Abstract
Background
Acute declines in kidney function occur in approximately 20–30% of patients with acute decompensated heart failure, but its significance is unclear and importance of its context is not known. This study aimed to determine the prognostic value of a decline in kidney function in the context of decongestion among patients admitted with acute decompensated heart failure.
Methods
Using data from patients enrolled in the Ultrafiltration in Decompensated Heart Failure with Cardiorenal Syndrome Study (CARRESS) and Diuretic Optimization Strategies Evaluation (DOSE) trials, we used multivariable Cox regression models to evaluate the association between decline in estimated glomerular filtration rate (eGFR) and change in N-terminal pro-b-type natriuretic peptide (NT-proBNP) with the composite outcome, as well as testing for an interaction between the two.
Results
Among 435 patients, in-hospital decline in eGFR was not significantly associated with death and re-hospitalization (HR=0.89 per 30% decline, 95% CI 0.74, 1.07) while decline in NT-proBNP was associated with lower risk (HR=0.69 per halving, 95% CI 0.58, 0.83). There was a significant interaction (p = 0.003 unadjusted; p = 0.03 adjusted) between decline in eGFR and change in NT-proBNP where a decline in eGFR was associated with better outcomes when NT-proBNP declined (HR=0.78 per 30% decline in eGFR, 95% CI 0.61, 0.99), but not when NT-proBNP increased (HR=0.99, 95% CI 0.76, 1.30).
Conclusions
Decline in kidney function during therapy for acute decompensated heart failure is associated with improved outcomes as long as NT-proBNP levels are decreasing as well, suggesting that incorporation of congestion biomarkers may aid clinical interpretation of eGFR declines.
Introduction
Approximately 20–30% of patients admitted for acute decompensated heart failure will experience a decline in kidney function during the hospitalization.(1, 2) The pathophysiology of this decline is not well understood and its association with outcomes is controversial. While some studies suggest that decline in function is associated with adverse outcomes,(1, 3, 4) others indicate that decline can sometimes be associated with improved outcomes.(5–7) Declines in kidney function in the setting of acute decompensated heart failure can have multiple causes, and understanding the mechanism responsible for decline is likely to assist clinical decision making.
There have been many proposed mechanisms of kidney function decline in acute decompensated heart failure, including renal congestion from volume overload.(8–10) Improvements in kidney function have been observed following decongestion,(11) however, decongestion itself can sometimes lead to acute declines in estimated glomerular filtration rate (eGFR) as well.(12) Congestion and volume overload can be evaluated by biomarkers including brain natriuretic peptide (BNP) and its prohormone N-terminal prohormone of brain natriuretic peptide (NT-proBNP), which are released from cardiac myocytes in response to stretch from volume overload. A prior study did not find that the relation of eGFR decline and mortality was modified by baseline NT-proBNP level.(7) There are no data however, evaluating the relation between changes in kidney function and clinical outcomes in the context of changes in natriuretic peptide levels.
Because changes in congestion during therapy for acute decompensated heart failure may contribute to trends in renal function, we utilized patient data from the Ultrafiltration in Decompensated Heart Failure with Cardiorenal Syndrome Study (CARRESS) and the Diuretic Optimization Strategies Evaluation (DOSE) trials to evaluate whether an interaction exists between in-hospital changes in eGFR and NT-proBNP and subsequent clinical outcomes among patients being treated for acute decompensated heart failure.
Methods
Study Population and Design
The National Heart Lung and Blood Institute (NHLBI)-funded CARRESS(13) and DOSE(14) trials were two randomized controlled trials that investigated multiple decongestion strategies in patients with acute decompensated heart failure. Both studies enrolled patients hospitalized with manifestations of congestion, and had 60-day follow-up with similar intervals of biomarker measurement, allowing for pooling of the two patient cohorts to increase statistical power, as done in previous studies.(15, 16) Detailed enrollment criteria have been published previously.(14, 17) Serum creatinine thresholds for exclusion were >3.5 mg/dl in CARRESS and >3 mg/dl in DOSE. Importantly, patients enrolled in CARRESS were required to have evidence of declining kidney function at the time of enrollment (increase in serum creatinine by >0.3 mg/dl). Both trials excluded patients requiring vasoactive medications. In CARRESS, patients were randomized to either a stepped pharmacologic diuretic strategy or to ultrafiltration. In DOSE, patients were randomized in a 2×2 factorial fashion to either bolus or continuous intravenous furosemide, and to either low or high dose regimens. Serum creatinine and NT-proBNP levels were measured at randomization, as well as 96 hours into hospitalization for CARRESS and 72 hours for DOSE and then again on Day 7 or day of discharge if discharged prior to Day 7.(17) Patients without a baseline or follow-up creatinine (n=9) were excluded from the analysis.
Exposure
Estimates of GFR were determined using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula with serum creatinine.(18) Serum creatinine measurements in the original trials were analyzed at either a core or local laboratory, with labs drawn at the baseline visit measured at both facilities concomitantly. Deming regressions were used to relate local and core lab measurements separately for each trial, allowing determination of a correction factor (slope=1.06, intercept= −0.22, for CARRESS; slope=0.99, intercept= −0.06 for DOSE) which were applied to local lab results to allow for both consistency and inclusion of all local laboratory measurements. While the CKD-EPI equation has not yet been validated in the heart failure population, prior literature has shown its accuracy as compared to the Modification of Diet in Renal Disease Study equation in chronic kidney disease, the general population and heart failure.(18–20) Values of eGFR were transformed on the log scale given non-normal distribution and modeled as a continuous exposure. For comparison of baseline characteristics, patients were categorized according to whether there was a decline or increase in eGFR from baseline to hospitalization discharge values. Change was determined as the difference between the log-transformed baseline eGFR and the last in-hospital eGFR prior to discharge. Log transformation by base 10/7 enabled hazard ratios to be assessed per a 3/10 decline, synonymous to a 30% decline which has been demonstrated as a surrogate endpoint in chronic kidney disease literature.(21, 22)
As for NT-proBNP, all NT-proBNP samples were analyzed at the core laboratory facility. Baseline and follow-up values were transformed using log base 2 given the skewed distribution of the data. Change was determined as the difference between the log-transformed baseline NT-proBNP and last in-hospital NT-proBNP measurement obtained prior to discharge.
Outcomes
The primary outcome of interest was a composite endpoint of death from any cause or rehospitalization from any cause over the course of follow-up through day 60. The time at risk for the composite outcome began at the time of discharge from the initial hospitalization.
Covariates
Several covariates were selected as potential confounding variables in our regression analyses based on review of the literature and clinical relevance, including demographic characteristics (age, sex, race, body mass index [BMI]), comorbid conditions (hypertension, diabetes), medications (angiotensin-converting enzyme inhibitors [ACEI] or angiotensin II receptor blockers [ARB], loop diuretics), trial assignment (CARRESS, DOSE) as well as randomization arm for each trial. In-hospital change in weight was also included as a covariate, as were baseline eGFR and baseline NT-proBNP.
Statistical Analysis
Differences in the baseline characteristics of patients by whether they had an increase or decrease in their eGFR were examined using χ2 tests and the t-test for categorical and continouous variables, as appropriate. The functional relationship between change in eGFR, and change in NT-proBNP with the endpoint were evaluated using restricted cubic splines. Multivariable Cox proportional hazards regression models were used to evaluate the association between decline in eGFR on the continuous scale and the composite outcome, as well as the association between changes in NT-proBNP with the composite outcome. To evalate for effect modification, interaction testing between decline in eGFR and change in NT-proBNP was performed with the outcome. Hazards ratios of mortality were calculated based on a fixed eGFR decline of 30%, chosen on the basis of it being associated with increased risk of mortality and considered as a surrogate end point in trials of chronic kidney disease.(21, 22) As a sensitivity analysis, patients who were randomized to ultrafiltration (n=92) were excluded from the model.
Results
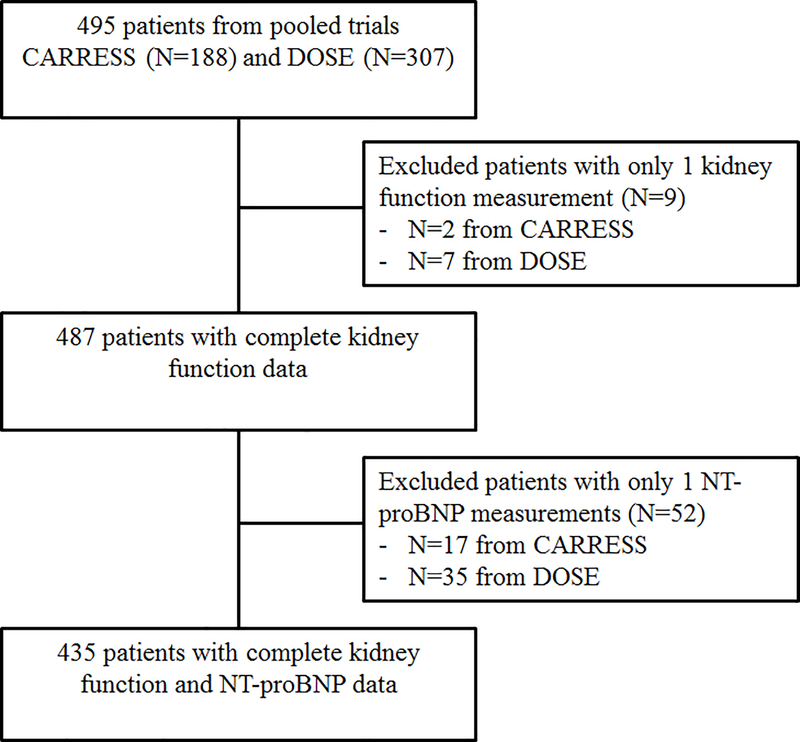
There were 435 patients with complete kidney function and NT-proBNP data available (Figure 1). Median (IQR) follow-up was 59 days (52, 64).
Figure 1.
Study patient population
Our analysis included 435 patients with complete data, including baseline and follow-up measurements of both NT-proBNP and of kidney function. CARRESS: Cardiorenal Rescue Study in Acute Decompensated Heart Failure; DOSE: Diuretic Optimization Strategies in Acute Heart Failure.
Baseline Patient Characteristics
Baseline characteristics for the pooled studies as well as separately by change in eGFR are presented in Table 1. Baseline characteristics by change in NT-proBNP are available in Supplemental Table S1. Median baseline serum creatinine was 1.67 mg/dl (IQR 1.21, 2.11), with median eGFR of 40.0 ml/min/1.73 m2 (29.4, 59.2) and 75.4% of patients had a baseline eGFR < 60 ml/min/1.73 m2. Baseline median NT-proBNP was 4509 pg/ml (IQR 2323, 9985). Among the pooled studies, 81.4% patients had hypertension and 58.2% had diabetes, which were similar in patients with and without in-hospital declines in eGFR. Most patients had baseline use of loop diuretics and 57.7% were taking either an ACEI or an ARB; there were no differences in baseline use of these medications amongst those with and without changes in eGFR (Table 1). There were similar distributions of both decline and increase in eGFR among all trial arms.
Table 1.
Baseline characteristics (total) and by change in estimated glomerular filtration rate (eGFR).
| Overall N=435 | In-Hospital eGFR Decline N=232 | In-Hospital eGFR Increase N=203 | |
|---|---|---|---|
| Age, years | 67.0 ± 13.2 | 66.6 ± 12.7 | 67.4 ± 13.9 |
| Female | 108 (24.8) | 66 (28.4) | 42 (20.7) |
| Black | 104 (23.9) | 53 (22.8) | 51 (25.1) |
| BMI, kg/m2 | 33.9 ± 9.5 | 35.2 ± 10.2 | 32.4 ± 8.4 |
| Ejection Fraction, % | 35.2 ± 17.6 | 38.0 ± 17.7 | 34.2 ± 17.4 |
| Ischemic etiology | 259 (59.5) | 138 (59.5) | 121 (59.6) |
| NYHA Functional Class | |||
| 2 | 10 ( 2.5) | 6 ( 2.8) | 4 ( 2.2) |
| 3 | 248 (61.8) | 131 (60.9) | 117 (62.9) |
| 4 | 143 (35.7) | 78 (36.3) | 65 (34.9) |
| Hypertension | 354 (81.4) | 193 (83.2) | 161 (79.3) |
| Diabetes | 253 (58.2) | 149 (64.2) | 104 (51.2) |
| Baseline Laboratory Results | |||
| NT-proBNP, pg/ml | 4509 [2323, 9985] | 4080 [2125, 7885] | 5178 [2511, 12905] |
| Creatinine, mg/dl | 1.67 [1.21, 2.11] | 1.52 [1.14, 2.04] | 1.75 [1.37, 2.22] |
| eGFR, ml/min/1.73 m2 | 40.0 [29.4, 59.2] | 41.7 [31.4, 65.1] | 37.3 [28.4, 53.9] |
| Hemoglobin, g/dl | 11.4 ± 1.9 | 11.4 ± 1.9 | 11.5 ± 2.0 |
| Medications | |||
| ACEI or ARB | 251 (57.7) | 131 (56.5) | 120 (59.1) |
| Aldosterone antagonist | 108 (24.8) | 66 (28.4) | 42 (20.7) |
| Loop diuretic | 423 (97.2) | 226 (97.4) | 197 (97.0) |
| CARRESS Trial | |||
| Ultrafiltration Arm | 83 (19.1) | 42 (18.5) | 41 (20.2) |
| Medical Therapy Arm | 86 (19.8) | 43 (18.5) | 43 (21.2) |
| DOSE Trial | |||
| Bolus, low dose | 67 (15.4) | 42 (18.1) | 41 (20.2) |
| Bolus, high dose | 69 (15.9) | 42 (18.1) | 27 (13.3) |
| Continuous, low dose | 64 (14.7) | 28 (12.1) | 36 (17.7) |
| Continuous, high dose | 66 (15.2) | 35 (15.1) | 31 (15.3) |
Values are represented by mean ± standard deviation, or median [25th and 75th interquartile range], or n (%).
Abbreviations: BMI, body mass index; NYHA, New York Heart Association; NT-proBNP, N-terminal prohormone of brain natriuretic peptide; eGFR, estimated glomerular filtration rate; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker
There were 61 patients in whom follow-up NT-proBNP data were missing, and among these, 9 were also missing follow-up kidney function measurements. Compared to patients with complete data, those with missing data had greater tendency to be women and self-reported non-white race and had higher baseline ACEI or ARB use (Supplemental Table S2). There were no differences in follow-up length of time between those with and without missing NT-proBNP data and these patients were similarly distributed between CARRESS and DOSE.
Outcomes
Over a median follow-up of 59 days (IQR 52, 64), there were 58 (12%) deaths and 217 patients (44%) experienced the composite outcome of death or hospitalization. Outcome rates were similar in the cohort of patients with missing follow-up NT-proBNP data.
In-hospital Changes in Biomarkers
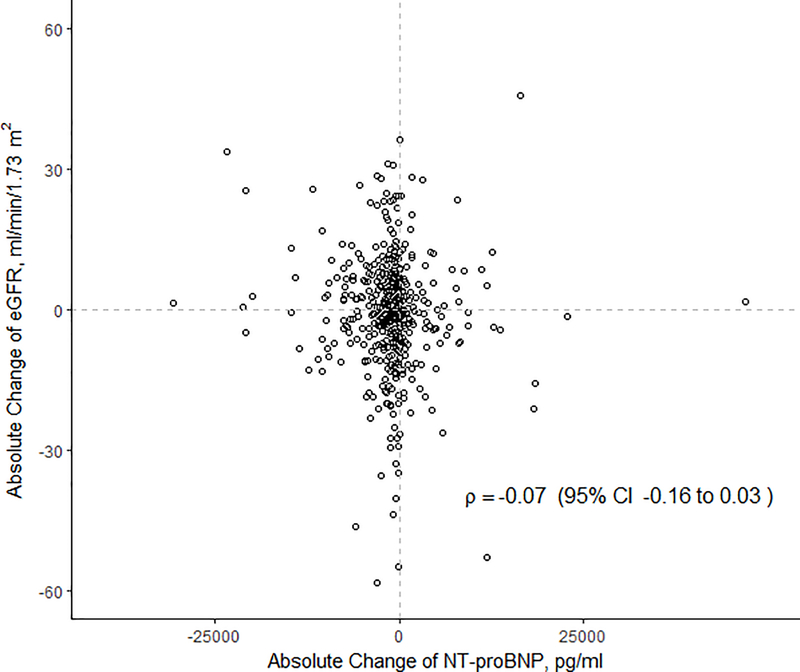
Median change in eGFR was −0.7 ml/min/1.73 m2 (IQR −6.8, 6.1 ml/min/1.73 m2) for the entire cohort, with 232 (53%) patients experiencing a decline in eGFR. Of those who had a decline, the median absolute decline was −6.1 ml/min/1.73m2 (IQR −11.9, −2.6). Median change in NT-proBNP was −841.3 pg/ml (IQR −2765.0, 106.5), with 71.3% of patients experiencing a decline in NT-proBNP levels. There was no correlation between changes in eGFR with changes in NT-proBNP (ρ = −0.07, p = 0.13) (Figure 2).
Figure 2.
Scatterplot for in-hospital changes in eGFR and NT-proBNP. Changes were calculated on the absolute scale from baseline to last measurement prior to discharge. Correlation coefficient ρ=−0.07, p=0.13. NT-proBNP: N-terminal prohormone of brain natriuretic peptide; eGFR: estimated glomerular filtration rate.
In-hospital Decline in eGFR
An in-hospital decline in eGFR was not significantly associated with the composite outcome in either univariate or multivariable models (HR=0.86 per every 30% decline in eGFR; 95% CI 0.72, 1.02; p=0.09 in univariate model; HR=0.89 per every 30% decline in eGFR; 95% CI 0.74, 1.07; p=0.21 in adjusted model) (Table 2). In-hospital declines in NT-proBNP were associated with lower hazard for the composite outcome, with a HR= 0.75 per halving of the change in NT-proBNP (95% CI 0.65, 0.86; p<0.001) in univariate analysis and HR=0.70 (95% CI 0.58, 0.83; p<0.001) in multivariable adjusted model.
Table 2.
Hazard ratio for composite outcome of death and re-hospitalization according to in-hospital changes in eGFR and changes in NT-proBNP
| N | N of Events | Unadjusted HR (95% CI) |
p-value | Adjusted HR‡ (95% CI) |
p-value | |
|---|---|---|---|---|---|---|
| IN-HOSPITAL DECLINE | ||||||
| Decline eGFR (per 30% decrease) | 435 | 197 | 0.86 (0.72, 1.02) | 0.09 | 0.89 (0.74, 1.07) | 0.21 |
| Decline NT-proBNP (per halving) | 435 | 197 | 0.75 (0.65, 0.86) | <0.001 | 0.70 (0.58, 0.83) | <0.001 |
| Decline eGFR x Decline NT-proBNP | 435 | 197 | - | 0.002 | - | 0.03 |
| eGFR DECLINE (per 30%) | ||||||
| Concomitant Decline NT-proBNP | 435 | 197 | 0.71 (0.57, 0.90) | 0.004 | 0.78 (0.61, 0.99) | 0.04 |
| Concomitant Increase NT-proBNP | 435 | 197 | 1.07 (0.84, 1.36) | 0.59 | 0.99 (0.76, 1.29) | 0.94 |
Cox proportional hazards models used to evaluate association between decline in biomarkers (eGFR and NT-proBNP) with composite outcome of death and re-hospitalization. Model was also fitted with an interaction term between decline in eGFR and decline in NT-proBNP (p=0.03 for interaction in adjusted models). Log transformation of eGFR by base 10/7 enabled hazard ratios to be assessed per a 3/10 decline, synonymous to a 30%.
adjusted for age, race, sex, BMI, trial, randomization arm, baseline NT-proBNP, use of ACEI or ARBs, and baseline eGFR, change in weight.
Abbreviations: BMI, body mass index; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; NT-proBNP, N-terminal prohormone of brain natriuretic peptide; eGFR, estimated glomerular filtration rate;
Interaction between Decline in eGFR and Change in NT-proBNP
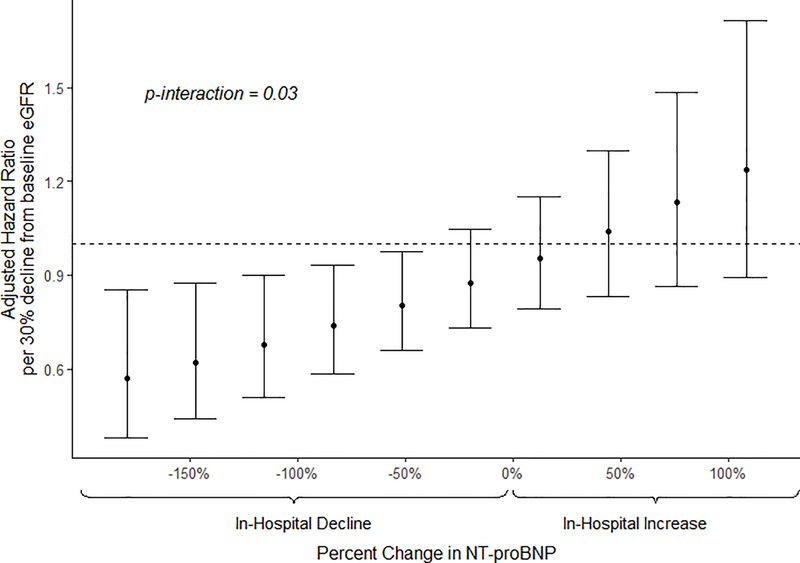
There was a significant interaction between in-hospital decline in eGFR and change in NT-proBNP (p=0.002 in unadjusted model; p=0.03 in adjusted model). A decline in eGFR was associated with significantly lower hazard of the composite outcome when NT-proBNP also declined (Table 2). When NT-proBNP declined, each 30% decline in eGFR was associated with a 22% reduction in the composite outcome (HR=0.78; 95% CI 0.61, 0.99; p=0.04). In contrast, when NT-proBNP increased, each 30% decline in eGFR had no association with outcomes (HR=0.99; 95% CI 0.76, 1.29; p=0.94). Larger declines in NT-proBNP were associated with progressively decreased risks of the composite outcome for each 30% decline in eGFR (Figure 3).
Figure 3.
Adjusted hazard ratios for composite outcome of death or re-hospitalization for each 30% decline in eGFR by varying levels of change in NT-ProBNP.
Multivariable adjusted Cox regression model including the interaction (p=0.03) between decline in eGFR and change in NT-proBNP. Both eGFR and NT-proBNP were log-transformed; eGFR by base 10/7 enabling hazard ratios to be assessed for a 3/10 decline, synonymous to a 30% decline. HR: hazard ratio; eGFR: estimated glomerular filtration rate; NT-proBNP: N-terminal prohormone of brain natriuretic peptide.
When patients randomized to ultrafiltration were removed from the analysis (n=92), the interaction remained significant and direction of the relationship the same (HR=0.66 for every 30% decline in eGFR as long as NT-proBNP decreased [95% CI 0.47, 0.91]; HR=0.95 for every 30% decline in eGFR if NT-proBNP increased [95% CI 0.68, 1.33]).
Discussion
This study demonstrates that acute decline in eGFR during hospitalization for acute decompensated heart failure was not significantly associated with death and re-hospitalization in the two months following discharge. However, there was a significant interaction between decline in eGFR and change in NT-proBNP such that decline in eGFR was associated with lower risk for death or readmission as long as there was also a decline in NT-proBNP. Progressively greater degrees of decline in NT-proBNP levels were associated with greater reductions in the risk of death and re-hospitalization associated with a 30% eGFR decline. This supports the notion that decongestion needs to be taken into account when evaluating the prognostic importance of a declining eGFR during therapy for acute decompensated heart failure.
These current findings add to the growing body of literature supporting the concept that the mechanism underlying the acute decline in eGFR is vital to interpreting the prognostic implications of the decline. These data are consistent with those from an analysis of the Placebo-Controlled Randomized Study of the Selective A1 Adenosine Receptor Antagonist Rolofylline for Patients Hospitalized With Acute Decompensated Heart Failure and Volume Overload to Assess Treatment Effect on Congestion and Renal Function (PROTECT) trial, which found that among patients who had an increase in serum creatinine of ≥ 0.3 mg/dl during their hospital admission, only those that were volume-overloaded at the time of the measurement were found to have higher risk of death or readmission over 30 days.(23) An analysis of the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness trial (ESCAPE) also demonstrated that hemoconcentration, as a proxy for decongestion, was associated with acute decline in kidney function as well as with lower mortality.(24) In the current analysis, decline in eGFR was associated with decreased risk of a composite outcome of death and re-hospitalization as long as there was a concominant decline in NT-proBNP, supporting the notion that decongestion, even at the cost of a decline in eGFR, is an important priority for outcomes in patients with acute decompensated heart failure.
Understanding the relation between kidney function and biomarkers of congestion could potentially guide management of patients with acute decompensated heart failure who have acute eGFR changes. Natriuretic peptides, which are released from cardiac myocytes in response to stretch from volume overload, are routinely measured and readily available. High levels of circulating BNP and NT-proBNP have been associated with higher rates of mortality and re-hospitalization,(25) and lack of reduction in natriuretic peptides levels during decongestion therapy is associated with worse survival.(26, 27) The present findings suggest that decline in eGFR could be considered a benign event as long as the NT-proBNP levels are also decreasing, thus providing reassurance to the treating physician and helping to guide care and subsequent clinical decision making. The finding of a significant interaction between decline in eGFR and change in NT-proBNP could also explain prior conflicting results regarding the impact of decline in kidney function, as some studies had found that it was associated with poor clinical outcomes(1–4) and others with improved outcomes.(24) A separate analysis of the DOSE trial noted that an in-hospital decline in kidney function from day 1 to 3 similarly was not associated with worse outcomes. Tests for interaction in that analysis were performed between decline in eGFR and baseline NT-proBNP levels, in contrast to the current study which utilized in-hospital change in NT-proBNP levels.(7) While baseline NT-proBNP levels were not found to have a modifying effect on decline in kidney function, our study suggests that changes in in-hospital NT-proBNP levels do have a significant modifying effect on decline in eGFR.
Natriuretic peptides are established biomarkers that aid in the diagnosis of acute decompensated heart failure, with levels correlating with severity of heart failure.(29, 30) Other methods to assess for changes in congestion status are limited, and clinical assessment of physical exam findings have been shown to have poor sensitivity especially if cardiac valvular disease is present.(31, 32) In clinical practice, some patients may have invasive hemodynamic monitoring via pulmonary artery catheterization, but for the majority of patients admitted with acute decompensated heart failure, these data may not be readily available. Direct methods of measuring plasma volume are available, but challenging to obtain and not yet widely used. Daily weights are also difficult to obtain accurately, especially in patients who are critically ill and bed-bound, as can often be the case for patients with chronic heart failure. Use of hemoconcentration, such as was examined in the analysis of the ESCAPE trial data,(24) can serve as a proxy of decongestion but changes can be subtle and are influenced by a multitude of other factors that occur frequently in this population such as bleeding, iron deficiency or bone marrow suppression.
There are a number of limitations to this analysis. The composite outcome is predominantly driven by recurrent hospitalizations and is underpowered to evaluate the impact of changes in kidney function on mortality alone. Nevertheless, available data supports that re-hospitalizations are closely tied to increased mortality in patients with advanced heart failure.(33, 34) The patient populations comprising CARRESS and DOSE were slightly different given that patients from CARRESS required demonstration of decline in kidney function to meet inclusion criteria. We performed a complete case analysis without imputation for missing follow-up NT-proBNP data, which can have potential for bias; however, patients with missing follow-up data did not have differing lengths of follow-up or different event rates from those with complete follow-up data, suggesting that missingness was not conditional on other variables.
Conclusions
Declines in kidney function in the setting of targeted decongestion for acute decompensated heart failure appears to be a benign event as long as decongestion is being achieved. Incorporation of objective measures of biomarkers of congestion, such as NT-proBNP, may add to clinical decisions regarding interpretation and management of acute declines in eGFR. Both serum creatinine and natriuretic peptides such as BNP and NT-proBNP are readily available and could provide guidance for patients with acute decompensated heart failure and changes in eGFR.
Supplementary Material
Acknowledgements
The research was supported by NIH/NIDDK training grant T32-DK07777. Manuscript was prepared using data set provided by the NHLBI and does not necessarily reflect the opinions or views of CARRESS, DOSE, or the NHLBI.
Sources of Funding:
NIH Training Grant T32 DK007777
Footnotes
Declarations of Interest: none
Authorship: All authors had access to the data and a role in writing this manuscript
References
- 1.Forman DE, Butler J, Wang Y, et al. Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. Journal of the American College of Cardiology. 2004;43(1):61–7. Epub 2004/01/13. [DOI] [PubMed] [Google Scholar]
- 2.Damman K, Navis G, Voors AA, et al. Worsening renal function and prognosis in heart failure: systematic review and meta-analysis. Journal of cardiac failure. 2007;13(8):599–608. Epub 2007/10/10. doi: 10.1016/j.cardfail.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Fonarow GC, Adams KF Jr., Abraham WT, Yancy CW, Boscardin WJ. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. Jama. 2005;293(5):572–80. Epub 2005/02/03. doi: 10.1001/jama.293.5.572. [DOI] [PubMed] [Google Scholar]
- 4.Hillege HL, Girbes AR, de Kam PJ, et al. Renal function, neurohormonal activation, and survival in patients with chronic heart failure. Circulation. 2000;102(2):203–10. Epub 2000/07/13. [DOI] [PubMed] [Google Scholar]
- 5.Testani JM, Kimmel SE, Dries DL, Coca SG. Prognostic importance of early worsening renal function after initiation of angiotensin-converting enzyme inhibitor therapy in patients with cardiac dysfunction. Circulation Heart failure. 2011;4(6):685–91. Epub 2011/09/10. doi: 10.1161/circheartfailure.111.963256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiernan MS, Gregory D, Sarnak MJ, et al. Early and late effects of high- versus low-dose angiotensin receptor blockade on renal function and outcomes in patients with chronic heart failure. JACC Heart failure. 2015;3(3):214–23. Epub 2015/03/07. doi: 10.1016/j.jchf.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Brisco MA, Zile MR, Hanberg JS, et al. Relevance of Changes in Serum Creatinine During a Heart Failure Trial of Decongestive Strategies: Insights From the DOSE Trial. Journal of cardiac failure. 2016;22(10):753–60. Epub 2016/07/05. doi: 10.1016/j.cardfail.2016.06.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mullens W, Abrahams Z, Francis GS, et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. Journal of the American College of Cardiology. 2009;53(7):589–96. Epub 2009/02/14. doi: 10.1016/j.jacc.2008.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burnett JC Jr., Knox FG. Renal interstitial pressure and sodium excretion during renal vein constriction. The American journal of physiology. 1980;238(4):F279–82. Epub 1980/04/11. doi: 10.1152/ajprenal.1980.238.4.F279. [DOI] [PubMed] [Google Scholar]
- 10.Nohria A, Hasselblad V, Stebbins A, et al. Cardiorenal interactions: insights from the ESCAPE trial. Journal of the American College of Cardiology. 2008;51(13):1268–74. Epub 2008/03/29. doi: 10.1016/j.jacc.2007.08.072. [DOI] [PubMed] [Google Scholar]
- 11.Testani JM, McCauley BD, Chen J, et al. Clinical characteristics and outcomes of patients with improvement in renal function during the treatment of decompensated heart failure. Journal of cardiac failure. 2011;17(12):993–1000. Epub 2011/11/30. doi: 10.1016/j.cardfail.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greene SJ, Gheorghiade M, Vaduganathan M, et al. Haemoconcentration, renal function, and post-discharge outcomes among patients hospitalized for heart failure with reduced ejection fraction: insights from the EVEREST trial. European journal of heart failure. 2013;15(12):1401–11. Epub 2013/07/13. doi: 10.1093/eurjhf/hft110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bart BA, Goldsmith SR, Lee KL, et al. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. The New England journal of medicine. 2012;367(24):2296–304. Epub 2012/11/08. doi: 10.1056/NEJMoa1210357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Felker GM, Lee KL, Bull DA, et al. Diuretic strategies in patients with acute decompensated heart failure. The New England journal of medicine. 2011;364(9):797–805. Epub 2011/03/04. doi: 10.1056/NEJMoa1005419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mentz RJ, Stevens SR, DeVore AD, et al. Decongestion strategies and renin-angiotensin-aldosterone system activation in acute heart failure. JACC Heart failure. 2015;3(2):97–107. Epub 2014/12/30. doi: 10.1016/j.jchf.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lala A, McNulty SE, Mentz RJ, et al. Relief and Recurrence of Congestion During and After Hospitalization for Acute Heart Failure: Insights From Diuretic Optimization Strategy Evaluation in Acute Decompensated Heart Failure (DOSE-AHF) and Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARESS-HF). Circulation Heart failure. 2015;8(4):741–8. Epub 2015/06/05. doi: 10.1161/circheartfailure.114.001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bart BA, Goldsmith SR, Lee KL, et al. Cardiorenal rescue study in acute decompensated heart failure: rationale and design of CARRESS-HF, for the Heart Failure Clinical Research Network. Journal of cardiac failure. 2012;18(3):176–82. Epub 2012/03/06. doi: 10.1016/j.cardfail.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. The New England journal of medicine. 2012;367(1):20–9. Epub 2012/07/06. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manzano-Fernandez S, Flores-Blanco PJ, Perez-Calvo JI, et al. Comparison of risk prediction with the CKD-EPI and MDRD equations in acute decompensated heart failure. Journal of cardiac failure. 2013;19(8):583–91. Epub 2013/08/06. doi: 10.1016/j.cardfail.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 20.Oh J, Kang SM, Hong N, et al. The CKD-EPI is more accurate in clinical outcome prediction than MDRD equation in acute heart failure: data from the Korean Heart Failure (KorHF) Registry. International journal of cardiology. 2013;167(3):1084–7. Epub 2012/11/30. doi: 10.1016/j.ijcard.2012.10.054. [DOI] [PubMed] [Google Scholar]
- 21.Coresh J, Turin TC, Matsushita K, et al. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. Jama. 2014;311(24):2518–31. Epub 2014/06/04. doi: 10.1001/jama.2014.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levey AS, Inker LA, Matsushita K, et al. GFR decline as an end point for clinical trials in CKD: a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2014;64(6):821–35. Epub 2014/12/03. doi: 10.1053/j.ajkd.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 23.Metra M, Cotter G, Senger S, et al. Prognostic Significance of Creatinine Increases During an Acute Heart Failure Admission in Patients With and Without Residual Congestion: A Post Hoc Analysis of the PROTECT Data. Circulation Heart failure. 2018;11(5):e004644 Epub 2018/05/12. doi: 10.1161/circheartfailure.117.004644. [DOI] [PubMed] [Google Scholar]
- 24.Testani JM, Chen J, McCauley BD, Kimmel SE, Shannon RP. Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation. 2010;122(3):265–72. Epub 2010/07/08. doi: 10.1161/circulationaha.109.933275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santaguida PL, Don-Wauchope AC, Oremus M, et al. BNP and NT-proBNP as prognostic markers in persons with acute decompensated heart failure: a systematic review. Heart failure reviews. 2014;19(4):453–70. Epub 2014/07/27. doi: 10.1007/s10741-014-9442-y. [DOI] [PubMed] [Google Scholar]
- 26.Michtalik HJ, Yeh HC, Campbell CY, et al. Acute changes in N-terminal pro-B-type natriuretic peptide during hospitalization and risk of readmission and mortality in patients with heart failure. The American journal of cardiology. 2011;107(8):1191–5. Epub 2011/02/08. doi: 10.1016/j.amjcard.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 27.Kubler P, Jankowska EA, Majda J, et al. Lack of decrease in plasma N-terminal pro-brain natriuretic peptide identifies acute heart failure patients with very poor outcome. International journal of cardiology. 2008;129(3):373–8. Epub 2007/12/07. doi: 10.1016/j.ijcard.2007.07.126. [DOI] [PubMed] [Google Scholar]
- 28.Felker GM, Anstrom KJ, Adams KF, et al. Effect of Natriuretic Peptide-Guided Therapy on Hospitalization or Cardiovascular Mortality in High-Risk Patients With Heart Failure and Reduced Ejection Fraction: A Randomized Clinical Trial. Jama. 2017;318(8):713–20. Epub 2017/08/23. doi: 10.1001/jama.2017.10565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maisel AS, Krishnaswamy P, Nowak RM, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. The New England journal of medicine. 2002;347(3):161–7. Epub 2002/07/19. doi: 10.1056/NEJMoa020233. [DOI] [PubMed] [Google Scholar]
- 30.Kazanegra R, Cheng V, Garcia A, et al. A rapid test for B-type natriuretic peptide correlates with falling wedge pressures in patients treated for decompensated heart failure: a pilot study. Journal of cardiac failure. 2001;7(1):21–9. Epub 2001/03/27. doi: 10.1054/jcaf.2001.23355. [DOI] [PubMed] [Google Scholar]
- 31.Wang CS, FitzGerald JM, Schulzer M, Mak E, Ayas NT. Does this dyspneic patient in the emergency department have congestive heart failure? Jama. 2005;294(15):1944–56. Epub 2005/10/20. doi: 10.1001/jama.294.15.1944. [DOI] [PubMed] [Google Scholar]
- 32.Butman SM, Ewy GA, Standen JR, Kern KB, Hahn E. Bedside cardiovascular examination in patients with severe chronic heart failure: importance of rest or inducible jugular venous distension. Journal of the American College of Cardiology. 1993;22(4):968–74. Epub 1993/10/01. [DOI] [PubMed] [Google Scholar]
- 33.Solomon SD, Dobson J, Pocock S, et al. Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation. 2007;116(13):1482–7. Epub 2007/08/29. doi: 10.1161/circulationaha.107.696906. [DOI] [PubMed] [Google Scholar]
- 34.Lee DS, Austin PC, Stukel TA, et al. “Dose-dependent” impact of recurrent cardiac events on mortality in patients with heart failure. The American journal of medicine. 2009;122(2):162–9.e1. Epub 2008/12/23. doi: 10.1016/j.amjmed.2008.08.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.