Abstract
Background:
The purpose of the present study was to determine (1) whether the current literature supports the choice of using autologous chondrocyte implantation over other cartilage procedures with regard to clinical outcome, magnetic resonance imaging, arthroscopic assessment, and durability of treatment, (2) whether the current literature supports the use of a specific generation of autologous chondrocyte implantation, and (3) whether there are patient-specific and defect-specific factors that influence outcomes after autologous chondrocyte implantation in comparison with other cartilage repair or restoration procedures.
Methods:
We conducted a systematic review of multiple databases in which we evaluated Level-I and II studies comparing autologous chondrocyte implantation with another cartilage repair or restoration technique as well as comparative intergenerational studies of autologous chondrocyte implantation. The methodological quality of studies was evaluated with use of Delphi list and modified Coleman methodology scores. Effect size analysis was performed for all outcome measures.
Results:
Thirteen studies (917 subjects) were included. Study methodological quality improved with later publication dates. The mean modified Coleman methodology score was 54 (of 100). Patients underwent autologous chondrocyte implantation (n = 604), microfracture (n = 271), or osteochondral autograft (n = 42). All surgical techniques demonstrated improvement in comparison with the preoperative status. Three of seven studies showed better clinical outcomes after autologous chondrocyte implantation in comparison with microfracture after one to three years of follow-up, whereas one study showed better outcomes two years after microfracture and three other studies showed no difference in these treatments after one to five years. Clinical outcomes after microfracture deteriorated after eighteen to twenty-four months (in three of seven studies). Autologous chondrocyte implantation and osteochondral autograft demonstrated equivalent short-term clinical outcomes, although there was more rapid improvement after osteochondral autograft (two studies). Although outcomes were equivalent between first and second-generation autologous chondrocyte implantation and between open and arthroscopic autologous chondrocyte implantation, complication rates were higher with open, periosteal-cover, first-generation autologous chondrocyte implantation (four studies). Younger patients with a shorter preoperative duration of symptoms and fewer prior surgical procedures had the best outcomes after both autologous chondrocyte implantation and microfracture. A defect size of >4 cm2 was the only factor predictive of better outcomes when autologous chondrocyte implantation was compared with a non-autologous chondrocyte implantation surgical technique.
Conclusions:
Cartilage repair or restoration in the knee provides short-term success with microfracture, autologous chondrocyte implantation, or osteochondral autograft. There are patient-specific and defect-specific factors that influence clinical outcomes.
Level of Evidence:
Therapeutic Level II. See Instructions to Authors for a complete description of levels of evidence.
Hyaline articular cartilage plays a vital role in the function of the knee joint. Chondral injury that does not violate the subchondral bone lacks an inherent ability to heal spontaneously1. The natural history of an isolated articular cartilage lesion is not completely understood, nor is it understood which lesions become symptomatic. Nevertheless, these defects have the potential to lead to substantial patient morbidity and may progress to diffuse osteoarthritis2. Patients with isolated focal chondral defects in the knee have considerable impairment in their quality of life, similar to that of patients selecting knee replacement or tibial osteotomy for the treatment of end-stage arthritis and worse than that of patients with chronic anterior cruciate ligament-deficient knees3.
A wide variety of surgical procedures have been developed to address this problem. Repair techniques (marrow-stimulation techniques such as abrasion arthroplasty, drilling, and microfracture) penetrate the subchondral bone and induce the formation of fibrocartilage repair tissue4. Although excellent short-term clinical outcomes have been demonstrated after marrow stimulation, the clinical durability of marrow-stimulated repair tissue has shown an objective and functional decline with further follow-up5-8. Restoration techniques such as osteochondral autograft, mosaicplasty, and osteochondral allograft attempt to replace the cartilage defect with host or donor articular cartilage in a single stage. Clinical outcomes after osteochondral autograft have been good to excellent after as much as seventeen years of follow-up in >90% of patients with defects measuring 1 to 5 cm2, although donor-site morbidity remains a concern9. Restoration with autologous chondrocyte implantation attempts to generate hyaline or hyaline-like cartilage but typically requires two surgical procedures. Long-term follow-up (up to twenty years) after autologous chondrocyte implantation has shown as much as a 92% rate of patient satisfaction, with sustained improvement in clinical outcomes and magnetic resonance imaging findings10-12. It is unknown which cartilage repair or restoration technique provides the best long-term clinical outcome.
Autologous chondrocyte implantation was first reported in 1994 for the treatment of focal chondral injury in the tibiofemoral and patellofemoral compartments13. Since then, chondrocyte-based therapy has utilized a periosteal cover, a collagen-membrane cover, and a variety of three-dimensional scaffolds with different methods of fixation. Although arthrotomy and a two-stage procedure are most commonly used, all-arthroscopic techniques14 and one-stage application of minced articular cartilage15 have emerged as potentially viable options. Pre-implantation chondrocyte phenotype manipulation also has shown excellent outcomes5. Long-term durability and success has been reported after as many as eleven years of follow-up16.
The purpose of the present systematic review was to address three questions. First and second, does the current literature support the use of autologous chondrocyte implantation over other cartilage procedures and the use of one specific generation of autologous chondrocyte implantation over another? We hypothesize that autologous chondrocyte implantation is equivalent to other cartilage procedures with regard to clinical outcomes, magnetic resonance imaging findings, arthroscopic assessment, and durability of treatment. We also hypothesize that there is no difference in outcomes associated with different generations of autologous chondrocyte implantation. Finally, a secondary purpose of the present review was to determine if there are patient-specific and defect-specific factors that may predict better outcomes after autologous chondrocyte implantation compared with other cartilage repair or restoration procedures.
Materials and Methods
To address our hypothesis, we conducted a systematic review of the available literature. Four independent reviewers (J.D.H., R.A.S., X.P., and D.C.F.) separately completed the search, and the results were duplicated three times by each reviewer. The initial search was performed on November 16, 2009, and it was repeated on November 17, 2009, to ensure accuracy. A second search was performed on February 25, 2010. Two additional studies were identified by repeating the search17,18. A search was performed with use of the following databases: MEDLINE, EMBASE, CINAHL, PubMed, SPORTDiscus, and Cochrane Collaboration systematic reviews. Search terms included autologous chondrocyte implantation, ACT, autologous, autogenous, chondrocyte, cartilage, implantation, and transplantation. All studies with Level-I and II evidence (according to the Oxford Centre for Evidence-Based Medicine used by the American volume of The Journal of Bone and Joint Surgery)19 that met criteria were included. Potentially inclusive papers were manually reviewed and were discussed among the authors, and a decision was made regarding inclusion. If there was any disagreement among authors regarding the inclusion of an article, the senior author (D.C.F.) made the final decision. Bibliographies of all reviewed papers were also referenced to assess for potentially inclusive papers that were missed by the initial search. The heterogeneity of studies and their associated outcomes precluded meta-analysis.
The inclusion criteria included (1) comparison of any generation of autologous chondrocyte implantation with any cartilage repair or restoration technique, with reporting of validated clinical outcome measures, (2) comparison of any generation of autologous chondrocyte implantation with a different generation of autologous chondrocyte implantation, with reporting of validated clinical outcome measures, (3) evaluation of both arthroscopic and open arthrotomy autologous chondrocyte implantation, (4) evaluation of Outerbridge/ICRS (International Cartilage Repair Society) Grade-III or IV focal cartilage defects, (5) Level-I and II evidence (randomized controlled trials with >80% follow-up; randomized controlled trials with <80% follow-up, prospective cohort studies), (6) a minimum duration of follow-up of twelve months, (7) use of the English language or any language for which successful medical translation was achievable, (8) evaluation of human subjects, (9) performance of the study from January 1, 1950 through February 25, 2010, and (10) evaluation of the knee joint only (including medial and lateral femoral condyles, trochlea, patella, and medial and lateral tibial plateaus).
The exclusion criteria included (1) case-control studies, all retrospective studies, case series, expert opinion (Level-V evidence), commentary, surgical techniques, letters to the editor, basic science, or animal studies, (2) studies utilizing surgical techniques that were not considered standard practice at the time of the writing of the present manuscript, (3) studies that did not use any validated clinical outcome measures, (4) a duration of follow-up of less than twelve months, (5) use of a language for which successful medical translation was impossible, (6) evaluation of any joint other than the knee (including talus, humeral head, femoral head, and acetabulum), and (7) diffuse osteoarthritis (as defined by a radiographic atlas of osteoarthritis20 Grade ≥2 or Kellgren and Lawrence21 Grade ≥2).
Initial application of inclusion criteria yielded 424 citations. Figure 1 illustrates the application of inclusion and exclusion criteria. Limitation with the term knee yielded 250 citations. Limitation to Level-I and II evidence yielded forty-six citations. Twelve systematic reviews were identified22-28. No meta-analyses were identified. Multiple studies were identified in languages other than English (Spanish, French, German, Czech, Chinese, Norwegian) but were translated with the assistance of a medical/surgical translator. Thirteen studies were deemed appropriate for inclusion.
Fig. 1.
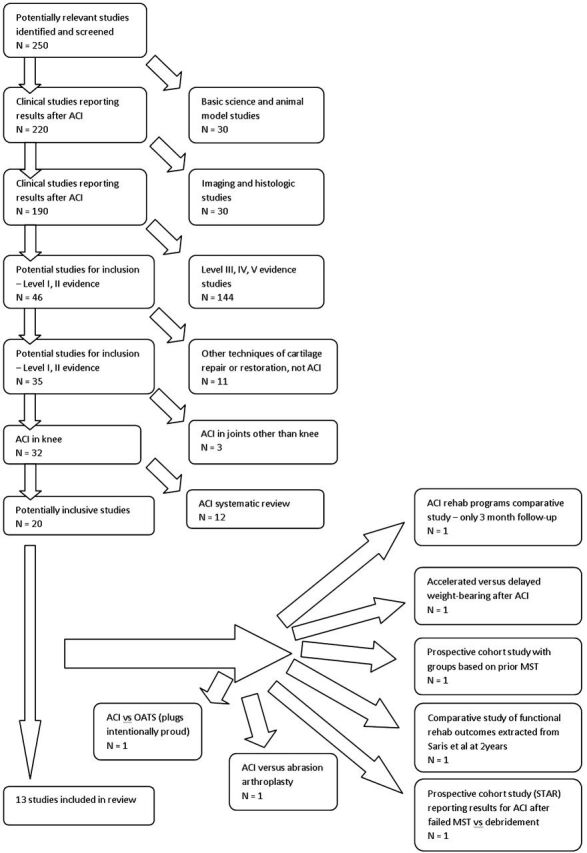
Exclusion criteria flow chart. ACI = autologous chondrocyte implantation, OATS = osteochondral autograft, STAR = Study of Treatment of Articular Repair, and MST = marrow-stimulation technique.
For the assessment of the quality of the randomized controlled trials, we utilized the Delphi list29 and a modification of the Coleman methodology score30-32 (see Appendix). The Delphi consensus29 established a criteria list for quality assessment of randomized controlled trials for conducting systematic reviews. The resultant Delphi list is a reference standard for randomized controlled trials on many different research topics. Consensus among epidemiologic and statistical experts allowed for the creation of a set of nine generic core items for quality assessment of randomized clinical trials. A quality score is the sum of the individual components, with 1 point given for a “yes,” 1 point deducted for a “no,” and 0 points for “don’t know.” Quality scores generated from the application of the Delphi list to each study allow for comparison among studies. The Coleman methodology score was designed for the grading of clinical studies on patellar30 and Achilles33 tendinopathy and assesses methodology with use of ten criteria, giving an overall score between 0 and 100, with 100 signifying a study that optimally limits chance, bias, and confounders. The modified Coleman methodology score31 is the sum of fifteen components, with a scaled potential score between 0 and 100, assessing the quality of study reporting. Scores were classified as excellent (85 to 100 points), good (70 to 84 points), fair (55 to 69 points), or poor (<55 points).
An attempt was made to identify common outcome measures among studies. However, several different clinical outcome measures were used across all studies, including the Tegner activity score (n = 8), the Lysholm knee score (n = 7), the IKDC (International Knee Documentation Committee) subjective and objective scores (n = 6), the modified Cincinnati knee score (n = 2), the SF-36 (Short Form-36) score (n = 3), the KOOS score (Knee Injury and Osteoarthritis Outcomes Score) (n = 2), the Stanmore functional score (n = 1), the Bentley functional score (n = 1), and the Meyers score (n = 1). No clinical outcome measure defines durability of treatment. Durability must be inferred by the longevity of results of validated outcomes scores. For clinical outcome measures, the effect size and 95% confidence interval were calculated.
Effect size is used to standardize the magnitude of differences between two groups across different studies and measures34. Effect size35 is the difference between the means from the two groups (M1 - M2) divided by the within-group standard deviation (S): (M1 - M2)/S. The accuracy of the estimated effect size is dependent on the sample size. The sampling error of the estimated effect size can be quantified with use of its confidence interval. The standard error and 95% confidence interval for an effect size can be calculated with a simple formula36. If the confidence interval of the estimated effect size includes 0, it can be normally interpreted that there is no significant difference between the two groups. The absolute magnitude of the effect size indicates its strength. Thus, an effect size of 0.2 suggests a small effect, whereas an effect size of 1.2 indicates a large effect.
Source of Funding
The authors received no external funding in support of this study.
Results
Thirteen studies were identified for inclusion. There were 917 subjects who underwent one of three relevant surgical techniques: 604 underwent autologous chondrocyte implantation, 271 underwent microfracture, and forty-two underwent osteochondral autograft. Studies that duplicated patient populations twice, with one of the two studies providing additional follow-up, were included twice for the purpose of data analysis; two pairs of studies involved a duplicate patient population. In one pair of studies, eighty patients (forty managed with microfracture, forty managed with autologous chondrocyte implantation) were reported twice, for a total of 1607,37. In another pair of studies, 118 patients (sixty-one managed with microfracture, fifty-seven managed with autologous chondrocyte implantation) were reported twice, for a total of 2365,38. Thus, 198 patients were included twice in our analysis. These studies evaluated clinical, radiographic, and arthroscopic outcomes. No studies evaluated outcomes after osteochondral allograft or metallic knee arthroplasty. Six studies were Level-I evidence, and seven were Level-II evidence. Financial conflicts of interest were declared in four studies7,37,39,40, not declared in six studies5,8,18,38,41,42, and not reported in three studies17,43,44.
Study Methodological Quality
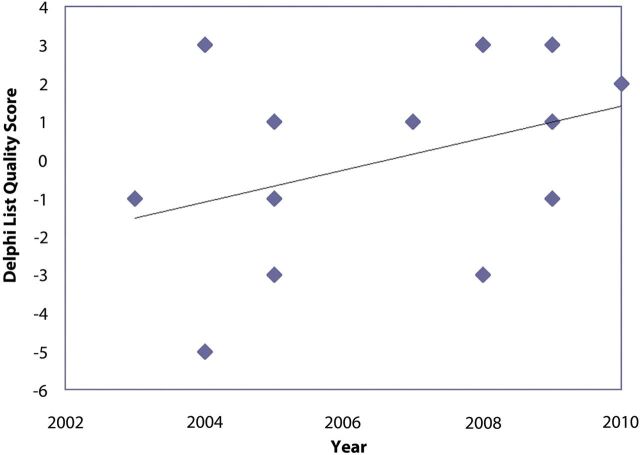
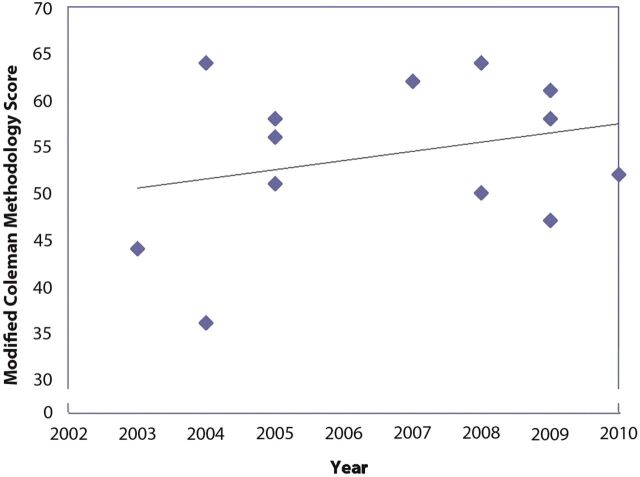
Delphi list quality scores and modified Coleman methodology scores were calculated for all thirteen studies (see Appendix). A general trend was observed that, with later years of publication, study quality improved (Figs. 2-A and 2-B). Based on a scaled maximum of 100, the mean overall study quality based on modified Coleman methodology scores was 54. On the basis of a categorical rating system established by Cowan et al.31, no studies were considered good or excellent, seven were considered fair, and six were considered poor.
Fig. 2-A Fig. 2-B.
Fig. 2-A Delphi score. There is a general trend of increasing score quality with later date of publication. Fig. 2-B Modified Coleman methodology score. Although the score quality increased to its peak in 2007, followed by a decline, the general trend line indicates increasing score quality overall.
Patient Populations
Patients undergoing cartilage repair or restoration in this review tended to be young (range of mean ages, 28.7 to 34.2 years); to have a long preoperative duration of knee symptoms (range of mean duration of symptoms, twenty-one to 103 months); and to have had multiple previous surgical procedures (range, zero to thirteen) (excluding arthroscopic cartilage biopsy). The defects treated in this population were moderate-sized (range of mean sizes, 1.9 cm2 to 6.2 cm2), full-thickness (Outerbridge III/IV or International Cartilage Repair Society III/IV/osteochondritis dissecans [100%]), and isolated, single defects (range, 80% to 100%). The defects were primarily located on the medial femoral condyle (range, 38% to 89% of all defect locations). The minimum duration of follow-up ranged from twelve to sixty months. Of the nine studies in which autologous chondrocyte implantation was compared with either microfracture or osteochondral autograft, two evaluated outcomes after less than twenty-four months of follow-up. Of the four studies comparing different generations of autologous chondrocyte implantation, one evaluated outcomes after less than twenty-four months of follow-up. All patient and defect demographic data are given in the Appendix.
Four studies included a declaration of a financial conflict of interest, but only one demonstrated a significant difference between compared surgical techniques37. Of the six studies in which the authors did not declare a conflict of interest, all demonstrated significant differences between compared surgical techniques5,8,18,38,41,42. Three studies did not include a conflict of interest statement and therefore, any financial interest influence was unable to be analyzed17,43,44.
Surgical Interventions
Seven studies compared autologous chondrocyte implantation with a marrow-stimulation technique5,7,8,17,37,38,44. Two studies compared autologous chondrocyte implantation with osteochondral autograft40,42. Four studies compared two separate generations of autologous chondrocyte implantation18,39,41,43. Nine studies utilized a periosteal cover for autologous chondrocyte implantation5,7,18,37,38,40-43. Two studies utilized a Type I/III collagen-membrane cover39,43. Two studies involved the use of ChondroCelect (TiGenix, Leuven, Belgium) via characterized chondrocyte implantation under a periosteal cover5,38. This latter technology (characterized chondrocyte implantation) uses a genetic profile marker score to predictably optimize the phenotype of the cartilage tissue produced with each autologous chondrocyte implantation cell batch. Six studies used second-generation, three-dimensional scaffold-chondrocyte products: two studies8,41 utilized Hyalograft C (HYAFF-11; Fidia Advanced Biopolymers, Abano Terme, Italy), and four studies used matrix-induced autologous chondrocyte implantation (MACI; Verigen, London, United Kingdom)17,18,39,44. All microfracture comparative studies5,7,8,17,37,38,44 used the Steadman technique4, with debridement of all unstable cartilage, including the calcified cartilage zone, the formation of perpendicular healthy defect rims, and the placement of multiple holes within the defect, 3 to 4 mm apart, with an arthroscopic awl. Two studies40,42 utilized osteochondral transplants but could not be compared with each other due to differences in surgical techniques. One study involved the use of a large diamond bone-cutting system with twin harvesting cylinders (10.0 to 17.0-mm-diameter cylinders) (DBCS; Merck, Darmstadt, Germany) for press-fit transplantation42, whereas the other study40 did not describe details of the technique.
Concurrent Surgical Interventions
Five studies reported the use of additional surgical procedures concurrent with the primary cartilage repair or restoration technique5,8,17,18,38. Four5,8,17,38 of the five studies reporting concurrent procedures were comparisons of autologous chondrocyte implantation and microfracture. In the studies reporting concomitant procedures, partial meniscectomy was the most commonly performed procedure (18%; fifty of 279), followed by anterior cruciate ligament reconstruction (16%; forty-six of 279). Partial meniscectomy was performed in twenty-nine (24%) of 121 patients managed with microfracture and twenty-one (13%) of 158 patients managed with autologous chondrocyte implantation in these studies. Anterior cruciate ligament reconstruction was performed in twenty-six (21%) of 121 patients managed with microfracture and twenty (13%) of 158 patients managed with autologous chondrocyte implantation. Within two studies, there were significant differences between microfracture and autologous chondrocyte implantation in terms of the number of subjects undergoing concomitant surgery (with a greater number of meniscal procedures in patients undergoing microfracture as opposed to autologous chondrocyte implantation)5,38. Within the other three studies reporting surgical procedures concurrent with either autologous chondrocyte implantation, osteochondral autograft, or microfracture, there were no significant differences between the analyzed groups8,17,18.
Clinical Outcome Measures, Primary Outcomes
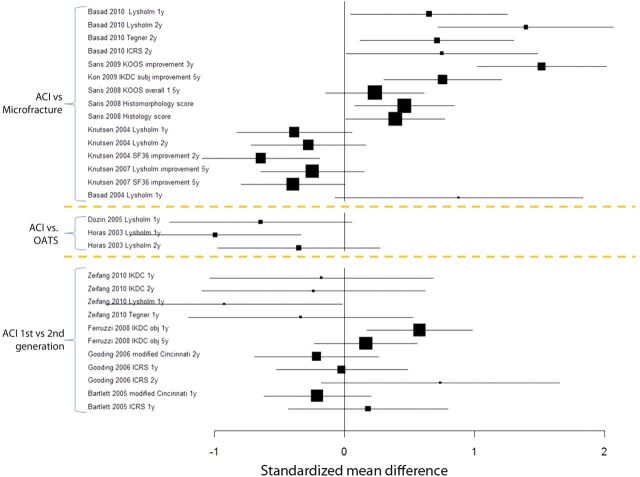
All studies showed improvement in clinical outcomes after all cartilage techniques (as compared with baseline). Figure 3 and tables in the Appendix summarize effect sizes and their corresponding confidence intervals for each study on the basis of various outcome measurements. The data used for the effect size calculation either were given in the text and tables or were estimated from the tables and figures in the papers. In the tables in the Appendix, large, positive effect sizes provide evidence in favor of the group of interest (autologous chondrocyte implantation), whereas large, negative effect sizes indicate evidence against the group of interest.
Fig. 3.
Forest plot demonstrating standardized mean difference between autologous chondrocyte implantation (ACI) and microfracture, between autologous chondrocyte implantation and osteochondral autograft (OATS), and between first and second-generation autologous chondrocyte implantation. Large boxes indicate large effect sizes, whereas small boxes indicate small effect sizes. A positive mean difference (on the right side of zero) favors autologous chondrocyte implantation, whereas a negative mean difference (on the left side of zero) favors either microfracture or osteochondral autograft (where applicable).
Autologous Chondrocyte Implantation versus Microfracture
Basad et al.17 randomized sixty patients to either matrix-induced autologous chondrocyte implantation (n = 40) or microfracture (n = 20) and reported both clinically important and statistically significant improvements and absolute outcomes in terms of Lysholm, Tegner, and ICRS patient and surgeon scores at one and two years. Saris et al.5 randomized 118 patients to either characterized chondrocyte implantation (n = 57) or microfracture (n = 61) and reported a mean improvement from baseline to thirty-six months in terms of the overall KOOS score that was greater in the characterized chondrocyte implantation group. Although improvement was statistically significant, the difference in absolute overall KOOS (78 compared with 75) does not represent clinical importance. Significant differences were demonstrated supporting characterized chondrocyte implantation over microfracture in terms of the absolute overall KOOS score (p = 0.048) and the subdomains of pain (p = 0.044) and quality of life (p = 0.036). Treatment responder status was defined as an increase of at least 10% in the overall KOOS score or decrease of at least 20% in the visual analog pain score. Although there were more KOOS-based (83% versus 62%) and visual analog score-based (83% versus 66%) treatment responders after characterized chondrocyte implantation than after microfracture, the differences were not significant (p = 0.084 and 0.165, respectively). Kon et al.8 performed a nonrandomized prospective cohort study of eighty subjects (forty managed with second-generation autologous chondrocyte implantation and forty managed with microfracture) with a minimum of five years of follow-up. When the groups were compared, better improvement in IKDC objective (p < 0.001) and subjective (p = 0.003) scores was seen after autologous chondrocyte implantation at the time of the five-year follow-up. The ability to return to sports was similar at the time of the two-year follow-up in both groups and remained constant after five years in the autologous chondrocyte implantation group, whereas it worsened in the microfracture group.
Knutsen et al.37 reported clinical outcomes (Lysholm, Tegner, ICRS, and SF-36) in a randomized trial of eighty subjects in which first-generation autologous chondrocyte implantation (n = 40) was compared with microfracture (n = 40) after two years of follow-up. Although both groups had significant clinical improvement two years after surgery, patients managed with microfracture had significantly greater improvement in SF-36 scores (p = 0.004) than did those managed with autologous chondrocyte implantation. No other significant differences were observed. The five-year follow-up of these two groups by Knutsen et al.7 demonstrated no significant clinical or radiographic differences. While the number of failures after autologous chondrocyte implantation was greater at two years after surgery, the number of failures after microfracture (n = 9) continued to increase to equal that after autologous chondrocyte implantation (n = 9) at five years postoperatively. Although SF-36 physical component scores showed superiority of microfracture over autologous chondrocyte implantation at two years, this trend was gone by five years.
Overall, comparison of autologous chondrocyte implantation and microfracture often demonstrates better short and intermediate-term clinical results with autologous chondrocyte implantation. Three of seven studies demonstrated a significantly better overall clinical result following autologous chondrocyte implantation as compared with microfracture5,8,17 in terms of one and two-year Lysholm scores (effect size = 0.66 [95% confidence interval, 0.05 to 1.25] and 1.42 [95% confidence interval, 0.72 to 2.07], respectively)17, three-year overall KOOS scores5 (effect size = 1.52; 95% confidence interval = 1.03 to 2.01), and five-year improvement in IKDC scores8 (effect size = 0.76; 95% confidence interval = 0.31 to 1.21). Two studies showed positive but not significantly better results for autologous chondrocyte implantation compared with microfracture in terms of one-year improvement in Lysholm scores44 (effect size = 0.92; 95% confidence interval = –0.06 to 1.82), and 1.5-year overall KOOS scores38 (effect size = 0.23; 95% confidence interval = –0.14 to 0.61). Two studies referred to the same population and suggested that microfracture was significantly superior to autologous chondrocyte implantation in terms of the SF-36 physical component at two years (effect size = –0.65; 95% confidence interval = –1.09 to –0.19)37; however, these differences were not significant at five years of follow-up (effect size = –0.40; 95% confidence interval = –0.79 to 0.01)7.
Autologous Chondrocyte Implantation versus Osteochondral Autografts
Comparison between autologous chondrocyte implantation and osteochondral autograft has demonstrated equivalent clinical outcomes; however, a slower treatment response is seen after autologous chondrocyte implantation. In both studies evaluating osteochondral autograft, the surgical technique involved congruent joint surfaces with plugs placed perpendicular to the joint surface. Horas et al.42 randomized forty subjects to either first-generation autologous chondrocyte implantation (n = 20) or osteochondral autograft (n = 20). Significantly better Lysholm scores (effect size = –1.0; 95% confidence interval = –1.65 to –0.34) were seen after mosaicplasty as compared with autologous chondrocyte implantation at one year42. However, these outcomes were not evident at two years42 (effect size = –0.36; 95% confidence interval = –0.97 to 0.28). Dozin et al.40 randomized forty-four patients to either autologous chondrocyte implantation or mosaicplasty. Although outcomes were presented as an intent-to-treat analysis, a large proportion of patients (32%) were “clinically cured” after arthroscopic debridement and biopsy or were lost to follow-up and therefore did not undergo the intended surgical technique of autologous chondrocyte implantation or mosaicplasty. This large attritional bias alters the conclusions drawn from the study population.
First-Generation versus Second-Generation Autologous Chondrocyte Implantation and Open versus Arthroscopic Autologous Chondrocyte Implantation
Comparison of first and second-generation autologous chondrocyte implantation has, in large part, shown equivalent short-term clinical outcomes, with similar complications and a similar rate of reoperation. Bartlett et al.39 randomized ninety-one patients to either collagen-covered, first-generation autologous chondrocyte implantation (n = 44) or second-generation, matrix-induced autologous chondrocyte implantation (n = 47) and reported significant improvement at the time of the one-year follow-up in terms of modified Cincinnati scores but reported no difference with regard to clinical outcome, complications, or the rate of reoperation. Comparison of open versus arthroscopic autologous chondrocyte implantation has shown more rapid improvement with fewer complications and a lower rate of reoperation with arthroscopic autologous chondrocyte implantation. Zeifang et al.18 compared the outcomes after periosteal autologous chondrocyte implantation with those after open second-generation autologous chondrocyte implantation (matrix-induced autologous chondrocyte implantation). While IKDC, Tegner, and SF-36 scores were equivalent at one and two years of follow-up, the study demonstrated significant differences between periosteal autologous chondrocyte implantation and matrix-induced autologous chondrocyte implantation in terms of absolute values and improvements in Lysholm scores, with better outcomes following periosteal autologous chondrocyte implantation. Ferruzzi et al.41 performed a nonrandomized prospective cohort study comparing open (n = 48) and arthroscopic (n = 50) autologous chondrocyte implantation at a minimum of five years of follow-up and reported significantly better IKDC subjective, objective, and functional scores in the arthroscopic group as compared with the open group at six (p = 0.001), twelve (p < 0.0005), and eighteen (p = 0.022) months. While the arthroscopic group improved for as long as eighteen months after surgery and then remained stable until the time of the latest follow-up, the open group continued to improve beyond eighteen to twenty-four months.
Arthroscopic and Histologic Outcomes
Of the 917 patients who were analyzed (including study duplicates), 376 (41%) underwent second-look arthroscopy (and biopsy, when possible). There were 255 second-look arthroscopies after autologous chondrocyte implantation (six were performed because of symptoms, whereas all of the others were planned a priori). There were 116 second-look arthroscopies after microfracture (one was performed because of symptoms, whereas all others were a priori) and five after osteochondral autograft (all planned).
There is conflicting evidence on arthroscopic and histologic outcomes following autologous chondrocyte implantation. One study demonstrated significantly better outcomes after autologous chondrocyte implantation than after microfracture (effect size for histologic scores = 0.39 [95% confidence interval, 0.01 to 0.77]; effect size for histomorphometric scores = 0.46 [95% confidence interval, 0.08 to 0.85]) at the time of the one-year follow-up38. However, four other studies showed no significant difference in arthroscopic (ICRS) or histologic outcomes after autologous chondrocyte implantation as compared with microfracture37, osteochondral autograft42, or a different generation of autologous chondrocyte implantation39,43.
Durability of Autologous Chondrocyte Implantation versus Microfracture and Osteochondral Autograft
While the clinical outcomes of microfracture tended to either plateau or deteriorate at longer follow-up periods, the results of autologous chondrocyte implantation tended to remain stable or to continue to improve5,7,8. Continued improvements in the KOOS score have been documented at the time of the latest follow-up (thirty-six months) after characterized chondrocyte implantation as compared with an initial improvement followed by a decline and plateau after microfracture5. A trend toward progressive elevation of the subchondral bone plate, so-called “subchondral bone reaction,” was demonstrated on magnetic resonance imaging more often after microfracture than after characterized chondrocyte implantation (p = 0.056)5. Sports activity and return to the pre-injury level of sports, as illustrated by the Tegner activity score, was significantly improved after both microfracture and second-generation autologous chondrocyte implantation at two years of follow-up8. The level of sports in the autologous chondrocyte implantation group remained stable at five years of follow-up as compared with a significant decline in the microfracture group8. Of the group of twenty subjects who were able to achieve the pre-injury level of sports at two years after microfracture, only seven retained this ability at five years (as compared with eighteen of eighteen in the autologous chondrocyte implantation group)8. Although Lysholm and SF-36 (physical component) scores after microfracture had higher values at two years of follow-up as compared with those after autologous chondrocyte implantation, these results were not maintained at five years of follow-up7. The duration of follow-up within all included studies was longest for microfracture and all generations and techniques of autologous chondrocyte implantation (up to five years). Follow-up after osteochondral autograft was shorter (two to three years).
Patient Factors Influencing Outcomes
Patient age was a significant predictor of outcome following cartilage repair and restoration. Patients less than thirty years of age had significantly better Lysholm and physical component SF-36 scores at two and five years after both microfracture and autologous chondrocyte implantation than did those more than thirty years of age7. After matrix-induced autologous chondrocyte implantation and Type I/III collagen-membrane autologous chondrocyte implantation, significantly better modified Cincinnati knee scores were seen at the time of the one-year follow-up in those who were less than thirty-five years old as compared with those who were more than thirty-five years old39. Patient age was not predictive of better outcomes with one technique over another in either of the latter two studies. More active patients, as evidenced by the Tegner score, had significantly improved Lysholm, SF-36 physical component, and visual analog scale pain scores after both microfracture and autologous chondrocyte implantation than did less active patients at two years of follow-up37. However, activity levels were not predictive of better outcomes with one technique over another.
The duration of symptoms prior to cartilage repair or restoration was an important factor affecting outcomes following surgery. The mean symptomatic time period prior to surgical intervention was highly variable between studies, ranging from twenty-one months to more than 103 months. The mean modified Cincinnati knee scores after matrix-induced autologous chondrocyte implantation or Type I/III collagen-membrane autologous chondrocyte implantation were significantly greater in subjects who had been symptomatic for less than fifty months as compared with those who had been symptomatic for more than fifty months (70.1 compared with 55.8)39. In the group of subjects managed within twelve months (n = 7), the outcome was even better (modified Cincinnati score, 79.1)39. After characterized chondrocyte implantation, the mean overall improvement in the KOOS score was 28% and 71% higher among patients who had been symptomatic for less than two and three years prior to surgery, respectively5. Nevertheless, the preoperative duration of symptoms was not predictive of better outcomes with one technique over another.
Surgical procedures prior to cartilage repair or restoration had a significant impact on postoperative outcomes in one study39. Although this metric was not analyzed in all studies, all patients in this study who were managed with matrix-induced autologous chondrocyte implantation or Type I/III collagen-membrane autologous chondrocyte implantation following a previous failure of carbon-fiber grafting, mosaicplasty, or autologous chondrocyte implantation had a poor postoperative clinical outcome (mean modified Cincinnati score, 35)39. Again, this factor was not predictive of better outcomes with one technique over another.
Defect Factors Influencing Outcomes
No study has shown that the location of intra-articular defects (medial or lateral femoral condylar weight-bearing defects or patellofemoral defects) predicts better outcomes after any of the analyzed surgical techniques. Small sample sizes of intra-articular location-specific differences precluded statistical analysis of the effects that these areas have on the response to surgery. No other significant associations between lesion location and outcome were observed across all studies.
The only size-based clinical outcome association was shown when autologous chondrocyte implantation was compared with microfracture17,37. Although the mean defect size ranged from 1.9 to 6.2 cm2 between studies, the mean defect sizes of individual groups within each study were not significantly different prior to cartilage repair or restoration. At two years after microfracture, patients with defects measuring <4 cm2 had significantly better Lysholm, visual analog scale, and SF-36 physical component scores than did patients with larger defects37. Furthermore, patients with lesions measuring >4 cm2 had better results following autologous chondrocyte implantation as compared with microfracture. Nevertheless, this size association was not demonstrated at five years of follow-up7. An inclusion criterion in another study was the presence of relatively large defects, specifically, an isolated defect measuring >4 cm2 (range, 4 to 10 cm2)17. That study demonstrated better clinical outcomes after matrix-induced autologous chondrocyte implantation as compared with microfracture. These latter two studies were the only two studies that were able to identify a patient-specific or defect-specific factor (defect size, >4 cm2) that predicted a better outcome following autologous chondrocyte implantation as compared with a non-autologous chondrocyte implantation cartilage technique.
Complications
Complications after autologous chondrocyte implantation, microfracture, and osteochondral autograft were reported in all studies. When reported, graft hypertrophy occurred in fifty-four (22%) of 243 cases after periosteal autologous chondrocyte implantation, in six (6%) of 108 cases after collagen-membrane autologous chondrocyte implantation, in two (4%) of fifty cases after Hyalograft C autologous chondrocyte implantation, and in four (7%) of fifty-eight cases after matrix-induced autologous chondrocyte implantation. The rate of symptomatic hypertrophy was not typically reported. Most commonly defined as reoperation due to persistent symptoms, “failure” was reported differently among all studies. Although this definition was variable, it occurred in seventeen (2.8%) of 604 cases after autologous chondrocyte implantation, ten (3.7%) of 271 cases after microfracture, and three (7.1%) of forty-two cases after mosaicplasty. Arthrofibrosis occurred in fifteen cases (2.5%) after autologous chondrocyte implantation (including six cases of periosteal autologous chondrocyte implantation, six cases of Type I/III collagen-membrane autologous chondrocyte implantation, and three cases of matrix-induced autologous chondrocyte implantation), in seven cases (17%) after mosaicplasty, and in one case (0.4%) after microfracture. Less common complications included deep-vein thrombosis (n = 2), superficial wound infection (n = 3), septic arthritis (n = 1) (although there was no growth on culture), and reflex sympathetic dystrophy (n = 2). Comparison between arthroscopic and open autologous chondrocyte implantation revealed a significantly lower rate of complications and a lower rate of reoperation in the arthroscopic group41.
Discussion
We hypothesized that autologous chondrocyte implantation was equivalent to other cartilage procedures with regard to clinical outcome, magnetic resonance imaging and arthroscopic assessment, and durability of treatment. Analysis of the current body of high-level evidence suggests that there is a trend for autologous chondrocyte implantation to demonstrate improved outcomes in comparison with microfracture but does not allow us to conclude that there is any difference between autologous chondrocyte implantation and osteochondral autograft transplantation. Also, there is no significant difference between first and second-generation autologous chondrocyte implantation. Our review demonstrated that there are patient-specific and defect-specific factors that do influence clinical outcome after autologous chondrocyte implantation. Although the methodological quality of studies on these procedures was initially poor, recent studies have demonstrated substantive improvement in study quality (Figs. 2-A and 2-B). Younger, more active patients, with a shorter duration of preoperative symptoms, fewer surgical procedures prior to cartilage repair or restoration, smaller isolated defects on the medial femoral condyle, and no concomitant ligamentous instability, meniscal deficiency, or tibiofemoral or patellofemoral malalignment, can expect the best outcome regardless of technique.
Despite several Level-III and IV studies demonstrating unambiguous improvement after autologous chondrocyte implantation, Level-I and II studies included in other systematic reviews23,25,26,45,46 have failed to show a clear superiority of any cartilage repair or restoration technique. Furthermore, the generally low methodological quality of cartilage-repair studies overall precludes confident interpretation of their results32. This finding has been further supported in several recent systematic reviews22-28. Those reviews have analyzed cartilage techniques, including autologous chondrocyte implantation, and have shown short-term improvement in knee function23,25,26,45,46. Despite the theoretical and proven advantage of an increased proportion of hyaline cartilage after autologous chondrocyte implantation and osteochondral autograft with its associated improved histologic scores, the lack of long-term data in these other reviews precluded a statement of superiority of one cartilage repair or restoration technique over another.
Previous systematic reviews have lacked consensus because of the heterogeneity of included studies, the inclusion of comparative studies not utilizing autologous chondrocyte implantation, and a focus on commercial funding and economic issues of autologous chondrocyte implantation. The recent addition of high-quality randomized trials not included in previous reviews supplements the existing literature and may show a trend toward better outcomes after autologous chondrocyte implantation5,8,17,38. Our current review of Level-I and II studies and our statistical analysis indicate that autologous chondrocyte implantation demonstrates sustained clinical outcomes after as much as five years of follow-up. The theoretical potential benefit of autologous chondrocyte implantation over microfracture due to a more durable structural tissue was not observed at the time of intermediate-term follow-up but may be seen with longer-term follow-up. It must be noted that, of the thirteen studies included in our review, three had less than twenty-four months of follow-up. The short-term assessment of the clinical outcomes after any surgical technique clearly does not allow a sufficient time period for a definitive statement regarding the efficacy or durability of the technique. Although biopsies after autologous chondrocyte implantation continue to show maturation for as long as twenty-four months47, the timing of maturation of cartilage repair and restoration tissue and its clinical correlation have yet to be definitively determined. The inclusion of studies with short-term follow-up was deemed appropriate as there may be clinical differences as the tissue evolves and the patients have already returned to activities of daily living and high-impact sports. Nevertheless, these results must be interpreted cautiously.
Other reviews of cartilage repair and restoration include a recent study23 that demonstrated an increasing quality of studies with time after second-generation autologous chondrocyte implantation, but there was an inverse relationship between study outcome and study quality. This relationship was also noted by Jakobsen et al.32, confirming that superior outcomes may be inherently more biased. Nonetheless, heterogeneous outcome measures, lack of a control group across all studies, analysis of multiple different techniques of autologous chondrocyte implantation, and inherent limitations within many of the individual studies still limit the conclusions one may draw from comparison of these “high-level evidence” clinical studies.
The findings of this systematic review have confirmed those of several recent systematic reviews23,25-28,45,46,48. Although many of those recent reviews had small differences regarding study inclusion and exclusion criteria, study methodological assessment tools, and surgical techniques analyzed, their conclusions were quite similar. The unique strengths of our systematic review include the inclusion and discussion of high-level (Level-I and II) evidence with inclusion and exclusion criteria developed to properly achieve the aims of the study, comparison of autologous chondrocyte implantation with non-autologous chondrocyte implantation cartilage techniques, comparison of different generations of autologous chondrocyte implantation, the identification of factors that influence outcomes after autologous chondrocyte implantation, the identification of factors that predict better outcomes with autologous chondrocyte implantation as compared with non-autologous chondrocyte implantation techniques, and effect size statistics of studies analyzed.
Three studies that were included in our review demonstrated significantly better clinical outcomes following autologous chondrocyte implantation in comparison with other techniques. Autologous chondrocyte implantation has demonstrated significantly better clinical outcomes than microfracture (in terms of KOOS5, IKDC8, Lysholm17, Tegner17, ICRS17, and return to sports8) and better histologic38 outcomes than microfracture. A genetic profile score indicative of chondrocyte quality, used in characterized chondrocyte implantation, was shown by Saris et al. to be a significant predictor of outcome. This is the only study in the literature to document ex vivo chondrocyte manipulation and implantation of the preserved articular cartilage phenotype5. Although microfracture demonstrated significantly better improvement as compared with autologous chondrocyte implantation at the time of short-term follow-up, the results did not endure with longer follow-up, with equivalent clinical outcomes being demonstrated, indicating deterioration with time after microfracture7. This deterioration was also observed in two other Level-I studies5,8. Effect-size analysis of microfracture versus autologous chondrocyte implantation indicates that the initial benefit of microfracture peaks early and then declines with time, eliminating any potential benefit over autologous chondrocyte implantation, especially for larger lesions. A recent systematic review of twenty-eight clinical studies (3122 subjects) on the clinical outcomes after microfracture confirmed that microfracture peaks early and deteriorates with time6. Progressively improved knee function generally was seen for as long as twenty-four months. Although seven studies in that review demonstrated deterioration in as many as 80% of patients between eighteen and thirty-six months after microfracture, limited long-term data and the quality of existing short and intermediate-term data precluded a statement of definitive efficacy of microfracture as compared with other cartilage-repair and restoration techniques6. A potential explanation for the poor durability of repair and the lack of long-term success after microfracture has been suggested to be related to the actual nature of the procedure, specifically, violation of subchondral bone38,49-52. Subsequent subchondral bone metabolic changes include the formation of intralesional osteophytes, tidemark advancement, and osseous overgrowth38,49-52. This subchondral bone change, easily visualized with magnetic resonance imaging or arthroscopy, has been shown to be a precursor to degenerative osteoarthritis50,51,53. In the studies in our review, microfracture was less likely to lead to arthrofibrosis than autologous chondrocyte implantation was. This finding may be due to the minimal insult to the knee imposed by the arthroscopy associated with microfracture as compared with the arthrotomy required with autologous chondrocyte implantation.
A slower clinical response has been observed after autologous chondrocyte implantation as compared with osteochondral autograft. This difference in response can be expected because of the immediate presence of hyaline cartilage with osteochondral autograft as compared with the longer-duration remodeling of liquid-based chondrocyte solution in first-generation autologous chondrocyte implantation and integration of scaffold-chondrocyte-based matrices in second-generation autologous chondrocyte implantation. A recent prospective study47 of histologic quality after autologous chondrocyte implantation showed a very strong dependency of clinical outcomes on the timing of the biopsy. If the time after implantation of chondrocytes doubles, then it is 4.21-times more likely that the histologic result will be consistent with hyaline-like tissue as opposed to fibrous tissue or fibrocartilage. Furthermore, on the basis of the results of their study, the authors proposed an optimal earliest timing of biopsy at twenty-four months as histologic outcome continues to improve as long as twenty-four months after surgery. The only high-level evidence to support a more rapid clinical response with osteochondral autograft as compared with autologous chondrocyte implantation was in the study by Horas et al.42, which showed significantly better Lysholm scores at one year after osteochondral autograft as compared with autologous chondrocyte implantation. By two years, however, these clinical scores were equivalent. That study involved the use of large plugs, and, thus, donor-site morbidity may become an issue with time. However, after as long as seventeen years after osteochondral autograft, patellofemoral pain related to donor-site morbidity resulting from graft harvest was seen in <5% of patients9.
Despite the Level-I and II studies included in our review, several limitations compromise their usefulness (see Appendix). Properly conducted randomization attempts to eliminate selection bias and to support the internal validity of a study54. Allocation of treatment was adequately concealed via randomization with use of sealed envelopes, random stratified lists balanced in permuted blocks of varying block size in random sequence, and an integrated voice response system (IVRS) computer minimization method in nine studies5,7,17,18,37-40,43. Alternating consecutive-selection randomization is a potential source of selection bias and was used in one study42. None of the study authors were involved in the randomization of subjects. Demographic data such as subject age, duration of follow-up, defect size, defect depth, and defect location were similar among studies. However, across all studies, the number of patients within each comparative surgical group was unequal, with four times as many subjects undergoing microfracture as compared with osteochondral autograft and three times as many subjects undergoing autologous chondrocyte implantation as compared with microfracture. Study subjects should be similar at the time of study onset and, although this is true in most studies, the presence of prior and concurrent surgical interventions (selection bias) alters all subsequent surgical interventions and their dependent clinical outcomes. Nearly all of the studies in this review included patients who had had previous operations to the affected knee. Although microfracture and other marrow-stimulation techniques traditionally have been regarded as low-morbidity, first-line, “non-bridge-burning” techniques, it has recently been shown that these procedures cause a three-fold increase in the rate of failure of subsequent autologous chondrocyte implantation55. This illustrates a strong negative effect on subsequent autologous chondrocyte implantation and warrants caution as marrow-stimulation techniques may truly be a “bridge-burning” procedure via their effect on the subchondral bone within the defect. Two of the studies demonstrated significant differences between the number of subjects within each group undergoing concurrent surgery to address meniscal lesions. Even small degrees of meniscal pathology and/or resection influence contact mechanics within the knee and thus influence the stress placed on cartilage repair or restoration and the bias in the outcomes reported.
Many dissimilar autologous chondrocyte implantation surgical techniques (open periosteal cover autologous chondrocyte implantation7,18,37,40-43, open periosteal cover autologous chondrocyte implantation with characterized chondrocytes5,38, open collagen-membrane cover autologous chondrocyte implantation39,43, open second generation autologous chondrocyte implantation17,18,39,44, and arthroscopic second generation autologous chondrocyte implantation8,41) are compared with osteochondral autograft transplant, which also used dissimilar techniques (different sizes of cylinders and instrumentation for osteochondral cylinder transplantation). This performance bias is analogous to comparing “apples to apples and oranges to oranges.”56 This likely had an impact on the findings between the different studies of osteochondral autograft. The use of smaller, proud osteochondral plugs can lead to more intervening fibrous tissue between plugs, increased pressure, and breakdown of the cartilage on the plugs57,58, all of which can negatively influence results. In addition to differences between like surgical procedures, concomitant surgical techniques (including meniscal, ligamentous, and realignment surgery), which were performed in five of thirteen studies5,8,17,18,38 in this review, add substantial performance bias. The natural history of an untreated, isolated focal chondral defect (control) is unknown; thus, the effect of treatment relative to control may be overestimated or underestimated.
Assessment of outcomes via an independent observer is necessary to minimize detection bias. This was done clinically in only four of thirteen studies18,37,38,43. However, histologic, magnetic resonance, and radiographic imaging was evaluated blinded in all studies that used these evaluation parameters. The obvious caveat that warrants independent post-treatment evaluation is that the surgeon cannot be excluded from knowledge of the treatment as he or she is the entity performing the technique being investigated. The assessment of studies ideally should be done with use of validated, reliable, responsive, disease-specific outcome measures across all studies. The KOOS and IKDC systems have been validated and are reliable and responsive for cartilage pathology and response to treatment59. These two measures were utilized in only seven of thirteen studies5,8,18,38,40,41,44 in this review, introducing detection bias. Nevertheless, the use of other outcome measures is warranted and supplements the IKDC and KOOS results. The use of multiple outcome measures allows the response to treatment to be fully appreciated.
A statement of significance does not equate to clinical importance. Therefore, the difference between the two must be made clear with high-level evidence. Basad et al.17 reported significant differences between autologous chondrocyte implantation and microfracture in terms of both absolute values and the degree of improvement of Lysholm scores. These differences are clinically relevant as the patient would be able to perceive the differences entailed with this outcome measure. Saris et al.5 reported significant differences between autologous chondrocyte implantation and microfracture in terms of the amount of improvement in KOOS scores at the time of the latest follow-up. However, the absolute difference in this clinical outcome measure (overall KOOS score, 78 compared with 75) precludes a statement of significantly better clinical outcomes as the patient would be unable to perceive a difference between the two different scores. Assessment of the methodological quality of a randomized controlled clinical trial via the Delphi list is appropriate. However, this simple, nine-question analysis may be too stringent for the evaluation of a prospective cohort, as three of the questions are directed at comparative groups within a randomized trial. As the table in the Appendix regarding the Delphi list illustrates, study quality has improved with later publication date. However, this finding simply may have been due to the increasing number of randomized comparative studies more recently and more prospective cohort studies earlier in the study analysis.
Multiple sources of heterogeneity within studies included in our review precluded the performance of a meta-analysis. With the different clinical outcome measures used in different studies, effect size was used as described. Effect size can summarize the result of a study based on various outcome measures. It allows numerically direct comparison of the results from different individual studies that used different outcome measurements. However, this analysis is limited by the quality of the data reported in each study. In the present study, some data used in our analysis were given in the text or tables of the original studies or the original data were provided by the authors of the studies. However, not all data could be obtained directly. Some data were directly measured from the figures in the papers or were roughly estimated from the tables by assigning a score to each item of categorical data, for example, by assigning a score of 80 to the category “good (70-90).” In these cases, the effect sizes may slightly differ if we could not use the original data. Nonetheless, most differences are small and will not markedly influence our conclusions. Our analysis describes the effect size on the basis of the data given.
To truly assess the efficacy and durability of autologous chondrocyte implantation in comparison with other techniques, future studies should attempt to limit the deficiencies mentioned within our discussion via proper and transparent subject enrollment with clearly stated inclusion and exclusion criteria; proper independently performed randomization techniques; no concurrent surgical interventions (anterior cruciate ligament reconstruction, realignment osteotomy, meniscal surgery, etc.); consistent surgical technique; longer clinical follow-up with an independent observer; the use of validated, responsive, and reliable outcome measures; and clear reporting of data with a statement of both clinical relevance and significance, as the two are not always coincident.
Conclusions
Cartilage repair and restoration with microfracture, autologous chondrocyte implantation, and osteochondral autograft has proven short-term and intermediate-term success. On the basis of our analysis, intermediate-term clinical outcomes after autologous chondrocyte implantation demonstrate a trend toward autologous chondrocyte implantation having improved outcomes as compared with microfracture but do not allow us to conclude that there is any difference between autologous chondrocyte implantation and osteochondral autograft transplant. Further, no differences in clinical outcomes were shown between first and second generation autologous chondrocyte implantation. Additional long-term follow-up with high-level evidence will be required to determine the degree of difference between autologous chondrocyte implantation and microfracture. Autologous chondrocyte implantation may be the best option for large defects in young, active patients with a short duration of symptoms and no previous cartilage surgery. Autologous chondrocyte implantation may provide a more durable repair tissue than microfracture, with preservation of clinical outcome success in the long term. Microfracture remains indicated for smaller defects in young, active patients. Osteochondral autograft may provide more rapid improvement in terms of clinical outcome than autologous chondrocyte implantation or microfracture but is limited by donor-site morbidity. The methodological quality of studies investigating these techniques has historically been poor but is improving. Additional high-quality studies will continue to provide evidence-based outcomes with which guidelines for the treatment of articular cartilage injury in the knee may be refined.
Appendix
Tables summarizing quality score criteria and presenting quality scores, demographic data, and outcomes for the individual studies are available with the electronic version of this article on our web site at jbjs.org (go to the article citation and click on “Supporting Data”).
Footnotes
Investigation performed at the Sports Medicine Center, The Ohio State University, Columbus, Ohio
Disclosure: The authors did not receive any outside funding or grants in support of their research for or preparation of this work. Neither they nor a member of their immediate families received payments or other benefits or a commitment or agreement to provide such benefits from a commercial entity.
References
- 1. Buckwalter J Mankin H. Articular cartilage. Part II: degeneration and osteoarthrosis, repair, regeneration, and transplantation. J Bone Joint Surg Am. 1997;79:612-32. [Google Scholar]
- 2. Alford JW Cole BJ. Cartilage restoration, part 1: basic science, historical perspective, patient evaluation, and treatment options. Am J Sports Med. 2005;33:295-306. [DOI] [PubMed] [Google Scholar]
- 3. Heir S Nerhus TK Røtterud JH Løken S Ekeland A Engebretsen L Arøen A. Focal cartilage defects in the knee impair quality of life as much as severe osteoarthritis: a comparison of knee injury and osteoarthritis outcome score in 4 patient categories scheduled for knee surgery. Am J Sports Med. 2010;38:231-7. [DOI] [PubMed] [Google Scholar]
- 4. Steadman JR Rodkey WG Briggs KK. Microfracture to treat full-thickness chondral defects: surgical technique, rehabilitation, and outcomes. J Knee Surg. 2002;15:170-6. [PubMed] [Google Scholar]
- 5. Saris DB Vanlauwe J Victor J Almqvist KF Verdonk R Bellemans J Luyten FP; TIG/ACT/01/2000&EXT Study Group. Treatment of symptomatic cartilage defects of the knee: characterized chondrocyte implantation results in better clinical outcome at 36 months in a randomized trial compared to microfracture. Am J Sports Med. 2009;37 Suppl 1:10S-9S. [DOI] [PubMed] [Google Scholar]
- 6. Mithoefer K McAdams T Williams RJ Kreuz PC Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med. 2009;37:2053-63. [DOI] [PubMed] [Google Scholar]
- 7. Knutsen G Drogset JO Engebretsen L Grøntvedt T Isaksen V Ludvigsen TC Roberts S Solheim E Strand T Johansen O. A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at 5 years. J Bone Joint Surg Am. 2007;89:2105-12. [DOI] [PubMed] [Google Scholar]
- 8. Kon E Gobbi A Filardo G Delcogliano A Zaffagnini S Marcacci M. Arthroscopic second-generation autologous chondrocyte implantation compared with microfracture for chondral lesions of the knee: prospective nonrandomized study at 5 years. Am J Sports Med. 2009;37:33-41. [DOI] [PubMed] [Google Scholar]
- 9. Hangody L Dobos J Baló E Pánics G Hangody LR Berkes I. Clinical experiences with autologous osteochondral mosaicplasty in an athletic population: a 17-year prospective multicenter study. Am J Sports Med. 2010;38:1125-33. [DOI] [PubMed] [Google Scholar]
- 10. Moseley JB Jr Anderson AF Browne JE Mandelbaum BR Micheli LJ Fu F Erggelet C. Long-term durability of autologous chondrocyte implantation: a multicenter, observational study in US patients. Am J Sports Med. 2010;38:238-46. [DOI] [PubMed] [Google Scholar]
- 11. Vasiliadis HS Danielson B Ljungberg M McKeon B Lindahl A Peterson L. Autologous chondrocyte implantation in cartilage lesions of the knee: long-term evaluation with magnetic resonance imaging and delayed gadolinium-enhanced magnetic resonance imaging technique. Am J Sports Med. 2010;38:943-9. [DOI] [PubMed] [Google Scholar]
- 12. Peterson L Vasiliadis HS Brittberg M Lindahl A. Autologous chondrocyte implantation: a long-term follow-up. Am J Sports Med. 2010;38:1117-24. [DOI] [PubMed] [Google Scholar]
- 13. Brittberg M Lindahl A Nilsson A Ohlsson C Isaksson O Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889-95. [DOI] [PubMed] [Google Scholar]
- 14. Marcacci M Kon E Zaffagnini S Filardo G Delcogliano M Neri MP Iacono F Hollander AP. Arthroscopic second-generation autologous chondrocyte implantation. Knee Surg Sports Traumatol Arthrosc. 2007;15:610-9. [DOI] [PubMed] [Google Scholar]
- 15. McCormick F Yanke A Provencher MT Cole BJ. Minced articular cartilage—basic science, surgical technique, and clinical application. Sports Med Arthrosc. 2008;16:217-20. [DOI] [PubMed] [Google Scholar]
- 16. Peterson L Brittberg M Kiviranta I Akerlund EL Lindahl A. Autologous chondrocyte transplantation. Biomechanics and long-term durability. Am J Sports Med. 2002;30:2-12. [DOI] [PubMed] [Google Scholar]
- 17. Basad E Ishaque B Bachmann G Sturz H Steinmeyer J. Matrix-induced autologous chondrocyte implantation versus microfracture in the treatment of cartilage defects of the knee: a 2-year randomized study. Knee Surg Sports Traumatol Arthrosc. 2010;18:519-27. [DOI] [PubMed] [Google Scholar]
- 18. Zeifang F Oberle D Nierhoff C Richter W Moradi B Schmitt H. Autologous chondrocyte implantation using the original periosteum-cover technique versus matrix-associated autologous chondrocyte implantation: a randomized clinical trial. Am J Sports Med. 2010;38:924-33. [DOI] [PubMed] [Google Scholar]
- 19. Obremskey W Pappas N Attallah-Wasif E Tornetta P 3rd Bhandari M. Level of evidence in orthopaedic journals. J Bone Joint Surg Am. 2005;87:2632-8. [DOI] [PubMed] [Google Scholar]
- 20. Altman RD Gold GE. Atlas of individual radiographic features in osteoarthritis, revised. Osteoarthritis Cartilage. 2007;15(Suppl A):A1-56. [DOI] [PubMed] [Google Scholar]
- 21. Schiphof D Boers M Bierma-Zeinstra SM. Differences in descriptions of Kellgren and Lawrence grades of knee osteoarthritis. Ann Rheum Dis. 2008;67:1034-6. [DOI] [PubMed] [Google Scholar]
- 22. Clar C Cummins E McIntyre L Thomas S Lamb J Bain L Jobanputra P Waugh N. Clinical and cost-effectiveness of autologous chondrocyte implantation for cartilage defects in knee joints: systematic review and economic evaluation. Health Technol Assess. 2005;9:1-101. [DOI] [PubMed] [Google Scholar]
- 23. Kon E Verdonk P Condello V Delcogliano M Dhollander A Filardo G Pignotti E Marcacci M. Matrix-assisted autologous chondrocyte transplantation for the repair of cartilage defects of the knee: systematic clinical data review and study quality analysis. Am J Sports Med. 2009;37 Suppl 1:156S-66S. [DOI] [PubMed] [Google Scholar]
- 24. Lubowitz JH Appleby D Centeno JM Woolf S Reid JB 3rd. The relationship between the outcome of studies of autologous chondrocyte implantation and the presence of commercial funding. Am J Sports Med. 2007;35:1809-16. [DOI] [PubMed] [Google Scholar]
- 25. Magnussen RA Dunn WR Carey JL Spindler KP. Treatment of focal articular cartilage defects in the knee: a systematic review. Clin Orthop Relat Res. 2008;466:952-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nakamura N Miyama T Engebretsen L Yoshikawa H Shino K. Cell-based therapy in articular cartilage lesions of the knee. Arthroscopy. 2009;25:531-52. [DOI] [PubMed] [Google Scholar]
- 27. Ruano-Ravina A Jato Díaz M. Autologous chondrocyte implantation: a systematic review. Osteoarthritis Cartilage. 2006;14:47-51. [DOI] [PubMed] [Google Scholar]
- 28. Wasiak J Clar C Villanueva E. Autologous cartilage implantation for full thickness articular cartilage defects of the knee. Cochrane Database Syst Rev. 2006;3:CD003323. [DOI] [PubMed] [Google Scholar]
- 29. Verhagen AP de Vet HC de Bie RA Kessels AG Boers M Bouter LM Knipschild PG. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol. 1998;51:1235-41. [DOI] [PubMed] [Google Scholar]
- 30. Coleman BD Khan KM Maffulli N Cook JL Wark JD. Studies of surgical outcome after patellar tendinopathy: clinical significance of methodological deficiencies and guidelines for future studies. Victorian Institute of Sport Tendon Study Group. Scand J Med Science Sports. 2000;10:2-11. [DOI] [PubMed] [Google Scholar]
- 31. Cowan J Lozano-Calderón S Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment of lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89:1693-9. [DOI] [PubMed] [Google Scholar]
- 32. Jakobsen R Engebretsen L Slauterbeck JR. An analysis of the quality of cartilage repair studies. J Bone Joint Surg Am. 2005;87:2232-9. [DOI] [PubMed] [Google Scholar]
- 33. Tallon C Coleman BD Khan KM Maffulli N. Outcome of surgery for chronic Achilles tendinopathy. A critical review. Am J Sports Med. 2001;29:315-20. [DOI] [PubMed] [Google Scholar]
- 34. Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed Hillsdale, NJ: Lawrence Erlbaum Assoc; 1988. [Google Scholar]
- 35. Rosnow R Rosenthal R. Computing contrasts, effect sizes, and counternulls on other people’s published data: general procedures for research consumers. Psych Meth. 1996;1:331-40. [Google Scholar]
- 36. Hedges L Olkin I. Statistical methods for meta-analysis. New York, NY: Academic Press; 1985. [Google Scholar]
- 37. Knutsen G Engebretsen L Ludvigsen TC Drogset JO Grøntvedt T Solheim E Strand T Roberts S Isaksen V Johansen O. Autologous chondrocyte implantation compared with microfracture in the knee. J Bone Joint Surg Am. 2004;86:455-64. [DOI] [PubMed] [Google Scholar]
- 38. Saris DB Vanlauwe J Victor J Haspl M Bohnsack M Fortems Y Vandekerckhove B Almqvist KF Claes T Handelberg F Lagae K van der Bauwhede J Vandenneucker H Yang KG Jelic M Verdonk R Veulemans N Bellemans J Luyten FP. Characterized chondrocyte implantation results in better structural repair when treating symptomatic cartilage defects of the knee in a randomized controlled trial versus microfracture. Am J Sports Med. 2008;36:235-46. [DOI] [PubMed] [Google Scholar]
- 39. Bartlett W Skinner JA Gooding CR Carrington RW Flanagan AM Briggs TW Bentley G. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomized study. J Bone Joint Surg Br. 2005;87:640-5. [DOI] [PubMed] [Google Scholar]
- 40. Dozin B Malpeli M Cancedda R Bruzzi P Calcagno S Molfetta L Priano F Kon E Marcacci M. Comparative evaluation of autologous chondrocyte implantation and mosaicplasty: a multicenter randomized clinical trial. Clin J Sports Med. 2005;15:220-6. [DOI] [PubMed] [Google Scholar]
- 41. Ferruzzi A Buda R Faldini C Vannini F Di Caprio F Luciani D Giannini S. Autologous chondrocyte implantation in the knee joint: open compared with arthroscopic technique. Comparison at a minimum follow-up of five years. J Bone Joint Surg Am. 2008;90:90-101. [DOI] [PubMed] [Google Scholar]
- 42. Horas U Pelinkovic D Herr G Aigner T Schnettler R. Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint. A prospective, comparative trial. J Bone Joint Surg Am. 2003;85:185-92. [DOI] [PubMed] [Google Scholar]
- 43. Gooding CR Bartlett W Bentley G Skinner J Carrington R Flanagan A. A prospective, randomized study comparing two techniques of autologous chondrocyte implantation for osteochondral defects in the knee: periosteum covered versus type I/III collagen covered. Knee. 2006;13:203-10. [DOI] [PubMed] [Google Scholar]
- 44. Basad E Sturz H Steinmeyer J. Die behandlung chondraler Defekte mit MACI oder Microfracture-erste Ergebnisse einer vergleichenden klinischen Studie. Orthopadische Praxis. 2004;40:6-10. [Google Scholar]
- 45. Bekkers J Inklaar M Saris DB. Treatment selection in articular cartilage lesions of the knee: a systematic review. Am J Sports Med. 2009;37 Suppl 1:148S-55S. [DOI] [PubMed] [Google Scholar]
- 46. Vasiliadis HS Wasiak J Salanti G. Autologous chondrocyte implantation for the treatment of cartilage lesions of the knee: a systematic review of randomized studies. Knee Surg Sports Traumatol Arthrosc. 2010 Feb 2 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 47. Gikas P Morris T Carrington R Skinner J Bentley G Briggs T. A correlation between the timing of biopsy after autologous chondrocyte implantation and the histological appearance. J Bone Joint Surg Br. 2009;91:1172-7. [DOI] [PubMed] [Google Scholar]
- 48. Brittberg M. Cell carriers as the next generation of cell therapy for cartilage repair: a review of the matrix-induced autologous chondrocyte implantation procedure. Am J Sports Med. 2010;38:1259-71. [DOI] [PubMed] [Google Scholar]
- 49. Henderson IJ LaValette DP. Subchondral bone overgrowth in the presence of full-thickness cartilage defects in the knee. Knee. 2005;12:435-40. [DOI] [PubMed] [Google Scholar]
- 50. Shapiro F Koide S Glimcher MJ. Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1993;75:532-53. [DOI] [PubMed] [Google Scholar]
- 51. Brown WE Potter HG Marx RG Wickiewicz TL Warren RF. Magnetic resonance imaging appearance of cartilage repair in the knee. Clin Orthop Relat Res. 2004;422:214-23. [DOI] [PubMed] [Google Scholar]
- 52. Mithoefer K Williams RJ 3rd Warren RF Potter HG Spock CR Jones EC Wickiewicz TL Marx RG. The microfracture technique for the treatment of articular cartilage lesions in the knee. A prospective cohort study. J Bone Joint Surg Am. 2005;87:1911-20. [DOI] [PubMed] [Google Scholar]
- 53. Potter HG Chong le R. Magnetic resonance imaging assessment of chondral lesions and repair. J Bone Joint Surg Am. 2009;91 Suppl 1:126-31. [DOI] [PubMed] [Google Scholar]
- 54. CONSORT. Randomization: generation; allocation concealment. CONSORT Transparent Reporting of Trials 2009. http://www.consort-statement.org/index.aspx?o=1025. Accessed 2009 Dec 1.
- 55. Minas T Gomoll AH Rosenberger R Royce RO Bryant T. Increased failure rate of autologous chondrocyte implantation after previous treatment with marrow stimulation techniques. Am J Sports Med. 2009;37:902-8. [DOI] [PubMed] [Google Scholar]
- 56. Best J. Damned lies and statistics: untangling numbers from the media, politicians, and activists. Berkeley, CA: University of California Press; 2001. [Google Scholar]
- 57. Koh JL Kowalski A Lautenschlager E. The effect of angled osteochondral grafting on contact pressure: a biomechanical study. Am J Sports Med. 2006;34:116-9. [DOI] [PubMed] [Google Scholar]
- 58. Koh JL Wirsing K Lautenschlager E Zhang LO. The effect of graft height mismatch on contact pressure following osteochondral grafting: a biomechanical study. Am J Sports Med. 2004;32:317-20. [DOI] [PubMed] [Google Scholar]
- 59. Hambly K Griva K. IKDC or KOOS? Which measures symptoms and disabilities most important to postoperative articular cartilage repair patients? Am J Sports Med. 2008;36:1695-704. [DOI] [PubMed] [Google Scholar]