Abstract
Background:
Postoperative sore throat (POST) is an important concern in surgical patients undergoing endotracheal intubation. Its prevalence after thyroidectomy is up to 80%. The current study aimed to assess the effect of dexmedetomidine and remifentanil on postoperative sore throat.
Methods:
Seventy-four patients who underwent thyroidectomy were randomized to receive either dexmedetomidine (group D) or remifentanil (group R). At anesthesia induction, group D received dexmedetomidine 1 μg/kg over 10 minutes, followed by continuous dexmedetomidine infusion at 0.3 to 0.6 μg/kg/hour during surgery. Group R received remifentanil of 3 to 4 ng/ml during induction, followed by 1.5 to 2.5 ng/ml remifentanil infusion during surgery. POST at rest and swallowing was assessed during the first 24 hours in serial time periods (0–1, 1–6, and 6–24 hours). Hoarseness and postoperative pain score were also assessed.
Results:
POST incidence at rest (0–1, 1–6, and 6–24 hours) and swallowing (1–6 and 6–24 hours) was lower in group D than in group R. POST severity was significantly lower in group D than in group R during each time period. The incidence of postoperative hoarseness was also lower in group D than in group R at 1 to 6 and 6 to 24 hours. The postoperative pain score was lower in group D than in group R during each time period.
Conclusion:
Intraoperative dexmedetomidine infusion reduced the incidence and severity of POST for 24 hours after thyroidectomy.
Keywords: dexmedetomidine, postoperative sore throat, remifentanil, thyroidectomy
1. Introduction
Postoperative sore throat (POST) is a common complaint after thyroidectomy, with a prevalence of up to 80%.[1] Endotracheal intubation can irritate the airway mucosa, which can cause POST.[2] Especially, during thyroid surgery, movement of the endotracheal tube by position change and mass manipulation can cause this unpleasant symptom.
Dexmedetomidine, a selective α2-adrenoreceptor (AR) agonist, has been used as a sedative, analgesic, and anxiolytic. Recently, instead of remifentanil as an anesthetic adjuvant during general anesthesia, its successful use has increased in various types of surgery.[3,4] Numerous studies have shown its anesthetic (inhalational or opioids) effect, its efficacy in attenuating postoperative unpleasant symptoms (pain and nausea), and its ability to maintain hemodynamic stability.[3,5,6] Moreover, the anti-inflammatory effect of dexmedetomidine has been reported; this effect is mediated by α2-AR.[7,8] Here, we focused on inflammation in the pathophysiology of POST. Steroids, such as dexamethasone,[9] and non-steroid anti-inflammatory drugs (NSAIDs), including benzydamine hydrochloride,[10] are the most commonly investigated drugs in the research of POST prevention.
In this study, we hypothesized that dexmedetomidine use as an anesthetic adjuvant during surgery is favorable in reducing POST; thus, we assessed the effect of dexmedetomidine on POST, hoarseness, and postoperative pain in patients undergoing thyroidectomy. In addition, we compared this effect to that of remifentanil, as remifentanil is one of the most currently used anesthetic adjuvants during surgery in clinical practice.
2. Materials and methods
We received an approval from the Institutional Review Board of our hospital, and written informed consent was obtained from all patients. This study was registered at clinicaltrials.gov (NCT03805568). A total of 74 patients (aged 20–65 years) with scheduled primary thyroidectomy were enrolled, and their physical status was determined to be American Society of Anesthesiologists (ASA) class 1 or 2. Patients with respiratory tract disease, previous head and neck surgery, preexisting steroid or NSAID use, and known or suspected difficult airway were excluded from this study. Patients with damage to the nerves supplying the vocal cord were also excluded from analysis.
After randomization using computer-generated random table, patients were assigned either to the remifentanil group (group R, n = 37) or the dexmedetomidine group (group D, n = 37). Standard ASA monitoring was installed. Without premedication, anesthesia was induced with propofol 1.5 to 2 mg/kg and rocuronium 0.6 mg/kg. Patients in group R received remifentanil of 3 to 4 ng/ml during induction, followed by remifentanil infusion (1.5–2.5 ng/ml) during the surgery. Patients in group D received dexmedetomidine infusion (loading dose of 1 μg/kg over 10 minutes and continuous infusion at 0.3–0.6 μg/kg/hour) during the surgery. A target-controlled infusion (TCI) device (Orchestra Base Primea; Fresenius Vial, France) was used for continuous infusion of both anesthetics (remifentanil and dexmedetomidine). Tracheal intubation was performed using a 7.0- and 7.5-mm Taperguard endotracheal tube for women and men, respectively; this procedure was performed by an experienced anesthesiologist who was blinded to the study protocol. During tracheal intubation, laryngeal view from direct laryngoscopy was graded by Cormack-Lehane classification.[11] Patients who showed grade 3 or higher, those who underwent more than 2 intubation attempts, and those who underwent unsuccessful intubation were excluded in this study. Intracuff pressure was set at 20 to 24 cm H2O using a manometer (Mallinckrodt Medical, Hennef, Germany), and it was periodically checked to maintain the optimal range throughout the surgery. Anesthesia was maintained with sevoflurane in 50% oxygen, and sevoflurane concentration was controlled to maintain the anesthetic depth, 40 to 60 of bispectral index (BIS). Along with sevoflurane, remifentanil (1.5–2.5 ng/ml) or dexmedetomidine (0.3–0.6 μg/kg/hour) was also permitted to maintain an appropriate hemodynamic response (<20% of preoperative baseline). Dexmedetomidine was discontinued at the point of skin suture, whereas remifentanil was discontinued at the end of surgery. Residual neuromuscular blockade was antagonized as necessary using pyridostigmine and glycopyrrolate. When patients had adequate spontaneous breathing, extubation was performed followed gentle oropharyngeal suction. Ramosetron 0.2 mg was administered to prevent postoperative nausea at the end of surgery. During emergence, eye opening time (from the cessation of sevoflurane to the first eye opening in response to verbal commands without touching of the body) and extubation time (from the cessation of sevoflurane to the removal of endotracheal tube when patients regained consciousness with spontaneous breathing) were recorded. In the PACU, fentanyl 50 to 100 μg was injected upon the patient's request for analgesics or when the patient had a pain score of 5 or higher according to the pain numerical rating scale (NRS; from 0, no pain to 10, the worst possible pain) in the recovery room.
At 1, 6, and 24 hours after surgery, POST, hoarseness, and postoperative pain were checked by an anesthesiologist who was unaware of the allocation group. The incidence of POST at rest and swallowing was assessed, and its severity was checked using a four-point scale (0 = none; 1 = mild; 2 = moderate; 3 = severe).[12] Hoarseness was also assessed using a four-point scale (0 = none; 1 = mild; 2 = severe; 3 = aphonia).[13] Postoperative pain was determined using an NRS score, and total dose of rescue analgesics during 24 hours after surgery was recorded.
2.1. Statistical analyses
Preliminary study showed that the incidence of POST was 80% in patients after thyroidectomy under sevoflurane and remifentanil. We hypothesized that a 40% reduction in the incidence of POST would have clinical relevance in patients who were administered dexmedetomidine. A total of 34 patients in each group were required at a set α = 0.05, power = 80%; thus, 37 patients per group were enrolled to consider the dropouts. Statistical analysis was performed using SPSS version 23 (Chicago, IL). Continuous data were analyzed using t test and are expressed as mean ± SD, whereas categorical data were analyzed using chi-squared test or Fisher's exact test as appropriate and are expressed as number (%). P < .05 was considered statistically significant.
3. Results
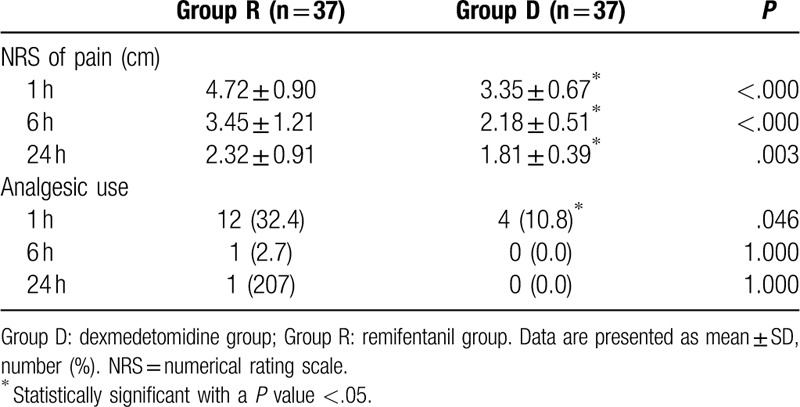
A total of 74 patients completed this study, and no patient was excluded in the final analysis (Fig. 1). The patients’ demographic and operative data were not different between the 2 groups (Table 1). The incidence of POST and hoarseness is shown in Table 2. At rest, the incidence of sore throat, which was assessed at serial predefined time points (0–1, 1–6, and 6–24 hours after surgery), was all significantly lower in group D than in group R (P = .024, P < .001, and P < .001, respectively). At swallowing, the incidence of sore throat was similar in the 2 groups at 0 to 1 hour, but significantly different at 1 to 6 and 6 to 24 hours (P = .041 and P < .001, respectively). The incidence of postoperative hoarseness was also lower in group D than in group R at 1 to 6 and 6 to 24 hours (P < .001 for both). The severity of POST and hoarseness is shown in Table 3. During rest and swallowing, the severity of POST, as assessed by a four-point scale, was significantly lower in group D than in group R during each time period (0–1, 1–6, and 6–24 hours after surgery) (P < .001 for both). The severity of hoarseness was also significantly lower in group D than in group R during each time period (0–1, 1–6, and 6–24 hours after surgery) (P < .001 for both). The other postoperative outcome, postoperative pain, is shown in Table 4. The NRS scores for postoperative pain severity were different between the 2 groups. It was lower in group D than in group R during each time period (0–1, 1–6, and 6–24 hours after surgery) (P < .001, P < .001, and P = .003, respectively), but additional analgesic requirement was lower in group D than in group R just at 1 hour after surgery (P = .046).
Figure 1.
Flow diagram of the study.
Table 1.
Demographic and baseline characteristics.
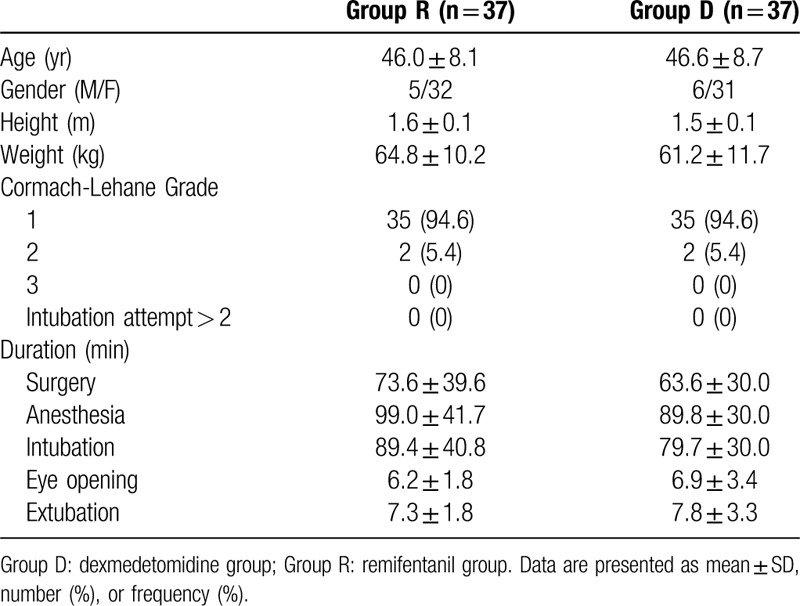
Table 2.
Incidence of sore throat and hoarsness in the first 24 h after thyroidectomy.
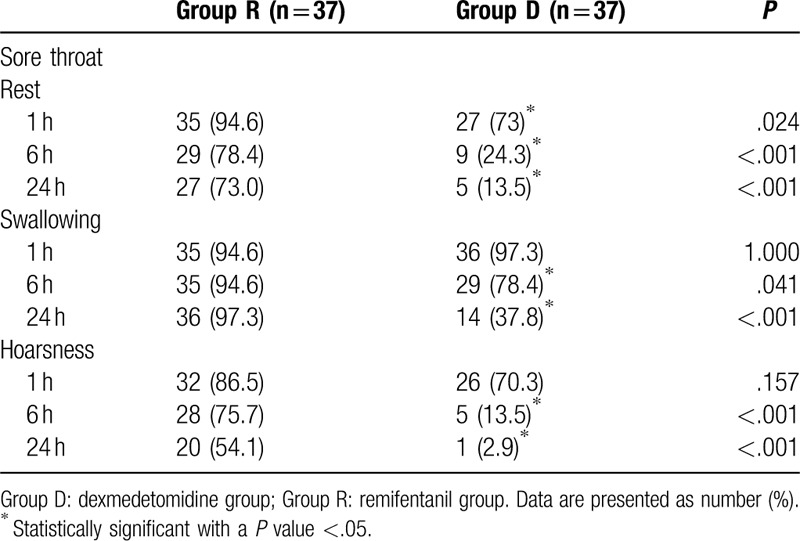
Table 3.
Severity of sore throat and hoarseness during the first 24 h after extubation.
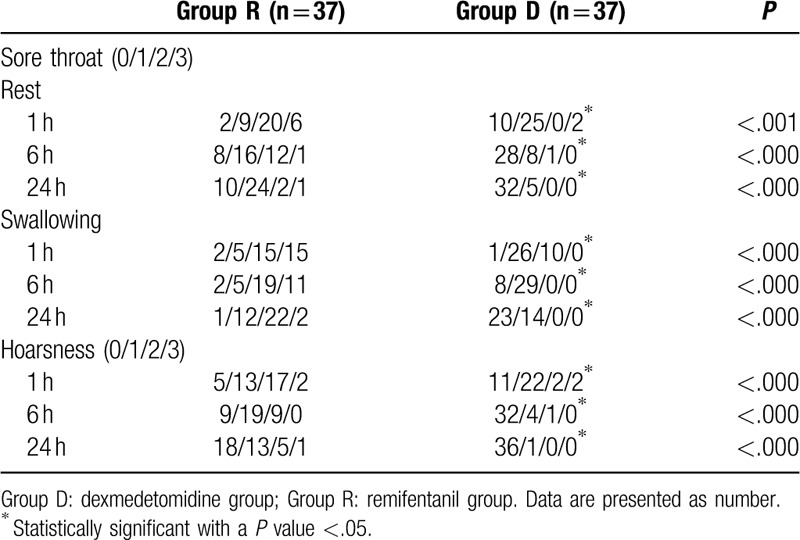
Table 4.
Postoperative pain in the first 24 h after thyroidectomy.
4. Discussion
This study showed that intraoperative dexmedetomidine infusion reduced the incidence and severity of POST and hoarseness for 24 hours after thyroidectomy. In addition, postoperative pain scores were lower in group D than in group R for 24 hours after surgery.
POST is an undesirable outcome following general anesthesia with tracheal intubation, which occurs in 30% to 80% of patients.[1,2] Its occurrence can increase the length of recovery and cost of care, as well as decrease patient satisfaction. Various etiologies that have been suggested to increase the likelihood of POST include inflammation of the airway, mechanical injury related to tracheal intubation, and mucosal damage due to cuff pressure of the endotracheal tube.[2] Moreover, the female sex, younger age, and prolonged duration of surgery are associated with a greatest risk for POST.[14] Some pharmacologic interventions, such as inhaled and topical steroids or NSAIDs, have been proposed to reduce the incidence and severity of POST, suggesting the potential role of airway inflammation in POST pathophysiology.
Dexmedetomidine, a highly selective α2-AR agonist, has analgesic, sympatholytic, and amnestic effects, as it binds to the transmembrane G protein binding receptor.[15] According to α2-AR location, dexmedetomidine has different actions; its sedative action may be explained by stimulation of AR in the brainstem, whereas its analgesic effect may be attributed to stimulation of AR in the supraspinal and spinal sites.[16] In addition, imidazoline receptors are related to the action of dexmedetomidine because it contains an imidazole moiety.[16] Besides the central and peripheral nervous systems, since wide distribution including blood vessels, heart, and white blood cells of receptors, dexmedetomidine may have extra-anesthetic effect such as anti-inflammatory action.
The protective effects of dexmedetomidine against inflammation have been shown in many models, including ischemia reperfusion model,[17,18] sepsis model,[19] and acute lung injury model.[20] This ample experimental and clinical evidence showed that dexmedetomidine mitigates inflammatory responses by inhibiting proinflammatory cytokines.[7,8] In terms of prevention of postoperative sore throat, we focused on the anti-inflammatory and anti-nociceptionary effect of dexmedetomidine. However, although evidence suggests that dexmedetomidine can be used for reducing inflammatory responses, there has been no research on the effectiveness of dexmedetomidine on postoperative airway sequalae, especially in patients undergoing thyroid surgery. In this study, as an anesthetic adjuvant, dexmedetomidine infusion showed better effect on POST than did remifentanil infusion. In addition, the NRS scores for postoperative pain were lower in group D than in group R, which may be explained by inhibition of the release of substance P through activation of α2-AR.[21]
Remifentanil, an ultra-short-acting μ-opioid agonist, is commonly used in clinical practice as an intraoperative analgesia and to maintain hemodynamic stability. In addition, remifentanil has been shown to attenuate inflammatory responses.[22,23] Nevertheless, there is a lack of controlled studies evaluating the effect of remifentanil on POST. Recently, Park et al showed that high-dose remifentanil increased the incidence of POST in patients undergoing orthopedic surgery.[14] The authors explained that enhancement of POST following treatment with high-dose remifentanil (infused at a rate of 0.25 mg/kg/minute and subsequently titrated at 0.05 mg/kg/minute) compared to that after low-dose remifentanil treatment (infused at a rate of 0.05 mg/kg/minute) may be caused by the central sensitization of nociceptive pathways and the descending pain modulation system of remifentanil.[24,25] However, as the biologic and clinical basis of the effect of remifentanil on POST remains obscure, large-scale trials should be conducted to explore the dose–response relationship and efficacy of remifentanil.
This study has some limitations. First, although the protective effect of dexmedetomidine against POST may be explained by its anti-inflammatory and anti-nociceptionary properties, we did not provide a biological evidence of its pathophysiologic mechanism through analysis of inflammatory cytokines. Second, the infusion dose may not be equipotent in terms of comparing the effect of both drugs on POST, although this approach was determined based on the estimated dose with clinical evidence, which needs to be further studied. Third, sedation scores were not measured in the assessments of outcomes. As dexmedetomidine has relatively longer context-sensitive half-time than does remifentanil, it was discontinued earlier than remifentanil (at the time point of skin suture). However, plasma dexmedetomidine concentration may be affect sedation in the early period after surgery.
In conclusion, POST is an important concern among surgical patients undergoing endotracheal intubation. Dexmedetomidine infusion as an anesthetic adjuvant during surgery appeared to exert protective effect on POST and postoperative pain in patients undergoing thyroid surgery. In addition, in this regard, dexmedetomidine showed better controlling effect than did remifentanil.
Author contributions
Conceptualization: Hyojin Kwon, Eun Kyung Choi.
Data curation: Hyojin Kwon, Sungmin Jeon, Eun Kyung Choi.
Formal analysis: Hyojin Kwon, Eun Kyung Choi.
Funding acquisition: Eun Kyung Choi.
Investigation: Hyojin Kwon, Sungmin Jeon.
Project administration: Hyuckgoo Kim.
Software: Sungmin Jeon.
Supervision: Hyuckgoo Kim, Eun Kyung Choi.
Validation: Sungmin Jeon.
Writing – original draft: Hyuckgoo Kim, Hyojin Kwon, Sungmin Jeon, Eun Kyung Choi.
Writing – review & editing: Eun Kyung Choi.
Footnotes
Abbreviations: AR = adrenoreceptor, BIS = bispectral index, NRS = numerical rating scale, NSAID = non-steroid anti-inflammatory drug, PONV = postoperative nausea and vomiting, POST = postoperative sore throat, TCI = target-controlled infusion.
How to cite this article: Kim H, Kwon H, Jeon S, Choi EK. The effect of dexmedetomidine and remifentanil on the postoperative sore throat after thyroidectomy. Medicine. 2020;99:29(e21060).
This work was supported by the 2018 Yeungnam University Research Grant.
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Jung TH, Rho JH, Hwang JH, et al. The effect of the humidifier on sore throat and cough after thyroidectomy. Korean J Anesthesiol 2011;61:470–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kalil DM, Silvestro LS, Austin PN. Novel preoperative pharmacologic methods of preventing postoperative sore throat due to tracheal intubation. AANA J 2014;82:188–97. [PubMed] [Google Scholar]
- [3].Choi EK, Seo Y, Lim DG, et al. Postoperative nausea and vomiting after thyroidectomy: a comparison between dexmedetomidine and remifentanil as part of balanced anesthesia. Korean J Anesthesiol 2017;70:299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hwang W, Lee J, Park J, et al. Dexmedetomidine versus remifentanil in postoperative pain control after spinal surgery: a randomized controlled study. BMC Anesthesiol 2015;15:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sun Y, Lu Y, Huang Y, et al. Is dexmedetomidine superior to midazolam as a premedication in children? A meta analysis of randomized controlled trials. Pediatr Anesth 2014;24:863–74. [DOI] [PubMed] [Google Scholar]
- [6].Gupta N, Rath GP, Prabhakar H, et al. Effect of intraoperative dexmedetomidine on postoperative recovery profile of children undergoing surgery for spinal dysraphism. J Neurosurg Anesthesiol 2013;25:271–8. [DOI] [PubMed] [Google Scholar]
- [7].Zhang J, Wang Z, Wang Y, et al. The effect of dexmedetomidine on inflammatory response of septic rats. BMC Anesthesiol 2015;15:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wang L, Zhang A, Liu W, et al. Effects of dexmedetomidine on perioperative stress response, inflammation and immune function in patients with different degrees of liver cirrhosis. Exp Ther Med 2018;16:3869–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lee JH, Kim SB, Lee W, et al. Effects of topical dexamethasone in postoperative sore throat. Korean J Anesthesiol 2017;70:58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kuriyama A, Aga M, Maeda H. Topical benzydamine hydrochloride for prevention of postoperative sore throat in adults undergoing tracheal intubation for elective surgery: a systematic review and meta-analysis. Anaesthesia 2018;73:889–900. [DOI] [PubMed] [Google Scholar]
- [11].Landucci F, Byrne A, Caldiroli D. Interpreting the Cormack and Lehane classification during videolaryngoscopy. Anaesthesia 2018;73:652–3. [DOI] [PubMed] [Google Scholar]
- [12].Stout DM, Bishop MJ, Dwersteg JF, et al. Correlation of endotracheal tube size with sore throat and hoarseness following general anesthesia. Anesthesiology 1987;67:419–21. [DOI] [PubMed] [Google Scholar]
- [13].Friedman PG, Rosenberg MK, Lebenbom-Mansour M. A comparison of light wand and suspension laryngoscopic intubation techniques in outpatients. Anesth Analg 1997;85:578–82. [DOI] [PubMed] [Google Scholar]
- [14].Park JH, Lee YC, Lee J, et al. The influence of high-dose intraoperative remifentanil on postoperative sore throat: a prospective randomized study: a CONSORT compliant article. Medicine (Baltimore) 2018;97:e13510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Piao G, Wu J. Systematic assessment of dexmedetomidine as an anesthetic agent: a meta-analysis of randomized controlled trials. Arch Med Sci 2014;10:19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Khan Z, Ferguson C, Jones R. Alpha-2 and imidazoline receptor agonists. Their pharmacology and therapeutic role. Anaesthesia 1999;54:146–65. [DOI] [PubMed] [Google Scholar]
- [17].Zeng X, Wang H, Xing X, et al. Dexmedetomidine protects against transient global cerebral ischemia/reperfusion induced oxidative stress and inflammation in diabetic rats. PLoS One 2016;11:e0151620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Liu G, Song H, Qiu L, et al. Dexmedetomidine preconditioning inhibits the long term inflammation induced by renal ischemia/reperfusion injury in rats. Acta Cir Bras 2016;31:8–14. [DOI] [PubMed] [Google Scholar]
- [19].Chen Y, Miao L, Yao Y, et al. Dexmedetomidine ameliorate CLP-induced rat intestinal injury via inhibition of inflammation. Mediators Inflamm 2015;2015:918361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Xu Y, Zhang R, Li C, et al. Dexmedetomidine attenuates acute lung injury induced by lipopolysaccharide in mouse through inhibition of MAPK pathway. Fund Clin Pharmacol 2015;29:462–71. [DOI] [PubMed] [Google Scholar]
- [21].Whittington RA, Virag L. Dexmedetomidine-induced decreases in accumbal dopamine in the rat are partly mediated via the locus coeruleus. Anesth Analg 2006;102:448–55. [DOI] [PubMed] [Google Scholar]
- [22].Minguet G, Brichant JF, Joris J. Opioids and protection against ischemia-reperfusion injury: from experimental data to potential clinical applications. Acta Anaesthesiol Belg 2012;63:23–34. [PubMed] [Google Scholar]
- [23].Vianna PTG, Castiglia YMM, Braz JRC, et al. Remifentanil, isoflurane, and preconditioning attenuate renal ischemia/reperfusion injury in rats. Transplant Proc 2009;41:4080–2. [DOI] [PubMed] [Google Scholar]
- [24].Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain 2009;10:895–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Vanegas H, Schaible H-G. Descending control of persistent pain: inhibitory or facilitatory? Brain Res Rev 2004;46:295–309. [DOI] [PubMed] [Google Scholar]


