Supplemental Digital Content is available in the text
Keywords: diabetes Mellitus, glycemic Load, lipids, meta-Analysis, niacin, type 2
Abstract
Background:
Lipid profiles and glycemic control play a critical role in subsequent atherosclerotic cardiovascular disease for patients with type 2 diabetes mellitus (T2DM). This study aimed to evaluate the effectiveness of niacin supplementation on lipid profiles and glycemic control for patients with T2DM.
Methods:
The PubMed, Embase, and the Cochrane Library databases were searched to identify randomized controlled trials (RCTs) that investigated the effects of niacin supplementation for patients with T2DM throughout December 2019. The weighted mean differences (WMDs) with 95% confidence intervals (CIs) were applied to calculate the pooled effect estimates using a random-effects model.
Results:
Eight RCTs comprised a total of 2110 patients with T2DM who were selected for final quantitative analysis. The patients’ niacin supplementation was associated with lower levels of total cholesterol (WMD, −0.28; 95% CI, −0.44 to −0.12; P = .001), triglyceride (WMD, −0.37; 95% CI, −0.52 to −0.21; P < .001), and low-density lipoprotein (WMD, −0.42; 95% CI, −0.50 to −0.34; P < .001). Moreover, the level of high-density lipoprotein was significantly increased when niacin supplementation (WMD, 0.33; 95% CI, 0.21 to 0.44; P < .001) was provided. However, niacin supplementation produced no significant effects on plasma glucose (WMD, 0.18; 95% CI, −0.14 to 0.50; P = .275) and hemoglobin A1c (HbA1c) levels (WMD, 0.39; 95% CI, −0.15 to 0.94; P = .158).
Conclusions:
This study found that niacin supplementation could improve lipid profiles without affecting the glycemic levels for patients with T2DM. Additional large-scale RCTs should be conducted to evaluate the long-term effectiveness of niacin supplementation.
1. Introduction
Type 2 diabetes mellitus (T2DM) involves a group of disorders of intermediary metabolism and is characterized by glucose intolerance.[1] Patients diagnosed with T2DM have increased risk of death, cardiovascular disease, blindness, kidney, and lower-limb amputation.[2] Presently, there are >400 million adults with T2DM worldwide; this number is increasing rapidly and causing substantial economic costs.[3] The increased prevalence of T2DM is correlated with increased body fat and inactivity,[4] which often accompanies atherogenic dyslipidemia and is associated with high plasma triglycerides (TG) and lower high-density lipoprotein (HDL) concentrations.[5] Statins are widely used for improving lipid profiles and further reducing cardiovascular risk, while residual risk remains due to the modest effects of statins on plasma TG and HDL concentrations.[6] Therefore, additional management strategies should be identified to further improve lipid profiles in patients with T2DM.
Niacin is an essential B vitamin that plays an important role in increasing HDL, lowering plasma TG, and low-density lipoprotein (LDL).[7–9] Studies have already proven that niacin supplementation could regress coronary atherosclerosis and prevent risk of cardiac death.[10,11] However, whether niacin supplementation could produce additional clinical benefits in patients with dyslipidemia treated with statins remains controversial.[12–14] Clarifying the effects of niacin supplementation is particularly important in patients with T2DM because of the high prevalence of dyslipidemia in these patients, and because this concern has not yet been addressed. Therefore, this meta-analysis based on randomized controlled trials (RCTs) was conducted to evaluate the effects of niacin supplementation on lipid profiles and glycemic control in patients with T2DM.
2. Materials and methods
2.1. Data sources, search strategy, and selection criteria
This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis.[15] RCTs investigated the effects of niacin supplementation on lipid profiles and glycemic control for patients with T2DM who were identified as eligible participants for this study, without restriction on published language. The effectiveness of niacin supplementation on total cholesterol (TC), TG, LDL, HDL, plasma glucose, and HbA1c was examined. The electronic databases of PubMed, Embase, and the Cochrane Central Register of Controlled Trials were systematically searched for eligible studies throughout December 2019, using the following search terms: (niacin or nicotinic acid or NIASPAN or vitamin B3) and diabetes. Moreover, the hand-searches were performed by reviewing the reference lists from the retrieved studies to select any potential eligible studies. Ethics statement is not applicable in this study.
The literature search and study selection were conducted by two authors following a standardized flow, and conflicts between authors were discussed until a consensus was reached. The inclusion criteria of this study based on Patients, Intervention, Control, Outcomes, and Study design (PICOS), and the details of the inclusion criteria are listed as follows: patients: all of the included patients diagnosed with T2DM; intervention: niacin supplementation, irrespective of dosage or other background therapies; control: placebo or no treatment, and the background therapies that were consistent with the intervention group; outcomes: at least 1 of TC, TG, LDL, HDL, plasma glucose, and HbA1c should be reported; and study design: RCT design.
2.2. Data collection and quality assessment
Two authors independently extracted the data from the included studies following a standardized protocol, and a third data check was conducted for disagreement by reading the original article. The collected variables were derived from the included studies, for example, first author's name, publication year, country, study design, sample size, mean age, percentage male, body mass index, HbA1c, fasting blood glucose, intervention, control, follow-up, and investigated outcomes. Moreover, the study quality was assessed using the Jadad scale.[16] The Jadad scale based on randomization, blinding, allocation concealment, withdrawals and dropouts, use of intention-to-treat analysis, and the coring system was in the range of 0 to 5. The study quality was conducted by 2 authors, and any inconsistency was settled by discussion until a consensus was reached.
2.3. Statistical analysis
The treatment effectiveness of niacin supplementation on lipid profiles and glycemic control for patients with T2DM was assigned as continuous data, and the weighted mean differences (WMDs) with 95% confidence intervals (CIs) were calculated before the data pooling. A random-effects model was applied for pooling the effect estimates of niacin supplementation.[17,18] A heterogeneity test was performed using I2 and Q statistics, and I2 > 50.0% or P < .10 was considered as significant heterogeneity.[19,20] The stability of pooled conclusions was tested using a sensitivity analysis,[21] and the sources of heterogeneity were explored using a subgroup analysis. Moreover, the difference between the subgroups was tested using interaction P test, which assumed that the data distribution met the normality.[22] Publication bias was tested using the funnel plot, Egger and Begg tests.[23,24] The inspection levels are 2-sided, and P < .05 was considered as the pooled conclusion with statistical significance. All of the statistical analyses were conducted using STATA software (version 10.0, Stata Corporation, College Station, TX).
3. Results
3.1. Literature search
A total of 541 records were identified by initial electronic database searches, and 352 were excluded because of duplicate topics. The remaining 189 studies were retrieved for review of titles and abstracts, and 138 articles were excluded because of irrelevant topics. A total of 51 studies were retrieved for full-text evaluations, and eight studies were selected for final pooled analyses.[25–32] The manual searches for the reference lists of retrieved studies did not yield new eligible studies. A Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart for study selection is shown in Figure 1.
Figure 1.
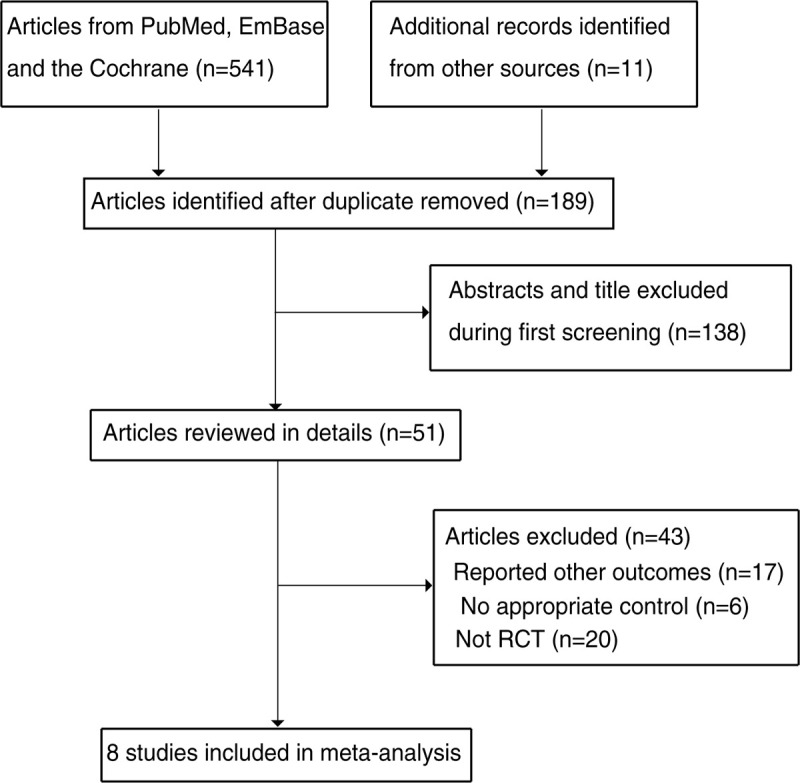
The PRISMA flowchart for the study selection process.
3.2. Study characteristics
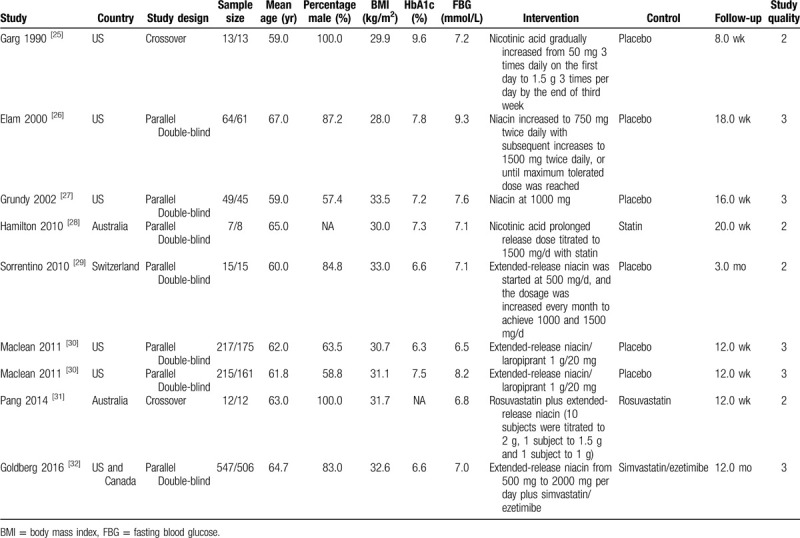
The baseline characteristics of studies and patients are shown in Table 1. Of the eight included studies, a total of 2110 patients with T2DM were recruited. Studies published ranged from 1990 to 2016, and 15 to 1053 patients were included in each individual trial. Six studies were designed as parallel double-blind trials, and the remaining 2 studies were designed as crossover trials. Five studies were conducted in the United States or Canada, 2 studies were conducted in Australia, and the remaining 1 study was conducted in Switzerland. Three studies combined niacin with statins, while the remaining 5 studies used niacin alone. The follow-up duration ranged from 8.0 weeks to 12.0 months. The study quality was assessed using the Jadad scale, 4 studies had 3 scores, and the remaining 4 studies had 2 scores.
Table 1.
Baseline characteristics of studies included in the meta-analysis.
3.3. TC
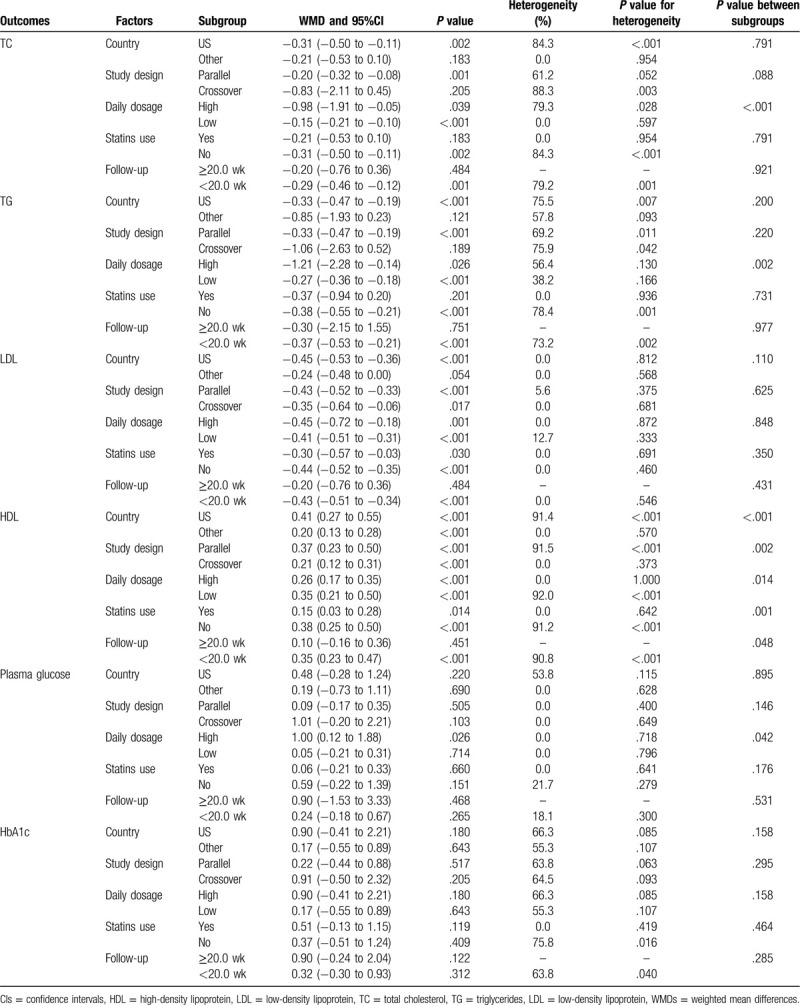
The data for the effect of niacin supplementation on TC were available in 5 trials. The pooled results suggested that niacin supplementation significantly reduced the level of TC (WMD, −0.28; 95% CI, −0.44 to −0.12; P = .001) (Fig. 2), and that significant heterogeneity was detected across the included trials (I2 = 74.0%; P = .002). The sensitivity analysis indicated that the pooled conclusion was stability (Supplemental Digital Content 1). The significant differences between the niacin and the control groups were observed in mostly the subgroups, while niacin supplementation was not associated with the level of TC if the studies were conducted in Australia or Switzerland, if the studies were designed as crossover studies, if they were combined with statins, or if follow-up duration was ≥20.0 weeks (Table 2). Moreover, the difference of TC between the niacin and the control groups could be affected by the niacin dosage (P < .001). No significant publication bias for TC was detected (P value for Egger, .091; P value for Begg, .452) (Supplemental Digital Content 2).
Figure 2.
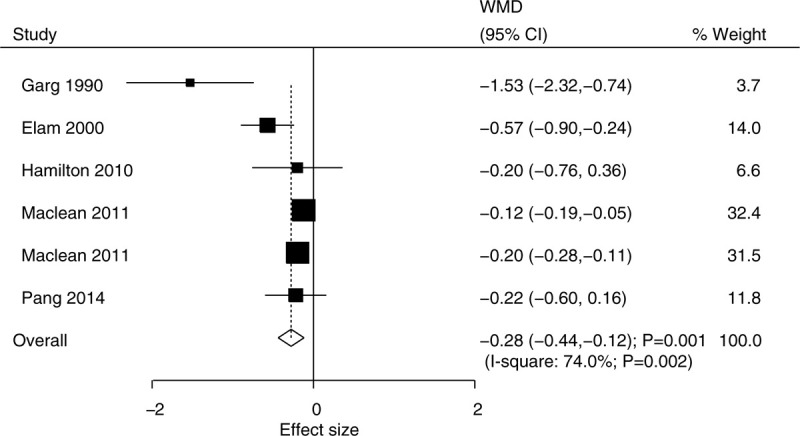
Effect of niacin supplementation on TC. TC = total cholesterol.
Table 2.
Subgroup analyses for lipid profiles and glucose.
3.4. TG
The data for the effect of niacin supplementation on TG were available in 6 trials. We noted that niacin supplementation was associated with lower TG level (WMD, –0.37; 95% CI, −0.52 to −0.21; P < .001) (Fig. 3), and that significant heterogeneity was shown among the included trials (I2 = 67.8%; P = .005). The pooled conclusion was stability and was not altered by excluding the individual trial (Supplemental Digital Content 1). There were significant differences between the niacin and the control groups on TG in mostly the subgroups, while there were no significant differences between the niacin and the control groups if the studies were conducted in Australia or Switzerland, if the studies were designed as crossover studies, if they were combined with statins, or if follow-up duration was ≥20.0 weeks (Table 2). Moreover, the effect of niacin supplementation on TG could be affected by the niacin dosage (P = .002). Although the Begg test indicated no significant publication bias for TG (P = .548), significant publication was detected by using the Egger test (P = .029). The conclusion was not changed after the adjustment of publication bias using the trim and fill method (Supplemental Digital Content 2).
Figure 3.
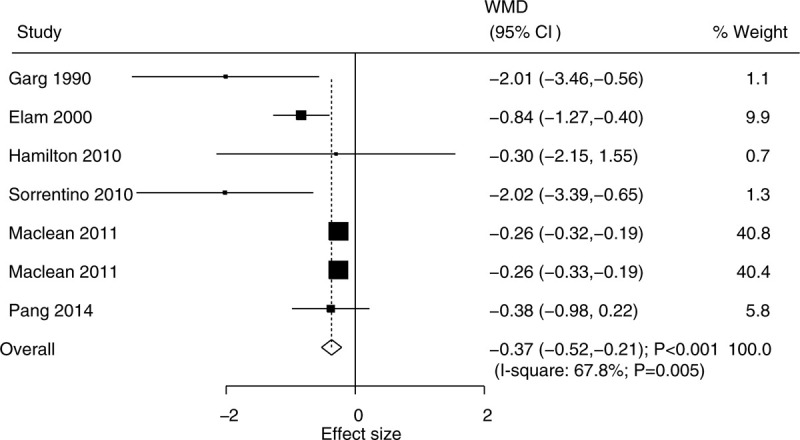
Effect of niacin supplementation on TG. TG = triglycerides.
3.5. LDL
The data for determining the effect of niacin supplementation on LDL were available in 6 trials. The pooled WMD suggested that niacin supplementation significantly reduced the level of LDL (WMD, −0.42; 95% CI, −0.50 to −0.34; P < .001) (Fig. 4), and that there was no evidence of heterogeneity (I2 = 0.0%; P = .590). The conclusion was not altered by sequentially excluding individual trial (Supplemental Digital Content 1). Although significant differences between the groups were observed in mostly the subgroups, we noted that niacin supplementation did not affect the level of LDL when the studies were conducted in Australia or Switzerland, or when there was follow-up duration of ≥ 20.0 weeks (Table 2). There was no significant publication bias for LDL (P value for Egger: .167; P value for Begg: .548) (Supplemental Digital Content 2).
Figure 4.
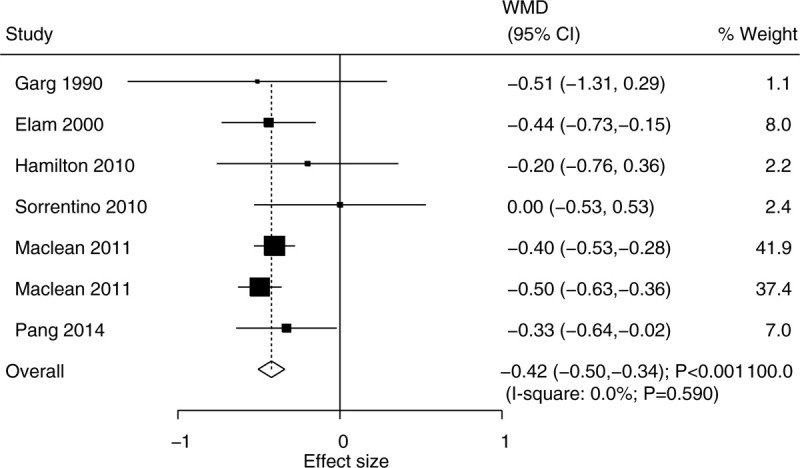
Effect of niacin supplementation on LDL. LDL = low-density lipoprotein.
3.6. HDL
The data for determining the effect of niacin supplementation on HDL were available in 7 trials. We noted that the HDL level was significantly increased when the patients were treated with niacin (WMD, 0.33; 95% CI, 0.21–−0.44; P < .001) (Fig. 5), and that there was significant heterogeneity across the included trials (I2 = 89.8%; P < .001). The pooled conclusion was robustness and was not affected by excluding any particular trial (Supplemental Digital Content 1). We noted that niacin supplementation significantly increased the HDL level in mostly the subgroups except for the follow-up duration of ≥20.0 weeks (Table 2). Moreover, the effect of niacin supplementation on HDL could be affected by country (P < 0.001), study design (P = .002), daily dosage (P = .014), use of statins (P = .001), and follow-up duration (P = .048). No significant publication bias for HDL was observed (P value for Egger: .571; P value for Begg: .319) (Supplemental Digital Content 2).
Figure 5.
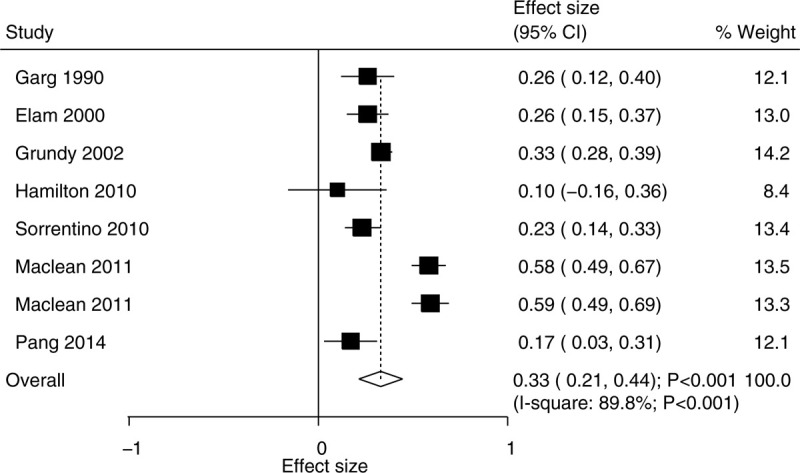
Effect of niacin supplementation on HDL. HDL = high-density lipoprotein.
3.7. Plasma glucose
The data for determining the effect of niacin supplementation on plasma glucose were available in 6 trials. There was no significant difference between the niacin and the control groups for the level of plasma glucose (WMD, 0.18; 95% CI, −0.14 to 0.50; P = .275) (Fig. 6), and there was unimportant heterogeneity across the included trials (I2 = 5.2%; P = .383). The sensitivity analysis suggested that the conclusion was not changed by sequentially excluding the individual trial (Supplemental Digital Content 1). Moreover, we noted that patients treated with a high dosage of niacin were associated with high levels of plasma glucose, and that the daily dosage of niacin could affect the niacin supplementation on the level of plasma glucose (P = .042). There was no significant publication bias for plasma glucose (P value for Egger: 0.098; P value for Begg: .707) (Supplemental Digital Content 2).
Figure 6.
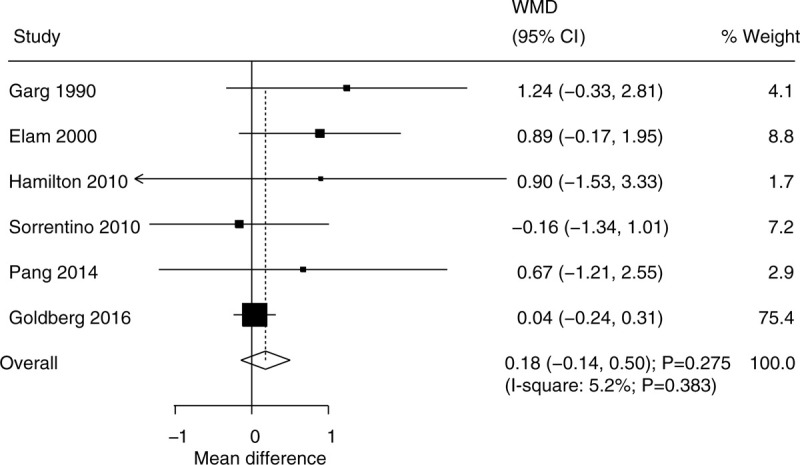
Effect of niacin supplementation on plasma glucose.
3.8. HbA1c
The data for determining the effect of niacin supplementation on HbA1c were available in 5 trials. Niacin supplementation has no significant effect on HbA1c levels (WMD, 0.39; 95% CI, −0.15 to 0.94; P = 0.158) (Fig. 7), and there was significant heterogeneity across the included trials (I2 = 57.6%; P = .051). The sensitivity analysis indicated that niacin supplementation might be associated with a high level of HbA1c when excluding the trial conducted by Sorrentino et al (Supplemental Digital Content 1). No significant differences between the subgroups were observed in all of the subgroups (Table 2). There was no significant publication bias for HbA1c (P value for Egger: .391; P value for Begg: .221) (Supplemental Digital Content 2).
Figure 7.
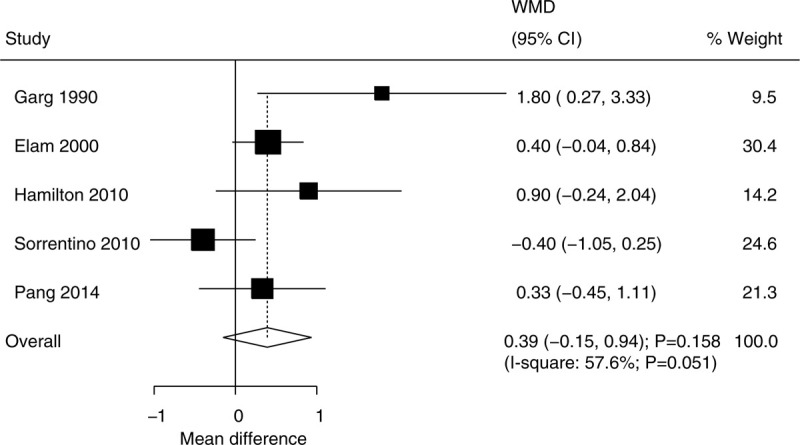
Effect of niacin supplementation on HbA1c.
4. Discussion
The effect of niacin supplementation on lipid profiles and glycemic control in patients with T2DM has not been confirmed. The current study based on RCTs explored the possible effects of niacin supplementation and the outcomes of TC, TG, LDL, HDL, plasma glucose, and HbA1c. This meta-analysis involved 2,110 patients with T2DM from 8 RCTs across a wide range of patients’ characteristics. The results of this study suggested that niacin supplementation significantly reduced the levels of TC, TG, and LDL, and increased the level of HDL. Moreover, there were no significant differences between the niacin and the control groups for the levels of plasma glucose and HbA1c.
A meta-analysis conducted by Ding et al involved 7 RCTs and suggested that niacin supplementation could improve lipid profiles for patients with T2DM, while the glucose should be monitored after long-term niacin supplementation.[33] Although the analysis of this study was based on the changes of lipid profiles and glucose, the data were transformed from pooled data, and the accuracy effect estimates were not reported. Sahebkar et al conducted a meta-analysis of 14 RCTs and found that niacin supplementation significantly reduced the level of lipoprotein(a).[34] However, this study did not report lipid profiles, and the involved patients were not restricted to T2DM. Therefore, the current study was conducted based on RCTs to evaluate the effects of niacin supplementation on lipid profiles and glycemic control in patients with T2DM.
The pooled results of this study demonstrated that the lipid profiles were significantly improved in patients with T2DM, which were consistent with previous studies.[7–9] Potential explanations for this included pleiotropic effects of niacin, dosage titration, and background therapies. Moreover, niacin plays a important role on the synthesis of TG and LDL in the liver through inhibit the mobilization of free fatty acids to the liver by peripheral adipose tissue.[35] Furthermore, the increased HDL level in patients received niacin could be owing to the increased apolipoprotein A-I synthesis and blocked apolipoprotein A-I consumption in liver.[35] The subgroup analyses suggested that niacin supplementation could affect TC, and that TG could affect the dosage of niacin. Moreover, the level of HDL in patients treated with niacin could be affected by country, study design, daily dosage, use of statins, and follow-up duration. The potential reason for this included the following:
-
(1)
the background management strategies for patients with T2DM;
-
(2)
the small number of studies in several subgroups and the unstable conclusions;
-
(3)
the severity of the disease status; and
-
(4)
the difference in the effects of niacin between short- and long-term treatment duration.
The pooled results suggested that there were no significant differences between the niacin and the control groups for the plasma glucose and HbA1c levels. However, the sensitivity analysis suggested that the HbA1c level was significantly increased when the patients were treated with niacin. Moreover, the subgroup analyses found that a high dosage of niacin supplementation was associated with a high level of plasma glucose. Although the mechanism for the detrimental effects of niacin on glycemic control remains controversial, the synergistic effect might exist because statins are associated with an increased risk of diabetes.[36,37] Therefore, a high niacin supplementation for patients with T2DM should be cautiously administered because of the potential detrimental effect of niacin on glycemic control.
Several strengths and limitations in this study should be highlighted. First, the analysis was based on RCTs, and the selection and confounder biases were minimized as shown in observational studies. Second, the stratified analyses for lipid profiles and glycemic control were conducted based on the study or the patients’ characteristics. However, the analysis based on study-level and the individual patient's data were not available, which restricted us from conducting more detailed analyses. Moreover, the publication bias was inevitable because of the analysis based on published articles. Finally, the heterogeneity was not fully explained through sensitivity and subgroup analyses, which suggested that the effects of niacin supplementation on lipid profiles and glycemic control could be affected by unknown variables.
5. Conclusion
This study suggested that niacin supplementation could improve the lipid profiles, including TC, TG, LDL, and HDL for patients with T2DM. Moreover, although there were no significant differences between the niacin and the control groups for plasma glucose and HbA1c levels, the results of sensitivity and the subgroup analyses suggested that niacin supplementation might produce a harmful effect on plasma glucose and HbA1c levels. Further large-scale RCTs should be conducted to evaluate the long-term effects of niacin supplementation on the prognosis of patients with T2DM.
Author contributions
Dan Xiang developed the concept of this study and and drafted the manuscript. Qian Zhang did the literature research and data analysis. Yangtian Wang contributed to the experimental technique.
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: CIs = confidence intervals, HDL = high-density lipoprotein, LDL = low-density lipoprotein, RCTs = randomized controlled trials, T2DM = type 2 diabetes mellitus, TC = total cholesterol, TG = triglycerides, WMDs = weighted mean differences.
How to cite this article: Xiang D, Zhang Q, Wang YT. Effectiveness of niacin supplementation for patients with type 2 diabetes: a meta-analysis of randomized controlled trials. Medicine. 2020;99:29(e21235).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Adeghate E, Schattner P, Dunn E. An update on the etiology and epidemiology of diabetes mellitus. Ann N Y Acad Sci 2006;1084:1–29. [DOI] [PubMed] [Google Scholar]
- [2].Disease GBD, Injury I, Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1545–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Seuring T, Archangelidi O, Suhrcke M. The economic costs of type 2 diabetes: a global systematic review. Pharmacoeconomics 2015;33:811–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Collaborators GBDRF. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390:1345–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mooradian AD. Dyslipidemia in type 2 diabetes mellitus. Nat Clin Pract Endocrinol Metab 2009;5:150–9. [DOI] [PubMed] [Google Scholar]
- [6].Fruchart JC, Sacks FM, Hermans MP, et al. The Residual Risk Reduction Initiative: a call to action to reduce residual vascular risk in dyslipidaemic patient. Diab Vasc Dis Res 2008;5:319–35. [DOI] [PubMed] [Google Scholar]
- [7].Guyton JR, Goldberg AC, Kreisberg RA, et al. Effectiveness of once-nightly dosing of extended-release niacin alone and in combination for hypercholesterolemia. Am J Cardiol 1998;82:737–43. [DOI] [PubMed] [Google Scholar]
- [8].Zema MJ. Gemfibrozil, nicotinic acid and combination therapy in patients with isolated hypoalphalipoproteinemia: a randomized, open-label, crossover study. J Am Coll Cardiol 2000;35:640–6. [DOI] [PubMed] [Google Scholar]
- [9].Malik S, Kashyap ML. Niacin, lipids, and heart disease. Curr Cardiol Rep 2003;5:470–6. [DOI] [PubMed] [Google Scholar]
- [10].Canner PL, Furberg CD, Terrin ML, et al. Benefits of niacin by glycemic status in patients with healed myocardial infarction (from the Coronary Drug Project). Am J Cardiol 2005;95:254–7. [DOI] [PubMed] [Google Scholar]
- [11].Duggal JK, Singh M, Attri N, et al. Effect of niacin therapy on cardiovascular outcomes in patients with coronary artery disease. J Cardiovasc Pharmacol Ther 2010;15:158–66. [DOI] [PubMed] [Google Scholar]
- [12].Brown BG, Zhao XQ, Chait A, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med 2001;345:1583–92. [DOI] [PubMed] [Google Scholar]
- [13].Taylor AJ, Lee HJ, Sullenberger LE. The effect of 24 months of combination statin and extended-release niacin on carotid intima-media thickness: ARBITER 3. Curr Med Res Opin 2006;22:2243–50. [DOI] [PubMed] [Google Scholar]
- [14].Investigators A-H, Boden WE, Probstfield JL, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011;365:2255–67. [DOI] [PubMed] [Google Scholar]
- [15].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1–2. [DOI] [PubMed] [Google Scholar]
- [17].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [18].Ades AE, Lu G, Higgins JP. The interpretation of random-effects meta-analysis in decision models. Med Decis Making 2005;25:646–54. [DOI] [PubMed] [Google Scholar]
- [19].Deeks JJ, Higgins J, Altman DG. Higgins J, Green S. Analysing Data and Undertaking Meta-Analyses. Oxford, Cochrane Handbook for Systematic Reviews of Interventions. UK: 2008. [Google Scholar]
- [20].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tobias A. Assessing the influence of a single study in the meta-analysis. Stata Technical Bulletin 1999;47:15–7. [Google Scholar]
- [22].Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ 2003;326:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [25].Garg A, Grundy SM. Nicotinic acid as therapy for dyslipidemia in non-insulin-dependent diabetes mellitus. JAMA 1990;264:723–6. [PubMed] [Google Scholar]
- [26].Elam MB, Hunninghake DB, Davis KB, et al. Effect of niacin on lipid and lipoprotein levels and glycemic control in patients with diabetes and peripheral arterial disease: the ADMIT study: a randomized trial. Arterial disease multiple intervention trial. JAMA 2000;284:1263–70. [DOI] [PubMed] [Google Scholar]
- [27].Grundy SM, Vega GL, McGovern ME, et al. Efficacy, safety, and tolerability of once-daily niacin for the treatment of dyslipidemia associated with type 2 diabetes: results of the assessment of diabetes control and evaluation of the efficacy of niaspan trial. Arch Intern Med 2002;162:1568–76. [DOI] [PubMed] [Google Scholar]
- [28].Hamilton SJ, Chew GT, Davis TM, et al. Niacin improves small artery vasodilatory function and compliance in statin-treated type 2 diabetic patients. Diab Vasc Dis Res 2010;7:296–9. [DOI] [PubMed] [Google Scholar]
- [29].Sorrentino SA, Besler C, Rohrer L, et al. Endothelial-vasoprotective effects of high-density lipoprotein are impaired in patients with type 2 diabetes mellitus but are improved after extended-release niacin therapy. Circulation 2010;121:110–22. [DOI] [PubMed] [Google Scholar]
- [30].MacLean A, McKenney JM, Scott R, et al. Efficacy and safety of extended-release niacin/laropiprant in patients with type 2 diabetes mellitus. Br J Cardiol 2011;18:37–45. [Google Scholar]
- [31].Pang J, Chan DC, Hamilton SJ, et al. Effect of niacin on high-density lipoprotein apolipoprotein A-I kinetics in statin-treated patients with type 2 diabetes mellitus. Arterioscler Thromb Vasc Biol 2014;34:427–32. [DOI] [PubMed] [Google Scholar]
- [32].Goldberg RB, Bittner VA, Dunbar RL, et al. Effects of extended-release niacin added to simvastatin/ezetimibe on glucose and insulin values in AIM-HIGH. Am J Med 2016;129:753 e713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ding Y, Li Y, Wen A. Effect of niacin on lipids and glucose in patients with type 2 diabetes: a meta-analysis of randomized, controlled clinical trials. Clin Nutr 2015;34:838–44. [DOI] [PubMed] [Google Scholar]
- [34].Sahebkar A, Reiner Z, Simental-Mendia LE, et al. Effect of extended-release niacin on plasma lipoprotein(a) levels: a systematic review and meta-analysis of randomized placebo-controlled trials. Metabolism 2016;65:1664–78. [DOI] [PubMed] [Google Scholar]
- [35].Adiels M, Chapman MJ, Robillard P, et al. Niacin action in the atherogenic mixed dyslipidemia of metabolic syndrome: insights from metabolic biomarker profiling and network analysis. J Clin Lipidol 2018;12:810–821.e811. [DOI] [PubMed] [Google Scholar]
- [36].Swerdlow DI, Preiss D, Kuchenbaecker KB, et al. HMG-coenzyme A reductase inhibition, type 2 diabetes, and bodyweight: evidence from genetic analysis and randomised trials. Lancet 2015;385:351–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Goldie C, Taylor AJ, Nguyen P, et al. Niacin therapy and the risk of new-onset diabetes: a meta-analysis of randomised controlled trials. Heart 2016;102:198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


