016/j.colsurfb.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 24.Cseh R, Benz R. Biophys J. 1999;77:1477–1488. doi: 10.1016/S0006-3495(99)76995-X. [DOI] [PMC free article] [PubMed] [Google Scholar]


