Objective(s):
To evaluate 2-year audiological and clinical outcomes of a transcutaneous implant for bone conduction hearing of a previously published 6-month evaluation.
Design:
Fifty-four unilaterally implanted adult patients with conductive or mild mixed hearing loss or single-sided sensorineural deafness were included in this prospective multicenter study. Follow-up visits were scheduled post-surgery at 10 days; 4, 6, and 12 weeks; 6, 12, and 24 months. Main outcomes were audiological benefit, patient-reported outcomes (PROs), soft tissue status, pain, numbness, implant survival, and daily usage.
Results:
In the study population, the transcutaneous implant resulted in statistically significant improvement in objective hearing test and PROs compared with the unaided situation. Soft tissue complications were observed in 4.6% of the patients per visit. Pain/discomfort and numbness were initially reported in the majority of the patients, but declined over time; approximately 9% of patients reported some degree of numbness and 15% (slight) pain/discomfort after 2 years. During the 24-month period, two implant magnets were removed (3.7%), while two other implants were converted to the percutaneous counterpart (3.7%). At the final visit, 89.6% (n = 42 out of 47) of the patients used their sound processor, with a median daily usage of 6 h/d (range, 0–18 h/d).
Conclusions:
After 24 months, the transcutaneous implant provided statistically significant mean improvement in objective and subjective hearing performance as well as PROs compared with the preoperative unaided condition and had a low soft tissue complication rate. The test device could be considered as an alternative treatment option for appropriately selected and counseled patients.
Keywords: Attract, Baha, Bone conduction, Bone conduction devices, Bone-anchored hearing, Health related quality of life, Hearing loss, Implant loss, Soft tissue reactions, Transcutaneous
Traditional percutaneous implants for bone conduction hearing consist of a titanium fixture surgically placed into the temporal bone and a skin-penetrating abutment, onto which a sound processor is coupled. These implants have shown to be an effective hearing rehabilitation option in patients suffering from either conductive (CHL) or mixed hearing loss (MHL), or single-sided sensorineural deafness (SSD). Despite its audiological success and good levels of patient satisfaction, the skin-penetrating abutment of percutaneous implants implies an entry point for microorganisms potentially causing complications, e.g., recurrent skin infections and implant loss (1,2).
In 1986, Hough et al. (3) developed a transcutaneous system, the Xomed Audiant, using magnets instead of a skin-penetrating abutment to transmit the sound vibrations to the skull. As a result, the skin remained intact postoperatively, thus avoiding an entry point for microorganisms and, hence, potentially preventing skin infections and loss of the implant. For the sound processor to remain seated, the magnets had to provide sufficient retention force. However, the necessary retention force resulted in too high static pressure towards the skin causing pressure related complications. Combined with insufficient amplification, the transcutaneous device was withdrawn from the market.
Although recent modifications in percutaneous implant design and surgical techniques have resulted in a reduction of adverse skin reactions (observed in <6.3% of the visits) (4–7) and implant loss rates (occurring in approximately 4.2% of patients with up to 5-year follow-up) (8–10), the concept of transcutaneous coupling remained attractive, as the intact skin could potentially further diminish skin reactions and could be considered to be cosmetically appealing. As such, a new passive transcutaneous implant for bone conduction hearing was introduced in 2013. This device consists of an internal magnet fixed in the temporal bone and an external magnet, onto which the sound processor is coupled. To reduce pressure related complications, a soft pad is attached to the external magnet to distribute the pressure evenly over the underlying skin.
The primary aim of this multicenter study was to evaluate efficacy in terms of hearing performance of a transcutaneous implant for bone conduction hearing after 6 months of follow-up, as previously reported by den Besten et al. (11). The aim of this paper was to evaluate the long-term audiological and clinical performance after a total of 2 years of follow-up in the same population, and to compare patient-reported outcomes (PROs) over time.
METHODS
Implant and Study Design
The device was a Baha Attract System, consisting of: 1) a BI300 implant (osseointegrating implant fixture), 2) an attached BIM400 implant magnet, and 3) an external sound processor magnet (SP magnet) with a soft pad to distribute the pressure more evenly over the skin. Together, the magnets constitute the transcutaneous coupling. A sound processor can be attached to the SP magnet via a snap coupling. All parts were manufactured by Cochlear Bone Anchored Solutions AB (Mölnlycke, Sweden).
The study was designed as an international multicenter, open, prospective clinical investigation with a primary evaluation after 6 months and a secondary evaluation after 24 months of follow-up. The participating centers were Radboudumc (Nijmegen, The Netherlands), Queen Elizabeth University Hospital (Birmingham, United Kingdom), Manchester Royal Infirmary (Manchester, United Kingdom), Medical College of Wisconsin (Milwaukee, WI), and World Hearing Center, Institute of Physiology and Pathology of Hearing (Warsaw, Poland). All patients eligible for a bone-conduction device were fully informed about the different percutaneous and transcutaneous options. All patients preferring the transcutaneous option were informed about the trial. The patients then attended a screening and baseline visit, during which eligibility criteria (Table 1) were evaluated, medical history was collected, and baseline hearing tests were performed for the unaided hearing condition as well as with a sound processor on a Baha Softband (same sound processor type as to be used on the implant). The baseline visit was followed by a period of softband trial previous the surgical intervention. Follow-up visits were scheduled at 10 days; 4, 6, and 12 weeks; 6, 12, and 24 months after surgery. The sample size was calculated for improvement in audiometric thresholds pure-tone average PTA4 (mean of 500, 1000, 2000, and 4000 Hz) compared with the preoperative unaided hearing condition for the whole study population; for details, see den Besten et al. (11). Furthermore, a subgroup analysis per type of hearing loss (CHL/MHL and SSD) was performed. Audiological outcomes, i.e., free-field hearing thresholds (PTA4 and per frequency 250–8000 Hz), adaptive speech recognition in noise, and speech recognition in quiet (at 50, 65, and 80 dB SPL) were compared with the unaided situation and to preoperative performance with a softband. In case of significantly better hearing in the contralateral ear, data were obtained with the better ear blocked. Audiometric methods are described in detail in den Besten et al. (11).
TABLE 1.
Eligibility criteria

In addition to audiological outcomes, the focus of the current manuscript was to evaluate long-term safety and usability of the test implant regarding implant survival, soft tissue tolerability, pain/discomfort after sound processor loading, skin numbness, retention difficulties, and daily usage of the sound processor. Soft tissue tolerability encompassed the presence of signs of infection, inflammation, skin necrosis, and/or scar hypertrophy. The level of pain/discomfort was classified as follows: 0 = no pain/discomfort (normal daily usage SP); 1 = slight pain/discomfort (not significantly affecting daily usage SP); 2 = discomfort/pain (reducing daily usage SP); 3 = excessive pain/discomfort (preventing usage SP). Skin sensibility was assessed at randomly picked locations in and around the implant area, i.e., within and beyond 2 cm from the center of the implant magnet, and was tested on both gnostic (cotton swab) and vital (pin prick) sensibility. The following scale was used: 0 = no numbness; 1 = numbness within 2 cm from the implant center; 2 = numbness within and beyond 2 cm from the implant centre. Daily usage encompassed the average use of the sound processor in hours per day during the last month before the study visit as reported by the patient. Non-use was defined as wearing the sound processor on average 0 hours a day.
Patient-reported outcomes were measured using the health-related quality of life (HRQoL) questionnaire Health Utilities Index (HUI3) (12) as well as the hearing specific questionnaires Abbreviated Profile of Hearing Aid Benefit (APHAB) (13), and Speech, Spatial, and Qualities of Hearing Scale (SSQ) (14). HUI3 is a multi-attribute health-status classification system which consists of 15 individual questions on eight HRQoL attributes: vision, hearing, speech, walking, dexterity, emotion, cognition, and pain. Each category is scored from 0.00 (highest degree of impairment or disability) to 1.00 (no impairment). A comprehensive health state attribute is calculated from these separate attributes. The APHAB is a 24-item inventory to evaluate the amount of difficulty the patient experiences in daily life listening conditions. All items are scored on a 7-point scale indicating the frequency of difficulties experienced, ranging from 1 to 99%, with higher scores indicating more frequently occurring difficulties. Items are grouped and reported on the four domains ease of communication, reverberation, background noise, and aversiveness, as well as a global score.
The SSQ is composed of 49 questions that are scored on a visual analogue scale ranging from 0 (complete inability) to 10 (complete ability/no effort). The questionnaire measures auditory disability across the three subscales speech recognition (in a variety of contexts), spatial hearing (segregation, direction, distance, and movement of sound), and hearing qualities (ease of listening, naturalness, and clarity). For each subscale a mean score is calculated.
Ethical Considerations
The current study was performed in accordance with the guidelines established in the Declaration of Helsinki (Washington 2002), ISO 14155:2011 Good Clinical Practice, and was approved by all local ethics committees. The current study was registered at www.ClinicalTrials.gov under identifier NCT02022085.
Data Analysis
Monitoring at the four European sites was performed by external monitors (Factory-CRO, Bilthoven, The Netherlands), while at the US site monitors at Cochlear Americas (Denver, CO) performed the monitoring. Data management and statistical analysis were performed by independent external data managers and biostatisticians (Statistiska Konsultgruppen, Göteborg, Sweden) according to a predefined statistical analysis plan. For comparison over time, Fisher's non-parametric permutation test for paired observations was used for continuous variables. For paired analysis of dichotomous and ordered categorical variables the Sign test was used. All data are reported according to the intention-to-treat principle. All tests were two-tailed, conducted at 0.05 significance level, and performed using SAS v9.4 (Cary, NC).
RESULTS
Patients and Follow-Up
In total, 54 patients were included in the study and implanted unilaterally; 39 of the patients had CHL or mild MHL and 15 patients had SSD. Baseline and surgery characteristics as well as choice of sound processor are displayed in Table 2. Seven patients discontinued the study prematurely: two patients had their transcutaneous system converted to a percutaneous system (after 7 and 13 months, respectively; see section implant survival); in two patients the implant magnet was removed (after 75 days and 25 months, respectively; see section implant survival); one patient was unable to attend the last visit due to being abroad; one patient passed away during the study (after 14 months); and one patient chose to discontinue participation after the 12-month visit due to non-usage of the device; the patient reported persistent pain at the last attended visit. Data for these patients up until the moment of discontinuation were included in the analysis. Furthermore, in one patient, the 24-month visit was partly performed by phone, since the patient was not using the sound processor anymore due to Menière attacks; hence, only implant survival, pain/discomfort, retention, and daily usage were collected at this visit.
TABLE 2.
Patient characteristics and demographics
Audiology
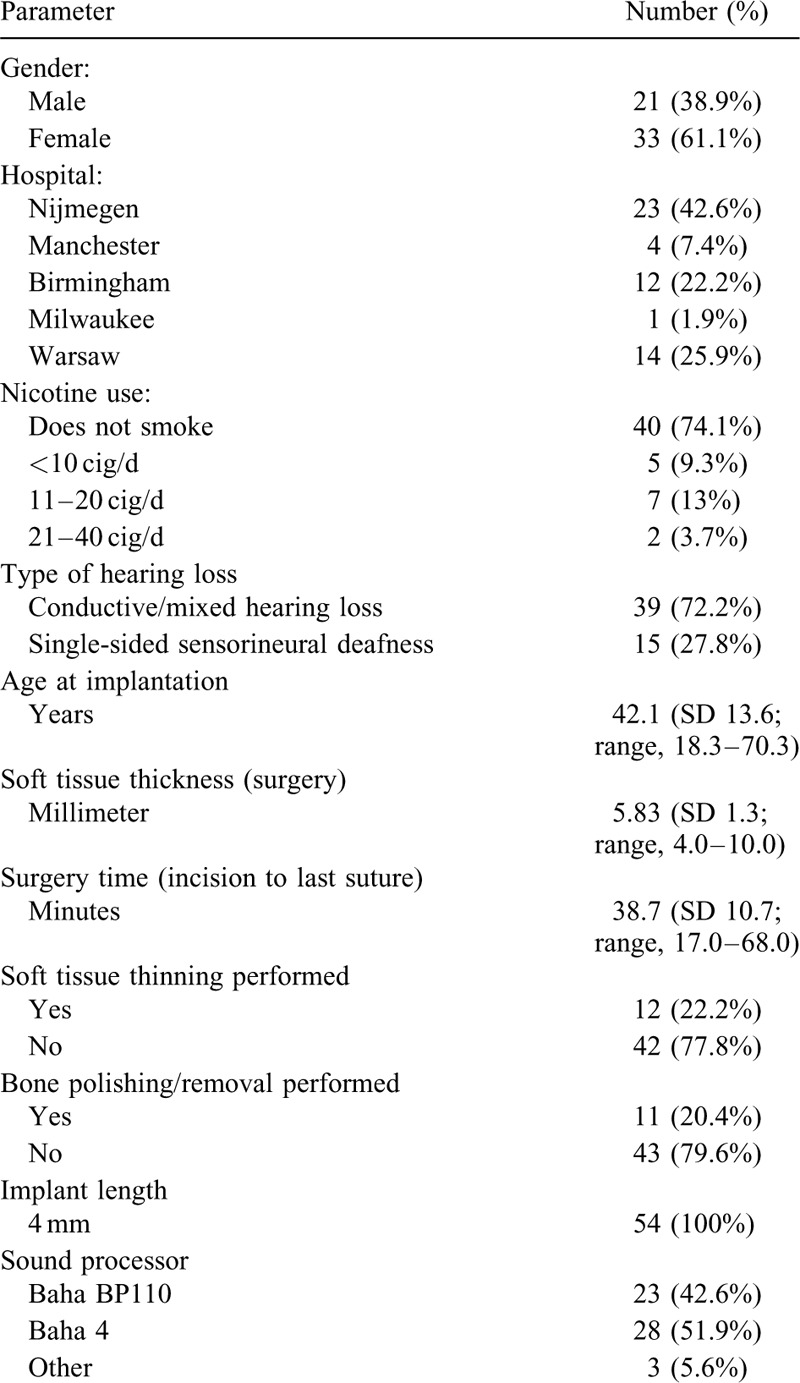
For the total cohort, the statistically significant improvement in audiological outcomes recorded at 6 months of follow-up as reported by den Besten et al. (11) was maintained also at 12 and 24 months and was numerically similar to the 6-month results. The mean improvement from baseline unaided PTA4 to the aided value at 24-month was –21.5 dB (SD 8.3, range, –45.0 to –3.8 dB, n = 46, p ≤ 0.0001). The mean improvement in speech recognition in noise at 24 months was –4.47 dB signal-to-noise ratio SNR (SD 6.11, range –25.2–4.20, n = 31, p ≤ 0.0001); data from two sites were excluded from the analysis due to invalid results for this specific test, as elaborated in den Besten et al. (11). In speech tests in quiet the mean improvement in % correctly repeated words from unaided to 24-month aided hearing was 40.4% at 50 dB SPL (SD 33.3, range, –65.0–90.0, n = 46, p ≤ 0.0001), 43.3% at 65 dB SPL (SD 29.9, range, –10.0–90.0, n = 46, p ≤ 0.0001) and 14.0% at 80 dB SPL (SD 20.7, range, –10.0 to 71.0, n = 46, p ≤ 0.0001). In line with the 6-month results, for the total study cohort no significant differences were observed at 24 months compared with baseline softband tests in terms of PTA4, speech recognition in noise, and speech in quiet at 80 dB SPL. However, while the improvements in speech recognition at 50 and 65 dB SPL compared with softband were not statistically significant at 6 months, the results at 24 months were statistically significantly better than softband scores.
The audiological outcomes per subgroups of patients with CHL/MHL and SSD, respectively, are displayed in Table 3. For the CHL/MHL group, the improvements compared with the unaided situation were statistically significant for all audiological tests at all time points (6, 12, and 24 mo). Compared with softband tests, the 24-month data showed statistically significant improvements for speech recognition in noise. For the smaller subgroup of patients with SSD, the improvement in all audiological tests compared with unaided hearing reached statistical significance or near-significance at all time points (6, 12, 24 mo), except for speech recognition in noise at 24 months. No statistically significant differences compared with softband were recorded in this patient group at any time point.
TABLE 3.
Mean change in audiometric results from to the preoperative Unaided and Softband test situation to the postoperative aided situation
Soft Tissue Tolerability
Over the entire follow-up, signs of inflammation, e.g., swelling or erythema or infection were observed in 4.6% (mean) of the patients per postoperative visit: in 1.8% (mean) of the patients per visit before fitting the external magnet/sound processor and of 5.8% (mean) of the patients per visit after fitting (6 weeks until 24 months; range mean 3.8–7.7%). Besides a patient who underwent implant magnet removal due to infection shortly after implantation (see section implant survival), all other observations were minor soft tissue inflammations or infections which resolved by local treatment.
Pain/Discomfort and Skin Numbness
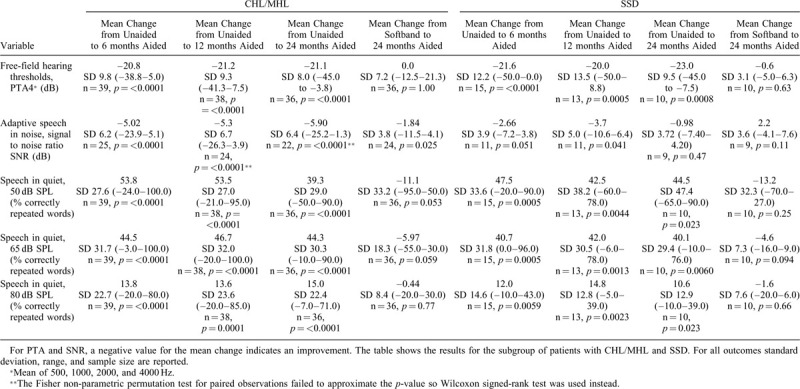
The presence of pain/discomfort and skin numbness per visit is displayed in Figure 1. Skin numbness was seen in 19.2% (vital sensibility) and 17.3% (gnostic sensibility) of the patients at the 6-month follow-up visit, most of whom had a numb area exceeding 2 cm in diameter. In the following 18 months, skin numbness declined steadily, and at the 24-month visit, numbness was reported in four patients (out of 46 patients, 8.7%), with only one patient having a numb area exceeding 2 cm in diameter.
FIG. 1.
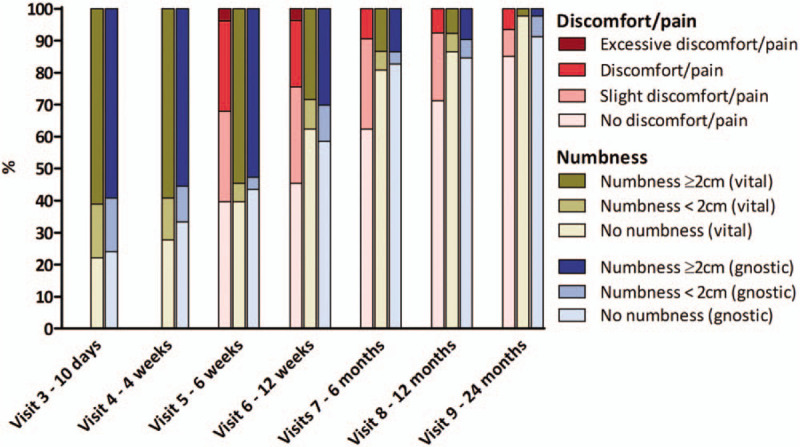
Stacked bar-chart displaying the percentage of patient with pain and its severity (left bar, from visit 5 and onwards), as well as numbness (middle bar is vital sensibility, right bar is gnostic sensibility) per visit around the implant site, i.e., within and beyond 2 cm from the center of the implant magnet. The latter was tested on both gnostic (cotton swab) and vital (pin) sensibility. Pain data were collected at visits after sound processor loading.
At the 6-month follow-up, five patients (out of 53 patients, 9.4%) experienced pain which significantly reduced daily use. Similar to the skin numbness outcomes, in the following 18 months, pain decreased steadily over time. At the last visit, three patients (out of 47 patients, 6.4%) experienced pain which significantly affected daily use.
Implant Survival
No spontaneous implants loss occurred and all patients had their osseointegrated implant in position, resulting in a 2-year implant survival of 100%. However, two patients (3.7%) had their implant magnet surgically removed (osseointegrated implant remained seated): in one patient due to persisting pain resulting in non-use, and the other due to infection shortly after surgery. In addition, two other patients (3.7%) had their transcutaneous system surgically converted to a percutaneous system. One of the conversions was performed due to insufficient audiological benefit experienced by the patient, and the other due to persisting pain combined with the sound processor frequently falling off. Conversion encompassed replacing the implant magnet with a skin-penetrating abutment, onto the seated osseointegrated implant.
Sound Processor Usage, Retention Difficulties, and Device Deficiency
Of the patients that attended the 24-month visit, 89.6% (n = 42 out of 47) used their sound processor on the transcutaneous implant. Grouped per indication, 97.2% (n = 35 out of 36) of the CHL/MHL patients used their sound processor, while 2.8% became a non-user (n = 1; due to insufficient benefit of the system). In contrast, 63.6% (n = 7 out of 11) of the SSD patients used their sound processor at the last follow-up, while 36.4% were non-users (n = 4; one due to pain and feedback issue, one due to pain/discomfort, one due to subjectively eliciting Menière attacks, and one due to insufficient benefit). The daily usage of the sound processor per visit is displayed in Figure 2. The median daily sound processor usage after 24 months was 6 h/d (range, 0–18 h/d) for the entire cohort (n = 47), 8 h/d (range, 0–18 h/d) in patients with CHL/MHL (n = 36), and 3 h/d (range, 0–17 h/d) in patients with SSD (n = 11). For patients that used their sound processor at the last visit, the median daily usage was 7.5 h/d for the total cohort (n = 42), 8 h/d for patients with CHL/MHL (n = 35), and 6 h/d for patients with SSD (n = 7).
FIG. 2.
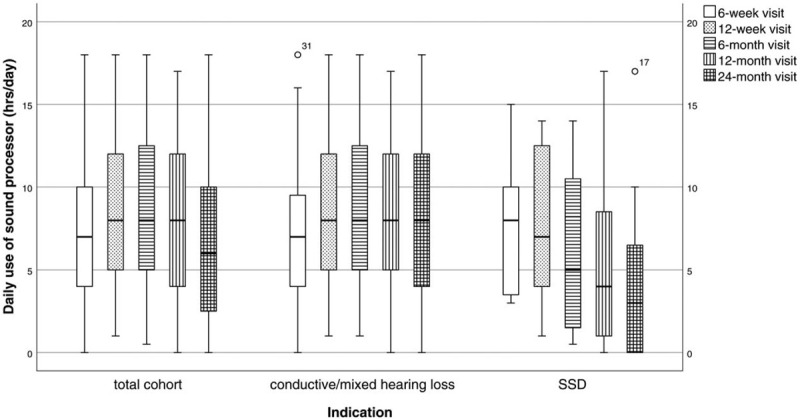
Daily use of sound processor per visit for the total cohort as well as per indication. The median (horizontal bar) is defined within each plot, boxes represent interquartile range, whiskers represent 95% range and dots represent outlier values.
At 6 weeks (first visit after sound processor loading), in 35.8% of the patients the sound processor fell off at least once a week (mean 4.53 times a week, range, 0–80). For the following visits it was reported as follows: 12 weeks—32.1% (mean 1.79 times a week, range, 0–30); 6 months—35.8% (mean 1.87 times a week, range, 0–50); 12 months—17.3% (mean 0.63 times a week, range, 0–10); 24 months—6.2% (mean 0.19 times a week, range, 0–7).
Twenty-four device deficiencies occurred during the 24-month follow-up period, of which almost all encompassed a broken snap coupling or battery door on the sound processor.
Patient-Reported Outcomes
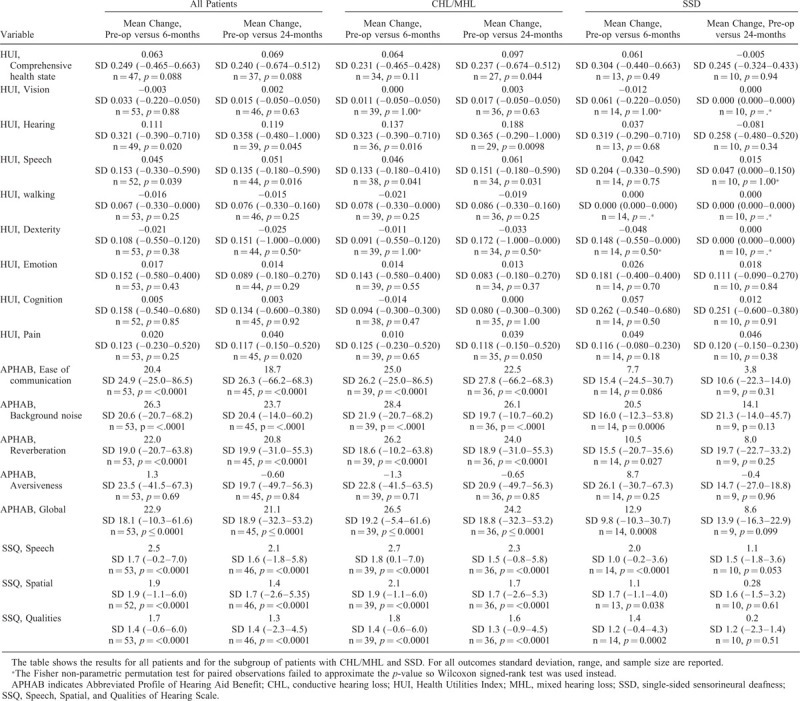
In the studied population, the transcutaneous system resulted in statistically significant improvement on the HUI3 attributes hearing, speech, and pain, the APHAB domains ease of communication, background noise, reverberation, and global score, and on all SSQ scales at the 24-month follow-up compared with the baseline situation. For the subgroup of patients with CHL/MHL a significant improvement was also reported on the HUI3 attribute Comprehensive Health State (Table 4). For patients with SSD, the statistically significant improvements seen at 6 months for APHAB (background noise, reverberation, global score) and SSQ (all subscales) were no longer statistically significant at 24 months; all HUI3 attributes failed to show improvements for the SSD population. No significant differences were found in either group comparing the aided situation at the 6-month visit to the 24-month visit, except for a significant deterioration on the SSQ quality scale in SSD patients (–1.05; p = 0.008).
TABLE 4.
Mean change in HUI3, APHAB, and SSQ from the preoperative situation to the postoperative aided situation after 6 and 24 months
DISCUSSION
Synopsis of Key/New Findings
In the current multicenter study, we evaluated the 2-year audiological and clinical performance of a new transcutaneous implant for bone conduction hearing, as well as patient-reported outcomes (by means of HRQoL and hearing specific questionnaires). The transcutaneous system provided significant improvement in all audiometric and patient-reported hearing outcomes compared with the unaided situation as well as in HRQoL. No implants were lost, although in four patients the implant magnet was either removed or replaced with an abutment due to complications or insufficient audiological benefit. The majority of the patients initially reported to experience both some degree of pain/discomfort and numbness; however, these complication rates declined over the following visits and were reported only sporadically at the last follow-up. In the subgroup analysis, the transcutaneous system provided both significant improvement in hearing outcomes compared with the unaided baseline condition as well as regarding PROs in patients with CHL/MHL. For patients with SSD, the transcutaneous system provided statistically significant or near-significant improvement compared with the unaided condition in all audiometric tests throughout the 24-month follow-up, except for speech recognition in noise at the 24-month visit. However, the statistically significant improvements in APHAB and SSQ recorded at 6 months were no longer present at 24 months. HUI failed to show statistically significant improvement at any time point in this small subgroup. At the last follow-up, 97.2% of the patients with CHL/MHL with the transcutaneous implant in place used their sound processor, compared with 63.6% of the SSD patients.
Strengths and Limitations of the Study
The results of the current study are considered to reliably reflect clinical outcomes of the transcutaneous system due to the prospective multicenter study design and data quality. The study design included one of the largest populations with the longest follow-up period to date with this device type. The study was designed as a within-subject evaluation with audiological benefit after 6 months compared with the unaided situation as primary outcome variable. The current long-term follow-up study, evaluating the clinical outcomes after 24 months, therefore, had the same within-subject design. The transcutaneous system was developed as an alternative to the percutaneous system, which is currently the gold standard in terms of transmission efficiency. While the high frequency sound transmission is less effective, passive transcutaneous devices are thought to offer other advantages in terms of non-audiological clinical outcomes. A direct comparison of such clinical outcomes (i.e., numbness, pain/discomfort, soft tissue tolerability, daily use, implant loss, and health-related quality of life) would have been desirable. Until now, prospective studies objectively comparing the clinical outcomes of the two systems are lacking. Another point that should be taken into consideration, is that the study was not powered for the subgroup analysis; hence, no firm conclusions could be drawn on these subgroup analyses, especially in the SSD population which at the 24-month visit only included 10 patients (nine patients for the speech in noise test). However, as discussed by den Besten et al. (11), since pooling of the data was not optimal due to differences between indications, the choice was made to also report data per indication. Last, a significant percentage of our implant recipient was smokers, which could have influenced complication rates. However, we did not include an analysis regarding the influence of smoking on postoperative complications in our predefined statistical analysis plan.
Comparisons With Other Studies and Clinical Applicability
In the current study, we observed signs of inflammation or infection with a mean of 5.8% of the patients per visit after sound processor loading. These were most likely the result of the constant pressure the magnets apply to the skin, since the prevalence was lower before loading (1.9%). Furthermore, almost all events resolved after switching to a weaker SP magnet. As aforementioned, soft tissue outcomes should ideally be compared with the percutaneous counterpart; however, accurate comparison to these implants is impossible due to differences in reporting soft tissue status and the nature of skin complications, i.e., infection-related versus pressure-related. Moreover, until recently, no systematic soft tissue scoring system for transcutaneous implants was available (15). This makes comparison to other studies evaluating the transcutaneous system difficult as well, since complications are not uniformly reported across studies.
However, previous studies have also reported on pressure-related issues: a too strong magnet resulted in pain and erythema, while a too weak magnet resulted in retention difficulties. The occurrence of various degrees of pain or discomfort in the first months after surgery varied across studies, ranging between 14.8 and 60% of the patients with the same transcutaneous system (16–19). In line with our observation, a general trend was seen that pain was most frequently reported in the first months after surgery, but declined over time. Retention difficulties were reported in 83% of the patients in Powell et al. (20) and in 20% of the patients in Carr et al. (18). As a result, finding the optimal magnet strength often required additional visits to the clinic, but was eventually almost always achieved. The issue, whether it was pain or retention difficulties, most often resolved once switched to a different magnet strength (17,18,20,21). Based on our experience, great attention should be given to carefully selecting a magnet strength that suits each individual patient. A too week magnet may fall off, and a too strong magnet may result in discomfort or skin soreness. It is important to regularly check the magnet, and change magnet strength when indicated due to insufficient retention or discomfort/soreness. In addition, patients should change the softpad regularly to warrant optimal pressure distribution and to avoid discomfort. Last, patients should be advised to use a safety line to avoid losing or damaging the sound processor in case it falls off.
The high rate of skin numbness after surgery followed by a decline over time was also reported in two other studies. Briggs et al. (17) reported skin numbness in 62.9% of the patients immediately following sound processor fitting and in 22.2% of the patients nine months after surgery. Godbehere et al. (22) reported slightly lower skin numbness rates: in 48.1% of patients following surgery, and in 29.6% 6 months postoperatively. In the current study, skin numbness declined from 77.8% (vital sensibility) and 66.7% (gnostic sensibility) of the patients at 10 days postoperatively, to 19.2% (vital sensibility) and 17.3% (gnostic sensibility) at 6 months, and finally to 2.2% (vital sensibility) and 8.7% (gnostic sensibility) at 24 months. In both studies, the same anterior based C-shaped incision was used as in the current study. Modifications to the incision technique to optimize soft tissue handling during surgery might play a pivotal role in improving clinical outcomes, and should therefore be further explored (23). From a clinical perspective, until then, patients should be informed before surgery, that the majority of patients will, to some extent, experience postoperative skin numbness, but that this will most likely resolve entirely over time.
In line with previous observations 6 months after implantation (11), the 12-month outcomes of the three PRO questionnaires showed that the transcutaneous implant system continues to provide significant improvement in subjective hearing benefit and HRQoL also over the longer term in the total studied population. At the 24-month follow-up, hearing and health-related PROs remained stable in CHL/MHL patients; in patients with SSD, however, no significant benefit could be seen in terms of HRQoL, and statistically significant improvements in terms of subjective hearing outcomes seen at 6 months were no longer statistically significant.
The daily usage of the transcutaneous system was previously presented by Briggs et al. (follow-up of 6 months) and Gawecki et al. (follow-up of 9 months) (17,19). For patients with CHL/MHL, daily sound processor usage seems comparable to our study at 6 months (8.3 h/d versus 7.6 h/d (17) and 10 h/d (19)). For patients with SSD the daily use recorded in the present study was slightly lower than in patients with CHL/MHL, but comparable to the other studies (6.5 h/d versus 6 h/d (17) and 9 h/d (19)). Furthermore, no patients in either of the two studies were non-users at the last follow-up. In our study, all patients used their sound processor to some extent at 6 months; however, among the patients with SSD who had the transcutaneous system in place, four stopped using the device for different reasons (including one case of insufficient benefit) and average daily usage declined to 3 h/d (including non-users) at 24 months. A diminished usage over time have also been observed in SSD patients with percutaneous systems (24,25). It should, however, be noted that patients with SSD may have sufficient hearing for normal communication, but typically experience difficulties with speech intelligibility in noise and sound localization (26). It has been suggested that these difficulties are challenging to overcome by means of any bone-conduction device (27). In addition, since the start of current study new, more powerful sound processors have been developed. Future research is needed to determine the effect of a more powerful hearing device on daily use, PROs, and audiological benefit.
CONCLUSION
The current multicenter study showed that after 24 months of follow-up, the transcutaneous implant for bone conduction hearing is safe to use and provides statistically significant improvement in hearing performance and patient-reported outcomes compared with the preoperative unaided condition in studied patients with CHL, mild MHL, or SSD. The transcutaneous test device did not necessitate daily skin care, although the magnetic coupling did result in pressure related symptoms, e.g., pain/discomfort and signs of inflammation. However, these symptoms were almost always relieved after switching to a weaker magnet strength. For the subgroup of patients with SSD, the improvement in speech understanding in noise and patient-reported outcomes was less outspoken than for patients with CHL/MHL and the percentage of non-users was higher. Since the sample size in the SSD group was too small to draw statistically supported conclusions, further research on a larger population is needed. Until then, for SSD patients an extra careful selection procedure may be needed. Based on the current results, the transcutaneous test device could be considered as an alternative treatment option for appropriately selected and counseled patients.
Acknowledgments
The authors thank all audiologists from participating centers for their contributions to the study; Jamie Jensen (Medical College of Wisconsin), Marion Atkin, June Jones, Gemma Mason (Queen Elizabeth Hospital Birmingham), and Rachel Andrew and Becki Gladdis (Manchester Royal Infirmary). Furthermore, the authors thank Rupan Banga (Queen Elizabeth Hospital Birmingham), Bartłomiej Krol and Kamila Kordowska (World Hearing Center Warsaw), Teja Repkes, Mieki Verbruggen, and Chrisje den Besten (Radboudumc Nijmegen) for their contributions in performing surgeries, data collection, and device fitting.
Footnotes
Funding: Cochlear Bone Anchored Solutions AB (Mölnlycke, Sweden) acted as sponsor for this study.
Conflicts of Interest: Cochlear Bone Anchored Solutions AB assumed the role as sponsor of the current study in accordance with ISO 14155:2011. In collaboration with all authors, the sponsor designed and managed the study and was responsible for data analysis and report writing. Data were recorded by the investigators and monitored by a Contract Research Organization (CRO) assigned by the sponsor. Data management and statistical analyses were completed by external data managers and biostatisticians also assigned by the sponsor. All authors had full access to the results. The authors and the sponsor had final responsibility for the content of the publication. I.K., A.B., E.M., and M.H. report financial support to their authors’ institution for conducting clinical studies from Oticon Medical AB (Askim, Sweden) and from Cochlear Bone Anchored Solutions AB (Molnlycke, Sweden), outside the submitted work. P.M. reports a consultancy fee from Oticon Medical AB (Askim, Sweden).
REFERENCES
- 1.Kiringoda R, Lustig LR. A meta-analysis of the complications associated with osseointegrated hearing aids. Otol Neurotol 2013; 34:790–794. [DOI] [PubMed] [Google Scholar]
- 2.Dun CA, Faber HT, de Wolf MJ, et al. Assessment of more than 1,000 implanted percutaneous bone conduction devices: skin reactions and implant survival. Otol Neurotol 2012; 33:192–198. [DOI] [PubMed] [Google Scholar]
- 3.Hough J, Vernon J, Johnson B, et al. Experiences with implantable hearing devices and a presentation of a new device. Ann Otol Rhinol Laryngol 1986; 95:60–65. [DOI] [PubMed] [Google Scholar]
- 4.Nelissen RC, Stalfors J, de Wolf MJ, et al. Long-term stability, survival, and tolerability of a novel osseointegrated implant for bone conduction hearing: 3-year data from a multicenter, randomized, controlled, clinical investigation. Otol Neurotol 2014; 35:1486–1491. [DOI] [PubMed] [Google Scholar]
- 5.Wazen JJ, Babu S, Daugherty J, et al. Three-week loading of the 4.5 mm wide titanium implant in bone anchored hearing systems. Am J Otolaryngol 2016; 37:132–135. [DOI] [PubMed] [Google Scholar]
- 6.Hultcrantz M. Stability testing of a wide bone-anchored device after surgery without skin thinning. Biomed Res Int 2015; 2015:853072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hogsbro M, Agger A, Johansen LV. Bone anchored hearing implant surgery: 1 year follow-up data shows no effect of hydroxyapatite coating on soft tissue reaction after loading at 1 week. Otol Neurotol 2017; 38:e152–e158. [DOI] [PubMed] [Google Scholar]
- 8.Nelissen RC, den Besten CA, Faber HT, et al. Loading of osseointegrated implants for bone conduction hearing at 3 weeks: 3-year stability, survival, and tolerability. Eur Arch Otorhinolaryngol 2016; 273:1731–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.den Besten CA, Stalfors J, Wigren S, et al. Stability, survival, and tolerability of an auditory osseointegrated implant for bone conduction hearing: long-term follow-up of a randomized controlled trial. Otol Neurotol 2016; 37:1077–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kruyt IJ, Nelissen RC, Mylanus EAM, et al. Three-year outcomes of a randomized controlled trial comparing a 4.5mm-wide to a 3.75mm-wide titanium implant for bone conduction hearing. Otol Neurotol 2018; 39:609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.den Besten CA, Monksfield P, Bosman A, et al. Audiological and clinical outcomes of a transcutaneous bone conduction hearing implant: 6-month results from a multicenter study. Clin Otolaryngol 2019; 44:144–157. [DOI] [PubMed] [Google Scholar]
- 12.Furlong WJ, Feeny DH, Torrance GW, et al. The Health Utilities Index (Hui) system for assessing health-related quality of life in clinical studies. Ann Med 2001; 33:375–384. [DOI] [PubMed] [Google Scholar]
- 13.Cox RM, Alexander GC. The abbreviated profile of hearing aid benefit. Ear Hear 1995; 16:176–186. [DOI] [PubMed] [Google Scholar]
- 14.Gatehouse S, Noble W. The speech, spatial and qualities of hearing scale (Ssq). Int J Audiol 2004; 43:85–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kruyt IJ, Nelissen RC, Johansson ML, et al. The Ips-Scale: a new soft tissue assessment scale for percutaneous and transcutaneous implants for bone conduction devices. Clin Otolaryngol 2017; 42:1410–1413. [DOI] [PubMed] [Google Scholar]
- 16.Iseri M, Orhan KS, Tuncer U, et al. Transcutaneous bone-anchored hearing aids versus percutaneous ones: multicenter comparative clinical study. Otol Neurotol 2015; 36:849–853. [DOI] [PubMed] [Google Scholar]
- 17.Briggs R, Van Hasselt A, Luntz M, et al. Clinical performance of a new magnetic bone conduction hearing implant system: results from a prospective, multicenter, clinical investigation. Otol Neurotol 2015; 36:834–841. [DOI] [PubMed] [Google Scholar]
- 18.Carr SD, Moraleda J, Procter V, et al. Initial Uk experience with a novel magnetic transcutaneous bone conduction device. Otol Neurotol 2015; 36:1399–1402. [DOI] [PubMed] [Google Scholar]
- 19.Gawecki W, Stieler OM, Balcerowiak A, et al. Surgical, functional and audiological evaluation of new Baha ((r)) attract system implantations. Eur Arch Otorhinolaryngol 2016; 273:3123–3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Powell HR, Rolfe AM, Birman CS. A Comparative study of audiologic outcomes for two transcutaneous bone-anchored hearing devices. Otol Neurotol 2015; 36:1525–1531. [DOI] [PubMed] [Google Scholar]
- 21.Sharma S, Reddy-Kolanu G, Marshall AH. Uk tertiary centre experience of outcomes from osseointegrated transcutaneous magnetic bone conduction hearing system implanted in twenty-five patients using a linear incision technique. Clin Otolaryngol 2017; 42:1041–1043. [DOI] [PubMed] [Google Scholar]
- 22.Godbehere J, Carr SD, Moraleda J, et al. A comparison study of complications and initial follow-up costs of transcutaneous and percutaneous bone conduction devices. J Laryngol Otol 2017; 131:667–670. [DOI] [PubMed] [Google Scholar]
- 23.Reddy-Kolanu G, Marshall A. Implantation of the Cochlear Baha((R)) 4 attract system through a linear incision. Ann R Coll Surg Engl 2016; 98:437–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Desmet JB, Wouters K, De Bodt M, et al. Comparison of 2 implantable bone conduction devices in patients with single-sided deafness using a daily alternating method. Otol Neurotol 2012; 33:1018–1026. [DOI] [PubMed] [Google Scholar]
- 25.Kompis M, Wimmer W, Caversaccio M. Long term benefit of bone anchored hearing systems in single sided deafness. Acta Otolaryngol 2017; 137:398–402. [DOI] [PubMed] [Google Scholar]
- 26.Lin LM, Bowditch S, Anderson MJ, et al. Amplification in the rehabilitation of unilateral deafness: speech in noise and directional hearing effects with bone-anchored hearing and contralateral routing of signal amplification. Otol Neurotol 2006; 27:172–182. [DOI] [PubMed] [Google Scholar]
- 27.Peters JP, Smit AL, Stegeman I, et al. Review: bone conduction devices and contralateral routing of sound systems in single-sided deafness. Laryngoscope 2015; 125:218–226. [DOI] [PubMed] [Google Scholar]


