Abstract
Background:
Coronary artery disease and aortic stenosis often coexist. Transcatheter aortic valve implantation (TAVI) has emerged as a valid therapeutic option for younger, lower-risk patients who may eventually require coronary artery disease treatment. Thus, post-TAVI coronary access (CA) and percutaneous coronary intervention are expected to increase. The purpose of this study was to retrospectively evaluate patients who were enrolled in the SOURCE 3 (SAPIEN 3 Aortic Bioprosthesis European Outcome) European registry for treatment with the balloon-expandable SAPIEN 3 transcatheter heart valve and underwent CA with or without percutaneous coronary intervention after TAVI.
Methods:
Baseline characteristics and clinical outcomes of patients with or without CA up to 3 years after TAVI were compared. A Kaplan-Meier estimate with a univariate model determined the impact of CA on cardiac mortality.
Results:
Of 1936 TAVI patients (mean age 81.6 years, 52% male), 68 (3.5%) had CA within 3 years (mean 441±332 days) after TAVI. At baseline, the logistic EuroSCORE was similar (20.2% versus 18.3%, P=0.2, CA and non-CA groups, respectively). Higher rates of coronary artery disease (76.5% versus 50.6%, P<0.001), myocardial infarction (20.6% versus 11.5%, P=0.03) and previous coronary artery bypass graft (22.1% versus 11.0%, P=0.01) were present in the CA group. In 100% of patients, CA was successfully achieved. The clinical success of percutaneous coronary intervention was 97.9%. Cardiovascular mortality was numerically higher in patients with CA than in those without CA.
Conclusions:
In the large SOURCE 3 European registry, CA was needed at 3-year follow-up after TAVI with a balloon-expandable valve in 3.5% of patients and was successful in all patients. The clinical success of percutaneous coronary intervention was 97.9%.
Registration:
URL: https://www.clinicaltrials.gov. Unique identifier: NCT02698956.
Keywords: coronary access, coronary artery disease, heart valves, mortality, myocardial infarction, percutaneous coronary intervention, transcatheter aortic valve
What Is Known
Coronary artery disease and aortic stenosis often coexist.
In the new era of low-risk transcatheter aortic valve implantation in younger patients, the need for coronary access after transcatheter aortic valve implantation will likely increase.
What the Study Adds
This is the first assessment of coronary access after transcatheter aortic valve implantation in a large, international, multicenter registry using the balloon-expandable SAPIEN 3 transcatheter heart valve.
At 3 years post transcatheter aortic valve implantation, the incidence of coronary access was 3.5% in an elderly, high-risk cohort.
Coronary access was successful in 100% of patients who received the balloon-expandable SAPIEN 3 transcatheter heart valve, and percutaneous coronary intervention successful in 97.9% of patients.
Transcatheter aortic valve implantation (TAVI) has emerged as the standard of care for severe aortic stenosis. Indications for TAVI are expected to expand following the positive outcomes from randomized clinical trials of transcatheter heart valves (THV) in younger and lower-risk patients.1–6 An estimated 40% to 75% of patients with severe aortic stenosis who undergo TAVI also have significant coronary artery disease (CAD).7 Younger patients are more likely to require coronary intervention after TAVI due to the progressive nature of CAD and their longer life expectancy. Thus, the need for coronary access (CA) for diagnostic angiography and percutaneous coronary intervention (PCI) after TAVI is expected to increase. However, scant data are available on incidence, feasibility, and success rate of coronary intervention in patients who underwent TAVI.8 Moreover, some evidence suggests that CA feasibility after TAVI may differ with different THV designs.8–11
We undertook a retrospective study of patients enrolled in the SOURCE 3 Registry (SAPIEN 3 Aortic Bioprosthesis European Outcome), an international, real-world registry, to determine the incidence, success rate, and effect on cardiovascular mortality up to 3 years post-TAVI of CA and PCI after TAVI with a balloon-expandable valve.
Methods
The SOURCE 3 registry is a post approval, observational registry of 1947 patients who underwent TAVI at 80 centers in 10 European countries. The aim of SOURCE 3 was to evaluate the safety and performance of the SAPIEN 3 THV (Edwards Lifesciences; Irvine, CA) up to 5 years postprocedure in patients with severe, symptomatic, calcific aortic stenosis at high risk for surgery in real-world practice. Registry design and 30-day and 1-year results have been previously described.12,13 Because of the sensitive nature of the data collected for this study, requests to access the dataset from qualified researchers trained in human subject confidentiality protocols may be sent to Edwards Lifesciences at Erin_Rogers@edwards.com.
The SOURCE 3 research protocol complied with the Declaration of Helsinki and was approved by local ethics committees and applicable health authorities in the participating countries. All enrolled patients provided written informed consent before study activities began.
Enrolled patients received the SAPIEN 3 THV (23, 26, or 29 mm) via a transfemoral approach or other routes. An independent clinical event committee reviewed and adjudicated significant clinical events according to Valve Academic Research Consortium 2 criteria.14 An independent neurologist reviewed all strokes to determine whether they were disabling.
Study Population
A heart team, comprising cardiac surgeons, interventional cardiologists, anesthetists, and imaging specialists, selected eligible patients based on clinical consensus that TAVI was the best treatment option. Selected patients conformed to the SAPIEN 3 indication for use and the European Society of Cardiology/European Association for Cardiothoracic Surgery guidelines.15
Inclusion/Exclusion Criteria
Patients from the database were selected if they underwent a coronary intervention post-TAVI, including CA for diagnostic angiography and PCI. Patients who had vascular interventions were excluded. Patients with acute coronary artery obstruction due to the valve prosthesis itself, native leaflets, or calcifications that occurred during or immediately after TAVI were also excluded.
To determine which patients met the inclusion criteria, the all-treated population (ie, all enrolled patients) in the database was searched by the following: adverse events up to 3 years, including congestive heart failure or worsening of congestive heart failure, coronary artery obstruction requiring intervention, death, myocardial infarction (MI), and other. The identified adverse events were then filtered by the following: PCI, coronary artery bypass graft, drug-eluting stent, stent, percutaneous transluminal coronary angioplasty, percutaneous transluminal angioplasty, and coronary angiography. Each case was reviewed to ensure it conformed to the inclusion/exclusion criteria. Patients with coronary artery events that occurred during TAVI were excluded.
Outcome Measures
Evaluated outcome measures were as follows: (1) successful CA (ie, ability to selectively cannulate the desired artery); (2) successful PCI (ie, ability to perform balloon angioplasty or stent implantation when needed); and (3) any complication, including death related to CA or PCI after TAVI.
Analysis
Baseline characteristics for patients with and without CA at 3 years post-TAVI in the all-treated population were compared using the Kruskal-Wallis test for continuous variables and Fisher exact test for categorical variables.
The first report of CA was used in the analysis. A Kaplan-Meier estimate and 95% confidence formulas, using Greenwood formula for CA and PCI occurrence are provided. Kaplan-Meier curves were used to compare the effect of CA on overall and cardiac mortality. No statistical testing was performed to compare mortality between the CA and non-CA groups due to the limited number of events and because the proportional hazard assumption was not met.
Statistical software R, v3.5.3 (R Foundation for Statistical Computing; Vienna, Austria) was used for the analysis.
Results
Patient Disposition
A total of 1936 were included in the analysis (Figure 1). Excluded patients received an alternative valve, had coronary intervention concomitant with TAVI, had coronary obstruction during or immediately after TAVI, or withdrew consent before receiving an implant. Of the included patients, 68 (3.5%) required CA within 3 years after TAVI.
Figure 1.
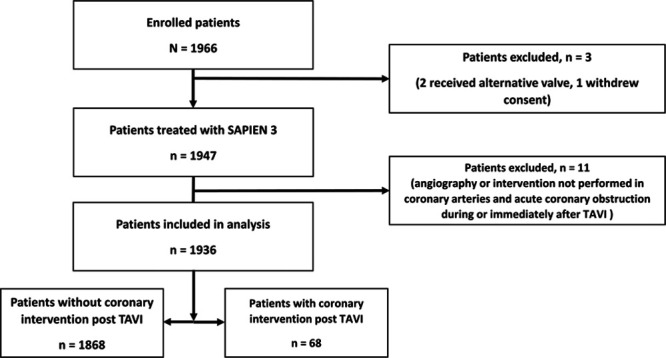
Study flow chart. TAVI indicates transcatheter aortic valve implantation.
Patient Characteristics
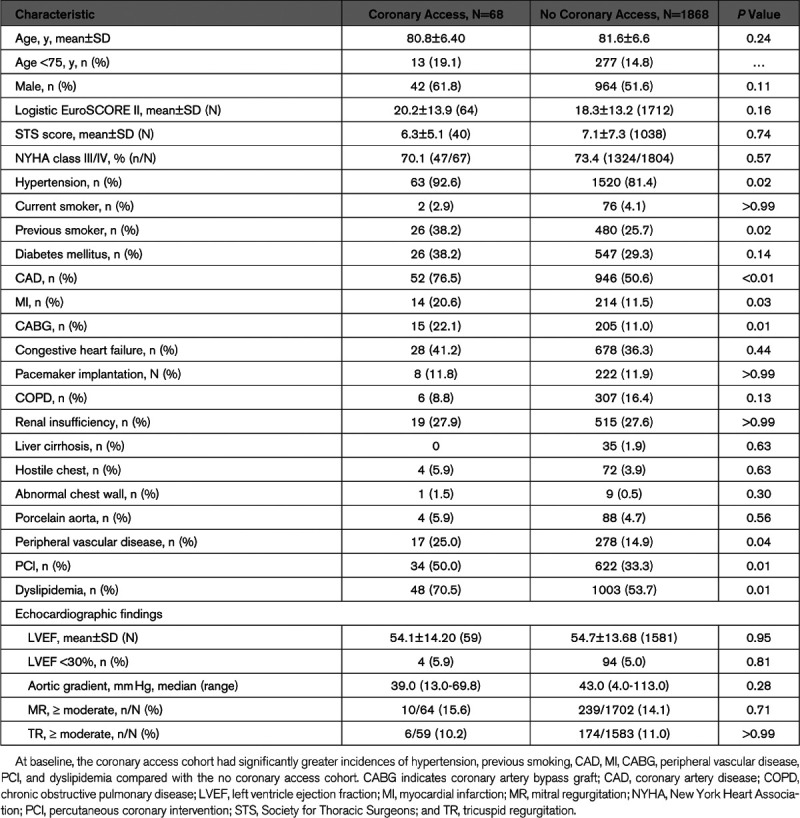
At baseline, most patients were male, and the mean age was 81 years (Table 1). The 2 cohorts were similar in terms of risk scores, New York Heart Association functional class, left ventricular ejection fraction, and other echocardiographic variables. At baseline, patients in the CA group had significantly higher incidences of MI, hypertension, CAD, peripheral vascular disease, dyslipidemia, previous coronary artery bypass graft, and PCI, and previous smoking compared with the non-CA group. Groups also differed in access sites for TAVI. More than twice as many patients in the CA group (26.5%) had TAVI via a transapical approach compared with the non-CA group (12.6%).
Table 1.
Baseline Characteristics
Coronary Intervention Outcomes
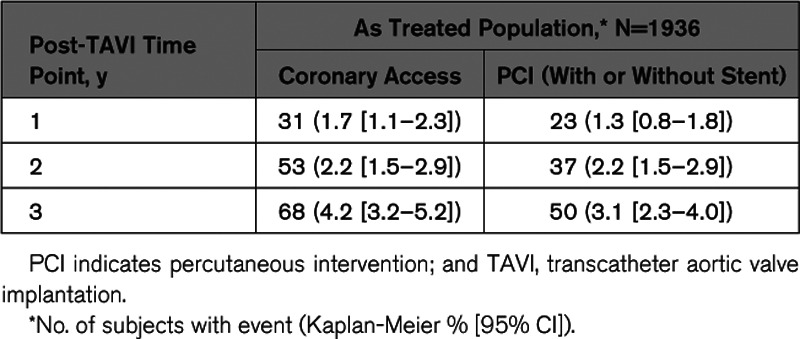
The mean time to CA after TAVI was 441 days, ranging from 1 to 1076 days. At 1 year, 31 patients underwent CA and 23 underwent PCI (with or without stent implantation; Table 2). At 2 years, an additional 22 and 14 patients underwent CA and PCI, respectively. And at 3 years, another 15 and 13 patients underwent CA and PCI, respectively.
Table 2.
Post-TAVI Coronary Intervention by Time
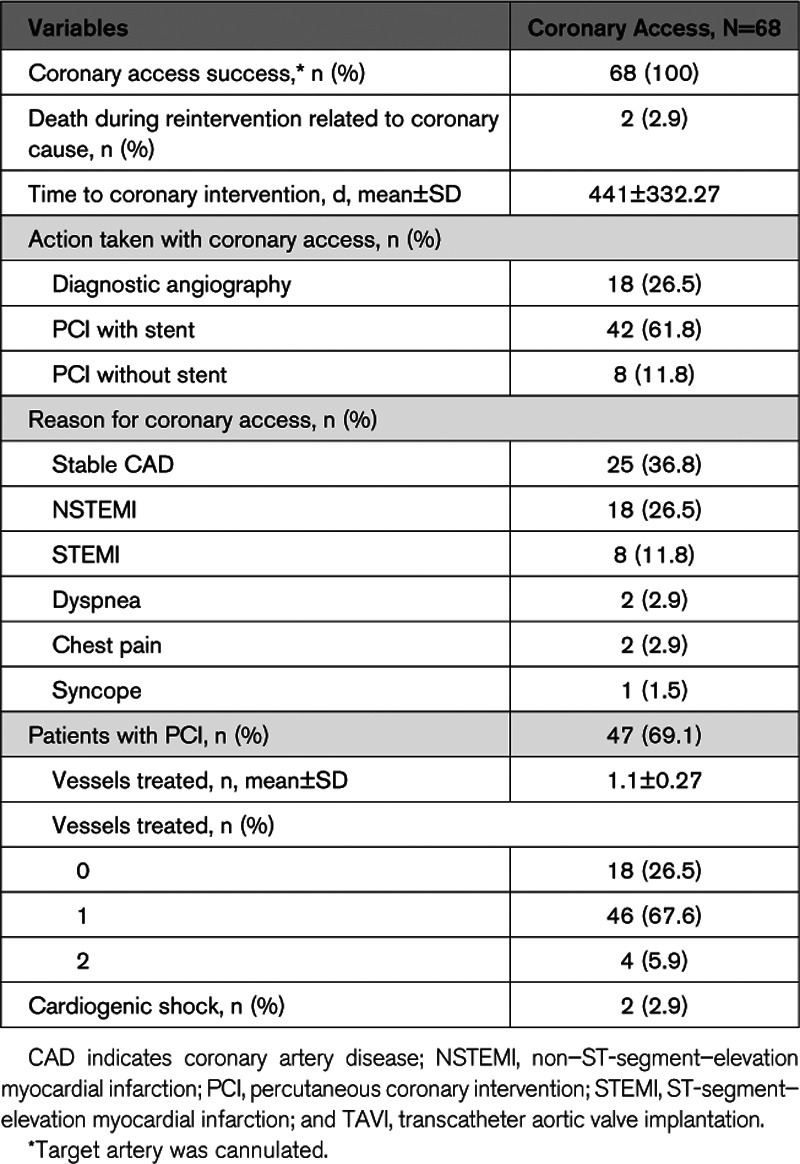
CA was successful in all cases (Table 3). Forty-seven patients had an indication for PCI. Of these, 89.4% underwent stent implantation, while 4.4% underwent balloon angioplasty only. The primary reason for CA was stable CAD, followed by non–ST-segment–elevation MI, ST-segment–elevation MI, syncope, dyspnea, and chest pain. Two patients were in cardiogenic shock at the time of PCI.
Table 3.
Coronary Access and Percutaneous Coronary Intervention After TAVI
The left anterior descending coronary artery was the most frequently treated vessel, followed by left circumflex artery, right coronary artery, saphenous vein graft, and left main trunk (Figure 2). Planned PCI was successful in all but 1 case that was complicated by coronary perforation followed by cardiac tamponade leading to patient’s death.
Figure 2.
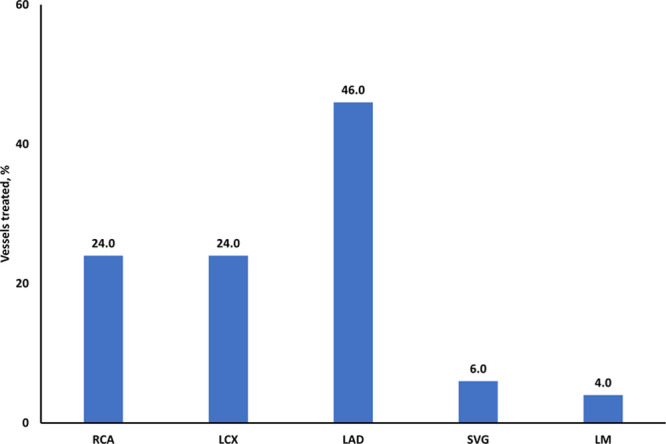
Coronary vessels treated after transcatheter aortic valve implantation. LAD indicates left anterior descending artery; LCX, left circumflex artery; LM, left main; SVG, saphenous vein graft; and RCA, right coronary artery.
The survival analysis showed a numerical increase in cardiovascular mortality in the period immediately after CA (Figure 3). Cardiovascular mortality appeared higher in patients with CA to 1-year post-CA than in those without CA but could not be statistically tested due to the limited number of events.
Figure 3.
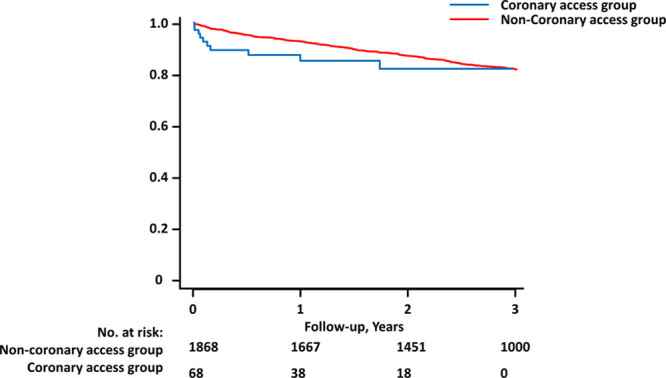
Cardiovascular mortality after coronary access post-transcatheter aortic valve implantation (TAVI). Cardiovascular mortality in the coronary intervention post-TAVI group (blue) and the group that did not have a coronary intervention post-TAVI (red). Baseline for the coronary intervention group is time of intervention. Baseline for the noncoronary intervention group is time of TAVI.
Discussion
The main findings of this study, the first to assess CA after TAVI in a large, real-world European registry, are as follows: (1) In the SOURCE 3 registry the incidence of CA up to 3 years after TAVI was 3.5%; (2) Patients who required CA more frequently had a history of coronary artery bypass graft or PCI, peripheral vascular disease, MI, hypertension, and dyslipidemia compared with the rest of the population; (3) The reasons for CA ranged from diagnostic to urgent (ST-segment–elevation MI and cardiogenic shock); and (4) CA was successful in all cases, and the success rate of PCI was 97.9%.
The need for CA after TAVI has not been adequately addressed by the current literature, with data mainly restricted to case series or single-center experiences. In our high risk, elderly cohort of patients treated with a balloon-expandable THV, the incidence of CA was 3.5%, with 69% of patients needing PCI. Mean time to CA after TAVI was 441 days. Of note, with the expansion of TAVI indication to lower risk and younger patients, these numbers are likely to increase, due to the progressive nature of CAD and the longer life expectancy of these patients.4 Accordingly, the possibility to easily engage the coronary arteries after THV implantation is of paramount importance.
In our study, selective CA was feasible in all patients requiring coronary angiography, while PCI was successfully performed in all but 1 case, which was complicated by coronary perforation leading to cardiac tamponade.
The first successful CA after TAVI was reported in 2007 by Zajarias et al16 who published a case study of an 85-year-old man who underwent a successful PCI 15 months after receiving an Edwards balloon-expandable THV. Since then, other small retrospective and observational studies have reported positive outcomes for coronary intervention in patient previously treated with self-expanding and balloon-expandable THV.17–19 While these studies demonstrated the feasibility and high success rate of CA and PCI post-TAVI, the procedures were not without challenges.8,9,19
The ability to traverse a THV to access the coronary vessels depends not only on anatomic factors such as coronary height and sino-tubular junction height and width but also on valve design. Self-expanding valves, such as CoreValve, may present technical challenges to CA because of its supra-annular position above the coronary ostia. Moreover, the asymmetrical skirt and closed-cell frame design often make CA difficult, particularly when a neocommissure rests in front of the coronary ostium.9,10,18,19 Conversely, balloon-expandable valves, such as SAPIEN 3, have a low frame height that frequently results in a sub-coronary implantation, large-cell frame design (4.4–6.8 mm) on the outflow side and commissure windows reducing frame material.20 All these features facilitate the passage of a coronary catheter and coronary ostium cannulation and make CA easier even when the SAPIEN 3 valve is implanted in a high position.9,21
Despite the lack of randomized clinical trials comparing CA among different valve types, our findings underscore the extremely high feasibility and success rates of CA and PCI after the implantation of the SAPIEN 3 THV, even in the urgent setting of ST-segment–elevation MI and cardiogenic shock. While this analysis was limited to the post-procedural CA in SOURCE 3, Wendler et al12 reported successful percutaneous treatment of 6 out of 7 cases of procedural coronary obstruction in the SOURCE registry, demonstrating the advantage of the open-cell valve design in allowing urgent CA and PCI.
Cardiovascular mortality was higher in patients with CA than in those without CA. Nevertheless, it is noteworthy that this does not seem to be related to CA itself. In fact, no cases of coronary ostium dissection, valve dislodgement, nor valve stent fracture were recorded, and only one case was complicated by coronary perforation, which was likely unrelated to the presence of the SAPIEN 3 THV.
While assessment and treatment planning of CAD in patients who are TAVI candidates is very important, interventional strategy and optimal timing of the coronary procedure is still a subject of debate. Some guidelines recommend treating severe CAD before TAVI, even in patients without angina symptoms.22 However, a systematic review of 9 studies evaluating CAD treatment for coronary stenosis before or during TAVI found no clinical advantage for either approach.23 The results of these studies are reassuring in terms of the ability to access the coronary arteries after implantation of a SAPIEN 3 THV and might allow a less aggressive approach when intermediate coronary artery stenoses are found in TAVI candidates, particularly in those with frailty or high bleeding risk.
Limitations
This analysis was limited by the observational, retrospective, and nonrandomized design of the study. The relatively small sample size of the CA population did not allow for further statistical analyses. Since CA was not a prespecified end point of the original SOURCE 3 study, we cannot exclude underreporting. However, all events—including MI—where adjudicated by an independent clinical event committee. Importantly, the results of this study should not be extended to patients treated for bicuspid aortic valve stenosis, for which THV implantation is usually higher, and CA might be more challenging.10,24
Conclusions
In this large multicenter study of patients treated with a balloon-expandable THV, the need for CA after TAVI was 3.5% at 3 years. CA was feasible in all patients, and clinical success of PCI was 97.9%. These results are reassuring for the future of TAVI with this type of device in younger and lower-risk patients, in whom the need for CA is expected to increase.
Acknowledgments
Tracey Fine, MS, ELS of Edwards Lifesciences provided medical writing services.
Sources of Funding
This study was funded by Edwards Lifesciences.
Disclosures
Dr Tarantini reports honoraria for lectures/consulting from Medtronic, Edwards Lifesciences, Boston Scientific, GADA, Abbott. T. Hovorka and Y.A. Navarro are employees of Edwards Lifesciences. Dr Bartorelli reports speaker and consulting fee from Abbott Vascular. Dr Wendler reports honoraria for lectures and consulting from Medtronic, Edwards Lifesciences, and Abbott. The other authors report no conflicts.
Footnotes
Nonstandard Abbreviations and Acronyms
- CA
- coronary access
- CAD
- coronary artery disease
- MI
- myocardial infarction
- PCI
- percutaneous coronary intervention
- SOURCE
- SAPIEN 3 Aortic Bioprosthesis European Outcome
- TAVI
- transcatheter aortic valve implantation
- THV
- transcatheter heart valve
For Sources of Funding and Disclosures, see page 6.
References
- 1.Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, Kapadia SR, Malaisrie SC, Cohen DJ, Pibarot P, et al. ; PARTNER 3 Investigators. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019; 380:1695–1705. doi: 10.1056/NEJMoa1814052 [DOI] [PubMed] [Google Scholar]
- 2.Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O’Hair D, Bajwa T, Heiser JC, Merhi W, Kleiman NS, et al. ; Evolut Low Risk Trial Investigators. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. 2019; 380:1706–1715. doi: 10.1056/NEJMoa1816885 [DOI] [PubMed] [Google Scholar]
- 3.Ueshima D, Fovino LN, D’Amico G, Brener SJ, Esposito G, Tarantini G. Transcatheter versus surgical aortic valve replacement in low- and intermediate-risk patients: an updated systematic review and meta-analysis. Cardiovasc Interv Ther. 2019; 34:216–225. doi: 10.1007/s12928-018-0546-5 [DOI] [PubMed] [Google Scholar]
- 4.Tarantini G, Nai Fovino L, D’Errigo P, Rosato S, Barbanti M, Tamburino C, Ranucci M, Santoro G, Badoni G, Seccareccia F; For the OBSERVANT Research Group. Factors influencing the choice between transcatheter and surgical treatment of severe aortic stenosis in patients younger than 80 years: results from the OBSERVANT study. Catheter Cardiovasc Interv. 2020; 95:e186–e195. doi: 10.1002/ccd.28447 [DOI] [PubMed] [Google Scholar]
- 5.Tarantini G, Nai Fovino L, Gersh BJ. Transcatheter aortic valve implantation in lower-risk patients: what is the perspective? Eur Heart J. 2018; 39:658–666. doi: 10.1093/eurheartj/ehx489 [DOI] [PubMed] [Google Scholar]
- 6.Tarantini G, Purita PAM, D’Onofrio A, Fraccaro C, Frigo AC, D’Amico G, Fovino LN, Martin M, Cardaioli F, Badawy MRA, et al. Long-term outcomes and prosthesis performance after transcatheter aortic valve replacement: results of self-expandable and balloon-expandable transcatheter heart valves. Ann Cardiothorac Surg. 2017; 6:473–483. doi: 10.21037/acs.2017.08.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goel SS, Ige M, Tuzcu EM, Ellis SG, Stewart WJ, Svensson LG, Lytle BW, Kapadia SR. Severe aortic stenosis and coronary artery disease–implications for management in the transcatheter aortic valve replacement era: a comprehensive review. J Am Coll Cardiol. 2013; 62:1–10. doi: 10.1016/j.jacc.2013.01.096 [DOI] [PubMed] [Google Scholar]
- 8.Yudi MB, Sharma SK, Tang GHL, Kini A. Coronary angiography and percutaneous coronary intervention after transcatheter aortic valve replacement. J Am Coll Cardiol. 2018; 71:1360–1378. doi: 10.1016/j.jacc.2018.01.057 [DOI] [PubMed] [Google Scholar]
- 9.Tang GHL, Zaid S, Ahmad H, Undemir C, Lansman SL. Transcatheter valve neo-commissural overlap with coronary orifices after transcatheter aortic valve replacement. Circ Cardiovasc Interv. 2018; 11:e007263 doi: 10.1161/CIRCINTERVENTIONS.118.007263 [DOI] [PubMed] [Google Scholar]
- 10.Tarantini G, Fabris T, Cardaioli F, Nai Fovino L. Coronary access after transcatheter aortic valve replacement in patients with bicuspid aortic valve: lights and shades. JACC Cardiovasc Interv. 2019; 12:1190–1191. doi: 10.1016/j.jcin.2019.03.031 [DOI] [PubMed] [Google Scholar]
- 11.Nai Fovino L, Scotti A, Massussi M, Fabris T, Cardaioli F, Rodinò G, Matsuda Y, Frigo F, Fraccaro C, Tarantini G. Incidence and feasibility of coronary access after transcatheter aortic valve replacement [published online January 8, 2020]. Catheter Cardiovasc Interv. 2020. doi: 10.1002/ccd.28720 [DOI] [PubMed] [Google Scholar]
- 12.Wendler O, Schymik G, Treede H, Baumgartner H, Dumonteil N, Ihlberg L, Neumann FJ, Tarantini G, Zamarano JL, Vahanian A. SOURCE 3 registry: design and 30-day results of the european postapproval registry of the latest generation of the SAPIEN 3 transcatheter heart valve. Circulation. 2017; 135:1123–1132. doi: 10.1161/CIRCULATIONAHA.116.025103 [DOI] [PubMed] [Google Scholar]
- 13.Wendler O, Schymik G, Treede H, Baumgartner H, Dumonteil N, Neumann FJ, Tarantini G, Zamorano JL, Vahanian A. SOURCE 3: 1-year outcomes post-transcatheter aortic valve implantation using the latest generation of the balloon-expandable transcatheter heart valve. Eur Heart J. 2017; 38:2717–2726. doi: 10.1093/eurheartj/ehx294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kappetein AP, Head SJ, Généreux P, Piazza N, van Mieghem NM, Blackstone EH, Brott TG, Cohen DJ, Cutlip DE, van Es GA, et al. ; Valve Academic Research Consortium (VARC)-2. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document (VARC-2). Eur J Cardiothorac Surg. 2012; 42:S45–S60. doi: 10.1093/ejcts/ezs533 [DOI] [PubMed] [Google Scholar]
- 15.Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Barón-Esquivias G, Baumgartner H, Borger MA, Carrel TP, De Bonis M, et al. ; Authors/Task Force Members. Guidelines on the management of valvular heart disease (version 2012): the Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Eur J Cardiothorac Surg. 2012; 42:S1–S44. doi: 10.1093/ejcts/ezs455 [DOI] [PubMed] [Google Scholar]
- 16.Zajarias A, Eltchaninoff H, Cribier A. Successful coronary intervention after percutaneous aortic valve replacement. Catheter Cardiovasc Interv. 2007; 69:522–524. doi: 10.1002/ccd.21028 [DOI] [PubMed] [Google Scholar]
- 17.Chakravarty T, Sharma R, Abramowitz Y, Kapadia S, Latib A, Jilaihawi H, Poddar KL, Giustino G, Ribeiro HB, Tchetche D, et al. Outcomes in patients with transcatheter aortic valve replacement and left main stenting: the TAVR-LM registry. J Am Coll Cardiol. 2016; 67:951–960. doi: 10.1016/j.jacc.2015.10.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Htun WW, Grines C, Schreiber T. Feasibility of coronary angiography and percutaneous coronary intervention after transcatheter aortic valve replacement using a Medtronic™ self-expandable bioprosthetic valve. Catheter Cardiovasc Interv. 2018; 91:1339–1344. doi: 10.1002/ccd.27346 [DOI] [PubMed] [Google Scholar]
- 19.Tanaka A, Jabbour RJ, Testa L, Agnifili M, Ettori F, Fiorina C, Adamo M, Bruschi G, Giannini C, Petronio AS, et al. Incidence, technical safety, and feasibility of coronary angiography and intervention following self-expanding transcatheter aortic valve replacement. Cardiovasc Revasc Med. 2019; 20:371–375. doi: 10.1016/j.carrev.2019.01.026 [DOI] [PubMed] [Google Scholar]
- 20.Tarantini G, Fabris T, Nai Fovino L. TAVR-in-TAVR and coronary access: the importance of pre-procedural planning. EuroIntervention. 2020;16:e129–e132 doi: 10.4244/EIJ-D-19-01094 [DOI] [PubMed] [Google Scholar]
- 21.Nai Fovino L, Badawy MRA, Fraccaro C, D’Onofrio A, Purita PAM, Frigo AC, Tellaroli P, Mauro A, Tusa M, Napodano M, et al. Transfemoral aortic valve implantation with new-generation devices: the repositionable Lotus vs. the balloon-expandable Edwards Sapien 3 valve. J Cardiovasc Med (Hagerstown). 2018; 19:655–663. doi: 10.2459/JCM.0000000000000705 [DOI] [PubMed] [Google Scholar]
- 22.Patel MR, Calhoon JH, Dehmer GJ, Grantham JA, Maddox TM, Maron DJ, Smith PK. ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2017 appropriate use criteria for coronary revascularization in patients with stable ischemic heart disease: a report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society of Thoracic Surgeons. J Nucl Cardiol. 2017; 24:1759–1792. doi: 10.1007/s12350-017-0917-9 [DOI] [PubMed] [Google Scholar]
- 23.Kotronias RA, Kwok CS, George S, Capodanno D, Ludman PF, Townend JN, Doshi SN, Khogali SS, Généreux P, Herrmann HC, et al. Transcatheter aortic valve implantation with or without percutaneous coronary artery revascularization strategy: a systematic review and meta-analysis. J Am Heart Assoc. 2017; 6:e005960 doi: 10.1161/JAHA.117.005960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ueshima D, Nai Fovino L, Brener SJ, Fabris T, Scotti A, Barioli A, Giacoppo D, Pavei A, Fraccaro C, Napodano M, et al. Transcatheter aortic valve replacement for bicuspid aortic valve stenosis with first- and new-generation bioprostheses: A systematic review and meta-analysis. Int J Cardiol. 2020; 298:76–82. doi: 10.1016/j.ijcard.2019.09.003 [DOI] [PubMed] [Google Scholar]


