Abstract
Purpose
High dietary magnesium intake may reduce insulin resistance (IR) and metabolic syndrome (MetS). The aim of the cross-sectional analysis was to evaluate the association between dietary magnesium intake, IR, and MetS using data from China Health and Nutrition Survey.
Methods
Dietary magnesium intake was defined as daily dietary magnesium intake divided by body weight. Logistic regression analysis was used to calculate the odds ratio (OR) for IR and the prevalence of MetS across the quartile categories of dietary magnesium intake. In addition, we used the macro PROCESS to perform the mediation analyses.
Results
A total of 8120 participants were included in the final analysis. We found a significant negative association between dietary magnesium intake and IR, the multivariable-adjusted OR for HOMA-IR comparing the highest to the lowest quartile of dietary magnesium intake was 0.435 (95% confidence intervals [CI] 0.376 to 0.502). The prevalence of the MetS was 38.6%, 28.9%, 22.5%, and 16.5% for increasing quartiles of dietary magnesium intake (p <0.001). The mediation model analysis displayed that insulin resistance mediated the effect of dietary magnesium on MetS. The direct effect and indirect effect of dietary magnesium on MetS were found significant, and the calculated percentage of mediation by insulin resistance was 19.6%.
Conclusion
Our study demonstrated a significant and independent negative relationship among weight-adjusted dietary magnesium intake, HOMA-IR, and MetS in a large Chinese population. IR partly mediated the relationship between dietary magnesium intake and MetS.
Keywords: diet magnesium intake, metabolic syndrome, insulin resistance, Chinese population, mediation effect
Introduction
Magnesium (Mg) is a cofactor required in more than 300 enzymatic reactions and is the fourth most abundant cation in the human body that is involved in both glucose metabolism and insulin homeostasis.1,2 Insulin resistance is an early-stage marker of diabetes mellitus (DM),3 and recent evidence has suggested that dietary Mg intake may play an important role in insulin resistance. However, population-based studies present conflicting evidence regarding the potential effects of dietary Mg intake. Several studies have reported a correlation between low dietary Mg intake and increased insulin resistance.3–5 Conversely, the results of other studies do not support the proposed protective effect of dietary Mg intake in attenuating the development of diabetes.6,7
Insulin resistance has been implicated in the development of metabolic syndrome (MetS).8 MetS is a widespread disease in both developed and developing countries and is characterized by a cluster of risk factors that threaten public health and increases disability, mortality, and health care costs.9,10 As low intake of dietary Mg may contribute to insulin resistance, it is also possible that inadequate Mg intake might play a role in the pathogenesis of MetS. A meta-analysis examining the role of Mg intake and risk of MetS consistently showed that dietary Mg intake was significantly and inversely associated with the risk of MetS.11–13 To further address the association between dietary magnesium intake and MetS, we quantified the role of insulin resistance as a potential pathway by which dietary magnesium intake influence MetS. To the best of our knowledge, no study has explored the potential mediating role of insulin resistance in the known effects of dietary Mg intake on MetS. Therefore, the present study aimed 1) to verify the relationship between dietary Mg intake, insulin resistance, and MetS in a large nationally representative sample of Chinese adults, and 2) to assess if insulin resistance mediated the effect of dietary Mg intake on MetS. We evaluated the association between dietary Mg intake, insulin resistance, and MetS considering several factors, including age, sex, energy intake, smoking status, alcohol consumption, and others.
Materials and Methods
Study Population
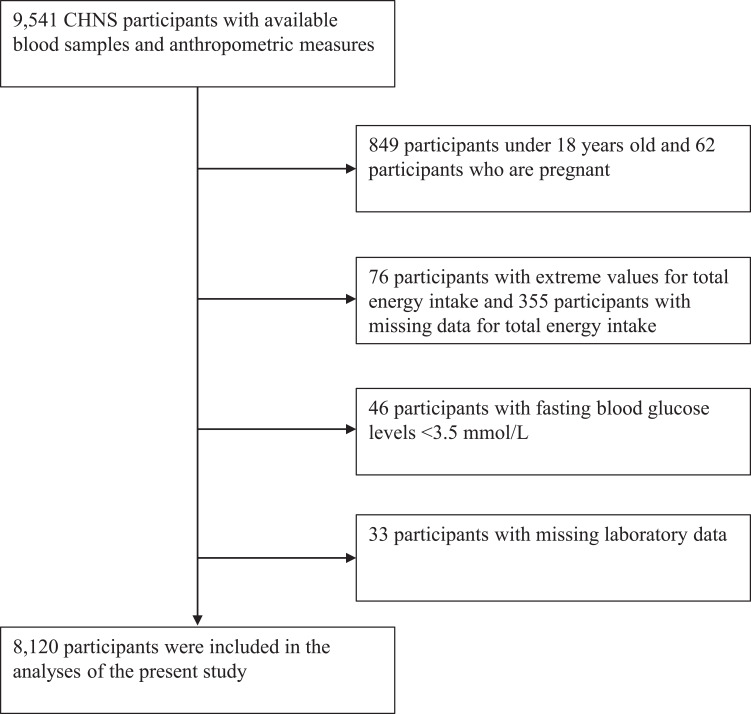
The China Health and Nutrition Survey (CHNS) was designed to examine how the social and economic transformation in China affects the health and nutritional status of the Chinese population.1 The survey used a multistage random-cluster sampling process to select samples from nine provinces (including Beijing, Chongqing, Guangxi, Guizhou, Heilongjiang, Henan, Hubei, Hunan, Jiangsu, Liaoning, Shaanxi, Shandong, Shanghai, Yunnan, Zhejiang),10,14 covering most of the north and south of China. Counties in the nine provinces were stratified by income (low, middle, and high), and a weighted sampling scheme was used to randomly select four counties in each province. In addition, the provincial capital and a lower income city were selected when feasible. Villages and townships within the counties and urban and suburban neighborhoods within the cities were selected randomly. In the present study, we used data from the 2009 CHNS, when the blood samples were first collected. At that time, data obtained from blood samples and anthropometric measures were available from 9541 CHNS participants. In the present study, the following exclusion criteria were applied: missing laboratory data (n=33), age <18 years (n=849), or pregnancy (n=62). In addition, participants with extreme values for total energy intake (male <800 or >4200 kcal/day, female <500 or >3500 kcal/day [n=76]), missing data for total energy intake (355) and fasting blood glucose levels <3.5 mmol/L were also excluded (n=46). Insulin resistance was defined as the upper quartile of HOMA-IR. After applying the exclusion criteria, a total of 8120 participants (85.1% of 9541) were included in the analyses of the present study (Figure 1).
Figure 1.
Study flow diagram.
Abbreviation: CHNS, the China Health and Nutrition Survey.
The China Health and Nutrition Survey (CHNS) is an ongoing open cohort, international collaborative project between the Carolina Population Center at the University of North Carolina at Chapel Hill and the National Institute for Nutrition and Health at the Chinese Center for Disease Control and Prevention.1 CHNS data is freely available on the website https://www.cpc.unc.edu/projects/china. The survey was approved by institutional review boards at the University of North Carolina, Chapel Hill (Chapel Hill, NC), and the National Institute of Nutrition and Food Safety, China Center for Disease Control and Prevention (Beijing, China), and each participant provided written informed consent.
Dietary Mg Intake Assessment and Other Relevant Variables
The 2009 CHNS combined data from three consecutive days of 24-hour dietary recall and a household food inventory to assess individual consumption.9 Individual dietary intake for three consecutive days (2 weekdays and 1 weekend day) was recorded for every household member. Interviewers were trained to use standard forms for administering dietary recalls in household interviews. The participants were asked to report all foods and beverages consumed both at home and away from home. In addition, household food intake was determined on a daily basis by calculating the changes in the food inventory. Individual daily intake values for each food item was assessed using data from the 24-hour dietary recall, which was enhanced using data from household measures. Additionally, edible oils and other common condiments (sugar, starch, soy sauce, salt) consumed in the household by each member were allocated based on the proportion of the reference man. The collection of both household and individual dietary intake allowed us to check the quality of data collection by comparing the two. Thus, each individual’s average daily dietary intake, calculated from the household survey, has been compared with his or her dietary intake based on 24-hour recall data,14 and the results of the above two methods are consistent. The number of Chinese people using nutrient supplements is very small, accounting for only 0.7% of the total,15 so we did not include the dietary supplements for nutrient intake.
In individuals who participated in the 2009 survey, blood samples were collected by venipuncture after overnight fasting and tested immediately for glucose and hemoglobin A1c (HbA1c) levels. Plasma and serum samples were then frozen and stored at −86°C for further laboratory analysis.10,14 All samples were analyzed in a national central laboratory in Beijing with strict quality control.
The questionnaire for adults collected data on participant background information, health history, physical measurements, and health-related behaviors.16 Standard procedures were followed by the trained interviewers. Weight was measured to the nearest 0.1 kg with lightweight clothing on a calibrated beam scale. Height was measured to the nearest 0.1 cm without shoes using a portable stadiometer. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters. Waist circumference (WC) was measured at a midpoint between the lowest rib and the iliac crest in a horizontal plane using a non-elastic tape.
Insulin resistance was determined with HOMA-IR, as described by Matthews et al.17 The formula for calculating HOMA-IR is HOMA-IR (mIU·mmol/L2)= fasting insulin (mIU/L) × fasting glucose (mmol/L)/22.5. Based on the Adult Treatment Panel III (ATP III) guidelines for Asian populations,18 MetS was defined as the presence of three or more of the following characteristics: abdominal obesity, WC ≥90 cm in men or ≥80 cm in women; elevated triglyceride (TG) levels, TG ≥150 mg/dL; low high-density lipoprotein (HDL) cholesterol levels, HDL cholesterol <40 mg/dL in men or <50 mg/dL in women; elevated blood pressure (BP), systolic blood pressure (SBP) ≥130 mmHg or diastolic blood pressure (DBP) ≥85 mmHg (antihypertensive drug treatment in a patient with a history of hypertension is an alternate indicator); and elevated fasting glucose levels, glucose ≥100 mg/dL (drug treatment of elevated glucose is an alternate indicator). We also applied the International Diabetes Federation (IDF) standard to recalculate.19
Statistical Analysis
We categorized dietary Mg intake per kilogram body weight into quartiles based on the distribution in the whole study population and then used the quartiles to compare nutrient intake, metabolic index, and other lifestyle factors. Summary statistics were presented using frequencies and proportions for categorical data and means (standard deviations) for continuous variables. Baseline characteristics of participants were expressed as mean and SD or percentages and were compared according to quartiles of Mg intake using analysis of variance (ANOVA) or a χ2 test as appropriate. HOMA-IR values and insulin, glucose, and TG levels were log-transformed to better approximate a normal distribution before the analysis was conducted. Logistic regression analysis was used to estimate adjusted geometric means and 95% confidence intervals (CIs) for these variables across the categories of dietary Mg intake per kilogram. The first model was adjusted for age (years) and male sex (yes or no), and the second model was further adjusted for smoking status (yes or no), alcohol consumption (yes or no), educational level (high school level and above or below high school), and residence (urban or rural). The third model was further adjusted for total energy intake (kcal/day), total protein intake (g/day), total carbohydrate intake (g/day) and total fat intake (g/day).11
We performed a mediation analysis to quantify the role of insulin resistance in the association between dietary Mg intake per kilogram and MetS. To do so, we used the macro PROCESS version 3.3.20 We calculated the indirect effect, which is a measure of the degree of mediation through the mediator.21 We also used the third model like the models above to adjust for the potential confounders. All variables were modelled as continuous variables. Regulatory hypotheses were tested via a bias-corrected bootstrap method with 5000 samples to calculate confidence intervals (95%). Statistical significance of the mediating effect was set at zero not encompassed in the 95% CI.
All data were analyzed using IBM SPSS Statistics, version 25 SPSS (Chicago, IL, USA). A two-sided p value <0.05 was used to indicate statistical significance. The authors have full access to and take full responsibility for the integrity of the data.
Results
Participant Characteristics
A total of 8120 adults were included in this study. Table 1 shows the characteristics of the participants according to daily dietary Mg intake per kilogram of body weight (mg/kg). Supplemental Table 1 shows the comparison of baseline data between the study population and the population with invalid calorie intake. The average quartiles of dietary Mg intake were 2.91±0.52, 4.09±0.28, 5.14±0.36, and 7.66±2.06 mg/kg. Participants with a higher quartile of dietary Mg intake were younger (P<0.001), had lower HbA1c levels (P<0.001) and had lower HOMA-IR values (P<0.001). No significant difference was found in the proportion of men and women in each group (P=0.207). The group that consumed more Mg also consumed more total energy, protein, fat, and carbohydrate. Participants in higher quartiles of dietary Mg intake were more likely to have a low educational level, be an urban resident, and to have smoked in their lifetime. Participants in a higher quartile also had lower BMI, WC, BP, and low-density lipoprotein (LDL) cholesterol levels and higher HDL cholesterol level.
Table 1.
Baseline Characteristics of the Study Population According to Quartiles of Dietary Magnesium Intake per Kilogram Body Weight
| Magnesium Intake/kg (Quartiles) | |||||
|---|---|---|---|---|---|
| 1 (Low) | 2 | 3 | 4 (High) | P* | |
| Age (years) | 52.77±15.72 | 50.82±14.71 | 50.03±14.77 | 50.55±14.71 | <0.001 |
| Male (%) | 45.1 | 46.0 | 47.9 | 49.3 | 0.034 |
| BMI (kg/m2) | 24.99±3.64 | 23.85±3.27 | 22.69±2.96 | 21.83±2.98 | <0.001 |
| Daily dietary intake | |||||
| Mg intake/kg (mg/kg) | 2.91±0.52 | 4.09±0.28 | 5.14±0.36 | 7.66±2.06 | <0.001 |
| Mg intake (mg) | 190.61±46.16 | 254.04±45.52 | 301.93±52.99 | 426.05±124.43 | <0.001 |
| Energy intake (kcal) | 1617.65±459.49 | 1935.12±472.97 | 2166.51±520.13 | 2600.07±587.39 | <0.001 |
| Protein intake (kcal) | 49.28±15.32 | 60.83±16.55 | 68.82±17.77 | 85.23±24.93 | <0.001 |
| Fat intake (kcal) | 59.69±31.52 | 65.32±31.00 | 70.40±33.67 | 79.08±34.82 | <0.001 |
| Carbohydrate intake (kcal) | 220.53±67.15 | 273.96±76.31 | 312.07±87.76 | 387.14±105.77 | <0.001 |
| Urban residence (%) | 37.6 | 36.5 | 32.3 | 25.7 | <0.001 |
| High school education or above (%) | 27.0 | 25.6 | 24.1 | 18.0 | <0.001 |
| Current drinker (%) | 30.2 | 32.2 | 34.4 | 35.2 | 0.020 |
| Ever smoked (%) | 29.1 | 29.6 | 31.4 | 34.4 | 0.003 |
| HOMA-IR | 4.56±8.61 | 3.93±7.37 | 3.50±6.12 | 3.11±6.20 | <0.001 |
| FINS (uIU/mL) | 16.50±24.75 | 14.70±20.80 | 13.92±24.15 | 12.49±19.62 | <0.001 |
| FBG (mmol/L) | 5.69±1.73 | 5.44±1.47 | 5.33±1.32 | 5.20±1.18 | <0.001 |
| WC (cm) | 86.73±10.28 | 83.66±10.00 | 80.73±9.47 | 79.36±9.59 | <0.001 |
| SBP (mmHg) | 128.49±20.39 | 125.49±18.52 | 123.77±18.66 | 122.12±17.96 | <0.001 |
| DBP (mmHg) | 82.30±11.61 | 80.95±11.07 | 80.01±11.44 | 79.14±11.35 | <0.001 |
| TC (mmol/L) | 5.04±1.04 | 4.91±1.00 | 4.77±0.98 | 4.73±0.95 | <0.001 |
| TG (mmol/L) | 1.87±1.51 | 1.79±1.72 | 1.61±1.41 | 1.45±1.27 | <0.001 |
| LDL (mmol/L) | 3.13±0.99 | 3.01±0.99 | 2.90±0.92 | 2.88±0.99 | <0.001 |
| HDL (mmol/L) | 1.38±0.41 | 1.41±0.42 | 1.46±0.65 | 1.50±0.46 | <0.001 |
Notes: Data are presented as means±SD, or %. *P values are for any difference across the quartiles of magnesium intake using ANOVA or χ2 test as appropriate. Mg intake/kg: dietary magnesium intake per kilogram body weight, Mg intake: dietary magnesium intake.
Abbreviations: BMI, body mass index; FINS, fasting insulin; FBG, fast blood glucose; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; TC, total cholesterol; TG, triglyceride; HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol.
Association Between Dietary Mg Intake per Kilogram Body Weight and HOMA-IR
The cutoff point that aligns with the highest quartile for HOMA-IR is 3.66. A logistic regression analysis was performed to adjust for confounding factors. The dietary Mg intake was negatively associated with the logarithm of HOMA-IR (Table 2). We observed a significant inverse association between dietary Mg intake per kilogram of body weight and HOMA-IR (P<0.001), with an odds ratio (OR) of 0.435 (95% CI: 0.376–0.502) for the highest versus lowest quartiles. After additional adjustment for age and male sex, the OR between extreme quartiles was 0.442 (95% CI: 0.383–0.511). After further adjustment for other non-dietary covariates, including educational levels, residence, smoking, and alcohol consumption, the OR between extreme quartiles was 0.435 (95% CI: 0.396–0.529). The ORs remained significant after the addition of dietary variables in the multivariate models (P<0.001), but the estimate of the association did not significantly change (OR comparing extreme quartiles 0.250, 95% CI: 0.204–0.306).
Table 2.
Prevalence Ratios (95% CI) for Insulin Resistance According to Quartiles of Dietary Magnesium Intake per Kilogram Body Weight
| Magnesium Intake/kg (Quartiles) | |||||
|---|---|---|---|---|---|
| 1 (Low) | 2 | 3 | 4 (High) | P* | |
| 1 | 0.690(0.604, 0.790) | 0.553(0.481, 0.635) | 0.435(0.376, 0.502) | <0.001 | |
| Model 1 | 1 | 0.651(0.567, 0.748) | 0.496(0.426, 0.576) | 0.347(0.290, 0.416) | <0.001 |
| Model 2 | 1 | 0.646(0.563, 0.743) | 0.493(0.424, 0.573) | 0.347 (0.289, 0.415) | <0.001 |
| Model 3 | 1 | 0.589(0.511, 0.678) | 0.421(0.360, 0.493) | 0.250(0.204, 0.306) | <0.001 |
Notes: Data are presented as coefficients (95% CI). Insulin resistance was defined by the upper quartile of HOMA-IR. *All models were constructed using the logistic regression analysis. Model 1: adjustment for total energy intake (kcal/day), age (years) and male (yes or no); model 2: model 1 with additional adjustment for ever smoking (yes or no), current alcohol consumption (yes or no), education level (high school degree or above, degree below high school), and residence (urban or not urban); model 3: model 2 with additional adjustment total energy intake (kcal/day), total protein intake (g/day), total carbohydrate intake (g/day), and total fat intake (g/day). Magnesium intake/kg: dietary magnesium intake per kilogram bodyweight; MetS: metabolic syndrome.
Association of Dietary Mg Intake per Kilogram Body Weight with MetS
A total of 2171 participants were classified as having MetS according to the NCEP-ATP III definition, which yielded an overall prevalence of 26.7%. Participants with the lowest quartile of dietary Mg intake per kilogram had the highest prevalence of MetS (Supplement Table 2). We also applied the IDF standard to recalculate and put and the results were put in the supplement (Supplement Table 3). The proportion of obesity was 9.5% in the overall study population and 24.5% in population who had MetS. One hundred and eighty-nine individuals (8.7% of individuals with MetS) have diabetes and 546 individuals (25.1% of individuals with MetS) have hypertension in population who had MetS. We observed a significant inverse association between dietary Mg intake per kilogram body weight and MetS (Table 3), showing unadjusted ORs (95% CI) of 0.646 (0.567–0.735), 0.462 (0.403–0.529), and 0.313 (0.271–0.363) for the second, third, and fourth quartiles, respectively, as compared with the first quartile. The first model was adjusted for age and male sex; the second model was further adjusted for education level, rural residence, smoking status, and alcohol consumption; and the third model was further adjusted for dietary variables. After the three-model adjustment, the association remained significant (P<0.001).
Table 3.
Prevalence Ratios (95% CI) for MetS According to Quartiles of Dietary Magnesium Intake per Kilogram Body Weight
| Magnesium Intake/kg (Quartiles) | |||||
|---|---|---|---|---|---|
| 1 (Low) | 2 | 3 | 4 (High) | P* | |
| MetS | 1 | 0.646 (0.567, 0.735) | 0.462 (0.403, 0.529) | 0.313 (0.271, 0.363) | <0.001 |
| Model 1 | 1 | 0.555 (0.483, 0.638) | 0.349 (0.297, 0.404) | 0.171 (0.141, 0.207) | <0.001 |
| Model 2 | 1 | 0.550 (0.479, 0.632) | 0.343 (0.294, 0.400) | 0.168 (0.139, 0.204) | <0.001 |
| Model 3 | 1 | 0.481 (0.417, 0.555) | 0.274 (0.233, 0.322) | 0.105 (0.085, 0.131) | <0.001 |
Notes: Data are expressed as coefficients (95% CI). A logarithmic transformation was used to improve the normality of distribution for dependent variables. *All models were constructed by logistic regression analysis. The adjusted covariates in the models were the same as those listed in Table 2. MetS: metabolic syndrome, Magnesium intake/kg: dietary magnesium intake per kilogram body weight.
Role of HOMA-IR in the Association Between Dietary Mg Intake per Kilogram Body Weight and MetS
The association between Mg levels, HOMA-IR, and MetS is shown in Figure 2. We confirmed that dietary Mg intake levels were significantly associated with HOMA-IR and that HOMA-IR was significantly associated with MetS. When studying the direct effect of dietary Mg per kilogram body weight on MetS adjusted for HOMA-IR, the effect estimates were attenuated, and the association was still statistically significant. The association between dietary Mg, insulin resistance, and MetS was modeled through a mediation analysis, with insulin resistance calculated as log HOMA-IR levels. Regression a (B =−0.09; P ≤ 0.001) indicated that dietary magnesium intake was negatively associated with HOMA-IR and regression b (B =1.13; P < 0.001) demonstrated that there was a significant positive association between HOMA-IR and Mets. Additionally, there appeared to be a direct link between magnesium intake and MetS (B =−0.41; P <0.001). Our working hypothesis was confirmed as the CIs for indirect effect did not involve the number zero (−0.12 to −0.08), which represents the effect of dietary Mg levels on MetS was mediated by HOMA-IR levels, and the calculated percentage of mediation was 19.6%.
Figure 2.
The role of insulin resistance in the association between dietary magnesium intake and MetS. Zero not included in the 95% CI represents statistical significance. MetS: Metabolic syndrome. Regression a indicated that dietary magnesium intake was negatively associated with HOMA-IR and regression b demonstrated that there was a significant positively association between HOMA-IR and Mets.
Discussion
The present investigation revealed a significant and independent negative relationship between weight-adjusted Mg intake and HOMA-IR in a large Chinese population, independent of age, sex, smoking status, alcohol consumption, educational level, residence, energy intake, protein intake, carbohydrate intake, and fat intake. We also found that the group with high Mg intake was less likely to have MetS. To the best of our knowledge, this study was the first to demonstrate that insulin resistance partly mediated the relationship between dietary Mg intake and MetS.
Consistent with the result of our research, previous studies have demonstrated an inverse association and a dose–effect relationship between dietary Mg intake and insulin resistance.22–25 However, there is a lack of relevant research in Asian populations, and most Asian studies have focused on the relationship between dietary Mg and the risk of diabetes, showing inconsistent results. Some studies showed that high dietary Mg intake could reduce the risk of type 2 diabetes,5,23,26 whereas others could not obtain a significant result.6 To fill the gap, our study aimed to explore the relationship between dietary Mg intake and insulin resistance in a nationally representative sample of Chinese adults. Our study found a significant association between total dietary Mg intake and HOMA-IR, but after adjusting for dietary factors, the results did not stay significant. Then, we decided to adjust for dietary Mg intake per kilogram body weight and found a significant association before and after the three-model adjustment. A study from Mexico also chose to adjust for Mg intake per kilogram body weight and found a significant result showing that the insulin sensitivity assessed by the Matsuda index was higher in the high dietary Mg intake group; however, the sample size of the study was small.27 Given that the previous studies, which did not show a statistically significant correlation between dietary Mg intake and insulin resistance, all used total dietary Mg intake instead of weight-adjusted dietary Mg intake, we assumed that the amount of dietary Mg an individual needed was related to his/her weight. In other words, the heavier the person, the more Mg intake might be needed in his/her diet. This is consistent with the Dietary Reference Intakes (DRI) from the French Food Safety Agency, which recommended 6 mg/kg body weight of Mg intake per day.28 A study from the United States also demonstrated that the dietary Mg intake requirement, which was 2.36 mg/kg per day, was related to body weight.29 More studies are necessary to confirm whether weight-adjusted Mg intake is a better indicator for Mg intake.
Our study found that increased intake of dietary Mg was negatively associated with the prevalence of MetS and its five components in the Chinese population. Studies have reported the beneficial effect of dietary Mg intake or Mg supplementation in reducing the prevalence of MetS,11,30–32 which was consistent with our findings. In those studies, dietary Mg intake was only relative with one to no more than five components of MetS, and there are few correlation studies involving Chinese individuals. In addition to the abovementioned findings, we also found that dietary Mg intake was significantly associated with all five components of MetS in a nationally representative sample of Chinese adults. According to our data, the risk of MetS was nearly 70% lower in the top quartile of dietary Mg intake than in the bottom quartile.
Insulin resistance is the basis of MetS,8 and Mg has been shown to be a key factor in insulin action through the activation of the b-subunit of the insulin receptor and the activation of substrates and proteins in the insulin-signaling pathway,33 It is easy to speculate that the effect of Mg on MetS was mediated by insulin resistance. In this study, we explored the mediation of insulin resistance in the effect of dietary Mg on MetS. The calculated percentage of mediation was 19.6%, indicating that there were other mechanisms involved in the relationship between dietary Mg intake and MetS besides insulin resistance. It has been previously suggested that higher Mg intake and intracellular Mg might have a role in insulin secretion by preserving pancreatic β-cell function via its effect on calcium homeostasis and oxidative stress.11 Mg also acts as a cofactor for several critical enzymes involving lipid metabolism. Mg has been reported to raise HDL cholesterol levels and reduce LDL cholesterol and TG levels by limiting the action of lecithin cholesterol acyltransferase and HMG-CoA reductase and by increasing lipoprotein lipase activity.11 It has been assumed that Mg in the intestine, by forming a non-absorbable soap with fatty acids and cholesterol, can decrease their absorption, reduce energy intake from the diet, and may have advantages for weight maintenance because of this tendency.34 Apart from the abovementioned mechanisms, the relationship between Mg and MetS might also be affected by genes.35,36
The strengths of the present investigation include the following: 1) it is the first study to explore the mediating role of insulin resistance in the relationship between Mg and MetS; 2) it is a large-scale investigation of the relationship between dietary Mg intake, insulin resistance, and MetS in a nationally representative sample of the Chinese population; and 3) in this study, standardized protocols and data collection procedures were used, data collectors were well trained, and quality control was assured, which can largely prevent measurement bias. The potential perceived limitations would be that we applied a cross-sectional research design to investigate associations; thus, we cannot establish causality in the present study. The 24-hour recall, which was the method used to calculate Mg intake, is considered to have a similar accuracy to that of semi-quantitative food frequency questionnaires, but it has the disadvantage of recall bias.
The present study is the first to demonstrate a significant and independent negative relationship between weight-adjusted Mg intake and HOMA-IR and MetS in a large Chinese population. Insulin resistance has a partial mediating role in the relationship between Mg intake and MetS. However, prospective longitudinal studies are needed to verify this relationship.
Acknowledgments
All authors thank the National Institute of Nutrition and Food Safety, China Center for Disease Control and Prevention, the Carolina Population Center, University of North Carolina at Chapel Hill, the National Institutes of Health, and the Fogarty International Center, NIH for financial support for the CHNS data collection and analysis files since 1989. We are grateful to these parties, the China–Japan Friendship Hospital and the Ministry of Health for supporting the CHNS survey.
Funding Statement
The work was supported in part by the National Natural Science Foundation of China (91846106), Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences Clinical, and Translational Medicine Research Fund (2019XK320029). The Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (CIFMS2016-I2M-4-001), Training Program for Excellent Talents in Dongcheng District, and the Education Reforming Program, Peking Union Medical College (No. 2018zlgc0119) also funded the research. These organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Abbreviations
Mg, magnesium; DM, diabetes mellitus; MetS, metabolic syndrome; CHNS, China Health and Nutrition Survey; HOMA-IR, Homeostatic Model Assessment of Insulin Resistance; HbA1c, hemoglobin A1c; BMI, Body mass index; WC, Waist circumference; TG, triglyceride; HDL, high-density lipoprotein cholesterol; BP, blood pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; CI, confidence intervals; LDL, low-density lipoprotein cholesterol; OR, odds ratio.
Ethics Approval
The China Health and Nutrition Survey (CHNS) is an ongoing open cohort, international collaborative project between the Carolina Population Center at the University of North Carolina at Chapel Hill and the National Institute for Nutrition and Health.1 This study was approved by the institutional review boards of the University of North Carolina at Chapel Hill and the National Institute for Nutrition and Health as well as the Chinese Center for Disease Control and Prevention.
Data Sharing Statement
The datasets generated and/or analyzed during the current study are available in the CHNS repository, http://www.cpc.unc.edu/projects/china.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Zhang J, Wang H, Wang Z, Zhang J, Zhang B. Association between Toenail Magnesium and Type 2 Diabetes in Chinese Adults. Nutrients. 2017;9(8):811. doi: 10.3390/nu9080811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodriguez-Moran M, Simental-Mendia LE, Gamboa-Gomez CI, Guerrero-Romero F. Oral Magnesium Supplementation and Metabolic Syndrome: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Adv Chronic Kidney Dis. 2018;25(3):261–266. doi: 10.1053/j.ackd.2018.02.011 [DOI] [PubMed] [Google Scholar]
- 3.Pan WH. Reply to “Lower intake of magnesium and dietary fiber increases the incidence of type 2 diabetes in Taiwanese”. J Formos Med Assoc. 2013;112(3):174. doi: 10.1016/j.jfma.2013.01.003 [DOI] [PubMed] [Google Scholar]
- 4.Hruby A, Meigs JB, O’Donnell CJ, Jacques PF, Mckeown NM. Higher magnesium intake reduces risk of impaired glucose and insulin metabolism and progression from prediabetes to diabetes in middle-aged Americans. Diabetes Care. 2014;37(2):419–427. doi: 10.2337/dc13-1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hata A, Doi Y, Ninomiya T, et al. Magnesium intake decreases Type 2 diabetes risk through the improvement of insulin resistance and inflammation: the Hisayama Study. Diabet Med. 2013;30(12):1487–1494. [DOI] [PubMed] [Google Scholar]
- 6.Nanri A, Mizoue T, Noda M, et al. Magnesium intake and type II diabetes in Japanese men and women: the Japan Public Health Center-based Prospective Study. Eur J Clin Nutr. 2010;64(10):1244–1247. doi: 10.1038/ejcn.2010.138 [DOI] [PubMed] [Google Scholar]
- 7.Kirii K, Iso H, Date C, Fukui M, Tamakoshi A. Magnesium intake and risk of self-reported type 2 diabetes among Japanese. J Am Coll Nutr. 2010;29(2):99–106. doi: 10.1080/07315724.2010.10719822 [DOI] [PubMed] [Google Scholar]
- 8.La SA, Lee JY, Kim DH, Song EL, Park JH, Ju SY. Low Magnesium Levels in Adults with Metabolic Syndrome: a Meta-Analysis. Biol Trace Elem Res. 2016;170(1):33–42. doi: 10.1007/s12011-015-0446-9 [DOI] [PubMed] [Google Scholar]
- 9.Cheng M, Wang H, Wang Z, Du W, Ouyang Y, Zhang B. Relationship between dietary factors and the number of altered metabolic syndrome components in Chinese adults: a cross-sectional study using data from the China Health and Nutrition Survey. BMJ Open. 2017;7(5):e014911. doi: 10.1136/bmjopen-2016-014911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song P, Yu J, Chang X, Wang M, Prevalence AL. Correlates of Metabolic Syndrome in Chinese Children: the China Health and Nutrition Survey. Nutrients. 2017;9(1):79. doi: 10.3390/nu9010079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarrafzadegan N, Khosravi-Boroujeni H, Lotfizadeh M, Pourmogaddas A, Salehi-Abargouei A. Magnesium status and the metabolic syndrome: A systematic review and meta-analysis. Nutrition. 2016;32(4):409–417. doi: 10.1016/j.nut.2015.09.014 [DOI] [PubMed] [Google Scholar]
- 12.Dibaba DT, Xun P, Fly AD, Yokota K, He K. Dietary magnesium intake and risk of metabolic syndrome: a meta-analysis. Diabet Med. 2014;31(11):1301–1309. doi: 10.1111/dme.12537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ju SY, Choi WS, Ock SM, Kim CM, Kim DH. Dietary magnesium intake and metabolic syndrome in the adult population: dose-response meta-analysis and meta-regression. Nutrients. 2014;6(12):6005–6019. doi: 10.3390/nu6126005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu X, Hall J, Byles J, Shi Z. Dietary pattern, serum magnesium, ferritin, C-reactive protein and anaemia among older people. Clin Nutr. 2017;36(2):444–451. doi: 10.1016/j.clnu.2015.12.015 [DOI] [PubMed] [Google Scholar]
- 15.Gong W, Liu A, Yao Y, et al. Nutrient Supplement Use among the Chinese Population: A Cross-Sectional Study of the 2010⁻2012 China Nutrition and Health Surveillance. Nutrients. 2018;10(11):1733. doi: 10.3390/nu10111733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J, Wang R, Luo D, Li S, Xiao C. Association between serum ferritin levels and risk of the metabolic syndrome in Chinese adults: a population study. PLoS One. 2013;8(9):e74168. doi: 10.1371/journal.pone.0074168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883 [DOI] [PubMed] [Google Scholar]
- 18.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645. [DOI] [PubMed] [Google Scholar]
- 19.International Diabetes Federation: The IDF consensus worldwide definition of the metabolic syndrome, 2006. Available at https://www.idf.org/component/attachments/attachments.html?id=705&task=download.
- 20.Kieboom BCT, Ligthart S, Dehghan A, et al. Serum magnesium and the risk of prediabetes: a population-based cohort study. Diabetologia. 2017;60(5):843–853. doi: 10.1007/s00125-017-4224-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–1182. doi: 10.1037/0022-3514.51.6.1173 [DOI] [PubMed] [Google Scholar]
- 22.Valente MJ, Pelham WE, Smyth H, MacKinnon DP. Confounding in statistical mediation analysis: what it is and how to address it. J Couns Psychol. 2017;64(6):659–671. doi: 10.1037/cou0000242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim DJ, Xun P, Liu K, et al. Magnesium intake in relation to systemic inflammation, insulin resistance, and the incidence of diabetes. Diabetes Care. 2010;33(12):2604–2610. doi: 10.2337/dc10-0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Villegas R, Gao YT, Dai Q, et al. Dietary calcium and magnesium intakes and the risk of type 2 diabetes: the Shanghai Women’s Health Study. Am J Clin Nutr. 2009;89(4):1059–1067. doi: 10.3945/ajcn.2008.27182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cutler DA, Pride SM, Cheung AP. Low intakes of dietary fiber and magnesium are associated with insulin resistance and hyperandrogenism in polycystic ovary syndrome: A cohort study. Food Sci Nutr. 2019;7(4):1426–1437. doi: 10.1002/fsn3.977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cahill F, Shahidi M, Shea J, et al. High dietary magnesium intake is associated with low insulin resistance in the Newfoundland population. PLoS One. 2013;8(3):e58278. doi: 10.1371/journal.pone.0058278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Konishi K, Wada K, Tamura T, Tsuji M, Kawachi T, Nagata C. Dietary magnesium intake and the risk of diabetes in the Japanese community: results from the Takayama study. Eur J Nutr. 2017;56(2):767–774. doi: 10.1007/s00394-015-1122-8 [DOI] [PubMed] [Google Scholar]
- 28.Moctezuma-Velazquez C, Gomez-Samano MA, Cajas-Sanchez MB, et al. High Dietary Magnesium Intake is Significantly and Independently Associated with Higher Insulin Sensitivity in a Mexican-Mestizo Population: A Brief Cross-Sectional Report. Rev Invest Clin. 2017;69(1):40–46. doi: 10.24875/ric.17002086 [DOI] [PubMed] [Google Scholar]
- 29.Nielsen FH. The Problematic Use of Dietary Reference Intakes to Assess Magnesium Status and Clinical Importance. Biol Trace Elem Res. 2019;188(1):52–59. doi: 10.1007/s12011-018-1573-x [DOI] [PubMed] [Google Scholar]
- 30.Hunt CD, Johnson LK. Magnesium requirements: new estimations for men and women by cross-sectional statistical analyses of metabolic magnesium balance data. Am J Clin Nutr. 2006;84(4):843–852. doi: 10.1093/ajcn/84.4.843 [DOI] [PubMed] [Google Scholar]
- 31.Guerrero-Romero F, Jaquez-Chairez FO, Rodriguez-Moran M. Magnesium in metabolic syndrome: a review based on randomized, double-blind clinical trials. Magnes Res. 2016;29(4):146–153. doi: 10.1684/mrh.2016.0404 [DOI] [PubMed] [Google Scholar]
- 32.Mirmiran P, Shab-Bidar S, Hosseini-Esfahani F, Asghari G, Hosseinpour-Niazi S, Azizi F. Magnesium intake and prevalence of metabolic syndrome in adults: tehran Lipid and Glucose Study. Public Health Nutr. 2012;15(4):693–701. doi: 10.1017/S1368980011002941 [DOI] [PubMed] [Google Scholar]
- 33.Gommers LM, Hoenderop JG, Bindels RJ, de Baaij JH. Hypomagnesemia in Type 2 Diabetes: A Vicious Circle? Diabetes. 2016;65(1):3–13. doi: 10.2337/db15-1028 [DOI] [PubMed] [Google Scholar]
- 34.Elin RJ. Assessment of magnesium status for diagnosis and therapy. Magnes Res. 2010;23(4):S194–S198. doi: 10.1684/mrh.2010.0213 [DOI] [PubMed] [Google Scholar]
- 35.Nair AV, Hocher B, Verkaart S, et al. Loss of insulin-induced activation of TRPM6 magnesium channels results in impaired glucose tolerance during pregnancy. Proc Natl Acad Sci U S A. 2012;109(28):11324–11329. doi: 10.1073/pnas.1113811109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corre T, Arjona FJ, Hayward C, et al. Genome-Wide Meta-Analysis Unravels Interactions between Magnesium Homeostasis and Metabolic Phenotypes. J Am Soc Nephrol. 2018;29(1):335–348. doi: 10.1681/ASN.2017030267 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- International Diabetes Federation: The IDF consensus worldwide definition of the metabolic syndrome, 2006. Available at https://www.idf.org/component/attachments/attachments.html?id=705&task=download.