Abstract
Introduction
Clinical adoption of genomic medicine has lagged behind the pace of scientific discovery. Practice-based resources can help overcome implementation challenges.
Methods
In 2015, the IGNITE (Implementing GeNomics In pracTicE) Network created an online genomic medicine implementation resource toolbox that was expanded in 2017 to incorporate the ability for users to create targeted implementation guides. This expansion was led by a multidisciplinary team that developed an evidence-based, structured framework for the guides, oversaw the technical process/build, and pilot tested the first guide, CYP2C19-Clopidogrel Testing Implementation.
Results
Sixty-five resources were collected from 12 institutions and categorized according to a seven-step implementation framework for the pilot CYP2C19-Clopidogrel Testing Implementation Guide. Five months after its launch, 96 CYP2C19-Clopidogrel Testing Implementation Guides had been created. Eighty percent of the resources most frequently selected by users were created by IGNITE to fill an identified resource gap. Resources most often included in guides were from the test reimbursement (22%), Implementation support gathering (22%), EHR integration (17%), and genetic testing workflow steps (17%).
Conclusion
Lessons learned from this implementation guide development process provide insight for prioritizing development of future resources and support the value of collaborative efforts to create resources for genomic medicine implementation.
Keywords: pharmacogenomics, CYP2C19, clopidogrel, implementation, precision medicine, personalized medicine, clinical pharmacogenomics, CYP2D6
Background
Accumulating evidence supports the benefit of using genotype to inform medication use and improve outcomes, with clinical guidelines currently available for nearly 60 gene-drug pairs from the Clinical Pharmacogenetics Implementation Consortium (cpicpgx.org).1 However, routine adoption of pharmacogenetic testing alone or as part of larger genomic medicine initiatives remains limited. Many barriers to clinical implementation, such as cost and accessibility to genetic testing, are being addressed in part by technological and scientific advancements.2–7 A significant challenge in this area is the limited number of examples of sustainable clinical practice models and practice-based resources from which health systems or clinicians interested in implementing such testing can learn.
The IGNITE Network (Implementing GeNomics In pracTicE) was established by NHGRI in 2013 to address genomic medicine implementation challenges, develop and test clinical practice models, and disseminate practice-based resources to advance this field.8 In an effort to meet the need for tested, practice-based implementation resources for genomic medicine, IGNITE developed and launched the online Supporting Practice through Application, Resources, and Knowledge (SPARK) Toolbox (https://ignite-genomics.org/) in 2015 as a searchable, open-access database of point-of-care resources (eg, clinical decision support examples, guidance on pharmacogenetic test reimbursement, patient education materials). The SPARK Toolbox houses more than 350 unique clinical and/or research resources for genomic medicine implementation that have been developed and/or employed in practice by 23 primary and affiliated IGNITE research sites.
Practice-based resources such as those available in the SPARK Toolbox can provide contextual guidance for implementing genomic medicine. However, it has been demonstrated that meaningful practice change calls for more than simply providing clinicians with new information.9,10 In addition to the availability of pragmatic resources, successful and sustainable translation of scientific knowledge into meaningful evidence-based practice also requires a supportive culture with extensive interdisciplinary engagement, a vision of how to support change, and the appropriate resources to enable change. These are also the key elements needed to address many of the process-oriented questions surrounding genomic medicine implementation.10,11 Recognizing the importance of these additional needs, in May 2017, the IGNITE Network began creating functionality for users to be able to build individualized genomic medicine implementation guides on the IGNITE website based on their own needs. This functionality was then pilot tested through development of a step-by-step guide to implement CYP2C19 genotyping in select patients taking the antiplatelet agent clopidogrel to guide treatment in a health-system setting. This manuscript outlines the process for building the online IGNITE Implementation Guide functionality, describes our experiences developing the resources for the first guide, “CYP2C19-Clopidogrel Testing Implementation,” and summarizes usage characteristics of this guide after its launch.
Construction and Content
In June 2017, a multidisciplinary team of IGNITE clinicians and researchers from 12 large academic institutions (five primary and seven affiliate sites) was formed to develop the IGNITE Implementation Guide functionality and compile resources to pilot test the CYP2C19-Clopiodogrel Testing Implementation Guide. This group included members of the IGNITE Pharmacogenetics and Provider Adoption Barriers and Education Working Groups and the IGNITE Website Team, led by investigators from the University of Florida. Implementation of CYP2C19 testing to guide antiplatelet therapy after percutaneous coronary intervention (PCI) was selected as the topic for the pilot guide because of the strength of the evidence supporting improvement in clinical outcomes with genotype-guided therapy, availability of clinical guidelines to inform dosing recommendations, and extensive investigator experience and collaborations on clinical implementation and outcomes research with CYP2C19 testing.5,6,12-14
The IGNITE Implementation Guide project team met in person or via conference call on a weekly to biweekly basis from June 2017 through April 2018, and on an ad hoc basis after the CYP2C19-Clopidogrel Testing Implementation guide was launched in May 2018 to achieve the following:
Review published literature to identify an implementation framework that could be adapted to inform development of new clinical pharmacogenetic and genetic testing services;
Identify and compile resources from IGNITE Network sites to include in the initial CYP2C19-Clopidogrel Testing Implementation guide; and
Oversee the technical build, functionality, usability testing, peer-review and usage characteristics of the CYP2C19-Clopidogrel Testing Implementation guide.
Utility and Discussion
Selection of Implementation Framework for Clinical Pharmacogenetic and Genetic Testing
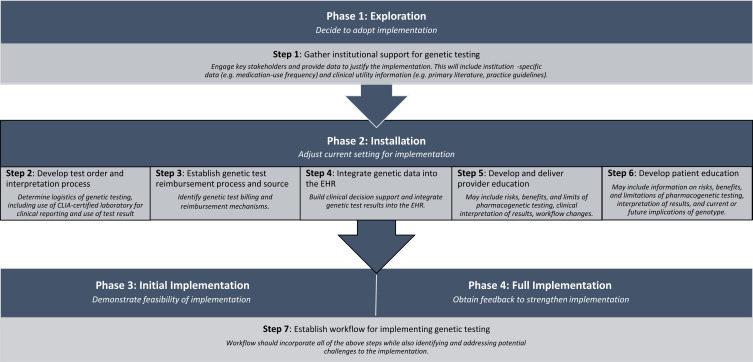
We first conducted a literature search to identify published descriptions of newly established clinical pharmacogenetic and genetic testing services that included discussion of implementation steps. Using the search terms “pharmacogenomics and/or pharmacogenetics and implementation” on PubMed, Web of Science, Embase, and Google Scholar databases, we identified 28 peer-reviewed papers published in 2017 or earlier that described 19 unique clinical implementation steps.6,7,15-41 These 19 steps were narrowed down through iterative discussion to a list of seven steps for clinical implementation of pharmacogenetic testing that were both unique and essential, based on the group’s experiences (Figure 1).
Figure 1.
Alignment of IGNITE implementation guide steps with implementation framework phases.
We then identified and evaluated ten published implementation frameworks on which the IGNITE implementation guide functionality could be based.42–52 We analyzed these implementation frameworks based on published implementation framework analyses (Table 1) and determined that the Stages of Implementation framework (National Implementation Research Network [NIRN]) most closely aligned with the desired structure and stepwise process for clinical implementation of pharmacogenetic and/or genetic testing.42,43,53-56 The NIRN framework contains critical elements consistently associated with successful and sustainable implementations, has been tested across diverse settings, including within health care systems, and is consistently utilized in practice and research.42,53-55 In addition, the NIRN framework phases aligned logically with the stepwise approach for implementing clinical pharmacogenetic and genetic testing previously identified by the group (Figure 1).
Table 1.
Selected Characteristics of Evaluated Implementation Frameworks
| Includes Stages/Steps | Includes Domains (Groups or Levels of Influence) | Includes Implementation Team | Addresses Sustainability | |
|---|---|---|---|---|
| National Implementation Resource Network Stages of Implementation42,43 | X | X | X | X |
|
EPIS:44 Exploration, Adoption/Preparation, Implementation, and Sustainment |
X | – | – | X |
|
CFIR:45 Consolidated Framework for Implementation Research |
– | X | – | – |
|
RE-AIM:46 Reach, Efficacy, Adoption, Implementation, and Maintenance |
– | X | – | X |
|
PRISM:47 Practical, Robust, Implementation and Sustainability Model |
– | X | X | X |
|
ISF:48 Interactive Systems Framework |
– | – | – | – |
|
ARC:49 Availability, Responsiveness, and Continuity |
– | X | X | – |
|
AIF:50 Active Implementation Framework |
X | – | X | X |
| The Learning Collaborative51 | X | – | X | X |
|
PPM:52 PRECEDE-PROCEED |
X | X | – | – |
Creation of the Pilot CYP2C19-Clopidogrel Testing Implementation Guide
The project team collected resources for clinical implementation of CYP2C19 testing from three sources: 1) those previously developed by IGNITE Network and affiliate sites when sites established clinical CYP2C19-clopidogrel testing (eg, site-specific Best Practice Advisory clinical decision support alert examples, patient education brochures); 2) resources created collectively by the IGNITE working groups to meet broad implementation needs that had been previously identified (eg, standardized slide set reviewing CYP2C19-clopidogrel outcomes literature; map of Center for Medicare and Medicaid Services pharmacogenetic test reimbursement policies by Medicare Administrative Contractor to assist clinicians with billing and reimbursement of CYP2C19 testing); and 3) freely available online resources for clinical CYP2C19 testing (eg, those available through PharmGKB, CPIC guidelines, FDA labeling). A total of 65 implementation resources were collected and were then categorized according to the NIRN phases and 7 implementation steps previously identified.
Although the number of contributed resources was relatively evenly distributed across the seven implementation steps, the majority of resources (n = 51 of 65; 78%) corresponded to a single phase (Installation) of the NIRN Implementation Framework. More than half of all contributed resources (n = 36 of 65; 55%) were created by IGNITE sites in the process of implementing CYP2C19-clopidogrel testing at their institution, with nearly all patient and provider education resources created by IGNITE sites (n = 20 of 21; 95%). Thirteen resources were created by IGNITE Network working groups to meet a previously identified need. Of these, 62% (n = 8 of 13) were created to assist with establishing reimbursement mechanisms for genetic testing (eg, Map of Pharmacogenetic Test Reimbursement).
Technical Build and Pre-Launch Usability Testing and Review of CYP2C19-Clopidogrel Implementation Guide
The IGNITE Website Team developed the workflow and technical build for the users to create an online implementation guide using jQuery, PHP, and WordPress custom post types. Users create their guide by completing a brief Implementation Readiness Assessment, in which they rate their completion for each of the seven implementation steps as “Not Yet Started,” “In Progress,” or “Completed,” for the target genomic medicine implementation.
Once this information is submitted, users are presented with possible resources for inclusion based on their current progress and asked to customize their implementation guide by previewing and selecting one or more resources for each step that they have not yet completed. The customized implementation guide is then automatically generated based on the user-selected resources. Once created, unique implementation guides can be bookmarked as static URLs (universal resource locators) or downloaded as PDFs (portable digital files). All user-generated guides include a brief explanation of each step, even if marked completed during the guide creation process, to ensure that the final guide provides a comprehensive listing of implementation steps.
Beta testing of implementation guide-creation process was conducted by members of the IGNITE Pharmacogenetics and Provider Adoption Barriers and Education Working Group members from February to March 2018. A questionnaire was used to assess the navigation and technical functionalities of the implementation guide creation process using different Internet browsers and computer operating systems. This group also provided additional feedback on included implementation resources and their categorization by implementation steps to identify and fill resource gaps, and further optimize the navigation and usability of the implementation guide creation process.
Early CYP2C19-Clopidogrel Testing Implementation Guide Usage
Once resources were collated and technical functionality built for users to create their online implementation guide, the pilot CYP2C19-Clopidogrel Testing Implementation Guide was launched for testing in May 2018. Between May and October 2018, the CYP2C19-Clopidogrel Implementation Guide landing page received 249 unique pageviews. A total of 96 completed CYP2C19-Clopidogrel Implementation Guides were created by end users during this time (usage data analyzed and refined to remove guides created for internal testing purposes from this count) that included an average of 19 resources per guide (1830 total resources selected across 96 guides).
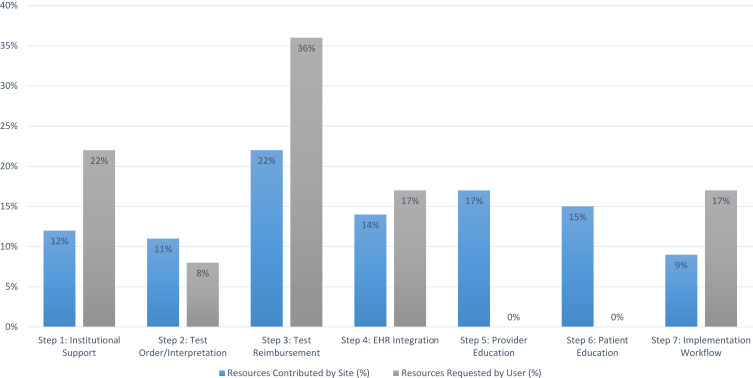
Of the top ten most frequently selected resources (Table 2), 80% were resources that had been created by the IGNITE Network working groups to meet identified implementation needs, with the remaining resources created by IGNITE sites during their implementation of CYP2C19 testing. The distribution of contributed resources did not differ significantly from the distribution of resources most frequently selected by users for inclusion in their CYP2C19-Clopidogrel Testing implementation guide, likely due to sample size limitations (P = 0.699; Figure 2).
Table 2.
Individual Resources That Were Most Often Included by Users in CYP2C19-Clopidogrel Implementation Guides During Pilot Testing
| Resource Name | Number of Times Resource Was Included in User Guide | Resource Contributor |
|---|---|---|
| Pharmacogenetic Test Reimbursement According to MAC | 53 | IGNITE Network |
| Payment and Reimbursement Glossary | 44 | IGNITE Network |
| Evidence Overview of CYP2C19-Clopidogrel Presentation | 41 | IGNITE Network |
| Publications: CYP2C19-Clopidogrel Evidence Overview | 34 | IGNITE Network |
| Publications: CYP2C19-Clopidogrel Clinical Decision Support | 31 | IGNITE Network |
| Workflow Diagram: Implementing CYP2C19 Testing | 31 | Individual Site |
| Summary of CYP2C19 Platforms and Variants by Site | 28 | IGNITE Network |
| Guidance: Use of CPT Codes for Molecular Pathology | 28 | IGNITE Network |
| Example: CYP2C19-Clopidogrel Clinical Decision Support | 28 | Individual Site |
| Publications: Implementation of CYP2C19-Clopidogrel Testing | 28 | IGNITE Network |
Abbreviations: NHGR, National Human Genome Research Institute; HER, electronic health record; IGNITE, Implementing GeNomics In pracTicE; SPARK, Supporting Practice through Applications, Research, and Knowledge; PCI, percutaneous coronary intervention; CPIC, Clinical Pharmacogenetics Implementation Consortium; FDA, Food and Drug Administration; NIRN, National Implementation Research Network; URL, universal resource locator; PDF, portable digital file; CPT, Current Procedural Terminology.
Figure 2.
Distribution of resources contributed by sites and requested by users according to implementation step.
Discussion
To our knowledge, this is the first published description of the development, technical build, and pilot testing of an online process for users to create implementation guides to support clinical use of pharmacogenetic and/or genetic testing. Although the first implementation guide that was created and pilot-tested focused on a single pharmacogenetic test (CYP2C19 testing for clopidogrel), the process used to develop the guide has applications beyond this single gene-drug pair. The guide incorporated established implementation frameworks, published descriptions of varied pharmacogenomic and genomic medicine implementations, and clinical experiences of successful early adopters in a variety of practice settings. As such, many of the insights and lessons learned during this process can be applied broadly to inform resource development to meet clinical implementation needs in genomic medicine overall (Box 1).
Box 1.
Lessons Learned for Implementation of Pharmacogenomics and Genomic Medicine
In the planning stages of an implementation, it is valuable to seek information and guidance from a variety of sources, including:
|
| Consider resources that can be used to engage and garner support from a range of stakeholders early in the implementation process, such as formal presentation and/or handout materials defining scientific, clinical, administrative, logistical, and reimbursement challenges and opportunities within your organization. |
| Brief summaries of published literature demonstrating benefits and limitations of a new clinical implementation in the context of existing standards of care and/or practice guidelines can help to demonstrate the use of an evidence-based, clinically relevant approach to non-genomics providers. |
| Objective data and guidance on reimbursement procedures and anticipated out-of-pocket patient costs for pharmacogenetic and/or genetic testing is critical to stakeholder engagement and sustainability of a clinical implementation. In our experience, users valued resources such as regional Medicare Administrative Contractor (MAC) reimbursement rates for testing and guidance on documenting tests using current procedure terminology (CPT) codes for billing, reimbursement, and patient care records. |
| Although provider and patient education are consistently identified as key implementation barriers, resources such as patient and provider brochures and patient education handouts were among the least-accessed resources by users. This finding suggests lower-than-anticipated need for externally developed educational materials, possibly due to availability from laboratories or other commercial databases or potentially a preference for internally-developed educational materials that can be customized with an institutional logo, standardized formatting, or boilerplate language. |
| Although many early clinical pharmacogenetic and/or genomic implementations have occurred in large, academic medical centers, there is a critical need for implementation experiences and resources to support diverse practice settings, such as in rural or underserved populations, community-based hospitals, private physician practices, managed care organizations, and community pharmacies. |
One distinguishing characteristic of this project in the genomic medicine implementation space is that all individuals on the project team had experience successfully implementing genetic and/or pharmacogenetic testing at their institution. Within genomic medicine, large-scale sustained clinical implementations are still rare, even for pharmacogenetic testing, which is often considered to be the “low-hanging” fruit of genomic medicine implementation. The IGNITE researchers and affiliate site members who participated in this project are early adopters of clinical pharmacogenetics, and as such, resources contributed by sites were developed and tested in a point-of-care implementation environment. We are not aware of any other clinical resource repositories for pharmacogenetics and/or genomic medicine that are based exclusively on pragmatic implementation experiences.
The collective experiences of the IGNITE Network overall brought additional diversity and value to the resources created. In addition to creating institution-specific implementation resources based on individual IGNITE site experiences, IGNITE researchers collaborated to develop Network-wide resources that address needs that have been repeatedly identified since the launch of IGNITE in 2013. In particular, IGNITE-Network resources such as standardized evidence overview slide sets to support stakeholder engagement, glossaries and interactive resources that help users understand and operationalize clinical genetic test ordering and reimbursement, and aggregated examples of genetic testing strategies, platforms, and clinical decision support resources that have been used in point-of-care settings can disseminate lessons learned and support early adoption of successful and sustainable implementation strategies in other settings. The value of these resources to end users is supported by CYP2C19-Clopidogrel Testing implementation guides created during the pilot-testing phase, in which 8 of the top 10 resources that users selected for in their guides inclusion were uniquely created by IGNITE to support an identified need, which suggests a perceived value in disseminating pragmatic resources for genomic medicine implementation.
Project findings also provide insight for prioritizing the development of resources to help individuals or institutions that desire to implement pharmacogenetic or genetic testing overcome common barriers. For example, provider knowledge and education is universally identified as a key implementation barrier.2,3,57,58 However, the most frequently selected resources for inclusion in user-created guides did not include any provider or patient education resources, in spite of a large number pragmatic examples of educational resources provided in the guide-creation process. This finding suggests that users’ needs for resources to help overcome barriers to obtaining reimbursement for process-oriented steps such as conducting and billing for genetic testing, securing institutional support, and creating a feasible clinical workflow for genetic testing may either outweigh or precede provider or patient education needs in a point-of-care implementation environment.
Although the differences in the distribution of contributed versus most frequently accessed resources for the pilot CYP2C19-Clopidogrel Testing Implementation guide did not differ significantly, likely due to sample size limitations, they do highlight an important disconnect that is consistent with our experiences implementing clinical pharmacogenetic and genetic testing. Because of variable insurance reimbursement for tests, limited knowledge of test reimbursement policies among different insurers, and a developing evidence base for clinical utility, point-of-care clinical implementation experiences remain limited. This creates a “chicken or the egg” dilemma in which clinicians and researchers are unable to implement pharmacogenetic/genetic testing clinically until its feasibility and clinical utility have been clearly demonstrated. However, practical challenges such as variable and vague test reimbursement policies among insurers limit our ability to test clinical practice models that could be used to demonstrate such feasibility and utility.59,60 Funding sources for research in this area should address these practical considerations to allow for investigation of point-of-care clinical implementations on a larger scale, similar to the IGNITE Network experiences. In addition, policy changes are needed to promote consistency, clarity, and an ongoing dialogue between insurers and the health care system at large regarding reimbursement for pharmacogenetic and genetic testing.
While there are potential benefits to the use of customizable resources to support implementation of a new service, there are also limitations in ensuring such resources are applicable to diverse practice settings. Although additional IGNITE implementation guides have already been launched and others are in development, this process may not be practical to provide solutions on a large scale. This finding is supported by overall IGNITE SPARK Toolbox usage data as compared with implementation guides usage data. There was a total of 249 unique views of the IGNITE implementation guide landing page during from May 2018 to October 2018. During this same time period, there were nearly 1500 unique pageviews of the SPARK Toolbox, a browsable database of implementation resources.
Additional limitations exist to the applications and lessons learned from this project. We describe development of an online process for users to create pharmacogenetic or genetic testing implementation guide. Resources and implementation strategies may not be applicable to other clinical scenarios. This project is also limited by its nature as a pilot, limitation of usability testing to a single pharmacogenetic testing example (versus broader genetic testing), and the lack of diversity of sites contributing resources. Nearly all contributing IGNITE Network and affiliate sites were large academic health systems, which creates a potential bias for our results since these sites are not representative of or generalizable to any given US institution. In addition, the sites that participated may inherently be more educated about or aware of pharmacogenomics and needs of other institutions may be significantly different.
Conclusion
The IGNITE Network genomic medicine implementation guides are unique in their integration of data from existing implementation frameworks, published evidence, and clinical implementation experiences. In addition, the IGNITE SPARK Toolbox continues to accept new implementation resources in an ongoing manner and as such creates a dynamic, continuously updated mechanism for disseminating genomic medicine implementation resources. While the initial guides have focused on implementation of pharmacogenomic gene-drug pairs, the process for creating the guides and technical functionality serve as a model for what we anticipate will be a growing resource to broadly support genomic medicine implementation.
Funding Statement
This work was supported by grants from the National Institutes of Health (U01 HG007269 [as part of the IGNITE Network]; U01 HG007775); the National Heart Lung and Blood Institute (RO1HL092173; 1K24HL133373), Clinical Translational Science Award (CTSA) program (UL1 TR001427; UL1TR000165), the University of Pennsylvania Center for Precision Medicine, NIH Cancer Center (P30-CA076292), the DeBartolo Family Personalized Medicine Institute Pilot Research Award in Precision Medicine, and substantial institutional support from the University of Florida.
Data Sharing Statement
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
Authors declare the following potential competing interests: NAL serves as a consultant for Admera Health; JKH receives clinical trial support from OneOme, LLC, reports personal fees from Novartis and grants from ASHP, and serves as a consultant for Quest Diagnostics; ALB and NP report grants from NIH; FF receives consulting fee or honoraria from Astra Zeneca and Sanofi. KW reports grants from National Human Genome Research Institute and Clinical Translational Science Award Program. The authors report no other conflicts of interest in this work.
References
- 1.Relling MV, Klein TE. CPIC: clinical pharmacogenetics implementation consortium of the pharmacogenomics research network. Clin Pharmacol Ther. 2011;89(3):464–467. doi: 10.1038/clpt.2010.279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sperber NR, Carpenter JS, Cavallari LH, et al. Challenges and strategies for implementing genomic services in diverse settings: experiences from the Implementing GeNomics In pracTicE (IGNITE) network. BMC Med Genomics. 2017;10(1):35. doi: 10.1186/s12920-017-0273-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levy KD, Blake K, Fletcher-Hoppe C, et al. Opportunities to implement a sustainable genomic medicine program: lessons learned from the IGNITE network. Genet Med. 2018;21(3):743–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schildcrout JS, Shi Y, Danciu I, et al. A prognostic model based on readily available clinical data enriched a pre-emptive pharmacogenetic testing program. J Clin Epidemiol. 2016;72:107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee CR, Sriramoju VB, Cervantes A, et al. Clinical outcomes and sustainability of using CYP2C19 genotype-guided antiplatelet therapy after percutaneous coronary intervention. Circ Genom Precis Med. 2018;11(4):e002069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Empey PE, Stevenson JM, Tuteja S, et al. Multisite investigation of strategies for the implementation of CYP2C19 genotype-guided antiplatelet therapy. Clin Pharmacol Ther. 2017;104(4):664–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavallari LH, Lee CR, Duarte JD, et al. Implementation of inpatient models of pharmacogenetics programs. Am J Health Syst Pharm. 2016;73(23):1944–1954. doi: 10.2146/ajhp150946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weitzel KW, Alexander M, Bernhardt BA, et al. The IGNITE network: a model for genomic medicine implementation and research. BMC Med Genomics. 2016;9(1):1. doi: 10.1186/s12920-015-0162-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellis I, Howard P, Larson A, Robertson J. From workshop to work practice: an exploration of context and facilitation in the development of evidence-based practice. Worldviews Evid Based Nurs. 2005;2(2):84–93. doi: 10.1111/j.1741-6787.2005.04088.x [DOI] [PubMed] [Google Scholar]
- 10.Kueny A, Shever LL, Lehan Mackin M, Titler MG. Facilitating the implementation of evidence- based practice through contextual support and nursing leadership. J Healthcare Leadersh. 2015;7:29–39. doi: 10.2147/JHL.S45077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tabak RG, Khoong EC, Chambers DA, Brownson RC. Bridging research and practice: models for dissemination and implementation research. Am J Prev Med. 2012;43(3):337–350. doi: 10.1016/j.amepre.2012.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cavallari LH, Lee CR, Beitelshees AL, et al. Multisite investigation of outcomes with implementation of CYP2C19 genotype-guided antiplatelet therapy after percutaneous coronary intervention. JACC Cardiovasc Interv. 2018;11(2):181–191. doi: 10.1016/j.jcin.2017.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cavallari LH, Beitelshees AL, Blake KV, et al. The IGNITE pharmacogenetics working group: an opportunity for building evidence with pharmacogenetic implementation in a real-world setting. Clin Transl Sci. 2017;10(3):143–146. doi: 10.1111/cts.12456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cavallari LH, Franchi F, Rollini F, et al. Clinical implementation of rapid CYP2C19 genotyping to guide antiplatelet therapy after percutaneous coronary intervention. J Transl Med. 2018;16(1):92. doi: 10.1186/s12967-018-1469-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pulley JM, Denny JC, Peterson JF, et al. Operational implementation of prospective genotyping for personalized medicine: the design of the Vanderbilt PREDICT project. Clin Pharmacol Ther. 2012;92(1):87–95. doi: 10.1038/clpt.2011.371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffman JM, Haidar CE, Wilkinson MR, et al. PG4KDS: a model for the clinical implementation of pre-emptive pharmacogenetics. Am J Med Genet C Semin Med Genet. 2014;166(1):45–55. doi: 10.1002/ajmg.c.31391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weitzel KW, Elsey AR, Langaee TY, et al. Clinical pharmacogenetics implementation: approaches, successes, and challenges. Am J Med Genet C Semin Med Genet. 2014;166(1):56–67. doi: 10.1002/ajmg.c.31390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shuldiner AR, Palmer K, Pakyz RE, et al. Implementation of pharmacogenetics: the University of Maryland personalized anti-platelet pharmacogenetics program. Am J Med Genet C Semin Med Genet. 2014;166(1):76–84. doi: 10.1002/ajmg.c.31396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Owusu-Obeng A, Weitzel KW, Hatton RC, et al. Emerging roles for pharmacists in clinical implementation of pharmacogenomics. Pharmacotherapy. 2014;34(10):1102–1112. doi: 10.1002/phar.1481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harada S, Zhou Y, Duncan S, et al. Precision medicine at the University of Alabama at Birmingham: laying the foundational processes through implementation of genotype-guided antiplatelet therapy. Clin Pharmacol Ther. 2017;102(3):493–501. doi: 10.1002/cpt.631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arwood MJ, Chumnumwat S, Cavallari LH, Nutescu EA, Duarte JD. Implementing pharmacogenomics at your institution: establishment and overcoming implementation challenges. Clin Transl Sci. 2016;9(5):233–245. doi: 10.1111/cts.12404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manolio TA, Chisholm RL, Ozenberger B, et al. Implementing genomic medicine in the clinic: the future is here. Genet Med. 2013;15(4):258–267. doi: 10.1038/gim.2012.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dunnenberger HM, Crews KR, Hoffman JM, et al. Preemptive clinical pharmacogenetics implementation: current programs in five US medical centers. Annu Rev Pharmacol Toxicol. 2015;55(1):89–106. doi: 10.1146/annurev-pharmtox-010814-124835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee JA, Lee CR, Reed BN, et al. Implementation and evaluation of a CYP2C19 genotype-guided antiplatelet therapy algorithm in high-risk coronary artery disease patients. Pharmacogenomics. 2015;16(4):303–313. doi: 10.2217/pgs.14.180 [DOI] [PubMed] [Google Scholar]
- 25.Vo TT, Bell GC, Owusu Obeng A, Hicks JK, Dunnenberger HM. Pharmacogenomics Implementation: considerations for selecting a reference laboratory. Pharmacotherapy. 2017;37(9):1014–1022. doi: 10.1002/phar.1985 [DOI] [PubMed] [Google Scholar]
- 26.O’Connor SK, Ferreri SP, Michaels NM, et al. Exploratory planning and implementation of a pilot pharmacogenetic program in a community pharmacy. Pharmacogenomics. 2012;13(8):955–962. doi: 10.2217/pgs.12.67 [DOI] [PubMed] [Google Scholar]
- 27.Moaddeb J, Mills R, Haga SB. Community pharmacists’ experience with pharmacogenetic testing. J Am Pharm Assoc (2003). 2015;55(6):587–594. doi: 10.1331/JAPhA.2015.15017 [DOI] [PubMed] [Google Scholar]
- 28.Haga SB, Allen LaPointe NM, Moaddeb J, Mills R, Patel M, Kraus WE. Pilot study: incorporation of pharmacogenetic testing in medication therapy management services. Pharmacogenomics. 2014;15(14):1729–1737. doi: 10.2217/pgs.14.118 [DOI] [PubMed] [Google Scholar]
- 29.Haga SB, LaPointe NM, Cho A, et al. Pilot study of pharmacist-assisted delivery of pharmacogenetic testing in a primary care setting. Pharmacogenomics. 2014;15(13):1677–1686. doi: 10.2217/pgs.14.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haga SB, Allen LaPointe NM, Moaddeb J. Challenges to integrating pharmacogenetic testing into medication therapy management. J Manag Care Spec Pharm. 2015;21(4):346–352. doi: 10.18553/jmcp.2015.21.4.346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Connor SK, Michaels N, Ferreri S. Expansion of pharmacogenomics into the community pharmacy: billing considerations. Pharmacogenomics. 2015;16(3):175–180. doi: 10.2217/pgs.14.183 [DOI] [PubMed] [Google Scholar]
- 32.Dunnenberger HM, Biszewski M, Bell GC, et al. Implementation of a multidisciplinary pharmacogenomics clinic in a community health system. Am J Health Syst Pharm. 2016;73(23):1956–1966. doi: 10.2146/ajhp160072 [DOI] [PubMed] [Google Scholar]
- 33.O’Donnell PH, Bush A, Spitz J, et al. The 1200 patients project: creating a new medical model system for clinical implementation of pharmacogenomics. Clin Pharmacol Ther. 2012;92(4):446–449. doi: 10.1038/clpt.2012.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Donnell PH, Danahey K, Jacobs M, et al. Adoption of a clinical pharmacogenomics implementation program during outpatient care – initial results of the University of Chicago “1200 patients project”. Am J Med Genet C Semin Med Genet. 2014;166(1):68–75. doi: 10.1002/ajmg.c.31385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenman MB, Decker B, Levy KD, Holmes AM, Pratt VM, Eadon MT. Lessons learned when introducing pharmacogenomic panel testing into clinical practice. Value Health. 2017;20(1):54–59. doi: 10.1016/j.jval.2016.08.727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levy KD, Decker BS, Carpenter JS, et al. Prerequisites to implementing a pharmacogenomics program in a large health-care system. Clin Pharmacol Ther. 2014;96(3):307–309. doi: 10.1038/clpt.2014.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hicks JK, Stowe D, Willner MA, et al. Implementation of clinical pharmacogenomics within a large health system: from electronic health record decision support to consultation services. Pharmacotherapy. 2016;36(8):940–948. doi: 10.1002/phar.1786 [DOI] [PubMed] [Google Scholar]
- 38.Ferreri SP, Greco AJ, Michaels NM, et al. Implementation of a pharmacogenomics service in a community pharmacy. J Am Pharm Assoc (2003). 2014;54(2):172–180. doi: 10.1331/JAPhA.2014.13033 [DOI] [PubMed] [Google Scholar]
- 39.Dolan ME, Maitland ML, O’Donnell PH, Nakamura Y, Cox NJ, Ratain MJ. Institutional profile: University of Chicago center for personalized therapeutics: research, education and implementation science. Pharmacogenomics. 2013;14(12):1383–1387. doi: 10.2217/pgs.13.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson JA, Elsey AR, Clare-Salzler MJ, Nessl D, Conlon M, Nelson DR. Institutional profile: University of Florida and Shands hospital personalized medicine program: clinical implementation of pharmacogenetics. Pharmacogenomics. 2013;14(7):723–726. doi: 10.2217/pgs.13.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gottesman O, Scott SA, Ellis SB, et al. The CLIPMERGE PGx program: clinical implementation of personalized medicine through electronic health records and genomics-pharmacogenomics. Clin Pharmacol Ther. 2013;94(2):214–217. doi: 10.1038/clpt.2013.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fixsen DNS, Blase K, Friedman R, Wallace F. Implementation Research: A Synthesis of the Literature. Tampa, FL: University of South Florida, Louis de la Parte Florida Mental Health Institute, The National Implementation Research Network; 2005. [Google Scholar]
- 43.Network NIR. National implementation research network’s active implementation hub. University of North Carolina at Chapel Hill’s FPG Child Development Institute; 2017 [Google Scholar]
- 44.Aarons GA, Hurlburt M, Horwitz SM. Advancing a conceptual model of evidence-based practice implementation in public service sectors. Adm Policy Ment Health. 2011;38(1):4–23. doi: 10.1007/s10488-010-0327-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4(1):50. doi: 10.1186/1748-5908-4-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. 1999;89(9):1322–1327. doi: 10.2105/AJPH.89.9.1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feldstein AC, Glasgow RE. A practical, robust implementation and sustainability model (PRISM) for integrating research findings into practice. Jt Comm J Qual Patient Saf. 2008;34(4):228–243. doi: 10.1016/s1553-7250(08)34030-6 [DOI] [PubMed] [Google Scholar]
- 48.Wandersman A, Duffy J, Flaspohler P, et al. Bridging the gap between prevention research and practice: the interactive systems framework for dissemination and implementation. Am J Community Psychol. 2008;41(3–4):171–181. doi: 10.1007/s10464-008-9174-z [DOI] [PubMed] [Google Scholar]
- 49.Glisson C, Schoenwald SK. The ARC organizational and community intervention strategy for implementing evidence-based children’s mental health treatments. Ment Health Serv Res. 2005;7(4):243–259. doi: 10.1007/s11020-005-7456-1 [DOI] [PubMed] [Google Scholar]
- 50.Metz A, Bartley L, Ball H, Wilson D, Naoom S, Redmond P. Active implementation frameworks for successful service delivery: Catawba county child wellbeing project. Res Soc Work Pract. 2015;25(4):415–422. doi: 10.1177/1049731514543667 [DOI] [Google Scholar]
- 51.Improvement IfH. The breakthrough series: IHI’s collaborative model for achieving breakthrough improvement; 2003. Available from: www.ihi.org. Accessed May27, 2020.
- 52.Howat P, Jones S, Hall M, Cross D, Stevenson M. The PRECEDE-PROCEED model: application to planning a child pedestrian injury prevention program. Inj Prev. 1997;3(4):282–287. doi: 10.1136/ip.3.4.282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hanson RF, Self-Brown S, Rostad WL, Jackson MC. The what, when, and why of implementation frameworks for evidence-based practices in child welfare and child mental health service systems. Child Abuse Negl. 2016;53:51–63. doi: 10.1016/j.chiabu.2015.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Skolarus TA, Lehmann T, Tabak RG, Harris J, Lecy J, Sales AE. Assessing citation networks for dissemination and implementation research frameworks. Implement Sci. 2017;12(1):97. doi: 10.1186/s13012-017-0628-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meyers DC, Durlak JA, Wandersman A. The quality implementation framework: a synthesis of critical steps in the implementation process. Am J Community Psychol. 2012;50(3–4):462–480. doi: 10.1007/s10464-012-9522-x [DOI] [PubMed] [Google Scholar]
- 56.Moullin JC, Sabater-Hernández D, Fernandez-Llimos F, Benrimoj SI. A systematic review of implementation frameworks of innovations in healthcare and resulting generic implementation framework. Health Res Policy Syst. 2015;13(1):16. doi: 10.1186/s12961-015-0005-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Owusu Obeng A, Fei K, Levy KD, et al. Physician-reported benefits and barriers to clinical implementation of genomic medicine: a multi-site IGNITE-network survey. J Pers Med. 2018;8(3):24. doi: 10.3390/jpm8030024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hauser D, Obeng AO, Fei K, Ramos MA, Horowitz CR. Views of primary care providers on testing patients for genetic risks for common chronic diseases. Health Aff (Millwood). 2018;37(5):793–800. doi: 10.1377/hlthaff.2017.1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Keeling NJ, Rosenthal MM, West-Strum D, Patel AS, Haidar CE, Hoffman JM. Preemptive pharmacogenetic testing: exploring the knowledge and perspectives of US payers. Genet Med. 2017;21(5):1224–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pezalla EJ. Payer view of personalized medicine. Am J Health Syst Pharm. 2016;73(23):2007–2012. doi: 10.2146/ajhp160038 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Improvement IfH. The breakthrough series: IHI’s collaborative model for achieving breakthrough improvement; 2003. Available from: www.ihi.org. Accessed May27, 2020.