Abstract
Objective:
To summarize the multi-specialty strategy and initial guidelines of a Case Review Committee in triaging oncologic surgery procedures in a large Comprehensive Cancer Center and to outline current steps moving forward after the initial wave.
Summary of Background Data:
The impetus for strategic rescheduling of operations is multifactorial and includes our societal responsibility to minimize COVID-19 exposure risk and propagation among patients, the healthcare workforce, and our community at large. Strategic rescheduling is also driven by the need to preserve limited resources. As many states have already or are considering to re-open and relax stay-at-home orders, there remains a continued need for careful surgical scheduling because we must face the reality that we will need to co-exist with COVID-19 for months, if not years.
Methods:
The quality officers, chairs, and leadership of the 9 surgical departments in our Division of Surgery provide specialty-specific approaches to appropriately triage patients.
Results:
We present the strategic approach for surgical rescheduling during and immediately after the COVID-19 first wave for the 9 departments in the Division of Surgery at The University of Texas MD Anderson Cancer Center in Houston, Texas.
Conclusions:
Cancer surgeons should continue to use their oncologic knowledge to determine the window of opportunity for each surgical procedure, based on tumor biology, preoperative treatment sequencing, and response to systemic therapy, to safely guide patients through this cautious recovery phase.
Keywords: case selection, coronavirus, pandemic, peak, triage
The initial onset of the coronavirus disease 2019 (COVID-19) pandemic forced cancer surgeons to make challenging decisions regarding the appropriate delay of potentially curative “elective” operations. However, “elective” cancer operations, whereas not “emergent,” have oncologic windows of opportunity that depend on tumor biology, treatment sequencing, and response to systemic therapy, and do not last indefinitely. There is a societal responsibility to balance the time pressures of individual oncologic surgical care against the societal goal of continued COVID-19 mitigation strategies, especially in the context of varied regional economic re-openings which began April 24. Herein, we present the strategic approach for surgical rescheduling during and immediately after the COVID-19 first wave for the 9 departments in the Division of Surgery at The University of Texas MD Anderson Cancer Center, in Houston, Texas.
INSTITUTIONAL PREPAREDNESS AND CREATION OF CASE REVIEW COMMITTEE
On March 4, 2020, our institution restricted employee travel and instituted intensive planning to reduce COVID-19 spread in our region and prepare to care for any potential surge. Each department within the Division of Surgery voluntarily evaluated scheduled operations and postponed them when oncologically reasonable.1 Rescheduling drastically cut the weekly operative volume from 460 operations during the week of March 8, to 258 the next week (Fig. 1). By the week of April 19, only 90 operations were scheduled, marking an 80% drop in usual volume. Downstream activity including clinic volume and inpatient census fell as well, allowing social distancing strategies inside the hospital. The Department of Surgical Oncology inpatient rounding list for 36 faculty members typically includes 60 to 75 patients. On April 10, this list had 5 patients. As of April 30, we are back to 16 inpatients.
FIGURE 1.
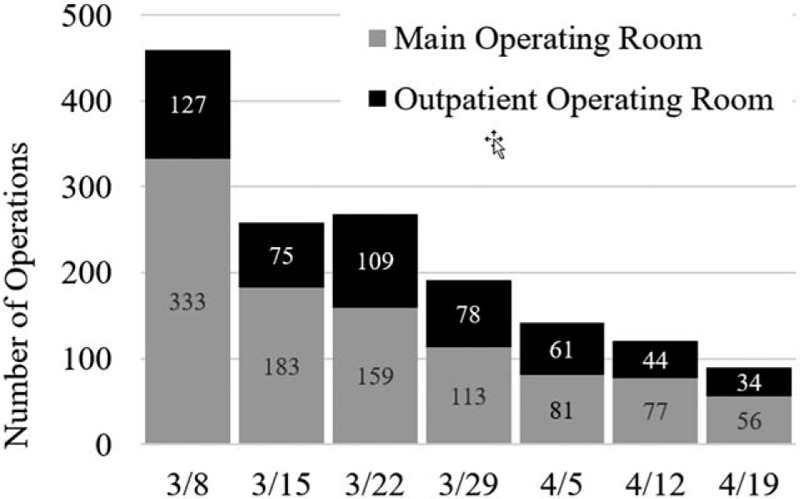
Total surgical case volume by week during early COVID-19 response, in which MD Anderson Cancer Center implemented goals to create a “moat” around hospitalized patients, to reduce workforce and visitor traffic, and to limit “elective” cases.
As recommended by national societies, a multispecialty, interdisciplinary Case Review Committee was created from 9 departmental quality officers and Division of Surgery leadership.2 Every afternoon, the Case Review Committee evaluated all scheduled operations and provided recommendations to departmental quality officers who had reviewed their faculty's cases in the morning, regarding which operations should proceed as scheduled and which should be postponed. In performing this work, the Committee balanced the competing requirements of patient safety and timely care, workforce protection and preservation, appropriate and limited exposure of trainees, limiting the need for transfusions, conserving personal protective equipment and critical care equipment, and preserving hospital ward and intensive care unit (ICU) capacity.
When the Case Review Committee was created on March 24, COVID-19 test kits were rare in the U.S.,3 and there were many unknowns in perioperative COVID-19 risk, including the prevalence of COVID-19 in our surgical patients (and visitors accompanying them) and in the workforce through community spread. There was an unknown postoperative mortality risk of operating unknowingly on a COVID-19 patient. Early case reports from China reported astoundingly high postoperative death rates of 20%,4 compared to contemporary 90-day mortality expectations of <1% in our institution. Healthcare providers have been exposed as well with notably age-correlated hospitalization rates of 5%–20% and death rates of 0.1%–4% in providers with documented COVID-19 infections.5 The Case Review Committee advised the individual surgeons on the potential consequences to the patient and hospital system across a spectrum of potential postoperative outcomes related to estimated transfusion needs, potential ICU need, and total hospital stay, all of which could potentially limit hospital capacity while preparing for a potential surge similar to New York City and northern Italy.6 As we enter May, with Texas re-opening for limited business, the Case Review Committee continues to review cases and adapt to a limited re-opening of our operating rooms.
COVID-19 MITIGATION STRATEGIES
As a cancer center with many immunocompromised and elderly patients, the institution created a “moat” to protect our uniquely vulnerable patients from excess hospital foot traffic.7 Based on early case reports, cancer patients with COVID-19 used greater hospital resources including ICU beds with higher mortality rates than the general population.7 To protect all parties, the institution moved quickly to 5-person limits on meetings (with 6 feet distance) utilizing virtual platforms almost exclusively (including fellowship interviews),8 and visitor restrictions culminating in a no-visitor policy on March 24, which will be continued indefinitely even as the state re-opens. Finally, as the pandemic affected surrounding states, a mandatory 14-day home quarantine for all patients traveling to MD Anderson Cancer Center (MDACC) from outside Texas was instituted, COVID-19 testing in a nonclinic building for out-of-state patients upon arrival on campus. Surgical trainees were no longer allowed to “double scrub” to limit exposure risk.9 Surgical departments moved toward rotational team-based care with “active duty” advanced practice providers, trainees, and faculty, with “reserve duty” counterparts encouraged and equipped to work remotely. Enhanced recovery protocols safely reduced hospital stays. Minimally invasive operations, with their known early discharge benefits, were part of this equation but with a balance taking into account avoiding longer (eg, robotic) operations which could be accomplished open or laparoscopically with less operating room utilization.
DEVELOPMENT OF DEPARTMENTAL GUIDELINES
After each department postponed elective cases and cases in which delay was oncologically appropriate, department quality officers, section chiefs, and chairpersons then developed internal guidelines for scheduling cases for the pre-peak period (April) and more importantly the post-peak period (May to summer).
Neurosurgery
The Department of Neurosurgery established a review board composed of 3 senior faculty.10 Preference was given to patients requiring urgent interventions and patients who would benefit most from surgical intervention, particularly newly diagnosed patients without pathologic verification of disease, younger patients who were considered less likely to be negatively impacted by COVID-19, and in-state patients who did not require a 14-day home quarantine. A separate faculty group reviewed all scheduled cases for oncologic necessity. This review group considered the aforementioned parameters and the status of systemic disease, prognosis, risk of neurologic deficit, possibility of nonsurgical treatment, and risk of progression to “unresectable” disease or development of an emergency situation during the initial wave.
Pituitary surgery was delayed in the early pre-peak period because of the increased risk associated with airway-related surgery. In addition, awake surgery was discouraged because of the theoretical risk of exposure for the anesthesia team and the staff assisting with intraoperative language assessments. Most of the cranial operations that were approved were for large malignant gliomas or large metastases that caused mass effect and progressive symptoms and neurologic deficits, including unremitting seizures despite use of multiple anticonvulsants. Patients with newly diagnosed intrinsic tumors or initial presentation with metastases were more commonly operated on than were patients with multiply recurrent tumors. Spine procedures were approved if patients had progressive neurologic deficit, severe unremitting pain from tumor involvement and nerve compression, or significant canal compromise with impending neurologic catastrophic symptoms.
As we re-open our operating room capacity, here are the priorities within Neurosurgery. Top priority patients remain those with large masses, progressive neurologic decline, severe pain, no nonsurgical options, or when diagnosis via surgery is required to initiate therapy. The next priority is posting previously deferred patients for whom no additional therapy was recommended but for whom surgery is required. In contrast, patients recommended to proceed with other nonsurgical therapy will be re-staged as indicated before re-scheduling. The third priority includes newly diagnosed patients with unbiopsied suspected malignant disease or those with diagnosis post-biopsy and requiring definitive resection. Also in this third priority are new patients with benign disease with pain, debilitating symptoms, radiographic evidence of brain(stem) compression, midline shift, ventriculomegaly, and spinal cord compression. The fourth priority includes patients with recurrent disease for whom surgery is indicated for cytoreduction, to obtain a diagnosis for clinical trial enrollment or adjuvant treatment, and symptom relief from mass effect.
Head and Neck Surgery
The Department of Head and Neck Surgery developed treatment and management guidelines by disease sites based on urgency as related to patient health, safety of healthcare personnel, and curative intent.11,12 Resection of tumors along mucosal surfaces of the upper aerodigestive tract increases the risk of aerosolization of COVID-19 virus particles, especially from the oral cavity, oropharynx, nasopharynx, larynx/hypopharynx, and paranasal sinuses and skull base.13 Thus, the guidelines developed by the department emphasized surgical treatment of intermediate-stage or advanced disease for which nonsurgical options were not available and disease progression would significantly affect patient function or disease outcome. Dental surgery and prosthodontic procedures performed in conjunction with head and neck operations or to prepare patients for adjuvant therapy were continued.
Salivary gland neoplasms and sarcomas were managed according to histologic grade: slow-growing low-grade and intermediate-grade disease was monitored, but high-grade carcinomas were resected. For salivary ductal carcinoma and carcinoma ex pleomorphic adenoma, neoadjuvant chemotherapy was considered. Similarly, neoadjuvant chemotherapy was considered for high-grade soft tissue sarcomas, but osteosarcomas were resected. Endocrine surgery proceeded for high-acuity situations, including progressive and biologically aggressive disease, such as anaplastic thyroid cancer and parathyroid carcinoma. Ophthalmologic surgery proceeded for higher-grade malignancies (eg, retinoblastoma, melanoma, choroidal metastasis) and diseases threatening sight or life.
Thoracic Surgery
The majority of thoracic oncology procedures, including resections of the lung parenchyma, airway, and esophagus, are considered aerosolizing procedures.14 Moreover, the preoperative tests for staging and quantifying pulmonary reserve (eg, bronchoscopy, endobronchial ultrasound, and pulmonary function tests) are also aerosolizing. This creates the dilemmas of whether or not to proceed with surgery in the absence of testing that might otherwise be considered standard of care. Further complicating decision-making is that substantial proportions of patients with primary lung and esophageal malignancies have comorbidities that render them at high risk for worse outcomes if they, unknowingly, are infected with COVID-19 perioperatively, including older age, smoking history, and concomitant cardiopulmonary disease. Another important consideration is that most thoracic oncologic procedures are operations for which there is a low but realistic potential for significant blood loss and need for postoperative ICU admission.
The following approach was decided. During the time of the initial wave up to our predicted late April/early May Texas peak, when few patients with COVID-19 were in the hospital and the majority of our workforce remained healthy, resection proceeded for patients with non-small cell lung cancer with predominantly solid appearance, especially patients with tumor stage of T1c or greater or positive nodes, and patients who completed induction therapy for lung or esophageal cancer, patients with chest wall tumors of high malignant potential, and patients with symptomatic thoracic malignancies. During the initial wave, deferral of resection was strongly considered for patients with predominantly ground glass nodules; small, minimally invasive thymomas; small, node-negative lung cancers; and well-differentiated carcinoids. For many patients with pulmonary metastatic disease, surgery was delayed or interval systemic therapy was offered, depending on tumor histology, location, and size. For patients with early-stage lung cancer, stereotactic radiation therapy was considered, with the caveat that it was also important to reduce hospital traffic for radiation oncology as well.
As we move beyond the first wave, repeat cross-sectional imaging can verify resectability and confirm lack of progression from previous clinical staging. As we slowly open up operative capacity, it will be of great importance to prioritize operative resources for non-small cell lung cancer and esophageal cancer. Diseases like thymoma and slow-growing ground glass lung nodules will continue to be suitable to delay until we see more clearly beyond the first wave.
Surgical Oncology
Because the Department of Surgical Oncology and MD Anderson Cancer Center have traditionally favored neoadjuvant therapy for many solid tumors, we strategically initiated or continued this treatment sequencing when possible to postpone surgery to beyond the late April peak of COVID-19 incidence in the Houston area. Each disease site group continues to formally review new patients to reach consensus regarding treatment plans even before patients take the risk of traveling to our institution. Patients with localized disease with potential for cure (eg, stage II colon cancer) and no indication for chemotherapy proceed to the operating room. Patients needing extensive gastrointestinal surgery, such as Whipple procedure for pancreatic adenocarcinoma, major hepatectomy for colorectal liver metastases, and retroperitoneal sarcomas, are carefully reviewed to balance the risks of delaying surgery versus excessive chemotherapy causing organ damage or performance status decline. However, with our extensive experience with neoadjuvant therapy, we are selectively extending neoadjuvant chemotherapy or chemoradiation, which pushes the surgery out another 2 months for many patients with gastrointestinal cancers, including cancers of the pancreas, stomach, and rectum, and liver metastases. Specific guidelines regarding selection and prioritization for each disease site have been outlined by several institutions and surgical societies.15–18
Gynecologic Oncology
Patients with pre-invasive disease and patients with genetic syndromes such as BReast CAncer (BRCA) mutations or Lynch syndrome who need risk-reduction surgery had their surgical procedures postponed beyond our late April peak. New patients with advanced ovarian cancer were triaged to neoadjuvant chemotherapy because data from phase III trials show equivalent survival for surgery and neoadjuvant chemotherapy.19 Patients with grade 1 endometrial cancer without deep myometrial invasion and no evidence of metastatic disease are being treated with progestin therapy.20
Patients considered to require surgery even in the initial wave include those with stage IB cervical cancer who are candidates for radical hysterectomy with low risk of needing adjuvant radiotherapy, patients with grade 2–3 endometrioid endometrial cancer, and patients with type 2 histologies with no evidence of metastatic disease.
A number of areas were considered “gray areas” and still require individual case review. Ovarian cancer patients with significant radiographic and tumor marker response after 3–4 cycles of neoadjuvant chemotherapy are considered for interval cytoreductive surgery if they have good performance status. Others are re-evaluated after additional chemotherapy. For patients with stage IA cervical cancer, patients who have had a conization with negative margins are generally having surgery postponed, and patients who have not had a conization are recommended to have outpatient cervical conization. For patients with stage IA2 cervical cancer with positive margins, we are considering immediate radical hysterectomy, but delayed radical hysterectomy is probably safe as well. Patients with a solitary adnexal/pelvic mass are evaluated with imaging and tumor markers and discussed at a multi-disciplinary conference to decide on surgery versus close surveillance and delayed surgery.
As of late April/early May, we are prioritizing patients with invasive cancers whose operations were delayed from April. Specifically, previously delayed early stage, low grade endometrial cancers, and solitary pelvic masses are now being scheduled. Additionally, advanced stage ovarian cancers that have received neoadjuvant chemotherapy and approaching their third or fourth cycle are being scheduled for their interval cytoreductive surgery if they have good response. During the first wave, newly diagnosed advanced ovarian cancers were almost exclusively being triaged to neoadjuvant chemotherapy. Now, we will be assessing them for upfront cytoreduction based on our operating room capacity and hospital resource utilization.
Urologic Oncology
For Urologic Oncology, 3 tiers of triage for case selection were created: “elective,” “move if possible,” and “urgent.” This guidance was used to evaluate existing operations until May 11.21 Cases in the middle tier and the highest urgent tier are evaluated weekly taking into account current hospital COVID-19 census and existing personal protective equipment (PPE) and related resources. The first (elective) tier included prostate cancer with low to favorable risk or patients already being treated with systemic therapy. Second tier (moved if possible) included unfavorable to high risk patients, especially those already scheduled for resection. Testis cancer was considered highest (urgent) tier if primary orchiectomy was required to start postoperative therapy or if resection of a residual mass with retroperitoneal lymphadenectomy was needed after neoadjuvant chemotherapy.
For kidney cancer, elective tier patients include those with masses <4 cm and those needing cytoreductive nephrectomy to undergo systemic therapy. Second tier kidney cancers included large masses without thrombus, those who are still <12 weeks from their final dose neoadjuvant chemotherapy, and those who can safely start chemotherapy to delay the need for surgery. Finally, the urgent tier included patients with renal vein or vena cava thrombus or patients with high grade disease after chemotherapy or those not candidates for chemotherapy.
For bladder cancer, operations which can wait include nonmuscle invasive cancer, muscle invasive cancer on chemotherapy, endoscopies for recurrence or while on chemotherapy, and diagnostic upper tract endoscopies. Patients in the mid-tier who can be delayed include those needing transurethral resection if the diagnosis is already established or those whose tissue biopsy is not needed to start chemotherapy. The urgent tier includes radical cystectomy within a 12-week time limit after chemotherapy and transurethral resection for high-grade pT1 tumors to determine intravesicular therapy versus cystectomy. True emergencies continue to include stents for pyelonephritis and refractory hematuria.
Orthopedic Oncology
Sarcomas are rare tumors that require multidisciplinary care best delivered at specialized sarcoma centers. Operations (particularly for spine and pelvic sarcomas) often require tremendous resources involving many specialists, significant transfusion volumes, and prolonged stays in intensive care, inpatient units, and rehabilitation centers. The decisions regarding extensive operations continue to be carefully reviewed by faculty at a weekly conference and then by the chair and departmental quality officer. All elective, nonurgent orthopedic operations, including those for benign diseases were postponed until elective operations were allowed in Texas on April 22. Priority was given to stabilization of lower extremity fractures and impending fractures, when bracing and activity modifications would be ineffective. We recommended preoperative radiotherapy for radiosensitive sarcomas, impending pathologic fractures, and metastatic epidural spinal cord compression, whenever feasible. We continue to recommend utilizing novel devices to decrease/contain aerosolized particles (ie, osteotomes and gigli saws instead of high-speed drills and saws; intubation boxes; and clear plastic enclosures while using high speed drills and saws).
Breast Surgical Oncology
Departmental consensus guidelines were developed balancing timing of surgery with likely oncologic outcome and availability of systemic therapy and informed by national recommendations.16,22,23 Patients proceeded to surgery if delay was associated with adverse outcome and no alternative treatments were available. These diagnoses included triple negative and inflammatory breast cancer after neoadjuvant chemotherapy, soft tissue sarcomas, and tumors with progression despite chemotherapy. Postponing surgery was recommended for benign diagnoses including atypia, prophylactic risk-reduction, ductal carcinoma in situ, and early-stage estrogen receptor (ER)-positive breast cancer treatable with neoadjuvant endocrine therapy. Less clear-cut situations were discussed daily for departmental recommendation, such as ER-negative, human epidermal growth factor receptor-positive disease after neoadjuvant chemotherapy, and ER-positive breast cancers in premenopausal women or after neoadjuvant chemotherapy for advanced disease.
Looking ahead to the recovery phase, breast surgical cases represent a high volume with low likelihood for utilization of significant hospital resources and capacity. Case prioritization for re-opening the operating room inversely followed the consensus guidelines for delay during the COVID-19 pandemic surge. The first priority are patients with invasive cancer diagnoses where surgery was postponed from April, followed by patients with ductal carcinoma in situ. In the first month after the peak, we will continue to postpone surgery for benign conditions and prophylactic surgery.
Plastic Surgery
Many reconstructions are performed quickly, with little or no hospital stay, transfusions, or intensive care, and relatively low PPE depletion. All non-emergent/urgent operations (e.g., delayed breast reconstruction, revisions, elective hernias, etc) were postponed starting in late March and continue to be delayed as the state cautiously re-opens. However, any immediate reconstruction that prevents/reduces major functional deformity and/or minimizes risk of major medical complications is considered “medically necessary,” and is proceeding.
Many head and neck resections require free flap reconstruction and were not delayed. Oncoplastic breast reconstruction after lumpectomy was permitted, as was placement of a tissue expander, implant, and/or acellular dermal matrix after mastectomy. However, contralateral symmetry procedures were delayed in March/April patients, but were allowed starting the week of April 27. Immediate autologous flap reconstruction after mastectomy was not allowed in April, but was allowed starting May 1. Autologous flap reconstructions elsewhere in the body were always permitted for coverage of exposed hardware, bone, and vital organs and structures. As we see beyond the first peak with improved clarity, the institution is allowing previously postponed reconstructions and revisions to be posted.
DISCUSSION
After the First Wave: Peak Versus Plateau
By flattening the curve with social distancing and forming the “moat” around our cancer hospital, our PPE and testing kits are very slowly catching up as of late April/early May. Preoperative COVID-19 testing remains mandatory, but the unknown false negative rate remains a reality, given the reported high rates of asymptomatic COVID-19 carriers. The prospect of contracting the virus in the weeks and months ahead, even on the downslope of the initial peak, or in a controlled plateau, or in secondary waves this year and next year, remains an impediment toward returning to pre-COVID-19 hospital practices. No-visitor policies will continue until we can ensure visitors are COVID-19-negative, thereby limiting family support for our postoperative patients, especially patients with greater needs (eg, pediatric, elderly, disabled, immunosuppressed). Serologic testing is a priority of research teams here and across the world, but with an unknown promise of immunity even among previously infected patients. We currently allow usage of our limited supply of N95 masks for certain high-risk exposure situations (eg, head and neck surgery, intubations, etc), but this will remain a concern for the perioperative workforce involved in putatively “low-moderate” exposure risk scenarios (eg, abdominal surgery).
Moving Forward With Surgical Cancer Care
We must face the reality that we will need to co-exist with COVID-19 for months, if not years. Our institution is likely similar to the majority of healthcare systems in the U.S. in that we are starting a cautious recovery process, slowly relaxing the restrictions detailed above in late April/early May. This recovery process includes daily assessment of the inpatient census (including suspected and confirmed COVID-19 patients), updated city/state COVID-19 incidence, optimizing testing and tracing capabilities, PPE burn rate, and workforce health/availability.
Through early mitigation strategies and cooperation within the Texas Medical Center, we avoided overloaded hospitals, but “business as usual” seems like both a distant memory and a faraway dream as local and state governments tiptoe into re-openings. As we transition from surge planning (which has been laid out and is ready for any second wave) toward re-opening business and society in a country without universal testing and case tracking,3 we propose a few practices that can help us move forward in the initial COVID-19 recovery period (May – summer 2020).
Remote Work and Virtual Visits
Remote work to protect the workforce and virtual visits for preoperative and postoperative patients are current practice and will remain necessary until we have effective treatments, a vaccine, or herd immunity.
Extra Precautions During Procedures
The surgical timeout now incorporates COVID-19 risks. Universal precautions should be employed during any procedure that entails COVID-19 aerosol risks. Personnel not needed to intubate a patient should leave the room and spare themselves the exposure risk. PPE should be distributed to personnel at high risk for exposure for all cases.24
Testing
Having both diagnostic and confirmatory tests could inform surgeons as to when an acute infection has resolved, so that cancer therapy or surgery planning can be resumed. A preoperative COVID-19 test to rule out infection has become as routine as a type and screen the day before surgery. Increasing the sensitivity and reducing the result time will increase confidence in the result and allow for less disruption to normal morning start times. Use of swab testing, serologic antibody testing, and even chest computed tomography could be the combination needed to inject confidence towards a return to normalcy.
Documenting the Patient's Wishes
Advanced care planning and documentation of goals of care should be required for all cancer patients. In the current and future COVID-19 era, knowing a patient's wishes in case they develop COVID-19 organ failure while undergoing cancer treatment is mandatory.
Strategic Operating Room Scheduling
Operation timing can be more carefully planned rather than bending to arbitrary surgeon preferences, especially because most surgeons are no longer tethered to heavy clinic and operating block days. Development of a collective strategy to prioritize previously delayed operations began on April 27. Our regional hospital cases were centralized to our main campus to consolidate resources and for COVID-19 testing. To balance hospital resources, operations can be distributed throughout the week (including weekends) to plan adequate but not excessive (to continue social distancing) daily staffing for the operating rooms, clinics, and inpatient wards.
CONCLUSIONS
Cancer surgeons can use their knowledge of tumor biology to schedule surgery appropriately for cancer patients at high risk for COVID-19 infections and sequelae, whereas fulfilling the societal responsibility to reduce COVID-19 dissemination. We hope that the early experience we have presented here will be useful to other cancer surgeons looking for disease-specific guidance for the remainder of this spring, for a potential second wave this summer or next year, and for future unforeseen crises that may strain our healthcare systems.
Acknowledgments
The authors acknowledge and thank Charles E. Butler, MD; Kelly K. Hunt, MD; Rosa Hwang, MD; Karen H. Lu, MD; and Neema Navai, MD; for helping create and review departmental guidelines. We thank Yujiro Nishioka, MD, for technical editing. We thank Stephanie Deming, MLS, for her scientific editorial review.
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1. Wick EC, Pierce L, Conte MC, et al. Operationalizing the Operating Room: Ensuring Appropriate Surgical Care in the Era of COVID-19. Ann Surg. 2020 May 1. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. American College of Surgeons. Create a Surgical Review Committee for COVID-19-Related Surgical Triage Decision Making. 2020. Available at: https://www.facs.org/covid-19/clinical-guidance/review-committee. Accessed April 16, 2020. [Google Scholar]
- 3. Dewan A, Petterson H, Croker N. As Governments Fumbled Their Coronavirus Response, These Four Got it Right. Here's how. 2020. Available at: https://www.cnn.com/2020/04/16/world/coronavirus-response-lessons-learned-intl/index.html. Accessed April 16, 2020. [Google Scholar]
- 4.Lei S, Jiang F, Wating S, et al. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. EClinicalMedicine 2020; 100331.Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.CDC COVID-19 Response Team. Characteristics of health care personnel with COVID-19 — United States, February 12–April 9. MMWR Morb Mortal Wkly Rep 2020; 69:477–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mazzaferro V, Danelli P, Torzilli G, et al. A Combined Approach to Priorities of Surgical Oncology During the COVID-19 Epidemic. Ann Surg. 2020 May 1. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol 2020; 21:335–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Day R, Taylor B, Bednarski B, et al. Virtual Interviews for Surgical Training Program Applicants During COVID-19: Lessons Learned and Recommendations. Ann Surg. 2020 May 20. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zarzaur BL, Stahl CC, Greenberg JA, et al. Blueprint for restructuring a department of surgery in concert with the health care system during a pandemic: the University of Wisconsin Experience. JAMA Surg 2020; Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 10.Burke JF, Chan AK, Mummaneni V, et al. Letter: the coronavirus disease 2019 global pandemic: a neurosurgical treatment algorithm. Neurosurgery 2020; Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MD Anderson Head and Neck Surgery Treatment Guidelines Consortium. Head and neck surgical oncology in the time of a pandemic: Subsite-specific triage guidelines during the COVID-19 pandemic. Head Neck 2020; Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiong KL, Guo T, Yao CMKL, et al. Changing practice patterns in head & neck oncologic surgery in the early COVID-19 era. Head Neck 2020; Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 13.Givi B, Schiff BA, Chinn SB, et al. Safety recommendations for evaluation and surgery of the head and neck during the COVID-19 pandemic. JAMA Otolaryngol Head Neck Surg 2020; Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 14.Thoracic Surgery Outcomes Research Network Inc. COVID-19 guidance for triage of operations for thoracic malignancies: a consensus statement from thoracic surgery outcomes research network. Ann Thorac Surg 2020; Apr 4;S0003-4975(20)30442-2. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartlett DL, Howe JR, Chang G, et al. Management of cancer surgery cases during the COVID-19 pandemic: considerations. Ann Surg Oncol 2020; 27:1717–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. American College of Surgeons. COVID-19: Elective Case Triage Guidelines for Surgical Care. 2020. Available at: https://www.facs.org/-/media/files/covid19/guidance_for_triage_of_nonemergent_surgical_procedures.ashx. Accessed April, 16, 2020. [Google Scholar]
- 17.Brindle ME, Doherty G, Lillemoe K, et al. Approaching surgical triage during the COVID-19 pandemic. Ann Surg 2020; May 1. doi: 10.1097/SLA.0000000000003992. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tzeng CW, Tran Cao HS, Roland CL, et al. Surgical decision-making and prioritization for cancer patients at the onset of the COVID-19 pandemic: a multidisciplinary approach. Surg Oncol. 2020;34:182–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vergote I, Trope CG, Amant F, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med 2010; 363:943–953. [DOI] [PubMed] [Google Scholar]
- 20.Shalowitz DI, Epstein AJ, Buckingham L, et al. Survival implications of time to surgical treatment of endometrial cancers. Am J Obstet Gynecol 2017; 216:268.e1–268.e18. [DOI] [PubMed] [Google Scholar]
- 21.Ficarra V, Novara G, Abrate A, et al. Urology practice during COVID-19 pandemic. Minerva Urol Nefrol 2020; Mar 23. doi: 10.23736/S0393-2249.20.03846-1. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 22. Oncology SoS. COVID-19 Resources: Disease-Site Specific Management Resources. 2020. Available at: https://www.surgonc.org/resources/covid-19-resources/. Accessed April 16, 2020. [Google Scholar]
- 23.Dietz JR, Moran MS, Isakoff SJ, et al. Recommendations for prioritization, treatment, and triage of breast cancer patients during the COVID-19 pandemic. The COVID-19 pandemic breast cancer consortium. Breast Cancer Res Treat 2020; 181:487–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stewart CL, Thornblade LW, Diamond DJ, et al. Personal protective equipment and COVID-19 – a review for surgeons. Ann Surg 2020; Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]


