Abstract
Purpose
The expression of programmed death-ligand 1 (PD-L1) is common in various solid human cancers and it is an important therapeutic target. However, the expression pattern, clinical significance and potential mechanism of PD-L1 in hypopharyngeal squamous cell carcinoma (HSCC) are still lacking.
Methods
PD-L1 expression in HSCC tumor tissues and paired adjacent hypopharyngeal mucosal tissues was detected using immunohistochemistry assay, and the clinical significance of PD-L1 in HSCC was characterized. In vitro assays including cell viability assays, migration assays, invasion assays as well as Western blot assays were performed to illuminate the biological functions and underlying molecular mechanisms of PD-L1 in HSCC development.
Results
PD-L1 expression was detected in HSCC samples but we found no positive expression in matched normal hypopharyngeal mucosal tissues. The levels of PD-L1 expression were significantly correlated with advanced clinical progression and poor patient survival. Multivariable analysis of Cox model showed that PD-L1 expression was an independent predictor for the prognosis of HSCC patients. Functional experiments showed that the ectopic expression of PD-L1 markedly influenced the proliferation, migration and invasion of FaDu cells in vitro. Mechanistically, investigations demonstrated that PD-L1 could promote the epithelial–mesenchymal transition of FaDu cells. Meanwhile, PD-L1 knockdown inhibited, while PD-L1 overexpression activated the Akt-mTOR signaling pathway in FaDu cells. The EMT induced by PD-L1 overexpression could be reversed by the Akt inhibitor.
Conclusion
In summary, the expression of PD-L1 can act as a significant biomarker for the adverse clinicopathological features and poor prognosis of patients with HSCC. PD-L1 can promote the proliferation, migration and invasion of FaDu cells and consequently enhance the aggressiveness. Moreover, PD-L1 induces EMT through AKT-mTOR signaling pathway. These suggest that PD-L1 has important tumor-intrinsic functions independent of its immunopathogenic effects.
Keywords: PD-L1, hypopharyngeal squamous cell carcinoma, prognosis, epithelial–mesenchymal transition
Introduction
Hypopharyngeal squamous cell carcinoma (HSCC) accounts for 3–5% of all head and neck squamous cell carcinomas.1 Advanced diseases were common in patients with HSCC and more than half of the patients had lymphatic metastasis when they came to medical attention.2 Despite the tremendous progress that has been made in the comprehensive treatments of HSCC, the prognosis of patients with HSCC is still poor.3 One important reason is that after the treatments up to 30% of patients with HSCC die within a year as a result of distant metastasis, which makes it a particularly devastating disease.4 Although there have been some studies on the pathogenesis of HSCC,5–7 investigations of new potential mechanisms related to the development and progression of HSCC are still urgent.
Programmed death-ligand 1 (PD-L1, also known as CD274 or B7-H1) is a coinhibitory molecule which belongs to B7 superfamily and plays a pivotal role in driving immune suppression and contributes to tumor immune evasion in the context of tumor progression.8 The expression of PD-L1 exhibits quite diverse patterns. PD-L1 is rarely expressed on normal tissues but up-regulated expression of PD-L1 is detected on many human cancers.9–19 The levels of PD-L1 expression on tumors are significantly associated with aggressive biological behaviors and poor prognosis.8,10,13 However, the contributions of PD-L1 to tumor pathogenicity, especially in HSCC, are incompletely understood. Furthermore, most studies associated with PD-L1 signals in cancers focused on its immunomodulatory effects on T cells.9 Nevertheless, recent work showed that tumor-expressed PD-L1 prevented tumor apoptosis,19 regulated cell growth and autophagy,20 and regulated tumor glucose metabolism in sarcomas.21 Thus, PD-L1 has important tumor-intrinsic functions which are still needed to be further elucidated.
Epithelial–mesenchymal transition (EMT), a process in which epithelial cells interact with surrounding mesenchymal cells and obtain mesenchymal phenotypes, is important for invasion and metastasis of carcinoma.22 Several authors have demonstrated that EMT had connections with PD-L1 expression in cancers.23–25 However, to our acknowledge, the analysis regarding the relationship between PD-L1 expression and EMT in HSCC has not yet been reported.
Here, we test the hypothesis that PD-L1 has important prognostic significance and tumor cell-autonomous functions in HSCC. Our data indicate that PD-L1 can act as a significant biomarker for the prognosis of patients with HSCC and has distinct tumor-intrinsic functions independent of its immunopathogenic effects.
Materials and Methods
Patients and Samples
Ninety-five patients with HSCC who underwent primary surgical resection and received post-surgical radiotherapy between January 2005 and November 2014 in the Shandong Provincial ENT Hospital were enrolled in this study. Tumor tissues and adjacent normal mucosal tissues were collected and all tumor samples were confirmed as squamous cell carcinomas by pathological analysis after surgical resection. Retrospective clinicopathological parameters are shown in Table 1. Tumor stage was determined using the seventh edition of TNM staging system for head and neck squamous cell carcinoma published by American Joint Committee on Cancer (AJCC). The lymphatic metastasis was confirmed according to the final pathological diagnosis, which defined the N stage. Follow-up data were available for all these patients, which were used for survival analysis. All patients provided written consents for the use of their specimens and disease information for future investigations. The study was conducted in accordance with the Declaration of Helsinki and was approved by the ethics committee of our institution.
Table 1.
Clinicopathological Features Associated with PD-L1 Expression in 95 HSCC Patients
| Clinicopathological Features | Total | PD-L1 Expression | P value | |
|---|---|---|---|---|
| Negative | Positive | |||
| Gender | 0.610 | |||
| Male | 90 | 50 | 40 | |
| Female | 5 | 3 | 2 | |
| Age (years) | 0.263 | |||
| <60 | 58 | 35 | 23 | |
| >60 | 37 | 18 | 19 | |
| Differentiation | 0.949 | |||
| High-moderate | 63 | 35 | 28 | |
| Poor | 32 | 18 | 14 | |
| T stage | 0.670 | |||
| T1–T2 | 20 | 12 | 8 | |
| T3–T4 | 75 | 41 | 34 | |
| N stage | <0.001 | |||
| N0 | 22 | 20 | 2 | |
| N+ | 73 | 33 | 40 | |
| Clinical stage | <0.001 | |||
| I–II | 9 | 9 | 0 | |
| III–IV | 86 | 44 | 42 | |
| Distant metastasis | <0.001 | |||
| No | 66 | 47 | 19 | |
| Yes | 29 | 6 | 23 | |
Abbreviations: PD-L1, programmed death-ligand 1; HSCC, hypopharyngeal squamous cell carcinoma.
Reagents
Rabbit anti-human PD-L1 monoclonal antibody (ab205921, 1:5000, Abcam, Cambridge, MA, USA) was used for the immunohistochemistry assay. The HPR-labeled goat polyclonal anti-rabbit secondary antibodies (SP9001, 1:500) were purchased from OriGene Technologies, Inc. (Rockville, MD, USA). In addition, antibodies used for Western blot analysis such as mouse monoclonal anti-human β-actin (ab8226, 1:20,000), rabbit monoclonal anti-human PD-L1 (ab205921, 1:15,000), mouse monoclonal anti-human E-cadherin (ab231303, 1:60,000), rabbit monoclonal anti-human N-cadherin (ab76011, 1:400), rabbit monoclonal anti-human Vimentin (ab92547, 1:2500), rabbit monoclonal anti-human p-Akt (ab81283, 1:5000), rabbit polyclonal anti-human Akt (ab8805, 1:20,000) and rabbit monoclonal anti-human p-mTOR (ab109268, 1:20,000) were also purchased from Abcam.
Immunohistochemical Staining and Analysis
Serial-3-um-thick sections were obtained from the paraffin-embedded tissue blocks and were dewaxed and rehydrated. Antigen retrieval was performed with an antigen retrieve solution (Abcam, Cambridge, MA, USA) and 0.3% hydrogen peroxide solution was used to block the activity of endogenous peroxidase. After blocking with 3% BSA solution at room temperature for 30 min, the sections were incubated with rabbit anti-human PD-L1 monoclonal antibody at 4°C overnight and subsequently incubated with HRP-labelled goat anti-rabbit secondary antibody in the next day. The chromogenic reaction was performed with a DAB substrate kit (Thermo Fisher Scientific, Inc., Waltham, MA, USA) and hematoxylin was used to perform the counterstain. Finally, the sections were dehydrated and mounted.
Two senior pathologists who were blind to this study examined the sections independently and membranal or cytoplasmic staining of cells was regarded as positive PD-L1 immunostaining. Staining intensity was scored as follows: 0, absent; 1, low; 2, medium; and 3, high. The extent of staining was scored as follows: 0, 0% stained; 1, 1–25% stained; 2, 26–50% stained; and 3, 51–100% stained. The final scores obtained by multiplying the scores of intensity with the extent of staining, which ranged between 0 and 9, were divided into four grades: 0, absent (-); 1–2, weak (+); 3–5, moderate (++); and 6–9, strong (+++). The positive expression was defined as final scores ≥3.
Cell Culture
The human HSCC cell line FaDu which was cultured in DMEM/F12 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS; Cromwell, CT, USA) was obtained from American Type Culture Collection (Manassas, VA, USA). The cells were incubated at 37°C with 5% CO2.
Cell Transfection
FaDu cells at a density of 3×105 cells/well were seeded into 6-well plates. The small interfering RNAs (siRNAs) against PD-L1 were synthesized by GenePharma Co., Ltd. (Shanghai, China). The siRNA sequences were as follows: sense, 5`-UUUGAAAGUAUCAAGGUCUTT-3`; antisense, 5`-AGACCUUGAUACUUUCAAATT-3`. We used Lipofectamine-RNA MAX Transfection Reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) to transfect siRNAs into FaDu cells following the manufacture’s protocol. The PD-L1 overexpressing plasmids constructed by GeneChem Co., Ltd. (Shanghai, China) were transfected into FaDu cells using Lipofectamine-RNA LTX Transfection Reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the manufacture’s protocol; cells were incubated with siRNAs or plasmids at 37°C for 48 hours. The transfection efficiency was evaluated by Western blot analysis.
Cell Viability Assay
Cells transfected with PD-L1 siRNAs or corresponding plasmids were seeded into 96-well plates at a density of 2000 cells/well and incubated at 37°C. The CCK-8 reagent (Beyotime Institute of Biotechnology, Shanghai, China) was added to the corresponding wells after incubation for 24, 48, 72 and 96 hours respectively. The cells added with the reagent were cultured for additional 3 hours and a microplate reader (BioTek Instruments, Inc., Winooski, VT, USA) was used to evaluate the absorbance at 450 nm. The graphs were generated by GraphPad Prism 8 (GraphPad Software, Inc., San Diego, CA, USA).
Transwell Assay
Transwell chambers (Costar; Corning, Inc., Corning, NY, USA) were used to conduct cell migration assays. The cells transfected with siRNAs or plasmids were resuspended in DMEM/F12 medium without FBS and placed into the upper chamber. Medium supplemented with 10% FBS was placed into the lower chamber as a chemo attractant. Cell invasion assays were performed in the same way as the migration assays, except that the upper chambers were pre-treated with Matrigel (BD Biosciences, San Jose, CA, USA) which was mixed with DMEM/F12 medium (1:3) 30 minutes before adding cells. After incubated at 37°C for 24 hours, the cells to the underside of the membrane were fixed and stained. Each experiment was repeated three times and five random microscope fields were counted under a microscope (magnification, ×100).
Western Blot
Total protein was obtained by lysing the transfected FaDu cells. A protein bicinchoninic acid assay kit (Beyotime Institute of Biotechnology, Shanghai, China) was used to measure the protein concentration. Equal amounts of protein were loaded onto the SDS-PAGE and then transferred to a polyvinyl fluoride membrane (EMD Millipore, Darmstadt, Germany). After blocking with 5% non-fat milk, the membranes were incubated with primary antibodies against PD-L1, E-cadherin, N-cadherin, Vimentin, Akt, p-Akt, p-mTOR and β-actin overnight at 4°C. Further, the membranes were incubated with appropriate secondary antibodies in the next day. The immunoreactive bands were detected using the enhanced chemiluminescence reagent (EMD Millipore, Darmstadt, Germany) and the gray values of protein bands were evaluated by Image J software (version 1.80; National Institutes of Health, Bethesda, MA, USA).
Statistical Analysis
SPSS 22.0 statistical analysis software (IBM, Armonk, NY, USA) was used to perform the analyses. Data obtained from three independent experiments were presented as the means ± standard deviation. The two-tailed Student’s t-test was performed for two-sample comparisons and one-way analysis of variance (ANOVA) was applied for the comparisons of multiple groups. The chi-squared or Fisher exact test was used to evaluate the association between PD-L1 and clinicopathological parameters. The Kaplan-Meier analysis and Log rank test were used to assess the differences of overall survival between groups with different levels of PD-L1 expression. The influence of each variable on survival was examined by the Cox-multivariate regression analysis. P<0.05 was considered to indicate a statistically significant difference.
Results
PD-L1 Expression in HSCC Tissues and Associations with Clinicopathological Findings and Prognosis
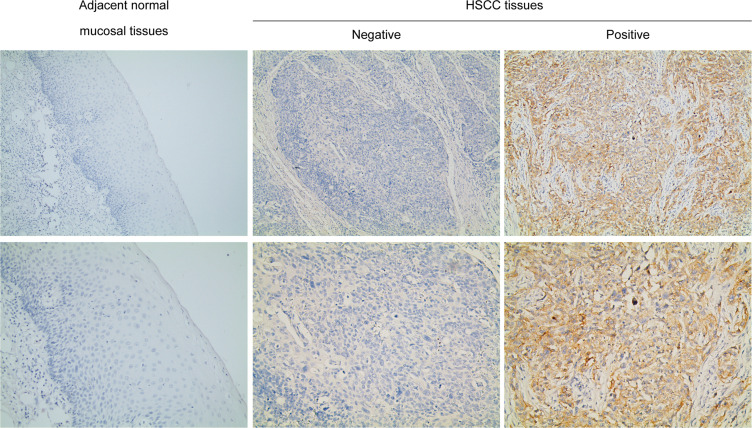
PD-L1 expression was validated by Immunohistochemistry. The results showed that PD-L1 was expressed in the cell membrane and cytoplasm of tumor cells in HSCC tissues while we found no positive expression of PD-L1 in adjacent normal mucosal tissues in the current study (Figure 1). Among the 95 HSCC cases, we identified 42 patients with positive expression of PD-L1. During the follow-up period, distant metastasis was detected in 29 patients, either by pathological analysis or imaging evaluation. The most common site of distant metastasis was the lung, followed by bone and brain. The correlations between PD-L1 expression and clinicopathological features assessed by the chi-squared or Fisher exact test are shown in Table 1, which demonstrated that positive expression of PD-L1 was significantly associated with advanced stage, lymph node metastasis and distant metastasis (P<0.001).
Figure 1.
PD-L1 expression in adjacent normal mucosal tissues and HSCC tissues. Representative immunohistochemical staining examples of PD-L1 protein expression are shown. In the current study there was no positive expression of PD-L1 in the mucosal tissues. Magnification, ×100 and ×200.
Abbreviations: PD-L1, programmed death-ligand 1; HSCC, hypopharyngeal squamous cell carcinoma.
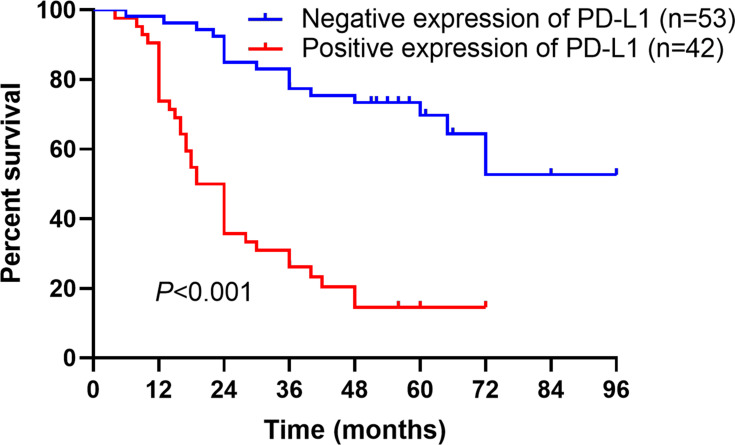
In prognostic analysis, it was shown that compared to those with negative expression, patients with positive PD-L1 expression had a worse prognosis according to the Kaplan-Meier analysis (P<0.001) (Figure 2). Furthermore, we identified three prognostic factors using univariate analysis: lymph node metastasis, distant metastasis and PD-L1 expression (Table 2). It was more important that PD-L1 expression was an independent prognostic factor for overall survival of HSCC patients based on the results of multivariate Cox regression analysis (Table 2).
Figure 2.
Kaplan-Meier analysis for overall survival of HSCC patients. Kaplan-Meier analysis of overall survival stratified by positive versus negative expression of PD-L1 in 95 HSCC patients (P<0.001). Log rank test was used for analyzing the differences.
Abbreviations: HSCC, hypopharyngeal squamous cell carcinoma; PD-L1, programmed death-ligand 1.
Table 2.
Univariate and Multivariate Analyses of Clinicopathological Factors for Overall Survival in 95 Patients with HSCC
| Risk Factors | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Gender | 0.757 | 0.235–2.438 | 0.641 | – | – | – |
| Age | 0.634 | 0.352–1.143 | 0.130 | – | – | – |
| Differentiation | 0.755 | 0.420–1.358 | 0.349 | – | – | – |
| T stage | 1.286 | 0.646–2.563 | 0.474 | – | – | – |
| N stage | 12.664 | 3.073–52.184 | <0.001 | 8.974 | 2.152–37.433 | 0.003 |
| Clinical stage | 25.581 | 0.817–801.187 | 0.065 | – | – | – |
| Distant metastasis | 5.386 | 2.990–9.072 | <0.001 | 3.501 | 1.873–6.543 | <0.001 |
| PD-L1 expression | 5.126 | 2.836–9.265 | <0.001 | 2.703 | 1.455–5.022 | 0.002 |
Abbreviations: HSCC, hypopharyngeal squamous cell carcinoma; HR, hazard ratio; CI, confidence interval; PD-L1, programmed death-ligand 1.
PD-L1 Expression Affected Proliferation, Migration and Invasion of FaDu Cells
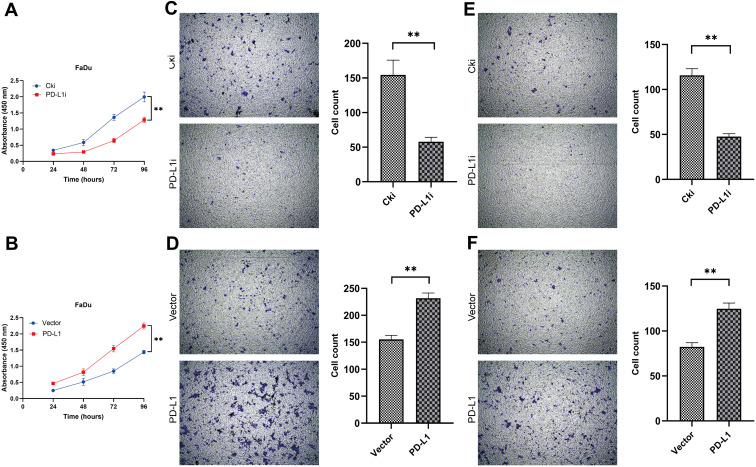
Cell Counting Kit-8 assay was performed to estimate the effects of PD-L1 on the proliferation of FaDu cells. The results showed that PD-L1 knockdown inhibited the proliferation of FaDu cells significantly, whereas the proliferation was promoted following PD-L1 overexpression (Figure 3A and B).
Figure 3.
PD-L1 promoted FaDu cells proliferation, migration and invasion in vitro. (A and B) Depletion of PD-L1 inhibited FaDu cells proliferation, whereas overexpression of PD-L1 stimulated FaDu cells proliferation as determined by CCK-8 assay. The data were presented as means ± standard deviation from three independent experiments. ** P<0.01. (C and D) The transwell migration assays showed that depletion of PD-L1 obviously inhibited the migration of FaDu cells. Conversely, overexpression of PD-L1 promoted the migration of FaDu cells. ** P<0.01. (E and F) The transwell invasion assays showed that depletion of PD-L1 obviously inhibited the invasion of FaDu cells. Conversely, overexpression of PD-L1 promoted the invasion of FaDu cells. The data were presented as means ± standard deviation from three independent experiments. **P<0.01.
Abbreviations: PD-L1, programmed death-ligand 1; CCK-8, Cell Counting Kit-8 assay; Cki, control siRNA; siRNA, small interfering RNA.
The effects of PD-L1 on the migration and invasion of FaDu cells were assessed by transwell assays. The migration assays indicated that PD-L1 knockdown led to a significant decrease of the number of migrated FaDu cells, whereas a significant increase of the number of migrated cells was observed following PD-L1 overexpression (Figure 3C and D); Similar results were obtained from the invasion assays (Figure 3E and F). These results suggested that PD-L1 positively regulated the migratory and invasive ability of FaDu cells.
PD-L1 Expression Promoted EMT in FaDu Cells
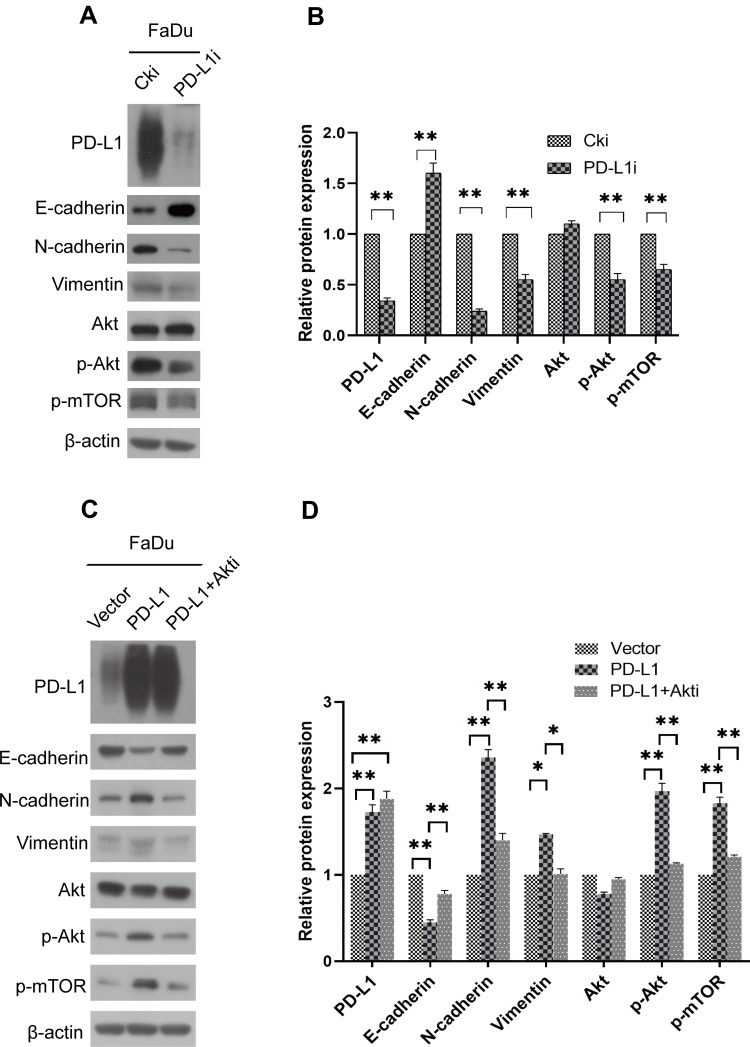
To investigate the relationship between PD-L1 and epithelial–mesenchymal transition in FaDu cells, related proteins were examined by Western blot. The assays demonstrated that in PD-L1-depletion FaDu cells, epithelial marker E-cadherin was up-regulated while the mesenchymal markers such as N-cadherin and Vimentin were suppressed (Figure 4A and B). The exact opposite results were obtained from the FaDu cells with PD-L1 overexpression (Figure 4C and D). These results demonstrated that PD-L1 induced EMT in HSCC cells.
Figure 4.
PD-L1 promoted EMT through the Akt-mTOR signaling pathway. (A) FaDu cells were transfected with PD-L1 siRNA or control siRNA, expression of PD-L1, E-cadherin, N-cadherin, Vimentin, Akt, p-Akt and p-mTOR was analyzed by Western blot. β-actin was used as an inner control. (B) Bands of Western blot were analyzed by Image J software. Results were obtained from the ratio of target band to β-actin. The data are presented as means ± standard deviation from three independent experiments. **P<0.01. (C) PD-L1-overexpressed or control FaDu cells were treated with Akt-inhibitor. The indicated protein levels were assayed by Western blot. β-actin was used as an inner control. (D) Bands of Western blot were analyzed by Image J software. Results were obtained from the ratio of target band to β-actin. The data are presented as means ± standard deviation from three independent experiments. *P<0.05, **P<0.01.
Abbreviations: PD-L1, programmed death-ligand 1; EMT, epithelial–mesenchymal transition; siRNA, small interfering RNA; Cki, control siRNA.
PD-L1 Expression Regulated EMT via Akt-mTOR Pathway
To further explore the molecular mechanism by which PD-L1 promotes HSCC development, we examined the effect of PD-L1 on Akt-mTOR pathway. The results revealed that a significant decrease of p-Akt and p-mTOR was observed in PD-L1-depleted FaDu cells (Figure 4A and B). Conversely, the expression of p-Akt and p-mTOR was significantly increased in cells transfected with the PD-L1 vector (Figure 4C and D). In order to further discuss whether the Akt-mTOR pathway affects the alteration of EMT genes in FaDu cells, an Akt inhibitor (MK2206; Gene Operation, Ann Arbor, MI, USA) was adopted to treat FaDu cells with PD-L1 overexpression. The inhibition was specific for Akt at a concentration of 5 µmol/l. The results showed that treatment with MK2206 significantly neutralized the effect of PD-L1 on the promotion of EMT (Figure 4C and D).
Discussion
The expression of PD-L1 and its clinical significance have been validated in various human cancers.26–29 Some clinical trials with applications of monoclonal antibodies targeting PD-L1 have been reported with promising results.30–32 Most notably, some authors demonstrated that only patients with positive expression of PD-L1 would benefit from the therapies targeting PD-1/PD-L1.33 Besides, the prognostic significance of PD-L1 in head and neck cancers is still controversial, with some reports linking PD-L1 expression to better rather than worse outcome.34,35 However, most subjects in these studies were patients with laryngeal and oropharyngeal cancers and some authors also mentioned that a possible distinct role of hypopharyngeal localization regarding immune activity and PD-L1 requires further clarification.35 So our study specially focused on the pattern and significance of PD-L1 expression in HSCC. We demonstrated that the expression of PD-L1 was common in HSCC and associated with advanced diseases as well as worse prognosis. Furthermore, we identified PD-L1 as an independent prognostic factor for patients with HSCC, which suggested that PD-L1 could act as a biomarker for HSCC progression and metastasis might be regulated by some mechanisms mediated by PD-L1.
It is generally considered that PD-L1 enhances the aggressive biological behaviors of tumor by establishing an immunosuppressive microenvironment in which anti-tumor T cells with PD-1 expression received suppressive signals delivered by PD-L1.9 But there have been evidences that PD-L1 also has some inherent functions.20,21,36 To elucidate the effect of PD-L1 in HSCC, we performed a serial of mechanistic investigations. In the current studies, we investigated the contribution of PD-L1 expression in regulating the biological behaviors of the tumor cells themselves. The results showed that the ability of proliferation, migration and invasion of Fadu cells could be significantly promoted by PD-L1, which supported the speculation that PD-L1 has some tumor-cell autonomous functions independent of its immunomodulatory effects.
Metastasis accounts for 90% of cancer treatment failures and one of the vital steps in the initial stages of metastasis is epithelial–mesenchymal transition. And more notably it has been reported that PD-L1 played a key role in regulating EMT in various tumors, including cancers of head and neck,23 lung,37 and colorectum.38 In the current studies, we established multiple cellular models using FaDu cell line, either by ablating or over-expressing PD-L1. Using these models we found that the ectopic expression of PD-L1 significantly influenced the expression of EMT-related proteins, which suggested that PD-L1 plays an important role in inducing the EMT phenotype in hypopharyngeal cancer cells. Interestingly, an initial study demonstrated that in a mouse lung cancer model PD-L1 could be promoted by zinc finger E-box binding homeobox 1 (ZEB 1), a transcription factor related to epithelial–mesenchymal transition.39 So, we speculated that PD-L1 and EMT may interact with each other and this cross-talk may enhance tumor aggressiveness and promote the progression of HSCC.
The Akt/mTOR pathway, which is involved in several processes related to invasion and metastasis of head and neck cancers,40,41 is one of the most important upstream signaling pathways that regulate epithelial–mesenchymal transition.42 It has also been reported that Akt/mTOR pathway could be activated by PD-L1 in sarcoma.21 So we speculated that PD-L1-induced EMT could be regulated by Akt/mTOR signaling pathway in HSCC. Our results suggested that PD-L1 knockdown inhibited, while PD-L1 overexpression activated the Akt-mTOR signaling axis in FaDu cells. The Akt inhibitor was adopted to treat the PD-L1-overexpressed FaDu cells for further verification. As expected, the Akt inhibitor neutralized the promotion of EMT induced by PD-L1 overexpression, suggesting that PD-L1-induced EMT was indeed mediated by the Akt-mTOR pathway in FaDu cells.
There were some limitations in the present study. Firstly, as mentioned above, HSCC only accounts for a minority of all head and neck cancers and HSCC cell lines available for research are rare, so investigations with a larger sample size and more cell lines are needed to further assess the role of PD-L1. Secondly, the functions of PD-L1 in HSCC need to be verified in in vivo assays.
Conclusion
Our study demonstrated that the expression of PD-L1 was closely correlated with adverse clinicopathological features and worse prognosis of HSCC patients. PD-L1 could significantly promote the proliferation, migration and invasion of FaDu cells. Besides, PD-L1 could drive EMT of FaDu cells via the Akt-mTOR signaling pathway. These findings suggest that PD-L1 has important tumor-intrinsic functions independent of its immunopathogenic effects. Patients with HSCC and positive PD-L1 expression might benefit more from the corresponding targeted therapies.
Acknowledgments
The authors thank the staff of the Key Laboratory of Otorhinolaryngology, National Health Commission (Shandong University), Department of Otorhinolaryngology Head and Neck Surgery, Shandong Provincial ENT Hospital for technical support.
Funding Statement
The present study was supported by grants from the National Natural Science Foundation of China (grant no. 81702679), the Natural Science Foundation of Shandong province (grant no. ZR2017BH060).
Disclosure
The authors declare that there is no conflict of interest in this work.
References
- 1.Cooper JS, Porter K, Mallin K, et al. National cancer database report on cancer of the head and neck: 10-year update. Head Neck. 2009;31(6):748–758. doi: 10.1002/hed.21022 [DOI] [PubMed] [Google Scholar]
- 2.Hashibe M, Brennan P, Benhamou S, et al. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. J Natl Cancer Inst. 2007;99(10):777–789. doi: 10.1093/jnci/djk179 [DOI] [PubMed] [Google Scholar]
- 3.Carvalho AL, Nishimoto IN, Califano JA, Kowalski LP. Trends in incidence and prognosis for head and neck cancer in the United States: a site-specific analysis of the SEER database. Int J Cancer. 2005;114(5):806–816. doi: 10.1002/ijc.20740 [DOI] [PubMed] [Google Scholar]
- 4.Denaro N, Russi EG, Adamo V, Merlano MC. State-of-the-art and emerging treatment options in the management of head and neck cancer: news from 2013. Oncology. 2014;86(4):212–229. doi: 10.1159/000357712 [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Wang B, Chen X, Li W, Dong P. AGO2 involves the malignant phenotypes and FAK/PI3K/AKT signaling pathway in hypopharyngeal-derived FaDu cells. Oncotarget. 2017;8(33):54735–54746. doi: 10.18632/oncotarget.18047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu P, Wu H, Tang Y, et al. Whole-exome sequencing reveals novel mutations and epigenetic regulation in hypopharyngeal carcinoma. Oncotarget. 2017;8(49):85326–85340. doi: 10.18632/oncotarget.19674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karpathiou G, Casteillo F, Giroult JB, et al. Prognostic impact of immune microenvironment in laryngeal and pharyngeal squamous cell carcinoma: immune cell subtypes, immuno-suppressive pathways and clinicopathologic characteristics. Oncotarget. 2017;8(12):19310–19322. doi: 10.18632/oncotarget.14242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8(6):467–477. doi: 10.1038/nri2326 [DOI] [PubMed] [Google Scholar]
- 9.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. doi: 10.1038/nm730 [DOI] [PubMed] [Google Scholar]
- 10.Wintterle S, Schreiner B, Mitsdoerffer M, et al. Expression of the B7-related molecule B7-H1 by glioma cells: a potential mechanism of immune paralysis. Cancer Res. 2003;63(21):7462–7467. [PubMed] [Google Scholar]
- 11.Strome SE, Dong H, Tamura H, et al. B7-H1 blockade augments adoptive T-cell immunotherapy for squamous cell carcinoma. Cancer Res. 2003;63(19):6501–6505. [PubMed] [Google Scholar]
- 12.Konishi J, Yamazaki K, Azuma M, Kinoshita I, Dosaka-Akita H, Nishimura M. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res. 2004;10(15):5094–5100. doi: 10.1158/1078-0432.CCR-04-0428 [DOI] [PubMed] [Google Scholar]
- 13.Ohigashi Y, Sho M, Yamada Y, et al. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res. 2005;11(8):2947–2953. doi: 10.1158/1078-0432.CCR-04-1469 [DOI] [PubMed] [Google Scholar]
- 14.Tsushima F, Tanaka K, Otsuki N, et al. Predominant expression of B7-H1 and its immunoregulatory roles in oral squamous cell carcinoma. Oral Oncol. 2006;42(3):268–274. doi: 10.1016/j.oraloncology.2005.07.013 [DOI] [PubMed] [Google Scholar]
- 15.Thompson RH, Kwon ED. Significance of B7-H1 overexpression in kidney cancer. Clin Genitourin Cancer. 2006;5(3):206–211. doi: 10.3816/CGC.2006.n.038 [DOI] [PubMed] [Google Scholar]
- 16.Hamanishi J, Mandai M, Iwasaki M, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104(9):3360–3365. doi: 10.1073/pnas.0611533104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nomi T, Sho M, Akahori T, et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res. 2007;13(7):2151–2157. doi: 10.1158/1078-0432.CCR-06-2746 [DOI] [PubMed] [Google Scholar]
- 18.Ghebeh H, Tulbah A, Mohammed S, et al. Expression of B7-H1 in breast cancer patients is strongly associated with high proliferative Ki-67-expressing tumor cells. Int J Cancer. 2007;121(4):751–758. doi: 10.1002/ijc.22703 [DOI] [PubMed] [Google Scholar]
- 19.Yao Y, Tao R, Wang X, Wang Y, Mao Y, Zhou LF. B7-H1 is correlated with malignancy-grade gliomas but is not expressed exclusively on tumor stem-like cells. Neuro Oncol. 2009;11(6):757–766. doi: 10.1215/15228517-2009-014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clark CA, Gupta HB, Sareddy G, et al. Tumor-intrinsic PD-L1 signals regulate cell growth, pathogenesis, and autophagy in ovarian cancer and melanoma. Cancer Res. 2016;76(23):6964–6974. doi: 10.1158/0008-5472.CAN-16-0258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang CH, Qiu J, O’Sullivan D, et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. 2015;162(6):1229–1241. doi: 10.1016/j.cell.2015.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2(6):442–454. doi: 10.1038/nrc822 [DOI] [PubMed] [Google Scholar]
- 23.Ock CY, Kim S, Keam B, et al. PD-L1 expression is associated with epithelial-mesenchymal transition in head and neck squamous cell carcinoma. Oncotarget. 2016;7(13):15901–15914. doi: 10.18632/oncotarget.7431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao Y, Zhang L, Kamimura Y, et al. B7-H1 overexpression regulates epithelial-mesenchymal transition and accelerates carcinogenesis in skin. Cancer Res. 2011;71(4):1235–1243. doi: 10.1158/0008-5472.CAN-10-2217 [DOI] [PubMed] [Google Scholar]
- 25.Alsuliman A, Colak D, Al-Harazi O, et al. Bidirectional crosstalk between PD-L1 expression and epithelial to mesenchymal transition: significance in claudin-low breast cancer cells. Mol Cancer. 2015;14:149. doi: 10.1186/s12943-015-0421-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Wu J, Guo W, et al. Alpha-Solanine modulates the radiosensitivity of esophageal cancer cells by inducing microRNA 138 expression. Cell Physiol Biochem. 2016;39(3):996–1010. doi: 10.1159/000447807 [DOI] [PubMed] [Google Scholar]
- 27.Li Z, Dong P, Ren M, et al. PD-L1 expression is associated with tumor FOXP3(+) regulatory T-cell infiltration of breast cancer and poor prognosis of patient. J Cancer. 2016;7(7):784–793. doi: 10.7150/jca.14549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen K, Cheng G, Zhang F, et al. Prognostic significance of programmed death-1 and programmed death-ligand 1 expression in patients with esophageal squamous cell carcinoma. Oncotarget. 2016;7(21):30772–30780. doi: 10.18632/oncotarget.8956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muller T, Braun M, Dietrich D, et al. PD-L1: a novel prognostic biomarker in head and neck squamous cell carcinoma. Oncotarget. 2017;8(32):52889–52900. doi: 10.18632/oncotarget.17547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berger R, Rotem-Yehudar R, Slama G, et al. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res. 2008;14(10):3044–3051. doi: 10.1158/1078-0432.CCR-07-4079 [DOI] [PubMed] [Google Scholar]
- 31.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–2028. doi: 10.1056/NEJMoa1501824 [DOI] [PubMed] [Google Scholar]
- 32.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vassilakopoulou M, Avgeris M, Velcheti V, et al. Evaluation of PD-L1 expression and associated tumor-infiltrating lymphocytes in laryngeal squamous cell carcinoma. Clin Cancer Res. 2016;22(3):704–713. doi: 10.1158/1078-0432.CCR-15-1543 [DOI] [PubMed] [Google Scholar]
- 35.Birtalan E, Danos K, Gurbi B, et al. Expression of PD-L1 on immune cells shows better prognosis in laryngeal, oropharygeal, and hypopharyngeal cancer. Appl Immunohistochem Mol Morphol. 2018;26(7):e79–e85. doi: 10.1097/PAI.0000000000000590 [DOI] [PubMed] [Google Scholar]
- 36.Kleffel S, Posch C, Barthel SR, et al. Melanoma cell-intrinsic PD-1 receptor functions promote tumor growth. Cell. 2015;162(6):1242–1256. doi: 10.1016/j.cell.2015.08.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim S, Koh J, Kim MY, et al. PD-L1 expression is associated with epithelial-to-mesenchymal transition in adenocarcinoma of the lung. Hum Pathol. 2016;58:7–14. doi: 10.1016/j.humpath.2016.07.007 [DOI] [PubMed] [Google Scholar]
- 38.Zhi Y, Mou Z, Chen J, et al. B7H1 expression and epithelial-to-mesenchymal transition phenotypes on colorectal cancer stem-like cells. PLoS One. 2015;10(8):e0135528. doi: 10.1371/journal.pone.0135528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen L, Gibbons DL, Goswami S, et al. Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nat Commun. 2014;5:5241. doi: 10.1038/ncomms6241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yan B, Broek RV, Saleh AD, Mehta A, Van Waes C, Chen Z. Signaling networks of activated oncogenic and altered tumor suppressor genes in head and neck cancer. J Carcinog Mutagen. 2013;Suppl 7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herzog A, Bian Y, Vander Broek R, et al. PI3K/mTOR inhibitor PF-04691502 antitumor activity is enhanced with induction of wild-type TP53 in human xenograft and murine knockout models of head and neck cancer. Clin Cancer Res. 2013;19(14):3808–3819. doi: 10.1158/1078-0432.CCR-12-2716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lamouille S, Derynck R. Cell size and invasion in TGF-beta-induced epithelial to mesenchymal transition is regulated by activation of the mTOR pathway. J Cell Biol. 2007;178(3):437–451. doi: 10.1083/jcb.200611146 [DOI] [PMC free article] [PubMed] [Google Scholar]