Dear Editor,
Mitochondrial diseases comprise a heterogeneous group of disorders due to dysfunction of mitochondrial respiratory chain caused by mutations in both mitochondrial and nuclear genes. EARS2 encodes mitochondrial glutamyl t-RNA synthetase responsible for attaching glutamate to its cognate mitochondrial t-RNA. Hence, EARS2 is critical for protein translation in mitochondria. Homozygous or compound heterozygous EARS2 pathogenic variants are associated with a neurological disorder characterized by leukoencephalopathy with thalamus and brain stem involvement and high lactate (LTBL) [1]. Patients with EARS2-related mitochondrial disease become symptomatic in infancy with characteristic MRI findings of diffuse white matter changes and symmetrical signal abnormalities in the thalamus and brain stem. Their clinical presentations fall into two groups- severe and mild. Patients in the severe group present before 6 months of age with marked neurological regression followed by clinical stagnation. Patients in the mild group present later in infancy with neurological regression, but they regain some milestones and follow a stable clinical course thereafter. We describe here a patient with an atypical presentation without the characteristic neuroimaging findings of LTBL.
1. Case presentation
The patient is a 2 year old girl who presented at 3 months of age with generalized tonic seizures. Her growth and development were age-appropriate at presentation. On physical examination, hepatomegaly was noted. Laboratory evaluation was significant for abnormal liver function tests: AST 237 U/L (reference range: 8–62), ALT 146 U/L (reference range:12–78), alkaline phosphatase 920 U/L (reference range 38–400), GGT 356 U/L (reference range 5–55), total bilirubin 3.3 mg/dl (reference range 0–1.1), direct bilirubin 2 mg/dl (reference range 0–0.3), and prothrombin time 20.6 s (reference range 12–15). Blood ammonia was 27 micromol/L (reference range 11–32). In addition, high lactate in the blood (9.8 mmol/L, reference range 0.4–2.2) and cerebrospinal fluid (9.4 mmol/L, reference range 0.6–2.2) was identified. Abdominal ultrasound showed a diffusely echogenic enlarged liver. CT head at 3 months of age was unremarkable and no calcifications or abnormal mass density were noted. The MRI of the brain obtained at 3 months of age showed normal basal ganglia, thalami, and brain stem. There was clear symmetrical abnormal signal and restricted diffusion in the cerebral white matter, including the corpus callosum, optic radiations, and centrum semiovale (Fig. 1A–C). MR spectroscopy showed abnormal lactate peak in the basal ganglia. Because of the high blood and cerebrospinal fluid lactate levels, a mitochondrial disease was suspected. Combined nuclear and mitochondrial gene panel testing for mitochondrial diseases showed two previously reported pathogenic variants in the EARS2 gene, c.322C > T (p.R108W) and c.328G > A (p.G110S) (NG_027752.2) [1]. The patient was started on an antiepileptic medication (Levetiracetam) and mitochondrial cocktail (riboflavin 50 mg daily, thiamine 50 mg daily, coenzyme Q10 20 mg twice a day, and levocarnitine 100 mg twice a day). Hypotonia and mild motor delay were noted on follow up at 6 months of age. Her physical growth, language and social development were normal. The patient was referred for physical therapy for minor motor delays. A repeat brain MRI at 13 months of age showed persisting normal appearance of the basal ganglia, thalami, and brainstem. There was progressing symmetrical, abnormal signal and restricted diffusion in larger areas of the supratentorial white matter sparing the subcortical U-fibers. There was relative preservation of a symmetrical rim of periventricular white matter (Fig. 1D–F). At 16 months of age, generalized hypotonia, motor delays, and speech delays were noted. The patient was able to crawl, but could not stand without support. The patient had no specific words other than “mama”. However, the patient was very interactive. She was receiving supportive therapies and making progress. Her physical growth was optimal. Blood lactate was trending down and liver enzymes had normalized. Levetiracetam was discontinued as the patient was seizure free for over a year.
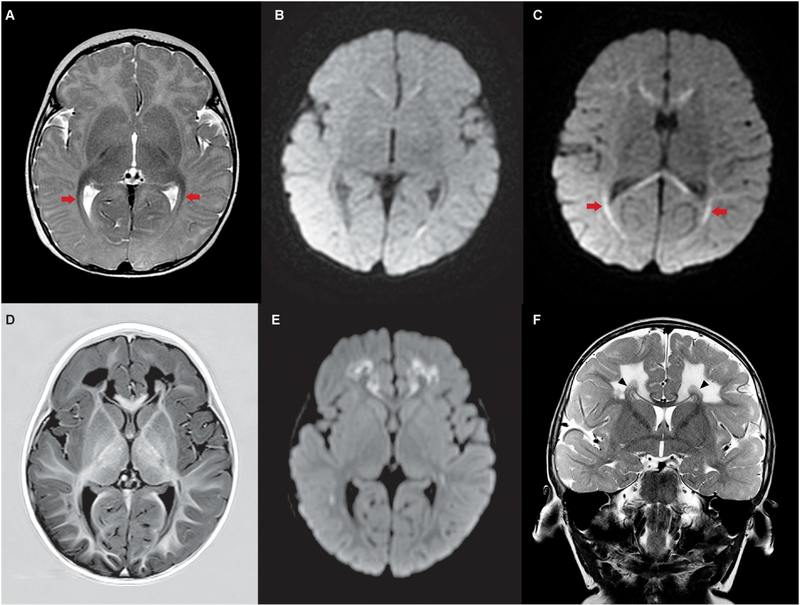
Fig. 1.
MR imaging of proband at 3 and 13 months reveals symmetrical white matter changes, but sparing of basal ganglia and thalamus.
A–C. MR images taken at 3 months of age. A. Axial T2 image demonstrates normal signal intensity in the multiple nuclei of the thalami and the basal ganglia. There is thickening and reduced T2 signal in the optic radiations (arrows). B–C. Two planes of axial diffusion-weighted imaging (DWI) demonstrate no abnormal diffusion in the basal ganglia or thalami, but clear restricted diffusion in the genu and splenium of the corpus callosum and in the optic radiations (C - arrows). D–F. MR images taken at 13 months. D–E. Axial T1 inversion recovery image (D) and corresponding axial diffusion weighted image (E) demonstrate persisting normal signal intensity in the multiple nuclei of the thalami and the basal ganglia. There is symmetric, markedly abnormal low T1 signal (D) and restricted diffusion (E) in the white matter of both frontal lobes sparing the subcortical U-fibers and rim of periventricular white matter. The optic radiations remain abnormal. F. Coronal T2 image demonstrates normal signal in the basal ganglia and high T2 signal in the white matter of both frontal lobes with sparing of the subcortical U-fibers. There is relative sparing of a periventricular rim of white matter (arrow heads). There is selective involvement of the fibers of the corpus callosum with sparing of early migrating fibers from the cingulate gyrus and greater involvement of the later migrating fibers.
2. Discussion
Here we report a patient with biallelic EARS2 pathogenic variants with developmental delay without typical “LTBL” presentation. The previously reported patients have presented with neuroregression and high lactate and characteristic neuroimaging finding of leukoencephalopathy with thalamus and brain stem involvement earning the acronym of “LTBL”. The patient described here had normal thalamus, basal ganglia and brain stem structures on neuroimaging. However, there was diffuse white matter involvement. Thalamus involvement has been reported in all previously described patients. Absence of thalamus involvement in our patient expands the spectrum of neruoradiological findings in EARS2-related mitochondrial disease. The first description of “LTBL” was based on targeted testing on a cohort of patient with similar neuroimaging findings [1]. The neuroradiological finding in our patient suggests that involvement of thalamus and brain stem are not pathognomonic for this condition and targeted testing for EARS2 in only typical “LTBL presentation” may miss the diagnosis. Although, diffuse white matter involvement was noted on first neuroimaging at 3 months of age, it was more severe in the repeat imaging at 13 months of age. The white matter disease in most of the previously described patients improved or remained unchanged on follow up [1]. In a patient with a severe form of EARS2-related disease, supratentorial white matter was normal at 2 months of age [2]. It is possible that white matter changes may be missed in very young infants due to immature myelination at this stage. Hence, neuroimaging findings are dependent on the age of the patient as well as the timing of the imaging following the initial metabolic crisis. On the other hand, presence of structural abnormalities, such as agenesis or dysgenesis of corpus callosum indicates antenatal insult and a severe presentation [1,3].
Hepatopathy has been described in patients with EARS2-related disease [2,4,5]. However, hepatopathy has always been associated with neuro-regression or severe neurological abnormalities such as severe generalized hypotonia and global developmental delay. The main concern at presentation in our patient was elevated liver enzymes, abnormal coagulation profile, and hepatomegaly. Although the patient did have seizures and abnormal neuroimaging, her development and neurological examination were normal at presentation. Hence, EARS2-related mitochondrial conditions may present primarily as hepatopathy. Association of high lactate, abnormal neurological findings such as seizures, or abnormal neuroimaging with unexplained hepatopathy should raise the concern for EARS2-related mitochondrial disorder.
EARS2 related LTBL are described to follow two clinical patterns-early onset severe neuroregression followed by stagnation, or late infantile onset neuroregression followed by partial recovery. Our patient’s clinical course does not fit either of these two patterns. The patient had early onset of symptoms before 6 months as in severe cases, but the main concerns were seizures and liver dysfunction rather than neuroregression. Global developmental delays manifested later. Thus, typical biphasic presentation seen with other EARS2 patients is not present. The clinical severity in this patient can be considered intermediate as the symptoms started before 6 months as in severe cases, but progression of symptoms followed the pattern of mild cases. A similar intermediate presentation but with characteristic neuroimaging finding of LTBL has been described before [6]. Prenatal presentation leading to absence of thalami has been reported in one patient with EARS2-related disease [7]. Another patient with EARS2-related disease presented shortly after birth with lactic acidosis and recurrent hypoglycemia [3]. A patient with homozygous EARS2 pathogenic variants presented at 16 months of age with delayed motor development [8]. Thus, EARS2-related disease is not confined to previously described infantile onset severe and mild LTBL, but encompasses a wide spectrum of prenatal to early childhood presentations. This is not unexpected as the phenotype associated with this gene continues to evolve because of increasing utilization of whole exome/genome sequencing in the current genomic era.
Of note, a patient with the same genotype as our patient has been previously described who presented at 8 months of age and was noted to have severe thalamus abnormalities in addition to extensive deep white matter changes and brain stem involvement on neuroimaging [1]. The clinical and radiological features of these two patients have been summarized in Supplementary Table 1. Of note, the presentations of these two patients are remarkably different despite the same genotype. Intrafamilial variability has also been described suggesting additional genetic or environmental modifiers [1,9].
In conclusion, the clinical spectrum of EARS2-related disease is wider than currently recognized. EARS2 deficiency should be considered in any child with developmental delay, neuroregression, or unexplained hepatopathy when associated with high lactate, even in the absence of characteristic neuroimaging findings.
Supplementary Material
Acknowledgements
We acknowledge Dr. Timothy A. Carlon for his help with the images.
Funding
Dr. Bryn D. Webb receives support from National Institutes of Health National Institute of Child Health and Human Development (K08HD086827).
Footnotes
Declaration of Competing Interest
None.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jns.2019.116448.
References
- [1].Steenweg ME, Ghezzi D, Haack T, Abbink TE, Martinelli D, van Berkel CG, et al. , Leukoencephalopathy with thalamus and brainstem involvement and high lactate ‘LTBL’ caused by EARS2 mutations, Brain. 135 (2012) 1387–1394. [DOI] [PubMed] [Google Scholar]
- [2].Oliveira R, Sommerville EW, Thompson K, Nunes J, Pyle A, Grazina M, et al. , Lethal neonatal LTBL associated with Biallelic EARS2 variants: case report and review of the reported neuroradiological features, JIMD Rep. 33 (2017) 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Danhauser K, Haack TB, Alhaddad B, Melcher M, Seibt A, Strom TM, et al. , EARS2 mutations cause fatal neonatal lactic acidosis, recurrent hypoglycemia and agenesis of corpus callosum, Metab. Brain Dis. 31 (2016) 717–721. [DOI] [PubMed] [Google Scholar]
- [4].Sellars EA, Balmakund T, Bosanko K, Nichols BL, Kahler SG, Zarate YA, Severe metabolic acidosis and hepatopathy due to leukoencephalopathy with thalamus and brainstem involvement and high lactate, Neuropediatrics. 48 (2017) 108–110. [DOI] [PubMed] [Google Scholar]
- [5].Talim B, Pyle A, Griffin H, Topaloglu H, Tokatli A, Keogh MJ, et al. , Multisystem fatal infantile disease caused by a novel homozygous EARS2 mutation, Brain. 136 (2013) e228. [DOI] [PubMed] [Google Scholar]
- [6].Biancheri R, Lamantea E, Severino M, Diodato D, Pedemonte M, Cassandrini D, et al. , Expanding the clinical and magnetic resonance spectrum of leukoencephalopathy with thalamus and brainstem involvement and high lactate (LTBL) in a patient harboring a novel EARS2 mutation, JIMD Rep. 23 (2015) 85–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kevelam SH, Klouwer FC, Fock JM, Salomons GS, Bugiani M, van der Knaap MS, Absent thalami caused by a homozygous EARS2 mutation: expanding disease spectrum of LTBL, Neuropediatrics. 47 (2016) 64–67. [DOI] [PubMed] [Google Scholar]
- [8].Taskin BD, Karalok ZS, Gurkas E, Aydin K, Aydogmus U, Ceylaner S, et al. , Early-onset mild type leukoencephalopathy caused by a homozygous EARS2 mutation, J. Child Neurol. 31 (2016) 938–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Şahin S, Cansu A, Kalay E, Dinçer T, Kul S, Çakır IM, et al. , Leukoencephalopathy with thalamus and brainstem involvement and high lactate caused by novel mutations in the EARS2 gene in two siblings, J. Neurol. Sci. 365 (2016) 54–58. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.