Abstract
Background.
Spirometry is the cornerstone of monitoring allograft function after lung transplantation (LT). We sought to determine the association of variables on best spirometry during the first year after bilateral LT with 3-year posttransplant survival.
Methods.
We reviewed charts of patients who survived at least 3 months after bilateral LT (n = 157; age ± SD: 54 ± 13 y, male:female = 91:66). Best spirometry was calculated as the average of 2 highest measurements at least 3 weeks apart during the first year. Airway obstruction was defined as forced expiratory volume in 1-second (FEV1)/forced vital capacity (FVC) ratio <0.7. Survival was compared based on the ventilatory defect and among groups based on the best FEV1 and FVC measurements (>80%, 60%–80%, and <60% predicted). Primary outcome was 3-year survival.
Results.
Overall, 3-year survival was 67% (n = 106). Obstructive defect was uncommon (7%) and did not have an association with 3-year survival (72% versus 67%, P = 0.7). Although one-half patients achieved an FVC>80% predicted (49%), 1 in 5 (19%) remained below 60% predicted. Irrespective of the type of ventilatory defect, survival worsened as the best FVC (% predicted) got lower (>80: 80.8%; 60–80: 63.3%; <60: 40%; P < 0.001). On multivariate logistic regression analysis, after adjusting for age, gender, transplant indication, and annual bronchoscopy findings, best FVC (% predicted) during the first year after LT was independently associated with 3-year survival.
Conclusions.
A significant proportion of bilateral LT patients do not achieve FVC>80% predicted. Although the type of ventilatory defect on best spirometry does not predict survival, failure to achieve FVC>80% predicted during the first year was independently associated with 3-year mortality.
INTRODUCTION
Monitoring protocols after lung transplantation (LT) include a combination of structural and functional assessments of the allograft. While a wide array of tools including radiologic, nuclear medicine, and bronchoscopy are deployed for structural assessments, the cornerstone of functional assessment continues to be the spirometry procedure. This includes serial assessments in pulmonary function laboratory during clinic visits, as well as self-monitoring at home by patients using a microspirometer. The natural history of lung functions after transplantation is well described, where spirometry tends to typically rise rapidly during the first 6–12 months followed by a plateau phase before the inevitable decline, albeit with significant differences between recipients with single and bilateral LT.1
Serial spirometry assessments are useful by way of early detection of clinically silent allograft dysfunction which can trigger prompt workup and management. The premise of pursuing a proactive approach in this regard is the potential to delay progression to chronic lung allograft dysfunction (CLAD), the clinical correlate encompassing the diverse histologic forms of chronic rejection which leads to irreversible allograft dysfunction. The role of spirometry in this regard cannot be overemphasized. In fact, previous studies have found an association of nonadherence to home spirometry with early progression to CLAD.2 Similarly, the pattern of ventilatory abnormality on office spirometry has been found to be associated with long-term outcomes.3,4
While previous studies have highlighted the prognostic significance of different patterns of lung function declines after LT, these data do not facilitate prognostication of individual patients in an effort to customize management strategies. Specifically, it is not known if the pattern of ventilatory defect or the absolute values on the best spirometry achieved during the first year (also referred to as the baseline spirometry) have an association with subsequent posttransplant outcomes. We hypothesized that a higher level of peak lung functions achieved during the first year would be associated with superior posttransplant survival. We also sought to investigate if the presence or type of ventilatory defect (obstructed versus nonobstructed) on the best spirometry measurements were associated with survival.
MATERIALS AND METHODS
This was a retrospective chart review study of patients who underwent LT at the University of Texas Southwestern Medical Center in Dallas, TX, between January 2012 and December 2015 (n = 255). Institutional review board granted approval for the study with a waiver of patient consent (Institutional Review Board #STU042017-036). Given the significant difference in the best spirometry based upon the procedure type, patients with single LT (n = 42) and those who did not survive to 3 months posttransplant (n = 14) were excluded. The group was split into a testing cohort consisting of patients transplanted between 2012 and 2014 (n = 157; age ± SD: 54 ± 13 y, male:female = 91:66) and validation cohort with patients transplanted during 2015 (n = 42; age±SD: 52 ± 14 y, male:female = 27:15).
Management Protocols
The patients are admitted to the lung transplant service after the transplant surgery. After they stabilize and are transferred out of the intensive care unit, in-house daily spirometry recordings are started. Discharge planning includes teaching sessions with the patient and caregiver regarding the outpatient monitoring and follow-up. All patients are managed using a uniform, protocol-based triple immunosuppressive regimen. This includes tacrolimus as the first choice calcineurin inhibitor with goal level of 10–15 ng/mL range in first year and 5–10 ng/mL beyond the first year. Antimetabolite (azathioprine or mycophenolate mofetil) are used with target WBC count <8000/µL. Prednisone dose is gradually tapered down to maintenance dose of 0.1 mg/kg weight at 1-year post lung transplant.
After discharge, patients are seen in the lung transplant clinic twice weekly for first 4 weeks, followed by every week for 4 weeks, every 2 weeks for next 4 weeks and monthly thereafter till the end of first year. A spirometry is done in the clinic with each visit according to the American Thoracic Society guidelines5 and typically a patient undergoes ~25 spirometry procedures during the first year after LT.
Best spirometry was calculated per the International Society for Heart and Lung Transplantation guidelines as the mean of 2 highest measurements at least 3 weeks apart during the first year.6 Airway obstruction was defined as forced expiratory volume in 1-second (FEV1)/forced vital capacity (FVC) ratio <0.7.
Variables
All the data were obtained directly from the electronic medical records. The variables recorded included patient demographics (age, gender, and race), underlying pulmonary disease as the transplant indication, pretransplant comorbidities and course, type of procedure, postoperative course including development of primary graft dysfunction (PGD) as well as the duration of intensive care unit (ICU) and hospital length-of-stay, postdischarge spirometry data and posttransplant survival. While development of PGD was determined based on retrospective review, patient charts were prospectively reviewed for the diagnosis and phenotype of CLAD at 3 year posttransplant. Per International Society for Heart and Lung Transplantation guidelines, the diagnosis of CLAD was based upon 20% or more decline in FEV1 for a duration of at least 3 weeks in the absence of other etiologies to explain the decline.
Outcome Parameters
The primary outcome parameter was all-cause mortality at 3-year posttransplant. Additionally, CLAD-free survival was analyzed as the secondary outcome variable.
STATISTICAL ANALYSIS
The study group was divided into 2 groups based upon the 3-year survival. Pretransplant and posttransplant characteristics were compared among the 2 groups using independent t-test, Mann-Whitney U and chi-square analysis as appropriate. The spirometric variables were compared as both continuous (absolute volume and percent-predicted) and categorical variables (using a priori defined cutoffs of percent predicted volumes on spirometry as follows: >80%, 60%–80%, and <60%). Variables significant on univariate analysis along with age, gender, transplant indication, and lung allocation score at match (to ensure that the associations identified were independent of these variables) were entered as covariates in a multivariate logistic regression model to identify variables with an independent association with 3-year mortality.
Finally, given the independent association of best FVC with posttransplant survival, we also compared the characteristics of patients with different levels of FVC impairment using the ANOVA, Kruskal-Wallis test and chi square test as appropriate. Kaplan-Meier analysis was conducted to compare the survival among patients with different levels of impairment on best spirometry.
Statistical significance was considered at P < 0.05 (2-tailed only). The analysis was done using SPSS statistical software (IBM Corp. Released 2012. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp.)
RESULTS
A majority of recipients had restrictive lung disease as the transplant indication (60.5%) followed by obstructive diseases (22.3%). Mean lung allocation score at match was 53.5 ± 18.9 with more than a quarter of all patients (n = 40) being hospitalized at the time of transplant and 8 patients (5.1%) were bridged to LT using extracorporeal membrane oxygenation support. At 3-year follow-up, all-cause mortality was 32.5% (n = 51) with a CLAD-free survival of 49% (n = 77).
Spirometry Variables During the First Year
Overall, an obstructive ventilatory defect was uncommon on the best spirometry (7%). Mean best FEV1 and FVC were 2.63 ± 0.9 L (84.6% ± 23.3% predicted) and 3.1 ± 1.1 L (78% ± 21% predicted). More patients achieved an FEV1 of >80% predicted (57.3%) when compared with FVC > 80% predicted (49.7%). Nearly 1 in 5 (19%) patients did not achieve an FVC of 60% predicted while the proportion of patients with predicted FEV1<60% predicted was 14%.The median time to achieving best FVC after transplant was 255 days (range 17–356 d, Figure 1) although this was not different among the 3 FVC groups (Table 1).
FIGURE 1.
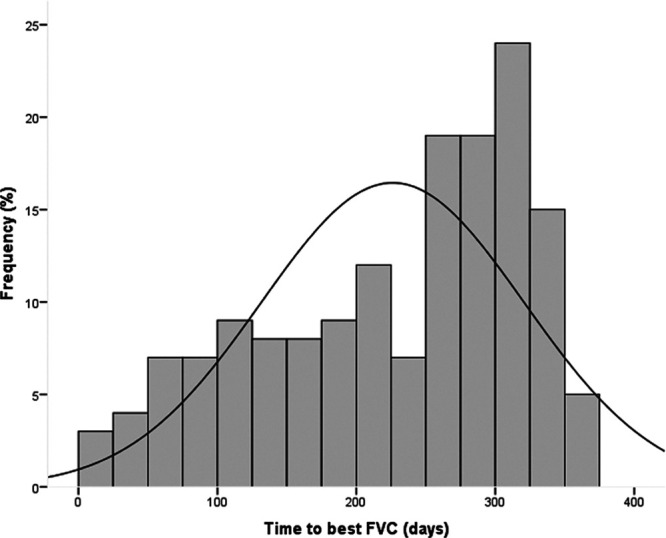
Histogram showing the frequency distribution of patients based upon the time since transplant to achieve the best FVC for the complete study group. FVC, forced vital capacity.
TABLE 1.
Characteristics of patients with different percent predicted forced vital capacity categories
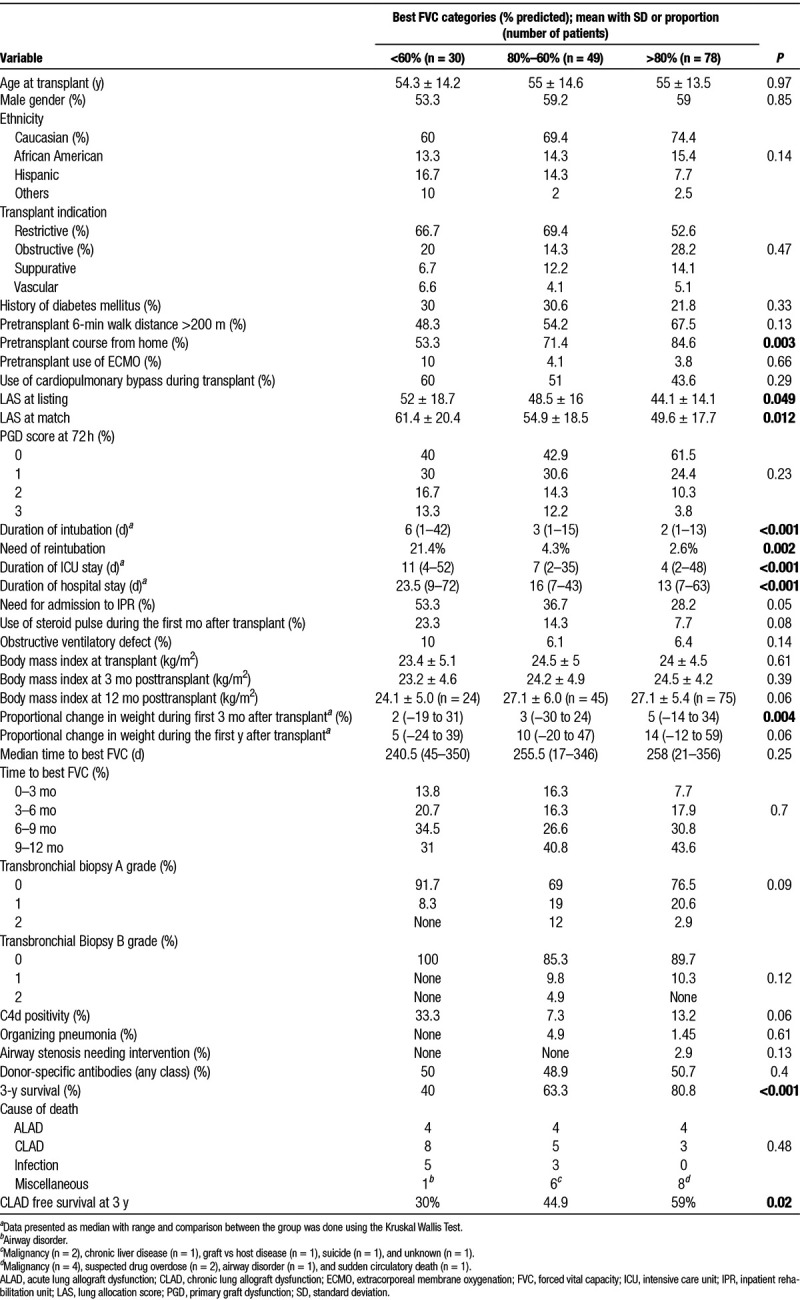
Comparison of patient characteristics among the 3 FVC groups is presented in Table 1. Several characteristics including higher LAS at listing and match, hospitalization at the time of transplant, development of PGD grade 2 or 3 at 72-hours posttransplant (27.8% among FVC ≤80% versus 14.1% FVC>80%; OR: 2.4, 1.1–5.3, P = 0.049), need of reintubation, higher duration of ventilator need, ICU, and hospital length-of-stay were all associated with low FVC during the first year. While the 3 groups started out with similar mean BMI during their last pretransplant visit as well as during early posttransplant period, patents with higher FVC had proportionally higher weight gain which was statistically significant during the first 3 months. Interestingly, despite an association between lower FVC and worse mortality, the risk of progression to CLAD at 3-year posttransplant was similar among the FVC groups (Table 1).
3-year Survival
Pretransplant and posttransplant characteristics among 3 year survivors and nonsurvivors are compared in Table 2. Several spirometry variables were associated with 3 year survival. While the absolute and %-predicted volumes (both FEV1 and FVC) on best spirometry were significantly lower among nonsurvivors, the FEV1/FVC ratio was similar. In other words, the presence of airway obstruction on best spirometry was not associated with 3-year survival. However, among patients without airway obstruction (FEV1/FVC ratio > 0.7), 3-year survival tended to worsen with increase in the ratio above 0.9 (57%) when compared with those with ratio between 07 and 0.9 (71.7%, P = NS). Irrespective of the type of ventilatory defect, 3-year survival worsened significantly as the best FVC (% predicted) achieved got lower (<60: 40%; 60–80: 63.3%; >80: 80.8%; P < 0.001). Kaplan-Meier analysis showed early and progressive separation of the survival curves for the 3 FVC categories (see Figure 2). Additionally, a longer median time to achieve best FVC after transplant was associated with a higher 3-year survival. In other words, patients who continued to gain FVC beyond the 6-month posttransplant period had a higher 3-year survival.
TABLE 2.
Variables associated with 3-y posttransplant survival: univariate and multivariable comparisons
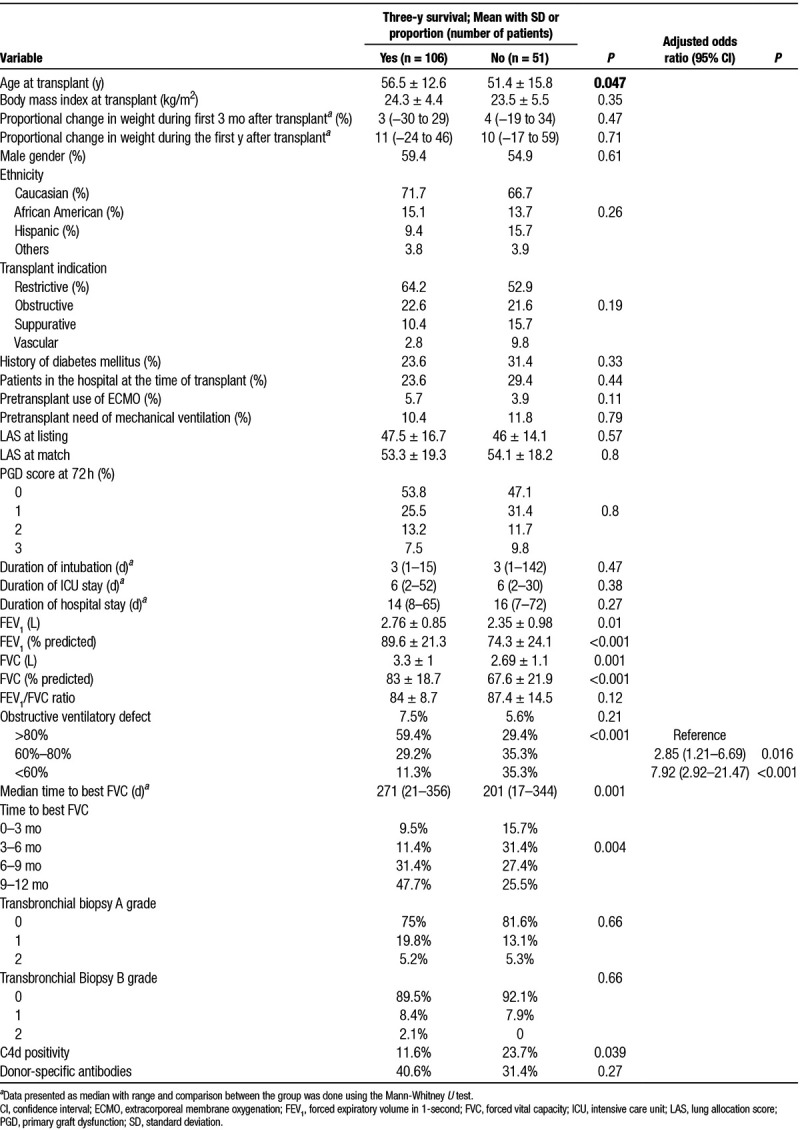
FIGURE 2.
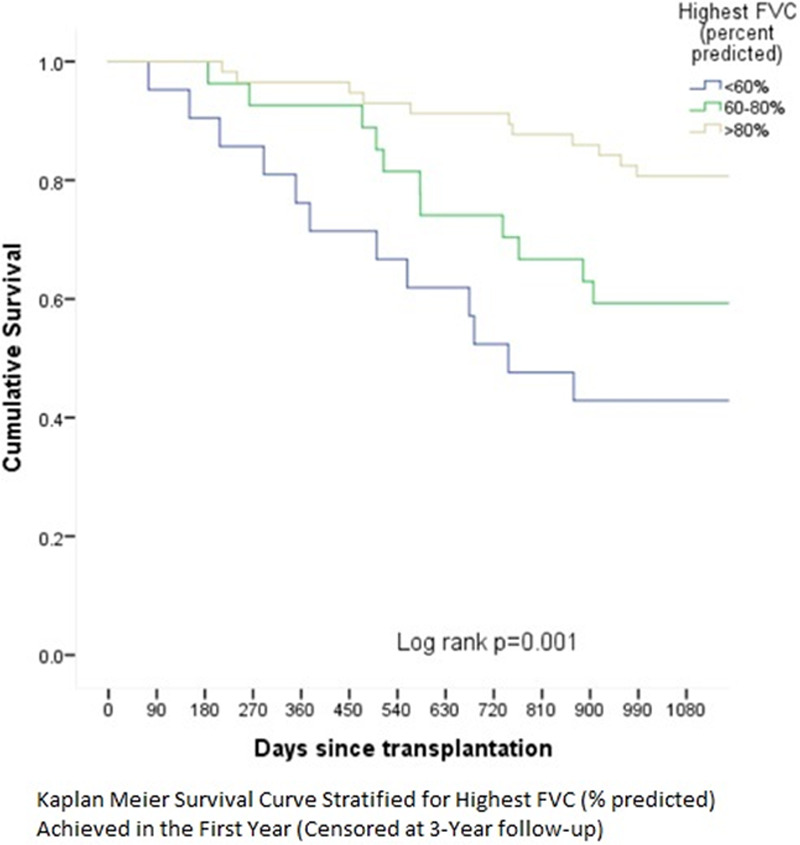
Three-y mortality post-lung transplant stratified by highest FVC (percent-predicted) achieved. FVC, forced vital capacity.
On multivariate analysis, after adjusting for age, gender, transplant indication, and LAS at match, best FVC (% predicted) during the first year after LT emerged as the independent predictor of 3-year survival (Table 2).
DISCUSSION
There is limited data in the literature regarding the role of best spirometry as a predictor of posttransplant survival. Previous studies have focused primarily on the prognostic significance of different patterns of decline in the lung functions.3,7,8 It has been reported that a concurrent FVC and FEV1 decline portends a much worse prognosis when compared with FEV1 decline alone. However, the prognostic significance of the ventilatory patterns or the magnitude of best lung functions after LT has not been as well studied. The current study sought to build on the existing literature on the prognostic significance of pulmonary physiology variables on the post-lung transplant outcomes.
While it is logical to strive for the highest possible lung functions after LT, the eventual best spirometry volumes tend to be highly variable among patients, even if we restrict the analysis to bilateral LT only.1 In consonance with previous articles, the current study also showed a wide variation in the best spirometry with a significant proportion of patients not even achieving 60% of predicted volumes. The findings from the current study were somewhat unexpected considering that only patients with bilateral LT were included. Apart from Mason et al1 who reported a peak FEV1 of 75% predicted at 1 year after LT, there is little in the published literature that could be used as a benchmark for the expected level of lung functions after bilateral LT. The lack of data regarding the “goal” as regards the best lung functions after bilateral LT makes it challenging to prognosticate patients based on their baseline spirometry before the inevitable decline in lung function eventually happens. Availability of this data could facilitate institution of potentially proactive strategies well before decline in lung functions starts to occur.
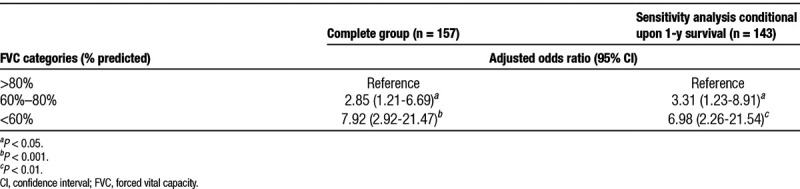
The independent association of best FVC during the first year with survival raises the possibility that patients who died during the first year may not have had the “time” or the “opportunity” to reach their peak lung functions which may atleast partly drive this association. To exclude the potential impact of patients who died during the first year on this association, we conducted additional sensitivity analysis among the subgroup of patients who survived the first year (n = 143). This analysis confirmed the strong and graded independent association of FVC groups with 3-year survival on multivariate logistic regression analysis (Table 3).
TABLE 3.
Sensitivity analysis for the independent association of peak FVC category with 3-y posttransplant survival
The reasons for failure to achieve high lung functions after LT can be diverse and may be related to pulmonary parenchymal or extrapulmonary factors.9 While we did not have access to the exact cause of low lung functions in the current study, a low incidence of obstructive ventilatory defect indicates pathologies sparing the airways as being the culprit in majority patients. These factors could include size mismatch (undersizing of the lungs),10 postoperative chest wall restriction, diffuse parenchymal processes such as organizing pneumonia or diffuse alveolar damage, pleural pathologies such as effusion or entrapment, diaphragm dysfunction, debility, neuromuscular weakness, and weight gain. We did have access to the temporal trends on body weight during the first year after transplantation in the testing cohort. The mean BMI among patients with best FVC > 80% predicted and ≤80% at 3, 6, 9, and 12 months was similar, thereby excluding weight gain as the etiology. Previous studies have found pathological findings of diffuse alveolar damage,11 and acute fibrinoid organizing pneumonia12 as strongly associated with poor outcomes and often these pathologies would lead to a restrictive ventilatory defect with a low FVC. It is also possible that low FVC resulting from extraparenchymal disorders such as chest wall restriction, or neuromuscular weakness may limit ability to track changes in FEV1, thereby limiting ability of spirometry to detect changes in FEV1 as a trigger for further evaluation. While we did not record specific indicators of frailty, there were trends indicating poor conditioning at baseline (pretransplant 6-min walk distance), early need of pulse dose corticosteroids and a tumultuous early postoperative course (longer duration of intubation, ICU and hospital length of stay as well as need of admission to the inpatient rehabilitation unit after discharge from the hospital) contributing to the lower peak FVC during the first year (Table 1). Irrespective of the etiology though, the strong, independent and graded association of low FVC with worsening 3-year survival highlights the prognostic significance of this simple to track variable. The cutoffs for best FVC identified in the current study, at the very least, can be useful in prognostication of patients with regard to their risk of 3 year mortality. Interestingly, we found the risk of CLAD at 3-year postlung transplant is similar across the FVC groups despite significantly lower CLAD free-survival. This is contrary to expectation that both rates of CLAD and mortality at 3-years would be higher in the group with the lowest FVC. This highlights the limitations in the sensitivity of spirometry in detecting allograft dysfunction especially among LT recipients with low FVC. In other words, among recipients with low FVC due to variety of reasons, allograft function may progressively deteriorate without getting detected eventually resulting in worse mortality risk. Further research is warranted to better define specific etiologies related to restrictive ventilatory defect which may portend worse posttransplant survival. Additionally, a customized management plan for each patient aimed at achieving a sustained level of FVC >80% predicted (for 3 wk or more) may also be worthwhile in an effort to improve the posttransplant survival.
Previous studies have looked at different outcome variables while evaluating the impact of lung functions.3,7,8 However, progression to CLAD (or Bronchiolitis obliterans syndrome) has been the most often used endpoint. While we included CLAD-free survival as one of the secondary outcome variables, we opted to analyze all-cause mortality at 3 years as the primary outcome variable. It is obvious that patients with low spirometry at baseline are at higher risk of progression to CLAD as a smaller absolute loss of lung function can result in these patients meeting the criteria for CLAD (in contrast to patients with higher baseline lung functions). However, the adverse impact of low lung functions is not limited to progression to CLAD alone. These patients, despite not meeting criteria for CLAD, have poor reserve in general and remain vulnerable to insults other than alloimmune processes such as infections, aspiration, and drug induced pneumonitis among others. Given these concerns, it was felt that all-cause mortality would be a more pertinent endpoint.
The comparative analysis of patients with low FVC revealed a combination of pretransplant and posttransplant variables that can contribute to allograft dysfunction leading to lower best lung functions. There was lack of association with demographic variables which is understandable given the comparisons were done for percent predicted volumes which accounts for the demographic variations. Similarly, lack of association with BMI at baseline is related to infrequent occurrence of obesity among these patients early after LT. In fact, while the absolute BMI at different time-points after transplant was similar among the 3 FVC groups, the proportional increase in median BMI was significantly more among patients with higher FVC perhaps indirectly reflecting better nutritional status and conditioning among these patients. The lack of association with diagnostic group was surprising although there appeared to be a trend favoring higher proportion of patients with obstructive disease in the higher FVC category. This is to be expected given the larger size of the chest cavities among these patients in comparison to restrictive diseases. Patients with lower listing and match LAS as well as those coming from home (in contrast to those in the hospital at the time of transplant) were likely to end up with higher FVC levels. This finding appears to be related to such patients being of higher acuity and severity of illness. Further, the interplay of grade 2–3 PGD, resulting in early and significant allograft dysfunction, complicated by the higher frequency of need for reintubation and longer ICU and hospital length of stay that lead to worse debility can all contribute towards limiting the level of FVC that can be achieved. Nevertheless, the association of PGD with low FVC may not be linear as the difference in the incidence of different PGD grades across the 3 groups did not reach statistical significance (Table 1). This is in consonance with the well-established link between late PGD with CLAD,13 which is believed to be due to the unmasking of epitopes on the alveolar epithelial lining due to the free radical injury which tends to trigger a premature, profound and sustained alloimmune response. In this regard, it is intriguing that development of grade 2–3 PGD is associated with early and persistent ill-effects on the allograft’s ability to achieve its full potential as assessed on spirometry.
The current study has some limitations. This was a retrospective chart review study evaluating associations that are vulnerable to interactions from hidden confounders. Such a design does not allow interpretations with regard to causality among the associations which must await further evaluation. While majority patients tend to achieve their best spirometry during the first year,1 sometimes the lung functions can continue to improve beyond the first year in which case some of the lung functions may not be truly “best” lung functions. Furthermore, FVC as a spirometry variable is nonspecific with regard to the etiology for low lung volume and markedly diverse conditions can impact it. For example, posttransplant debility or weight gain can both lead to lower FVC but can improve with time and effort with little contribution from the pulmonary parenchyma. However, neither of these flaws undermines the prognostic significance of the best FVC achieved during the first year and its utility as a potential target for patients to achieve after bilateral LT.
It is concluded that while a majority of patients with bilateral LT achieve >80% of their predicted lung functions on spirometry, a significant proportion do not. The best FVC achieved during the first year after bilateral LT has a strong and independent association with 3 year mortality. Efforts aimed at maximizing the FVC achieved during the first year may help to improve outcomes.
Footnotes
The authors declare no funding or conflicts of interest.
M.R.M. performed the research design, research, data analysis, and writing of article. R.K. performed the research design and research. H.G., S.B., J.M., J.J., V.K., and F.T. performed research. S.Z. performed research design and data analysis. A.B. performed research design, research, data analysis, and writing of article.
REFERENCES
- 1.Mason DP, Rajeswaran J, Li L, et al. Effect of changes in postoperative spirometry on survival after lung transplantation. J Thorac Cardiovasc Surg. 2012; 144:197–203 [DOI] [PubMed] [Google Scholar]
- 2.Kugler C, Fuehner T, Dierich M, et al. Effect of adherence to home spirometry on bronchiolitis obliterans and graft survival after lung transplantation. Transplantation. 2009; 88:129–134 [DOI] [PubMed] [Google Scholar]
- 3.Belloli EA, Wang X, Murray S, et al. Longitudinal forced vital capacity monitoring as a prognostic adjunct after lung transplantation. Am J Respir Crit Care Med. 2015; 192:209–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suhling H, Dettmer S, Rademacher J, et al. Spirometric obstructive lung function pattern early after lung transplantation. Transplantation. 2012; 93:230–235 [DOI] [PubMed] [Google Scholar]
- 5.Miller MR, Hankinson J, Brusasco V, et al. ; ATS/ERS Task Force Standardisation of spirometry. Eur Respir J. 2005; 26:319–338 [DOI] [PubMed] [Google Scholar]
- 6.Verleden GM, Glanville AR, Lease ED, et al. Chronic lung allograft dysfunction: definition, diagnostic criteria, and approaches to treatment-a consensus report from the pulmonary council of the ISHLT. J Heart Lung Transplant. 2019; 38:493–503 [DOI] [PubMed] [Google Scholar]
- 7.Todd JL, Jain R, Pavlisko EN, et al. Impact of forced vital capacity loss on survival after the onset of chronic lung allograft dysfunction. Am J Respir Crit Care Med. 2014; 189:159–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woodrow JP, Shlobin OA, Barnett SD, et al. Comparison of bronchiolitis obliterans syndrome to other forms of chronic lung allograft dysfunction after lung transplantation. J Heart Lung Transplant. 2010; 29:1159–1164 [DOI] [PubMed] [Google Scholar]
- 9.Arcasoy SM, Cordova FC. The fall in FVC after lung transplantation. A sentinel event. Am J Respir Crit Care Med. 2015; 192:127–128 [DOI] [PubMed] [Google Scholar]
- 10.Eberlein M, Permutt S, Chahla MF, et al. Lung size mismatch in bilateral lung transplantation is associated with allograft function and bronchiolitis obliterans syndrome. Chest. 2012; 141:451–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sato M, Hwang DM, Ohmori-Matsuda K, et al. Revisiting the pathologic finding of diffuse alveolar damage after lung transplantation. J Heart Lung Transplant. 2012; 31:354–363 [DOI] [PubMed] [Google Scholar]
- 12.Paraskeva M, McLean C, Ellis S, et al. Acute fibrinoid organizing pneumonia after lung transplantation. Am J Respir Crit Care Med. 2013; 187:1360–1368 [DOI] [PubMed] [Google Scholar]
- 13.Daud SA, Yusen RD, Meyers BF, et al. Impact of immediate primary lung allograft dysfunction on bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 2007; 175:507–513 [DOI] [PubMed] [Google Scholar]


