Abstract
In humans, the majority of sustained traumatic brain injuries (TBIs) are classified as ‘mild’ and most often a result of a closed head injury (CHI). The effects of a non-penetrating CHI are not benign and may lead to chronic pathology and behavioral dysfunction, which could be worsened by repeated head injury. Clinical-neuropathological correlation studies provide evidence that conversion of tau into abnormally phosphorylated proteotoxic intermediates (p-tau) could be part of the pathophysiology triggered by a single TBI and enhanced by repeated TBIs. However, the link between p-tau and CHI in rodents remains controversial. To address this question experimentally, we induced a single CHI or two CHIs to WT or rTg4510 mice. We found that 2x CHI increased tau phosphorylation in WT mice and rTg4510 mice. Behavioral characterization in WT mice found chronic deficits in the radial arm water maze in 2x CHI mice that had partially resolved in the 1x CHI mice. Moreover, using Manganese-Enhanced Magnetic Resonance Imaging with R1 mapping – a novel functional neuroimaging technique – we found greater deficits in the rTg4510 mice following 2x CHI compared to 1x CHI. To integrate our findings with prior work in the field, we conducted a systematic review of rodent mild repetitive CHI studies. Following Prisma guidelines, we identified 25 original peer-reviewed papers. Results from our experiments, as well as our systematic review, provide compelling evidence that tau phosphorylation is modified by experimental mild TBI studies; however, changes in p-tau levels are not universally reported. Together, our results provide evidence that repetitive TBIs can result in worse and more persistent neurological deficits compared to a single TBI, but the direct link between the worsened outcome and elevated p-tau could not be established.
Keywords: concussion, CTE, neurodegeneration, rodent behavior, tau, TBI
Introduction
Epidemiological evidence suggests that a single TBI may hasten the onset of neurodegenerative disorders and dementia, with as much as a 60% greater risk of developing dementia following a TBI compared with age-matched individuals with no history of TBI (Barnes et al., 2014). A conservative estimate for the number of Americans that suffer a mild TBI each year is 1.5 million, with mild TBI accounting for at least 75% of all TBIs (CDC, 2003; Faul et al., 2010). While not all blows to the head lead to neurodegeneration, evidence suggests that a mild TBI can result in progressive brain atrophy and persistent cognitive dysfunction (Gardner and Yaffe, 2015; Rabinowitz et al., 2015). Moreover, individuals who suffer a TBI are often at risk for multiple head injuries: because of hobbies, occupation, age, or medication use. Therefore, understanding the vulnerability of the brain to single vs. repetitive non-penetrating closed head injury (CHI) is vital for mitigating the enhanced risk for developing dementia associated with a TBI.
Abnormal tau hyperphosphorylation and aggregation is a pathological feature of TBI in athletes, military personnel, and aged individuals (Abisambra and Scheff, 2014). This suggests that conversion of tau into abnormally phosphorylated proteotoxic intermediates is a secondary event triggered by a single TBI and enhanced by repeated TBIs. TBI-related changes in tau mimic many aspects of those seen in Alzheimer’s diease (Abisambra and Scheff, 2014). In TBI, intracellular tau aggregates develop and progress over time; this feature is shared by at least 24 neurodegenerative disorders collectively termed tauopathies. In fact, much like other neurodegenerative disorders of tau aggregation such as AD, progressive supranuclear palsy, and frontotemporal dementia, small aggregates of hyperphosphorylated tau emerge early in the disease process and increase in size over time until they appear as NFTs (Abisambra and Scheff, 2014; Braak and Braak, 1991). Epidemiological and clinical-pathological correlation studies support the involvement of tau in the neuropathological changes following single and repetitive CHI, yet how CHI induces the conversion of tau into abnormally phosphorylated proteotoxic intermediates remains controversial (Bolton-Hall et al., 2019; Ojo et al., 2016b; Wojnarowicz et al., 2017). Therefore, we sought to test the hypothesis that two CHIs would induce worse phenotypic deficits than a single CHI, and that the increased phenotype seen after a 2x CHI would correspond to greater p-tau in the animals. To test this hypothesis, we used WT mice and the rTg4510 tauopathy mouse model.
Collectively, our current findings demonstrate that repeated CHI significantly increases phosphorylated tau (p-tau) species in the injured cortex of WT mice subacutely. Repeated CHI did not grossly exaggerate cognitive dysfunction at 14d post-injury (p.i.) compared to single injury; however, at 30d p.i., 2x CHI WT mice exhibited persistent cognitive deficits associated with learning and memory retention. Utilizing the same injury paradigm, we observed a robust decrease in MEMRI ΔR1 functional imaging in pre-pathological 2x CHI rTg4510 mice, relative to the single CHI cohort, suggesting abnormal voltage-gated calcium response to repeated trauma (Antkowiak et al., 2012; Fontaine et al., 2017; Vandsburger et al., 2012). Similar to our findings in WT mice, our data demonstrate that 2x CHI rTg4510 mice had increased p-tau; however, these changes were not significantly different from single CHI rTg4510 mice. Overall, our data demonstrate the increased susceptibility in terms of dysfunctional outcomes of the brain to repeated trauma. Our results, together with a systematic review of rodent mild repetitive CHI studies, found only modest evidence for abnormal tau species as a critical interaction point for the worse phenotype seen following repetitive vs. single CHI.
Methods and Materials
Animals:
All procedures were approved by the Institutional Animal Care and Use Committee of the University of Kentucky, and studies were conducted in accordance with the standards of proper experimentation in the Guide for the Care and Use of Laboratory Animals and ARRIVE guidelines. Experiments used either 4-month-old C57BL/6J mice or 2-month-old rTg4510 mice. Experiments included an equal ratio of male and female mice for all endpoints. Data for both sexes were combined after observing no differences between the responses of male and female mice tested. However, the study was not statistically powered to assess sex differences; thus, we cannot conclude that sex differences did not affect our results. The number of mice used for each endpoint is shown in the figure legend. One mouse died from apnea after the 2nd CHI surgery, and one mouse was excluded because of poor healing of the surgical incision; both mice were from the 2x CHI group (1d) in Figure 1. No other mice died or were excluded from the study. Mice were randomized and assigned groups before the start of the experiment. The study was completed with multiple batches of mice using a block experimental design, with each batch including all experimental groups and the order of the group was randomized for each block so that the order of sham and CHI surgery varied for each cage. Each cage of mice included more than one experimental group. Each subject was given a unique identification number, which does not identify the experimental group. The persons conducting the endpoint analysis were blinded to the treatment conditions.
Figure 1: Characterization of 2-hit CHI model in WT mice.
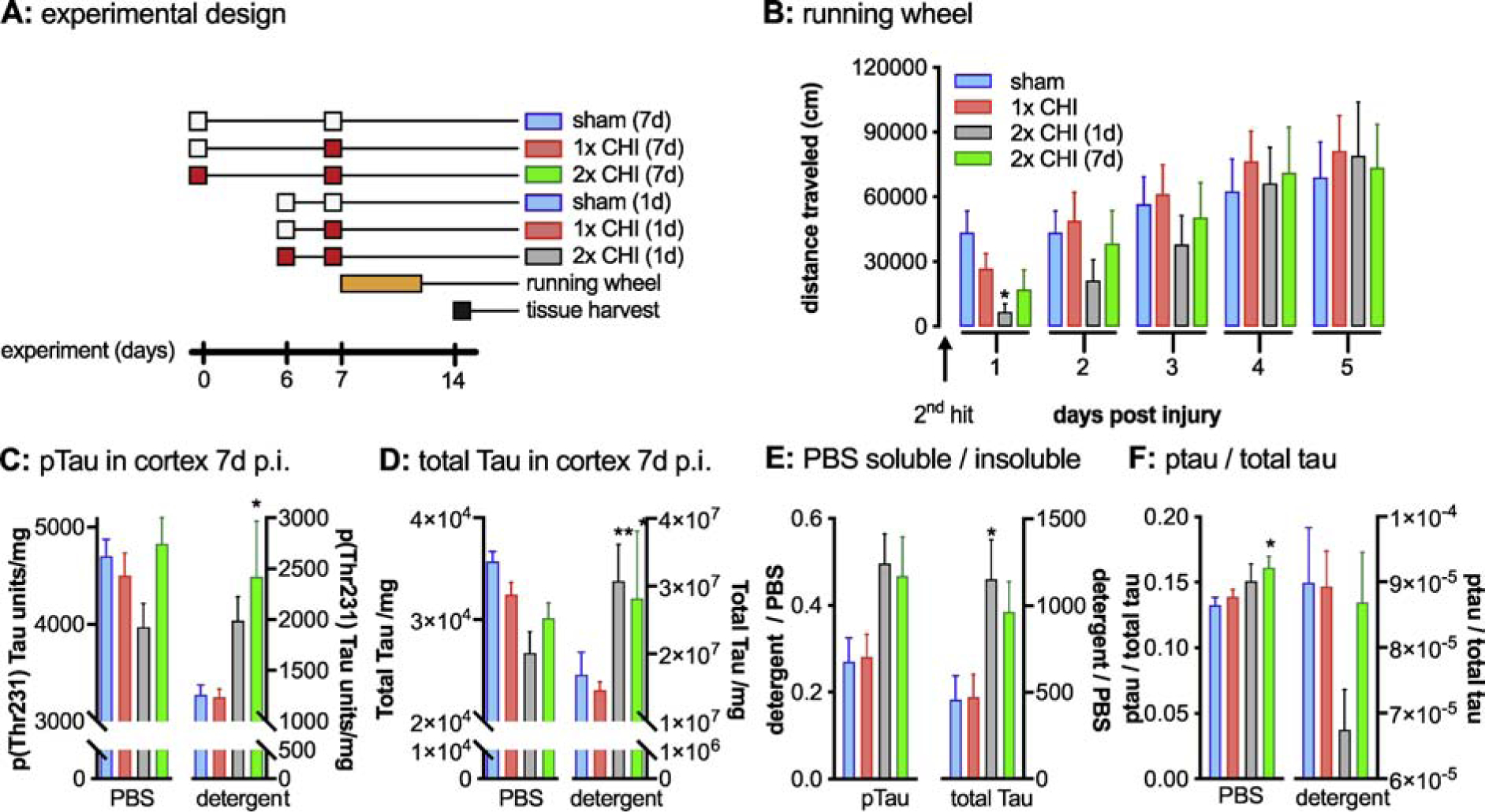
(A) Graphical summary of the overall experimental design is shown. The white boxes indicating a sham surgery, with the red boxes indicating a CHI surgery. (B) Mice were tested in the voluntary running wheel assay and the total distance traveled per day is shown in the bar graph. At 1d post injury (p.i.) the mice 2x CHI (1d) ran significantly less than the sham injured mice. *sham vs. 2x CHI (1d) p=0.032. A Mesoscale discovery Elisa was used to measure p-tau and total tau in a PBS soluble and detergent (TPER) soluble fraction of the cortex brain homogenate at 7d post injury (C) An increase in detergent soluble p-tau (*sham vs. 2x CHI (7d) p=0.017), (D) and total tau (**sham vs. 2x CHI (1d) p=0.0027. *sham vs. 2x CHI (7d) p=0.0257) was seen in the 2x CHI groups. (E) A shift in the ratio of detergent soluble to PBS was found in the 2x CHI groups. (*sham vs. 2x CHI (1d) p=0.045). (F) Ratio of p-tau to total tau is increased in the PBS soluble fraction (*sham vs. 2x CHI (7d) p=0.033). Sham n=11, 1x CHI n=12, 2x CHI (1d) n=4, 2x CHI (7d) n=8.
Closed head injury (CHI):
The CHI model was performed as previously described (Bachstetter et al., 2015; Webster et al., 2015). Briefly, mice were anesthetized with isoflurane (3–5%). The head was stabilized in a stereotaxic frame before a sagittal midline incision was made. A 1ml latex pipette bulb filled with water was placed under the head. The stereotaxic electromagnetic impactor used a 5.0mm flat steel tip (Leica Biosystems). It delivered a closed-skull midline impact (coordinates: mediolateral, 0.0mm; anteroposterior, 1.5mm) 1.0mm deep with a controlled velocity (5.0±0.2m/s) and a dwell time of (100ms). Sham mice received the incision, but not the impact. At time 0, all mice received a sham or CHI surgery. At 24h or 168h after the first surgery, the mice underwent a second sham or CHI surgery.
Nesting Behavior:
The nesting test followed established protocols as previously described (Webster et al., 2015). Briefly, a 5cm × 5cm pressed cotton square was added to the cage between 4–5pm. After 15–16h, two observers blind to the experimental conditions scored the quality of the nest following a semi-quantitative 5-point scale (1: >90% of nestlet intact; 2: 50–90% of nestlet intact; 3: 10–50% of nestlet intact but no identifiable nest site; 4: <10% of nestlet intact, nest is identifiable but flat; 5: <10% of nestlet intact, nest is identifiable with walls higher than the mouse body).
Running wheel behavior:
The running wheel assay followed our previously described protocol (Webster et al., 2015). Briefly, at 12–14h following the CHI or sham injury, the mice were introduced into the running wheels (Lafayette Instruments; Lafayette, IN) and data was recorded for 5 days. Computerized counting software (Lafayette Instruments; Lafayette, IN) automatically recorded the total distance run each hour by each animal, and the 1-hour blocks were combined into 1d blocks.
Radial Arm Water Maze (RAWM) behavior:
The six-arm RAWM followed the well-established protocol, as previously described (Bachstetter et al., 2015; Webster et al., 2015). Briefly, in block 1 (first 6 trials) and block 2 (6 trials), mice were trained to identify the platform location by alternating between a visible and a hidden platform (3 hidden platform trials and 3 visible platform trials for each block). Block 3 (3 trials) used only a hidden platform. The next one or two day, mice were tested in 3 blocks of 5 trials each (blocks 4–6; 15 total trials), all using only a hidden platform. Data are presented as the average errors per block during the hidden platform trials. RAWM performance was recorded and scored using EthoVision XT 8.0 video tracking software (Noldus Information Technology).
Brain tissue harvesting, biochemical endpoints:
Mice were deep anesthetized with 5% isoflurane prior to transcardial perfusion with ice-cold PBS for 5 min. The brains were rapidly removed, dissected, processed, and archived for subsequent biochemical analysis as previously described (Webster et al., 2015). Brain homogenates for tau levels were made as previously described (Webster et al., 2015). Levels of p-Thr231 and total tau were measured by V-Plex ELISA from Meso Scale Discovery (MSD) according to the manufacturer’s instructions. Western blots were performed as previously described with minor modifications (Koren et al., 2019; Meier et al., 2015). Briefly, protein levels were first determined by BCA. Western blotting was performed with SDS-PAGE using 10% tris-glycine gels (Biorad). Samples were then transferred onto polyvinylidene difluoride (PVDF) membrane. Following a 1 hour block in 5% milk in tris-buffered saline (TBS), blots were incubated with primary antibody in 5% milk/TBS overnight. The following day, blots were washed in TBS, incubated with horseradish peroxidase-labeled secondary antibody (ThermoFisher) for one hour, washed again, and then exposed using West Pico Plus chemiluminescent substrate (ThermoFisher) on the Amersham 680 Imager. Stripping of blots in between probes was performed with incubations in 0.5 M sodium hydroxide for 15 minutes followed by TBS washes. In this fashion, PHF1 (abcam), H150 (Santa Cruz), and Actin (Santa Cruz) antibodies were all serially applied to the same membrane. Western blot images were analyzed using imageJ software.
Magnetic Resonance (MR) Imaging:
MR imaging was performed in the University of Kentucky Magnetic Resonance Imaging and Spectroscopy Center (MRISC) on a 7-Tesla Clinscan scanner (Bruker, Billerica, MA, USA) using a cylindrical volume coil for excitation and a cryocoil for detection as previously described (Fontaine et al., 2017). Briefly, manganese chloride (MnCl2, 30 mM) prepared in saline was delivered to mice via intraperitoneal injection (66 mg/kg). Imaging was performed before the injection of MnCl2 (baseline) and repeated at 6 hours after injection. During the imaging, mice were anesthetized using isoflurane, and vital signs (core temperature and respirations) were monitored. Look-Locker imaging was performed following nonselective spin inversion in one slice of the brain containing large regions of hippocampus. Fifty images were acquired following inversion with image spacing of 100 ms (total sequence repetition time of 5s) to fully sample the T1 relaxation curve. Additional image parameters included TR/TE = 5500/1.9, Matrix = 128 × 128, number of averages = 3, field of view = 17 mm × 17 mm × 0.7 mm. T2-weighted images were acquired covering the entire brain (excluding the cerebellum and olfactory bulb) using a turbo-spin echo sequence with TR/TE = 3360/42, Slices = 21, Matrix = 448 × 336, number of averages = 2, field of view = 25 mm × 25 mm × 0.5 mm. The imaging procedures for scanning a mouse were completed in 45 minutes.
MR data analysis:
Image mapping and analysis were performed in MATLAB (Mathworks, Natick, MA, USA). Images from the Look-Locker series were used to reconstruct voxel-by-voxel signal relaxation curves which were fit to the equation S(TI) = So*(1−e−R1*TI), where S(t) represents the signal at a given inversion time (TI), So represents the steady-state signal at maximal TI, and R1 represents the longitudinal relaxation rate. Regions of interest (dentate gyrus [DG], cornu ammonis 1 [CA1], cornu ammonis 3 [CA3], and superior medial cortex [CTX]) were identified using the Allen Brain Mouse Atlas. Within each, the change in R1 relaxation rates (ΔR1) before and after MnCl2 exposure was calculated as ΔR1 = R16h − R1baseline.
Statistical Analysis:
JMP Software version 12.0 (SAS institute, Cary, NC, USA) was used for statistical analysis. A repeated-measures ANOVA was used for RAWM. For all other endpoints, a one-way ANOVA was used comparing injury groups. If a significant main effect was found, post hoc analysis was used to compare groups. Differences between mean were considered significant at α=0.05. Graphs were generated using GraphPad Prism version 7.0. Values are expressed as mean ± SEM, unless otherwise noted. Number of mice used for each endpoint are indicated in the figure or figure legend.
Systematic review:
Search Criteria:
Our search criteria were established to be specific for mild TBI models caused by a closed head injury (Bodnar et al., 2019). Following guidelines established by PRISMA (Moher et al., 2009), comprehensive searches (on 5/15/2018) of both PubMed and Web of Science were conducted using the following keyword search: mild TBI, concussion, closed head injury, rodent, mice, mouse, or rat; excluding controlled cortical impact, fluid percussion, or review papers. Using the advanced search tools on PubMed and Web of Science, we searched both title and abstract with the following Boolean search strategy: [(((((((rodent) OR rat) OR mouse) OR mice)) AND (((mild TBI) OR concussion) OR closed head injury))) NOT ((CCI) OR fluid percussion)]. From PubMed, 984 articles were given in the final results and Web of Science produced 1,336 articles. Both of these lists of articles were combined, and duplicate references were removed leaving 1,890 articles (Figure 1). The search was repeated on PubMed on 4/3/19 to identify additional articles published in the previous year. The following MESH Headings were used in the search [brain concussion, AND (mice or rats)], resulting in 411 articles.
Inclusion/Exclusion Criteria:
Abstracts and titles were screened by CNB to include only peer-reviewed primary research reports specific for mild, closed head traumatic brain injury only in rodents. All other types of articles were excluded. Blast injuries were excluded because although military related blast injuries are a major cause of TBI (DeWitt et al., 2013), the vast complexity of the different injury models used were beyond the scope of this review (Courtney and Courtney, 2015).
Results
Characterization of 2-hit CHI model.
A number of repetitive CHI models have been previously reported, with the range between impacts typically varying between minutes-to-weeks (for review see: (Bolton-Hall et al., 2019; Ojo et al., 2016b; Wojnarowicz et al., 2017)). To validate a repetitive CHI model in our hands, we started with only two impacts and selected either a 1 day (d) or 7d interval between impacts (Fig. 1A). As we did not find a difference between the sham groups with a 1d or 7d interval, or between the single CHI groups with a 1d or 7d interval, those groups are collapsed into a sham or 1x CHI group, respectively.
Voluntary running wheel behavior was monitored on day 1–5 p.i. to measure a composite functional outcome that includes variables of motor function, general activity levels, and anhedonia (Hibbits et al., 2009; Liebetanz et al., 2007), and thus provides a measure of overall function after the injury. As shown in Figure 1B, the group with two CHIs separated by 1d were found to travel significantly less distance in the running wheel, with the 2x CHI group being statistically different at 1d p.i. compared to sham injured mice (F3,67=3.294; p=0.0257).
A 2-hit CHI induces alterations in p-tau
A hallmark pathology associated with repetitive TBIs is the accumulation of post-translationally modified phosphorylated tau protein (Abisambra and Scheff, 2014). Importantly, elevated levels of phosphorylation of tau at specific epitopes are also seen in tauopathic neurodegenerative diseases, where it has been shown that these changes correlate with varying stages of tauopathic disease progression (Augustinack et al., 2002). We sought to determine if single or repetitive CHI had an effect on phosphorylation and solubility of tau. As shown in Fig 1C, p-tau levels in the cortex of mice at 7d p.i. were increased in the 2x CHI groups in the detergent fraction (F3,31=3.294; p=0.0077), whereas there was no significant change in p-tau levels in the PBS soluble fraction after injury. In addition to changes in phosphorylated tau levels, the 2x CHI groups showed a significant increase in the distribution of total tau in the detergent fraction (F3,31=6.119; p=0.0022), and both 2x CHI groups had less PBS soluble tau than the sham injured group (Fig 1D). As we saw in general a decrease in the amount of p-tau and total tau in the PBS soluble fraction and an increase in the PBS insoluble fraction, we determined the ratio of detergent soluble to PBS soluble tau (Fig 1E). For both p-tau and total tau, we found that the 2x CHI increased the ratio of PBS insoluble tau to PBS soluble tau. While the trend was similar for both p-tau and total tau, an overall statistical difference was found only for total tau (F3,30=3.916; p=0.018). Comparing the ratio of p-tau to total tau, we found a statistical difference in the PBS fraction (F3,31=3.157; p=0.0385) (Fig 1F).
Repetitive-CHI induces lasting cognitive deficits not seen with a single CHI.
We found only small differences between an inter-injury interval of 1d or 7d; with possibly the 1d interval showing greater behavioral and tau localization changes compared to the 7d inter-injury interval (Fig. 1). In addition, we found that re-exposing the skull at the 7d inter-injury interval required a new scalp incision, as the first incision had closed by 7d post-surgery, and this second scalp incision was not needed at the 1d inter-injury interval. Therefore, moving forward with the project, we selected the 1d inter-injury interval for the rest of the experiments.
We previously reported in WT mice that a single CHI produces robust deficits in the nesting behavior and the 6-arm radial arm water maze (RAWM), at 1d p.i. and 14d p.i., respectively (Bachstetter et al., 2015; Webster et al., 2015). As shown in Figure 2A, mice received a sham or CHI surgery on day 1. Twenty-four hours later, mice received a second sham, the first CHI, or the second CHI surgery. At 1d after the second surgery, nesting behavior was measured. Nest-building is a naturalistic mouse behavior, which has components of thermoregulation, exploration, and is used for camouflage in the wild. We found an overall effect of injury in the nesting behavioral assay (p=0.0005, Kruskal-Wallis test), with both the 1x CHI and 2x CHI groups building significantly lower quality nests compared to the sham-injured group. However, we found no difference between the 1x CHI or 2x CHI groups in the nesting behavior, suggesting a floor effect in the assay.
Figure 2: Cognitive deficit associated with 2-hit CHI model in WT mice.
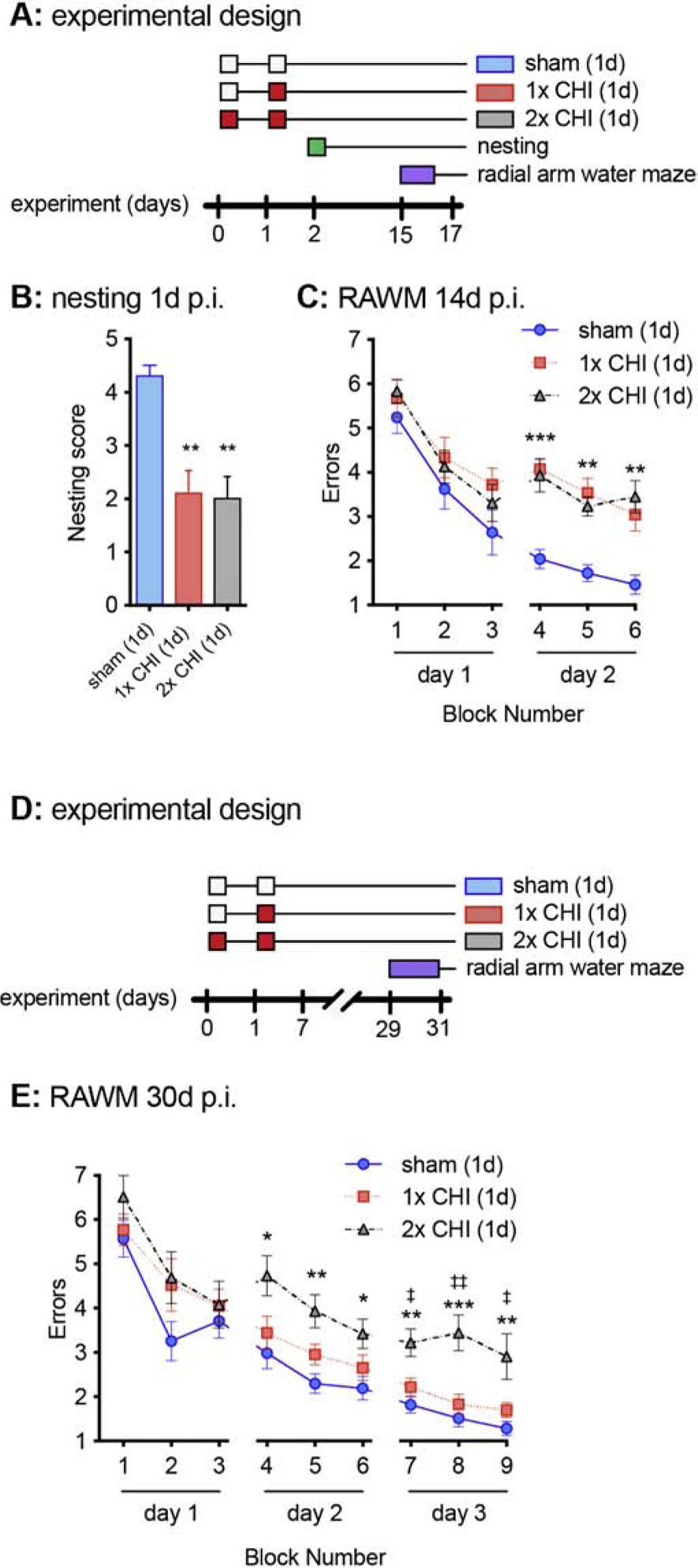
(A) Graphical summary of the overall experimental design is shown. The white boxes indicate a sham surgery, and the red boxes indicate a CHI surgery. (B) Nesting behavior was measured at 1d after the second surgery (p.i.). ** p<0.005, compared to sham (Dunn’s multiple comparisons test). (n=10 per group) (C) The 6-arm radial arm water maze (RAWM) was run over 2 consecutive days starting at day 14 after the second injury. The number of entries into arms, without finding the escape platform, was recorded as an error. ** p<0.005, *** p<0.0001 compared to sham (Tukey test). Sham n=15, 1x CHI n=14, 2x CHI n=14. (D) Graphical summary of the overall experimental design is shown. (E) The 6-arm RAWM was run over 3 consecutive days starting on day 28 after the second surgery. * p<0.05, ** p<0.005, *** p<0.0001 compared to sham (Tukey test). ‡ p<0.05, ‡‡ p<0.005 compared to 1x CHI (Tukey test). Sham n=14, 1x CHI n=13, 2x CHI n=9.
At 14d after the second surgery, mice were tested in the 6-arm RAWM. By a repeated measure ANOVA, we found an overall effect of the CHI (F2,39=18.57; p<0.0001). The group comparison analysis found that 1x CHI and 2x CHI groups performed more poorly in the RAWM compared to the sham-injured group (p<0.0001, Tukey test). No difference was observed between the mice that received a single versus repetitive CHI in the 6-arm RAWM at 14d p.i. (Fig 2C). The results of the 1x CHI group were in strong agreement with our previous study showing CHI-induced deficits in the RAWM (Bachstetter et al., 2015; Webster et al., 2015). The lack of a difference between the 1x and 2x CHI groups again suggested a floor effect in the assay. As we have previously found in WT mice the CHI-induced deficits in the 6-arm RAWM are mostly resolved by 30d p.i., we conducted a second experiment to determine if at 30d p.i. we could see a difference in behavioral performance between the 1x CHI and 2x CHI groups (Fig. 2D). In addition to the longer post-injury delay, we added a third day of testing to our RAWM protocol. By a repeated measure ANOVA, we found an overall effect of the CHI (F2,33=15.41; p<0.0001). We found that the 2x CHI group performed worse on the RAWM task compared to the sham group (p<0.0001, Tukey test), and the 1x CHI group (p<0.0042, Tukey test). In agreement with our previous work (Webster et al.), we found that the 1x CHI mice were able to perform the task almost as well as sham-injured mice (p=0.076, Tukey test).
Pathologically associated hyperphosphorylated tau is increased by CHI in 2mo rTg4510 mice.
In WT mice, we found changes p-tau at 7d p.i. as well as an increase in the PBS insoluble tau following a CHI. To directly test the effects of 1x or 2x CHI in a model predisposed to tauopathy, we used rTg4510 tauopathy mice (Santacruz et al., 2005). These mice develop aggressive tau pathology in the cortex and hippocampus beginning around 4 months of age (Ramsden et al., 2005; Santacruz et al., 2005) with profound changes in whole brain volume by 5.5 months of age (Ramsden et al., 2005). We chose to injure the rTg4510 mice at 2-months since this age represents a potential tipping point prior to the robust tau pathology and neurodegeneration. This enabled us to determine if a 1x CHI or 2x CHI would worsen progression of pathology in these mice (Fig 3A). At 13 days after the second surgery, we found no effect of 1x or 2x CHI on cortex or whole brain volume as measured by T2 MRI (Fig. 3B–C). Manganese-enhanced magnetic resonance imaging (MEMRI) is a sensitive in vivo neuroimaging method that can provide novel information about neuronal function and activity (Cloyd et al., 2018; Fontaine et al., 2017; Saar and Koretsky, 2018). We chose to use MEMRI, instead of other behavior assays, because of potential confounding behavior phenotypes seen in the rTg4510, including hyperactivity (Wes et al., 2014). The results of MEMRI revealed an attenuated increase of 52% in cortex R1 rates following MnCl2 infusion in the rTg4510 mice following 2xCHI compared to sham treated mice (Fig. 3D–E). A decrease in MEMRI-ΔR1 reflects a lower concentration of manganese accumulation in cells due to lower integrated manganese flux, and it is thought to correlate with the voltage-gated calcium channel activity (Antkowiak et al., 2012; Cloyd et al., 2018; Fontaine et al., 2017; Vandsburger et al., 2012). Using MEMRI, while an indirect measure of brain health, we found that the 2x CHI does have a greater effect than a 1x CHI. Future studies will be needed to determine if there is a change in abundance or activity of the voltage-gated calcium channel following a CHI.
Figure 3: Neuroimaging deficit associated with 2-hit CHI model in Tg4510 mice.
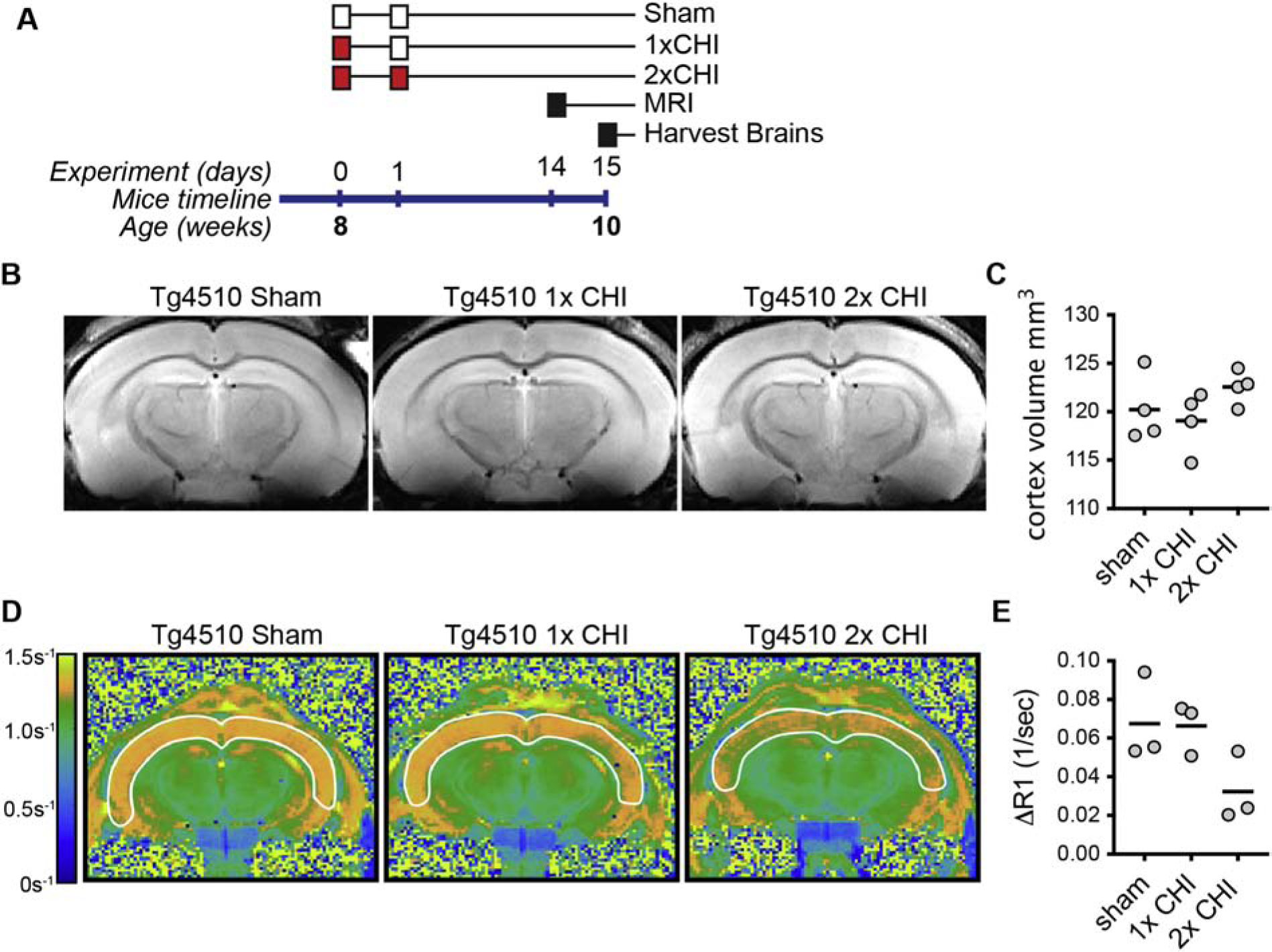
(A) Graphical summary of the overall experimental design is shown. The white boxes indicate a sham surgery, and the red boxes indicate a CHI surgery. (B) representative example of T2 MRI, and (C) quantification of the volume of the cortex in the mice (n= 4 per group). (D) representative example of the MEMRI, and (E) quantification of the ΔR1 relaxation rate in the cortex (n=3 per group).
To determine whether MEMRI deficits could be a result of worsened tau-dependent neuron degeneration we measured by immunoblot the amount of PHF1 (pS396/S404) positive tau, and total tau. We hypothesized that 2x CHI would enhance tau hyperphosphorylation compared to 1x CHI, and that increased p-tau levels would correlate with the MEMRI deficits. We found that compared to sham, 1x CHI (p=0.0496, Tukey test) or 2x CHI (p=0.0353, Tukey test) increased PHF1 levels in the cortex (Figure 4) (F2,21=4.402; p<0.0001). Total tau levels were also increased by 1x CHI or 2x CHI compared to sham levels; however, the increase was not statistically different. Strikingly, there was no difference between the 1x CHI or 2x CHI in the amount of PHF1 or total tau. There was no effect of CHI on the PHF1/total tau ratio. The lack of difference between the 1x CHI or 2x CHI in the amount of tau pathology suggests that a mechanism independent of direct effects on increasing p-tau burden must be associated with the differential effects of 1x CHI and 2x CHI seen by MEMRI.
Figure 4: Effects of 2-hit CHI on p-tau in the Tg4510 mice.
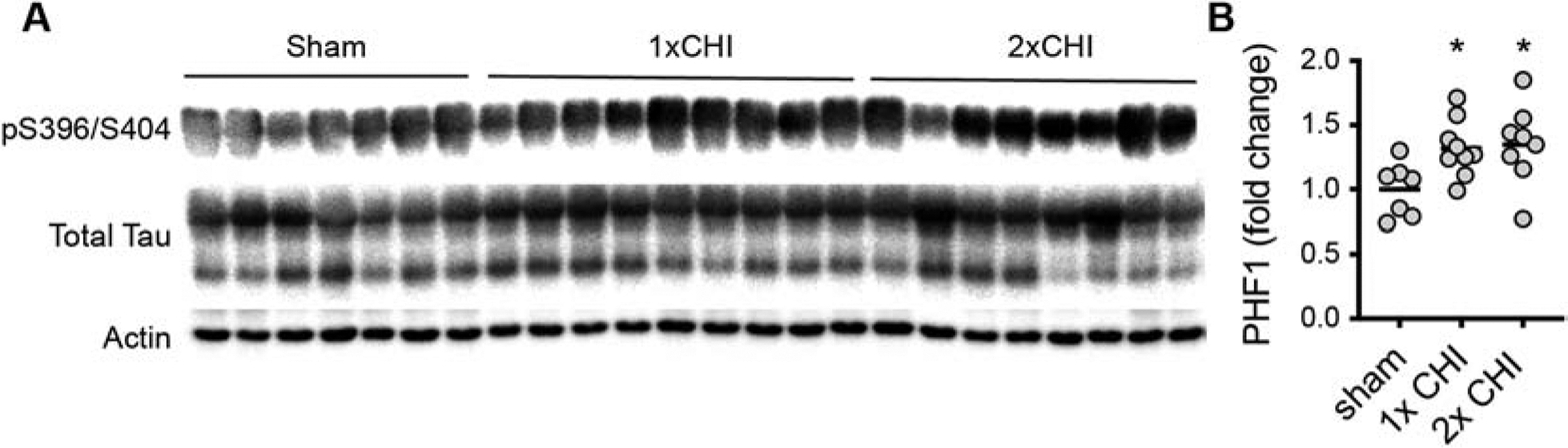
(A) Western blot for PHF1 (pS396/pS404 tau), and total tau in the PBS fraction of cortex homogenate. (B) Quantification of the PHF1 tau normalized to the actin loading control in the cortex of the Tg4510 mice. * p<0.05 compared to sham (Tukey test).
Discussion
We report here three key findings that deepen the understanding of the role of tau as a mediator of mechanisms associated with cognitive impairment following repetitive closed head TBI in mice. First, in support for a role of tau, we found that unlike a single CHI, 2x CHI was sufficient to increase the amount of p-tau in the detergent-soluble fraction of the cortex of WT mice. Correspondingly, learning and memory deficits were more persistent in the 2x CHI compared to the 1x CHI. Second, using the rTg4510 model of tauopathy, we found that 1x CHI and 2x CHI were both sufficient to increase PBS-soluble PHF1-tau (*p = 0.0496 and p=0.0353, respectively). Interestingly, 2x CHI, but not 1x CHI decreased an indirect measure of neuronal activity in the form of MEMRI. These results suggest that in a mouse prone to neuronal degeneration as a result of soluble tau accumulation, repetitive closed head injuries can worsen neuronal health. Importantly, the relationship between neuronal function and PBS-soluble PHF1-tau accumulation does not appear to be a linear one, as MEMRI was only decreased by 2x CHI, but 2x CHI did not induce a further significant increase in PHF1-tau beyond the increase caused by 1x CHI. This suggests that while tau may be affected as part of the pathological sequelae following CHI, it is likely not a reliable biomarker for severity of injury. Neurofibrillary tangles are known to correlate best with cognitive impairment in Alzheimer’s disease (Braak and Braak, 1991). However, tauopathy in the absence of amyloid beta plaques, as observed in primary age-related tauopathy (PART), is seen in most elderly individuals and is often not associated with profound cognitive changes (Crary et al., 2014); thus, neuronal tauopathy, as seen in PART, is not sufficient to present clinically with dementia.
Our study is not the first to explore the connection between p-tau and repetitive CHI in rodents. Therefore, to place our research in context with prior work in the field, and to understand differences in the common data elements associated with the injury models, we conducted a systematic review (Fig 5, and Table 1). Using broad search criteria, we identified over 2000 publications, which were screened for inclusion in our review. From this screen, we identified 132 original peer-reviewed publications of repetitive CHI models in rodents. Twenty five of these measured changes in tau. The primary goals of the review were to determine: (1) the time course of tau changes induced by repetitive CHI; (2) if there was a difference between a single vs. repetitive CHI; (3) if there was a difference dependent on the number of CHIs; and (4) what influence species difference or human tau transgenes have on tau changes.
Figure 5: Methods flow chart.
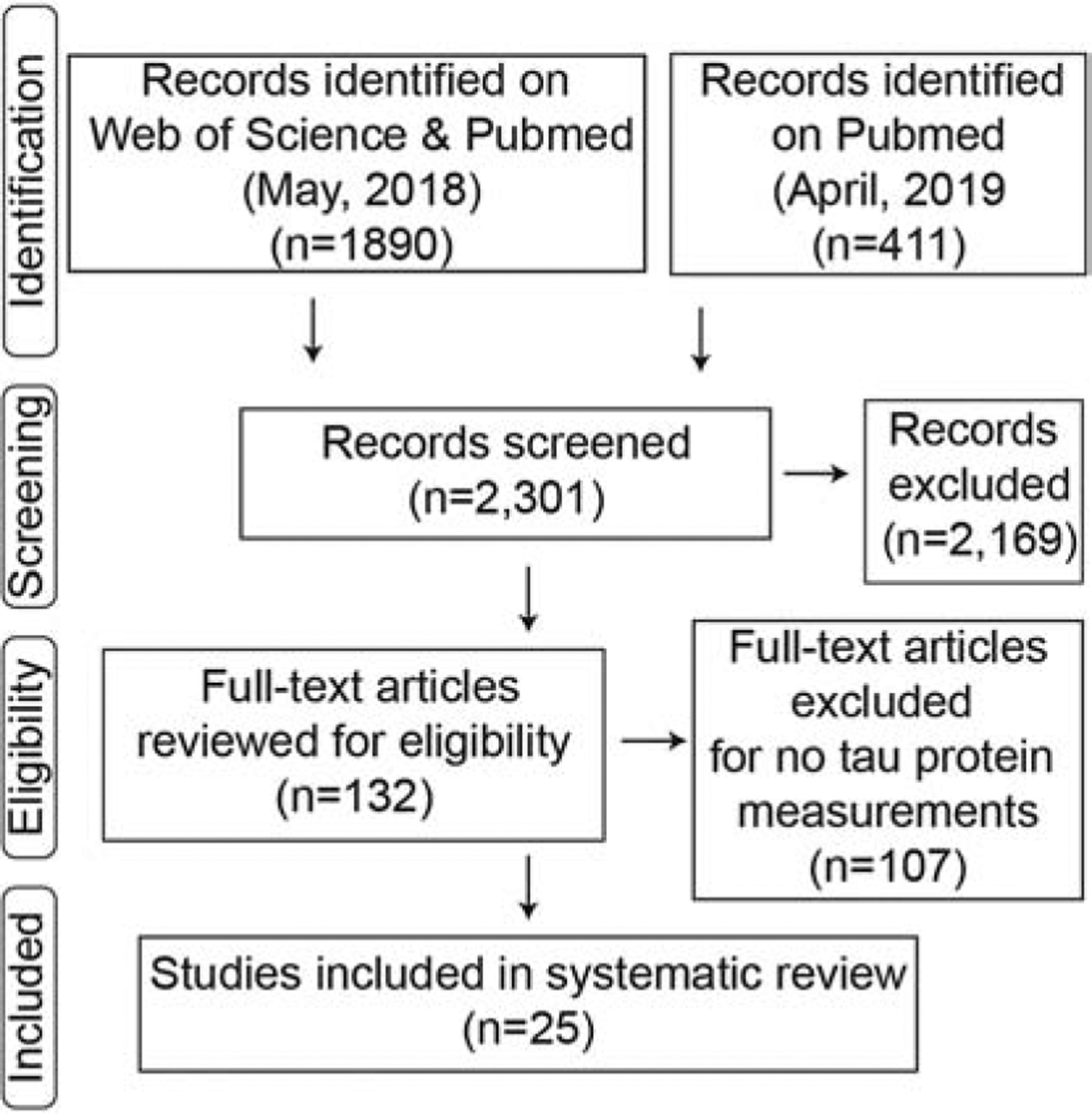
Identification through searches on two separate web-based platforms yielded 2,301 articles. Abstracts were screened with 2,169 articles excluded. The full-text examination of the remaining 132, resulted in 107 excluded for not tau protein measurements. A total of 25 repetitive mTBI articles that measured tau changes were included in our review.
Table 1:
systematic review of p-tau in rodent repetitive CHI studies.
| Ref | Tau Tg | Age | model | # of hits | HI | 0h- | 1d- | 1w- | 1m- | 3m- | 6m- | Method: tau antibody | n= |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rats | |||||||||||||
| (Corrigan et al., 2017) | n.d. | WD | 3 | 5d | ≈ | ↑* | IB: AT180, T22 | 4–6 | |||||
| (McAteer et al., 2016) | n.d. | WD | 3 | 5d | ↑*# | ↑*# | IHC AT180 | 5–6 | |||||
| (Mountney et al., 2017) | n.d. | proj | 4 | 1h | ≈ | IB: pTau202 | 6–8 | ||||||
| (Thomsen et al., 2017) | 60d | piston | 2–5 | 7d | ↑* | IHC: AT8 | 3–8 | ||||||
| (Thomsen et al., 2016) | n.d. | piston | 2–5 | 7d | ↑ | IHC: AT8 (n.q.) | 4–7 | ||||||
| Mice | |||||||||||||
| (Namjoshi et al., 2016) | 8w | chimera | 2 | 1d | ≈ | IB: CP13, RZ3, PHF1 | 4 | ||||||
| (Namjoshi et al., 2014) | 9–15m | chimera | 2 | 1d | ↑ | ↑* | ≈ | IB: CP13, RZ3, PHF1 | n.d. | ||||
| (Laurer et al., 2001) | 8–10w | piston | 2 | 1d | ↔ | ≈ | IHC: total Tau | n.d. | |||||
| (Luo et al., 2014) | 2–3m | piston | 2 | 1d | ≈ | IHC: AT8 | n.d. | ||||||
| Current (this study) | 4m | piston | 2 | 1–7d | ↑* | ELISA: pTau231 | 4–12 | ||||||
| (Zhang et al., 2015) | 6–10w | piston | 3 | 1d | ↑* | ↑* | IB: p-tau | 6 | |||||
| (Mei et al., 2018) | 8w | WD | 4 | 1d | ↑* | ≈ | IB: pTau231 | 8 | |||||
| (Yang et al., 2015) | 3–4m | piston | 4 | 2d | ↑ | ↑ | IHC CP13 (n.q.) | n.d. | |||||
| (Bolton and Saatman, 2014) | 3–4m | piston | 5 | 1–2d | ≈ | ≈ | IHC: PHF1 | n.d. | |||||
| (Bolton Hall et al., 2016) | 2–3m | piston | 5 | 1–2d | ≈ | IHC: PHF1 | n.d. | ||||||
| (Mouzon et al., 2014) | 9–15m | piston | 5 | 2d | ≈ | ≈ | ELISA: CP13, RZ3, PHF1 | 4 | |||||
| (Yu et al., 2017) | 8w | piston | 5 | 1d | ≈ | IHC: AT8 | |||||||
| (Mannix et al., 2013) | 3m | WD | 7 | 1–3d | ≈ | ELISA: pTau | 2–6 | ||||||
| (Xu et al., 2016) | 5w | WD | 4–12 | 4d | ≈ | ≈ | IHC: AT8, PHF1, CP13, Ser422 | 4 | |||||
| (Briggs et al., 2016) | 7–8w | WD | 30 | 1–3d | ↑* | IHC: AT8 | 2–5 | ||||||
| (Petraglia et al., 2014) | 12w | piston | 42 | 1–3d | ↑*# | ↑* | ↑*# | IHC: AT8 | n.d. | ||||
| Current (this study) | rTg4510 | 2m | piston | 2 | 1d | ↑* | IB: PHF1 | 7–9 | |||||
| (Xu et al., 2015) | P301S | 5w | WD | 4–12 | 4d | ↑*# | IHC: Ser-422 | 4 | |||||
| (Ferguson et al., 2017) | hTau | 2m, 11m | piston | 5 | 2 | ≈ | IB: CP13, PHF1 | 4 | |||||
| (Ojo et al., 2016a) | hTau | 12w | piston | 24–32 | 3–4d | ↑* | IB: DA9 RZ3, Toc1 | 6–8 | |||||
| (Tzekov et al., 2016) | hTau | 10–15w | piston | 5 | 2 | ↑ | IHC: CP13 (n.q.) | n.d. | |||||
| (Winston et al., 2016) | 3xTg AD | 18mo | piston | 30 | 1–3d | ≈ | ≈ | IB: AT270, CP13, Thr205, | 5–6 | ||||
| AT8, Tau1, Ser199, AT180, PHF1, Ser422 | |||||||||||||
reference (Ref); between hit interval (HI); not defined (n.d.); not quantified (n.q.); immunoblot (IB); immunohistochemistry (IHC); enzyme-linked immunosorbent assay (ELISA); weight drop (WD)
significant compared to sham,
significant compared to single hit;
increased;
not seen, or no change from sham
We identified 25 studies that were included in our review (Figure 5). In regards to the time course of tau changes after the injury, there was little consensus with 50% of the studies greater than 1-week post-injury finding an increase in tau with the CHI. Of the studies that investigated multiple time points post-injury, only one study did not see an increase in p-tau at an acute post-injury time point, while finding an increase in p-tau at a chronic post-injury time point (Corrigan et al., 2017). In contrast, one study found the opposite with only an acute change and no lasting changes in p-tau after the repetitive CHI (Mei et al., 2018). Most of the time course studies found that p-tau was consistently not seen or remained elevated at all the time points investigated (Table 1). Therefore, it is not possible to conclude if the p-tau changes seen after a repetitive CHI in rodents are progressive or will regress with time.
Next, we searched whether the increase in p-tau was greater following a repetitive CHI vs. a single CHI. We found that 9 of the 25 papers compared the effects of a single vs. repetitive CHI. Two studies did not quantify the changes in tau, so it is not possible to determine if there was a difference between single or repetitive CHI (Luo et al., 2014; Yang et al., 2015). In agreement with our study here in WT mice, three studies found that repetitive TBI increased tau levels more than a single TBI (McAteer et al., 2016; Petraglia et al., 2014; Xu et al., 2015). In contrast, five studies found that a single or repetitive TBI did not increase tau levels over sham-injured mice (Bolton and Saatman, 2014; Bolton Hall et al., 2016; Mountney et al., 2017; Mouzon et al., 2014; Xu et al., 2016). In the rTg4510 mice, we did not find a difference between a single vs. repetitive TBI, which was in contrast to our results in WT mice. It is possible that because of the aggressive pathology of the rTg4510 mice, there was a ceiling-effect in the 2x CHI mice. However, the P301S tau mouse line also has aggressive pathology, and a repetitive CHI increased the p-tau burden in this line compared to a single CHI (Xu et al., 2015). While there are many differences between the current study and Xu et al., (2015), one notable difference was the number of impacts. Xu et al., (2015), using a weight drop method of CHI, gave up to 12 impacts spaced four days apart, while we only gave two impacts. Therefore, it is possible that a large degree of pre-existing pathology is needed to detect differences between single vs. repetitive injury because there is a higher background of tau pathology associated with the tau transgene mutation. The increasing number of impacts, however, did not necessarily correspond to increasing hyperphosphorylated-Tau: even after 30 impacts, 3xTg-AD mice did not present changes in a comprehensive panel of pathological-associated tau markers (Winston et al., 2016).
An interesting finding that warrants a further investigation is the difference between WT mice and rats in terms of the likelihood that elevated p-tau would be seen after a repetitive CHI. While on average fewer than 50% of the studies using WT mice found increased p-tau after a repetitive TBI, 80% of the studies using WT rats found that repetitive CHI increased p-tau. The difference between the mice and the rats does not appear to be associated with differences in the number of hits, or injury mechanics as similar injury paradigms were used in both species. Rat tau, unlike mouse tau, is more similar to humans, in that rats express all six tau isoforms present in humans (Hanes et al., 2009; McMillan et al., 2008), while mice do not. The presence of six tau human isoforms may not be sufficient to explain the difference between the rats and mice, as CHI studies using the hTau mouse, which has all six human tau isoforms, did not consistently find increases in p-tau with the repetitive CHI.
In summary, our results suggest that tau may play a role in the pathogenic mechanisms leading to cognitive impairment, but the precise link remains unknown. Further studies are required to elucidate the mechanisms whereby increased p-tau caused by a CHI leads to pathological neurofibrillary tangles, neuronal loss and cogntive decline.
Highlights.
Chronic cognitive deficits are worsened following repetitive CHIs in WT mice.
Functional neuroimaging deficits were found in the rTg4510 mice following a repetitive but not single CHI.
Evidence that p-tau was a driver in the repetitive CHI phenotypes could not be established.
Acknowledgements:
Research reported in this publication was supported by National Institutes of Health under award numbers R00 AG044445 (ADB), P30 GM110787 (JFA & ADB), R01 NS103785 (ADB), P30 AG028383 (JFA & ADB), R01 NS091329 (JFA), UL1 TR000117 (JFA), and L32 MD009205 (JFA), and Department of Defense grant AZ140097 (JFA), The MRISC is supported by NIH S10 shared instruments grant number S10 RR029541. The content is solely the responsibility of the authors and does not represent the official views of the funding organizations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosure Statement:
No competing financial interests exist.
Reference:
- Abisambra JF, Scheff S, 2014. Brain injury in the context of tauopathies. J Alzheimers Dis 40, 495–518. [DOI] [PubMed] [Google Scholar]
- Antkowiak PF, Vandsburger MH, Epstein FH, 2012. Quantitative pancreatic beta cell MRI using manganese-enhanced Look-Locker imaging and two-site water exchange analysis. Magn Reson Med 67, 1730–1739. [DOI] [PubMed] [Google Scholar]
- Augustinack JC, Schneider A, Mandelkow EM, Hyman BT, 2002. Specific tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer’s disease. Acta Neuropathol 103, 26–35. [DOI] [PubMed] [Google Scholar]
- Bachstetter AD, Webster SJ, Goulding DS, Morton JE, Watterson DM, Van Eldik LJ, 2015. Attenuation of traumatic brain injury-induced cognitive impairment in mice by targeting increased cytokine levels with a small molecule experimental therapeutic. J Neuroinflammation 12, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes DE, Kaup A, Kirby KA, Byers AL, Diaz-Arrastia R, Yaffe K, 2014. Traumatic brain injury and risk of dementia in older veterans. Neurology 83, 312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar CN, Roberts KN, Higgins EK, Bachstetter AD, 2019. A Systematic Review of Closed Head Injury Models of Mild Traumatic Brain Injury in Mice and Rats. J Neurotrauma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton AN, Saatman KE, 2014. Regional Neurodegeneration and Gliosis Are Amplified by Mild Traumatic Brain Injury Repeated at 24-Hour Intervals. J Neuropathol Exp Neurol 73, 933–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton Hall AN, Joseph B, Brelsfoard JM, Saatman KE, 2016. Repeated Closed Head Injury in Mice Results in Sustained Motor and Memory Deficits and Chronic Cellular Changes. PLoS One 11, e0159442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton-Hall AN, Hubbard WB, Saatman KE, 2019. Experimental Designs for Repeated Mild Traumatic Brain Injury: Challenges and Considerations. J Neurotrauma 36, 1203–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E, 1991. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82, 239–259. [DOI] [PubMed] [Google Scholar]
- Briggs DI, Angoa-Perez M, Kuhn DM, 2016. Prolonged Repetitive Head Trauma Induces a Singular Chronic Traumatic Encephalopathy-Like Pathology in White Matter Despite Transient Behavioral Abnormalities. Am J Pathol 186, 2869–2886. [DOI] [PubMed] [Google Scholar]
- CDC, 2003. Report to Congress on mild traumatic brain injury in the United States: steps to prevent a serious public health problem. Atlanta, GA: US Department of Health and Human Services. CDC. [Google Scholar]
- Cloyd RA, Koren SA, Abisambra JF, 2018. Manganese-Enhanced Magnetic Resonance Imaging: Overview and Central Nervous System Applications With a Focus on Neurodegeneration. Front Aging Neurosci 10, 403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan F, Arulsamy A, Collins-Praino LE, Holmes JL, Vink R, 2017. Toll like receptor 4 activation can be either detrimental or beneficial following mild repetitive traumatic brain injury depending on timing of activation. Brain Behav Immun 64, 124–139. [DOI] [PubMed] [Google Scholar]
- Courtney A, Courtney M, 2015. The Complexity of Biomechanics Causing Primary Blast-Induced Traumatic Brain Injury: A Review of Potential Mechanisms. Front Neurol 6, 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crary JF, Trojanowski JQ, Schneider JA, Abisambra JF, Abner EL, Alafuzoff I, Arnold SE, Attems J, Beach TG, Bigio EH, Cairns NJ, Dickson DW, Gearing M, Grinberg LT, Hof PR, Hyman BT, Jellinger K, Jicha GA, Kovacs GG, Knopman DS, Kofler J, Kukull WA, Mackenzie IR, Masliah E, McKee A, Montine TJ, Murray ME, Neltner JH, Santa-Maria I, Seeley WW, Serrano-Pozo A, Shelanski ML, Stein T, Takao M, Thal DR, Toledo JB, Troncoso JC, Vonsattel JP, White CL 3rd, Wisniewski T, Woltjer RL, Yamada M, Nelson PT, 2014. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol 128, 755–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWitt DS, Perez-Polo R, Hulsebosch CE, Dash PK, Robertson CS, 2013. Challenges in the Development of Rodent Models of Mild Traumatic Brain Injury. Journal of Neurotrauma 30, 688–701. [DOI] [PubMed] [Google Scholar]
- Faul M, Xu L, Wald MM, Coronado VG, 2010. Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths. Atlanta (GA): Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. [Google Scholar]
- Ferguson SA, Mouzon BC, Lynch C, Lungmus C, Morin A, Crynen G, Carper B, Bieler G, Mufson EJ, Stewart W, Mullan M, Crawford F, 2017. Negative Impact of Female Sex on Outcomes from Repetitive Mild Traumatic Brain Injury in hTau Mice Is Age Dependent: A Chronic Effects of Neurotrauma Consortium Study. Front Aging Neurosci 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine SN, Ingram A, Cloyd RA, Meier SE, Miller E, Lyons D, Nation GK, Mechas E, Weiss B, Lanzillotta C, Di Domenico F, Schmitt F, Powell DK, Vandsburger M, Abisambra JF, 2017. Identification of changes in neuronal function as a consequence of aging and tauopathic neurodegeneration using a novel and sensitive magnetic resonance imaging approach. Neurobiol Aging 56, 78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner RC, Yaffe K, 2015. Epidemiology of mild traumatic brain injury and neurodegenerative disease. Mol Cell Neurosci 66, 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanes J, Zilka N, Bartkova M, Caletkova M, Dobrota D, Novak M, 2009. Rat tau proteome consists of six tau isoforms: implication for animal models of human tauopathies. J Neurochem 108, 1167–1176. [DOI] [PubMed] [Google Scholar]
- Hibbits N, Pannu R, Wu TJ, Armstrong RC, 2009. Cuprizone demyelination of the corpus callosum in mice correlates with altered social interaction and impaired bilateral sensorimotor coordination. ASN Neuro 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren SA, Hamm MJ, Meier SE, Weiss BE, Nation GK, Chishti EA, Arango JP, Chen J, Zhu H, Blalock EM, Abisambra JF, 2019. Tau drives translational selectivity by interacting with ribosomal proteins. Acta Neuropathol 137, 571–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurer HL, Bareyre FM, Lee VM, Trojanowski JQ, Longhi L, Hoover R, Saatman KE, Raghupathi R, Hoshino S, Grady MS, McIntosh TK, 2001. Mild head injury increasing the brain’s vulnerability to a second concussive impact. J Neurosurg 95, 859–870. [DOI] [PubMed] [Google Scholar]
- Liebetanz D, Baier PC, Paulus W, Meuer K, Bahr M, Weishaupt JH, 2007. A highly sensitive automated complex running wheel test to detect latent motor deficits in the mouse MPTP model of Parkinson’s disease. Exp Neurol 205, 207–213. [DOI] [PubMed] [Google Scholar]
- Luo J, Nguyen A, Villeda S, Zhang H, Ding ZQ, Lindsey D, Bieri G, Castellano JM, Beaupre GS, Wyss-Coray T, 2014. Long-term cognitive impairments and pathological alterations in a mouse model of repetitive mild traumatic brain injury. Front Neurol 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannix R, Meehan WP, Mandeville J, Grant PE, Gray T, Berglass J, Zhang J, Bryant J, Rezaie S, Chung JY, Peters NV, Lee C, Tien LW, Kaplan DL, Feany M, Whalen M, 2013. Clinical correlates in an experimental model of repetitive mild brain injury. Ann Neurol 74, 65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAteer KM, Corrigan F, Thornton E, Turner RJ, Vink R, 2016. Short and Long Term Behavioral and Pathological Changes in a Novel Rodent Model of Repetitive Mild Traumatic Brain Injury. PLoS One 11, e0160220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan P, Korvatska E, Poorkaj P, Evstafjeva Z, Robinson L, Greenup L, Leverenz J, Schellenberg GD, D’Souza I, 2008. Tau isoform regulation is region- and cell-specific in mouse brain. J Comp Neurol 511, 788–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei ZR, Qiu JH, Alcon S, Hashim J, Rotenberg A, Sun Y, Meehan WP, Mannix R, 2018. Memantine improves outcomes after repetitive traumatic brain injury. Behav Brain Res 340, 195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier S, Bell M, Lyons DN, Ingram A, Chen J, Gensel JC, Zhu H, Nelson PT, Abisambra JF, 2015. Identification of Novel Tau Interactions with Endoplasmic Reticulum Proteins in Alzheimer’s Disease Brain. J Alzheimers Dis 48, 687–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, Group TP, 2009. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Medicine 6, 1–6. [PMC free article] [PubMed] [Google Scholar]
- Mountney A, Boutte AM, Cartagena CM, Flerlage WF, Johnson WD, Rho C, Lu XC, Yarnell A, Marcsisin S, Sousa J, Vuong C, Zottig V, Leung LY, Deng-Bryant Y, Gilsdorf J, Tortella FC, Shear DA, 2017. Functional and Molecular Correlates after Single and Repeated Rat Closed-Head Concussion: Indices of Vulnerability after Brain Injury. J Neurotrauma 34, 2768–2789. [DOI] [PubMed] [Google Scholar]
- Mouzon BC, Bachmeier C, Ferro A, Ojo JO, Crynen G, Acker CM, Davies P, Mullan M, Stewart W, Crawford F, 2014. Chronic neuropathological and neurobehavioral changes in a repetitive mild traumatic brain injury model. Ann Neurol 75, 241–254. [DOI] [PubMed] [Google Scholar]
- Namjoshi DR, Cheng WH, Carr M, Martens KM, Zareyan S, Wilkinson A, McInnes KA, Cripton PA, Wellington CL, 2016. Chronic Exposure to Androgenic-Anabolic Steroids Exacerbates Axonal Injury and Microgliosis in the CHIMERA Mouse Model of Repetitive Concussion. PLoS One 11, e0146540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namjoshi DR, Cheng WH, McInnes KA, Martens KM, Carr M, Wilkinson A, Fan J, Robert J, Hayat A, Cripton PA, Wellington CL, 2014. Merging pathology with biomechanics using CHIMERA (Closed-Head Impact Model of Engineered Rotational Acceleration): a novel, surgery-free model of traumatic brain injury. Mol Neurodegener 9, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojo JO, Mouzon B, Algamal M, Leary P, Lynch C, Abdullah L, Evans J, Mullan M, Bachmeier C, Stewart W, Crawford F, 2016a. Chronic Repetitive Mild Traumatic Brain Injury Results in Reduced Cerebral Blood Flow, Axonal Injury, Gliosis, and Increased T-Tau and Tau Oligomers. J Neuropathol Exp Neurol 75, 636–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojo JO, Mouzon BC, Crawford F, 2016b. Repetitive head trauma, chronic traumatic encephalopathy and tau: Challenges in translating from mice to men. Exp Neurol 275 Pt 3, 389–404. [DOI] [PubMed] [Google Scholar]
- Petraglia AL, Plog BA, Dayawansa S, Dashnaw ML, Czerniecka K, Walker CT, Chen M, Hyrien O, Iliff JJ, Deane R, Huang JH, Nedergaard M, 2014. The pathophysiology underlying repetitive mild traumatic brain injury in a novel mouse model of chronic traumatic encephalopathy. Surg Neurol Int 5, 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz AR, Li X, McCauley SR, Wilde EA, Barnes A, Hanten G, Mendez D, McCarthy JJ, Levin HS, 2015. Prevalence and Predictors of Poor Recovery from Mild Traumatic Brain Injury. J Neurotrauma 32, 1488–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsden M, Kotilinek L, Forster C, Paulson J, McGowan E, SantaCruz K, Guimaraes A, Yue M, Lewis J, Carlson G, Hutton M, Ashe KH, 2005. Age-dependent neurofibrillary tangle formation, neuron loss, and memory impairment in a mouse model of human tauopathy (P301L). J Neurosci 25, 10637–10647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saar G, Koretsky AP, 2018. Manganese Enhanced MRI for Use in Studying Neurodegenerative Diseases. Front Neural Circuits 12, 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santacruz K, Lewis J, Spires T, Paulson J, Kotilinek L, Ingelsson M, Guimaraes A, DeTure M, Ramsden M, McGowan E, Forster C, Yue M, Orne J, Janus C, Mariash A, Kuskowski M, Hyman B, Hutton M, Ashe KH, 2005. Tau suppression in a neurodegenerative mouse model improves memory function. Science 309, 476–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen GM, Ko A, Harada MY, Ma A, Wyss L, Haro P, Vit JP, Avalos P, Dhillon NK, Cho N, Shelest O, Ley EJ, 2017. Clinical correlates to assist with chronic traumatic encephalopathy diagnosis: Insights from a novel rodent repeat concussion model. J Trauma Acute Care Surg 82, 1039–1048. [DOI] [PubMed] [Google Scholar]
- Thomsen GM, Ma AM, Ko A, Harada MY, Wyss L, Haro PS, Vit JP, Shelest O, Rhee P, Svendsen CN, Ley EJ, 2016. A model of recurrent concussion that leads to long-term motor deficits, CTE-like tauopathy and exacerbation of an ALS phenotype. J Trauma Acute Care Surg 81, 1070–1079. [DOI] [PubMed] [Google Scholar]
- Tzekov R, Phifer J, Myers A, Mouzon B, Crawford F, 2016. Inflammatory changes in optic nerve after closed-head repeated traumatic brain injury: Preliminary study. Brain Injury 30, 1428–1435. [DOI] [PubMed] [Google Scholar]
- Vandsburger MH, French BA, Kramer CM, Zhong X, Epstein FH, 2012. Displacement-encoded and manganese-enhanced cardiac MRI reveal that nNOS, not eNOS, plays a dominant role in modulating contraction and calcium influx in the mammalian heart. Am J Physiol Heart Circ Physiol 302, H412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster SJ, Van Eldik LJ, Watterson DM, Bachstetter AD, 2015. Closed head injury in an age-related Alzheimer mouse model leads to an altered neuroinflammatory response and persistent cognitive impairment. J Neurosci 35, 6554–6569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wes PD, Easton A, Corradi J, Barten DM, Devidze N, DeCarr LB, Truong A, He A, Barrezueta NX, Polson C, Bourin C, Flynn ME, Keenan S, Lidge R, Meredith J, Natale J, Sankaranarayanan S, Cadelina GW, Albright CF, Cacace AM, 2014. Tau overexpression impacts a neuroinflammation gene expression network perturbed in Alzheimer’s disease. PLoS One 9, e106050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston CN, Noel A, Neustadtl A, Parsadanian M, Barton DJ, Chellappa D, Wilkins TE, Alikhani AD, Zapple DN, Villapol S, Planel E, Burns MP, 2016. Dendritic Spine Loss and Chronic White Matter Inflammation in a Mouse Model of Highly Repetitive Head Trauma. Am J Pathol 186, 552–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojnarowicz MW, Fisher AM, Minaeva O, Goldstein LE, 2017. Considerations for Experimental Animal Models of Concussion, Traumatic Brain Injury, and Chronic Traumatic Encephalopathy-These Matters Matter. Front Neurol 8, 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Nguyen JV, Lehar M, Menon A, Rha E, Arena J, Ryu J, Marsh-Armstrong N, Marmarou CR, Koliatsos VE, 2016. Repetitive mild traumatic brain injury with impact acceleration in the mouse: Multifocal axonopathy, neuroinflammation, and neurodegeneration in the visual system. Exp Neurol 275 Pt 3, 436–449. [DOI] [PubMed] [Google Scholar]
- Xu LY, Ryu J, Nguyen JV, Arena J, Rha E, Vranis P, Hitt D, Marsh-Armstrong N, Koliatsos VE, 2015. Evidence for accelerated tauopathy in the retina of transgenic P301S tau mice exposed to repetitive mild traumatic brain injury. Exp Neurol 273, 168–176. [DOI] [PubMed] [Google Scholar]
- Yang ZH, Wang P, Morgan D, Lin D, Pan JC, Lin F, Strang KH, Selig TM, Perez PD, Febo M, Chang BG, Rubenstein R, Wang KKW, 2015. Temporal MRI characterization, neurobiochemical and neurobehavioral changes in a mouse repetitive concussive head injury model. Scientific Reports 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F, Shukla DK, Armstrong RC, Marion CM, Radomski KL, Selwyn RG, Dardzinski BJ, 2017. Repetitive Model of Mild Traumatic Brain Injury Produces Cortical Abnormalities Detectable by Magnetic Resonance Diffusion Imaging, Histopathology, and Behavior. J Neurotrauma 34, 1364–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Teng ZQ, Song YP, Hu M, Chen C, 2015. Inhibition of monoacylglycerol lipase prevents chronic traumatic encephalopathy-like neuropathology in a mouse model of repetitive mild closed head injury. J Cereb Blood Flow Metab 35, 443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]


