Abstract
To report the clinical characteristics and retinal abnormalities associated with orbital infarction syndrome after cerebral aneurysm clipping surgery.
In this retrospective case series, we evaluated 4 cases of orbital infarction syndrome using fluorescein angiography, optical coherence tomography, and computed tomography images from January 2011 to May 2014. The medical records of these patients including age, sex, laterality of the eyes, visual acuity, intraocular pressure, duration of the operation, location of the aneurysms, and surgical method with the type of approach used to reach the aneurysmal lesions were evaluated.
Aneurysms were located in either the anterior or the posterior communicating artery. Two patients had subarachnoid hemorrhage arising from a ruptured aneurysm, whereas 2 other patients had unruptured aneurysms. Clipping was performed by 3 different surgeons using the pterional craniotomy. The mean time interval from aneurysmal clipping to awareness of vision loss was 10.75 ± 13.8 days. In all patients, optic atrophy and irreversible deterioration of visual acuity ensued. Retinal edema, retinal vascular abnormality, or choroidal hypoperfusion was identified in these patients.
Orbital infarction syndrome is a rare but devastating complication of brain aneurysm clipping surgery. The associated retinal ischemia is not only due to the involvement of the retinal vessels, but also the choroidal circulation.
Keywords: fluorescein angiography, intracranial aneurysm, optical coherence tomography, orbital infarction syndrome
1. Introduction
Visual loss after brain surgery is a devastating complication. The incidence and mechanisms of visual loss after brain surgery remains difficult to determine. Visual disturbance following spinal surgery under prolonged prone position is defined as the perioperative visual loss (POVL) by the American Society of Anesthesiologist Task Force.[1] Although POVL after prolonged surgery in the prone position has been evaluated in many instances; studies evaluating patients with orbital ischemia who underwent surgery in the supine position, such as brain aneurysm clipping are scarce.[2–4] The suspected main causes of intraorbital and intraocular ischemia are ischemic optic neuropathy, central retinal artery occlusion, and occlusion of the ophthalmic artery or its branches.[5] This rare group of disorders resulting from ischemia is known as orbital infarction syndrome.[6] Orbital infarction syndrome is associated with ischemia of any intraorbital structure due to occlusion of the ophthalmic artery or its branches, such as the central retinal artery; and can happen with common carotid occlusion, orbital mucormycosis, giant cell arteritis, and myelofibrosis.[7]
Unlike any previously published report, we report 4 patients with visual loss and clinical findings consistent with orbital infarction syndrome after clipping of cerebral aneurysms. Few studies have investigated orbital infarction using ophthalmic imaging tools, such as optical coherence tomography (OCT). In this study, we evaluate all 4 cases using clinical features, fundus, radiologic, and angiographic characteristics. We also analyze OCT images for deteriorating visual acuity (VA) in the ipsilateral eye after brain aneurysm surgery.
2. Methods
We report 4 patients who presented with ipsilateral visual loss to our ophthalmology department from January 2011 to May 2014, following a clipping of cerebral aneurysms at our institution. This study was performed with approval from the Institutional Review Board (IRB) of Keimyung University Dongsan Hospital (2019-08-004-001). The aneurysms were clipped successfully under general anesthesia, without any intraoperative complications. None of the patients had reported a history of ocular surgery or ophthalmic diseases such as glaucoma and retinal disorders. We retrospectively reviewed the electronic medical records of these patients for age, sex, laterality of the eyes, initial VA, and intraocular pressure (IOP). The duration of surgery, location of the aneurysms, and surgical method, including the type of approach used to reach the aneurysmal lesions were also analyzed. The information on ocular abnormalities and alterations were obtained by carefully reviewing the findings from slit-lamp biomicroscopy, fundoscopy, fluorescein angiography (FA; FF450plus fundus camera, Carl Zeiss Inc., Berlin, Germany), and OCT (Spectral OCT/SLO; OTI, Ophthalmic Technologies, Toronto, Ontario, Canada). Statistical analysis was performed using SPSS Ver.22.0 (IBM, Armonk, NY).
3. Results
Our case series comprised, 3 male patients and 1 female patient. As shown in Table 1, the mean age of the patients was 54.5 ± 3.4 years (range, 52–61 years). Three patients presented with left eye (oculus sinister (OS)), and 1 with right eye (oculus dexter (OD)) involvement. In total, 3 patients were operated for brain aneurysms in the anterior communicating artery (COM), and 1 for aneurysm in the posterior COM. Aneurysm clipping surgery was performed using the pterional approach in all cases. During surgery, the patients were in the supine position, with their head fixed using a 3-point skull clamp. A craniotomy with myocutaneous flap was performed and flexed onto the ipsilateral eye without ocular protection. The flap was then drawn back using a retractor. Gauzes were inserted under the flap to prevent it from folding. Three experienced neurosurgeons performed the surgeries. The mean operation time was 6.11 ± 2.8 hours (range, 3.8–10.0 hours), and the mean time interval from aneurysm clipping surgery to awareness of vision loss was 10.75 ± 13.8 days (range, 0–29 days). VA testing and ophthalmic examination were performed when VA abnormality was recognized after surgery. Case 2 had light perception (LP) only, and the other cases were able to perceive hand motion (HM) at presentation. All of the patients had IOP within the normal range at that point. Two cases showed choroidal filling defect on FA, and the rest did not show choroidal filling abnormality. Each patient received intravenous methylprednisolone (Salon; Hanlim Pharm Co., Ltd., Korea) after ocular examinations at a dosage of 1 g/d for 3 sequential days followed by oral steroids (1 mg/kg daily) with gradual tapering weekly until 10 mg was reached.
Table 1.
Characteristics of the study patients.
4. Case summaries
4.1. Case 1
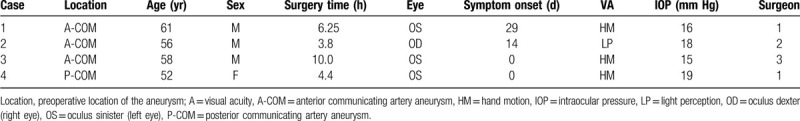
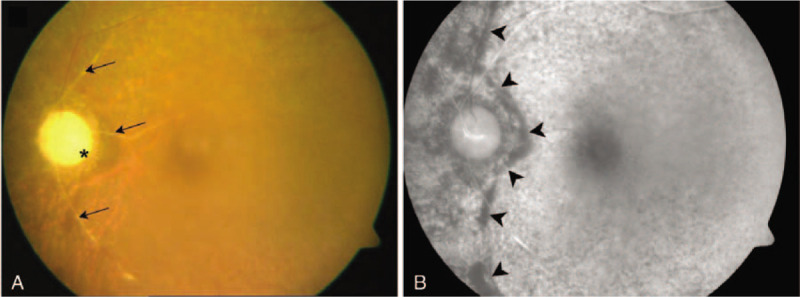
A 61-year-old man noted vision loss (OS) on day 29 after an urgent ruptured aneurysm clipping for the treatment of a subarachnoid hemorrhage (SAH). The aneurysm was located in the left anterior COM, and the surgery lasted 6.25 hours. Postoperatively, he experienced drowsiness for several days and complained of VA deterioration after surgical recovery. VA OS was HM, and the IOP was 16 mm Hg. On ocular examination, periocular swelling and chemosis were absent. Total ophthalmoplegia and ocular pain were not noted (OS). Fundus examination revealed optic atrophy and retinal vessel attenuation (Fig. 1A). FA showed a prolonged arterial phase (> 20 sec) and hypoperfusion of the nasal choroid (Fig. 1B). Computed tomography (CT) taken immediately and 3 days after the surgery showed proptosis without intraorbital hemorrhage (OS). On a later examination, it was noted that the optic nerve was straight, and the medial and lateral recti were enlarged (Fig. 2 A–C).
Figure 1.
Fundus photography and fluorescein angiography of Case 1. (A) Fundus photography shows pale optic disc due to atrophy (asterisk), and distinctive peripapillary sheathing of arteriolar vessels (arrows). Choroidal vessels are visible through the thinned retinal layers in the major vascular territories. (B) Fluorescein angiography shows nasal hypofluorescence forming a strong contrast with the temporal area and a definite boundary (arrowheads)between the hypoperfused and relatively well-perfused choroidal areas.
Figure 2.
Elapsed computed tomography (CT) after brain aneurysm clipping surgery. (A) An immediate postoperative CT image of case 1 shows no proptosis. (B) Repeat CT on postoperative day 3 shows proptosis (white arrow) and enlargement of the medial rectus muscle (red arrow) in case 1. (C) A coronal image shows enlargement of the medial and inferior rectus muscles (red arrows). (D) An immediate postoperative CT image of case 2 shows no proptosis. (E) Repeat CT 2 days later shows mild to moderate proptosis in case 2 (white arrow) and medial rectus enlargement. (F) A coronal sectional image shows enlargement of the medial and inferior rectus muscles (red arrows) in the affected eye.
4.2. Case 2
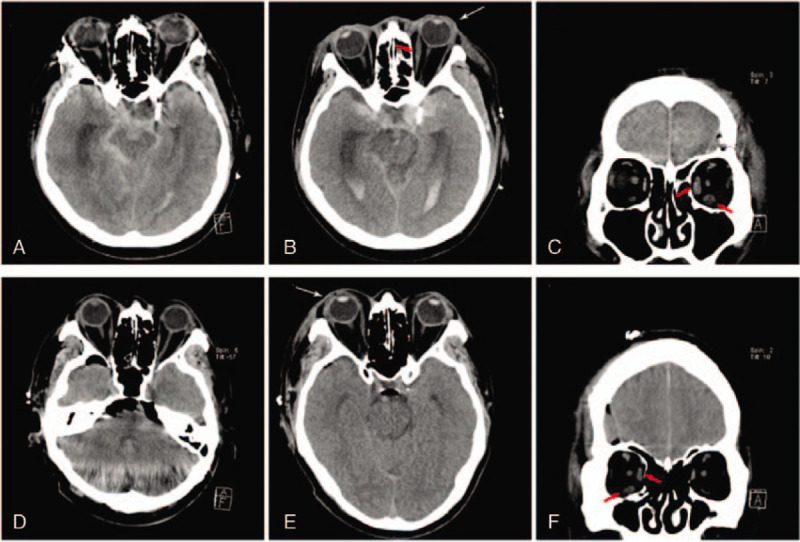
A 56-year-old man noted vision impairment (OD) on day 2 after urgent aneurysmal clipping for the treatment of SAH. The aneurysm was in the right anterior COM, and the surgery lasted 3.8 hours. VA OD was LP, and the IOP was 8 mm Hg. Proptosis and periocular swelling were evident, but intraorbital hemorrhage was absent. In CT images, the medial and lateral rectus muscles were found to be enlarged (Fig. 2 D–F). Total ophthalmoplegia with moderate ptosis, mild chemosis, and conjunctival injection was observed on ophthalmic examination performed on postoperative day 9. Fundoscopic examination at the time showed a slight atrophic change, with the blurring of the optic disc, retinal blot hemorrhages in the posterior pole, macular edema, and retinal artery attenuation (Fig. 3A). One month later, ophthalmoplegia and ptosis almost disappeared, but VA (OD) was no LP (NLP). At that time, FA revealed uneven choroidal filling in both the macular and peripapillary areas. The arterial phase and arteriovenous transit time (AVTT) were prolonged to 20 second and 40 second, respectively (Fig. 3B). OCT showed retinal thinning, and the layers were not distinguishable (Fig. 3C).
Figure 3.
Fundus photography, fluorescein angiography and optical coherence tomography (OCT) of case 2. (A) Fundus photography shows slight blurring and an atrophic optic disc with dot and blot retinal hemorrhages (arrows) in the posterior pole. The retinal arteries are attenuated, and vessels show sheathing (arrowheads). (B) Fluorescein angiography findings are consistent with fundus photography. Macular and peripapillary areas show uneven choroidal filling (asterisks). (C) OCT shows thin atrophic neurosensory retina with indistinct retinal layers (red arrows).
4.3. Case 3
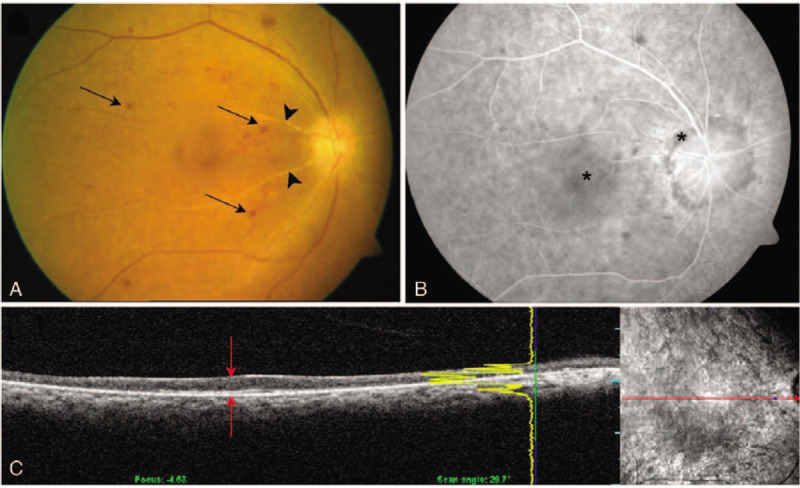
A 58-year-old man complained of impaired vision (OS) immediately after aneurysmal surgery. CT angiography revealed an unruptured left anterior COM. The surgery lasted for 10.0 hours. VA OS was HM, and the IOP was 15 mm Hg. The fundus examination showed thin vitreous opacities and a distinct cherry-red spot on the macula (Fig. 4 A). OCT showed hyperreflective and edematous inner retinal layers (Fig. 4 B). The arterial phase was within normal limits (5 sec), but AVTT was prolonged up to 50 second. After 1 month, VA improved to 20/400. However, the OCT showed inner retinal atrophy, thinning, and inter-layer boundaries that were difficult to discern (Fig. 4 C). On the final examination, VA was counting fingers at a distance of 10 cm. The fundus and OCT images remained unaltered.
Figure 4.
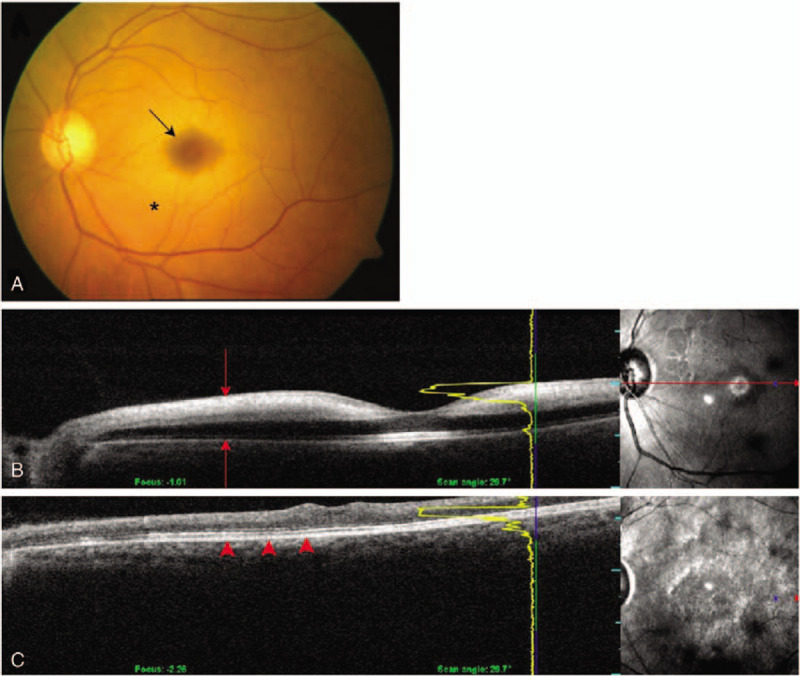
Fundus photography and serial optical coherence tomography (OCT) of case 3. (A) Fundus photography shows a cherry-red spot (arrow) caused by retinal edema (asterisk). (B) Initial OCT shows hyperreflective signals within edematous inner retinal layers (red arrows). (C) Repeat OCT at 1-mo follow-up shows atrophy and thinning of inner retinal layers. The outer retina is relatively preserved (red arrowheads).
4.4. Case 4
A 52-year-old woman complained of impaired vision (OS) immediately after left posterior COM clipping. The surgery lasted for 4.4 hours. VA OS was HM, and the IOP was 19 mmHg. Slit-lamp examination and fundoscopy on postoperative day 6 revealed a corneal epithelial defect and thin vitreous hemorrhage. Optic disc abnormalities or vascular changes were difficult to ascertain due to media opacity. Two months later, her VA improved to 20/100. Corneal epithelial defect and vitreous hemorrhage disappeared, but vitreous opacity after vitreous hemorrhage still existed. Four months after surgery, the VA decreased to 20/200. At the time, FA revealed focal leakages with scattered retinal hemorrhage. The arterial phase and AVTT were prolonged to 15 second and over 20 second, respectively (Fig. 5 A). OCT showed hyperreflectivity in the inner retinal layers and a slightly persistent retinal edema (Fig. 5 C).
Figure 5.
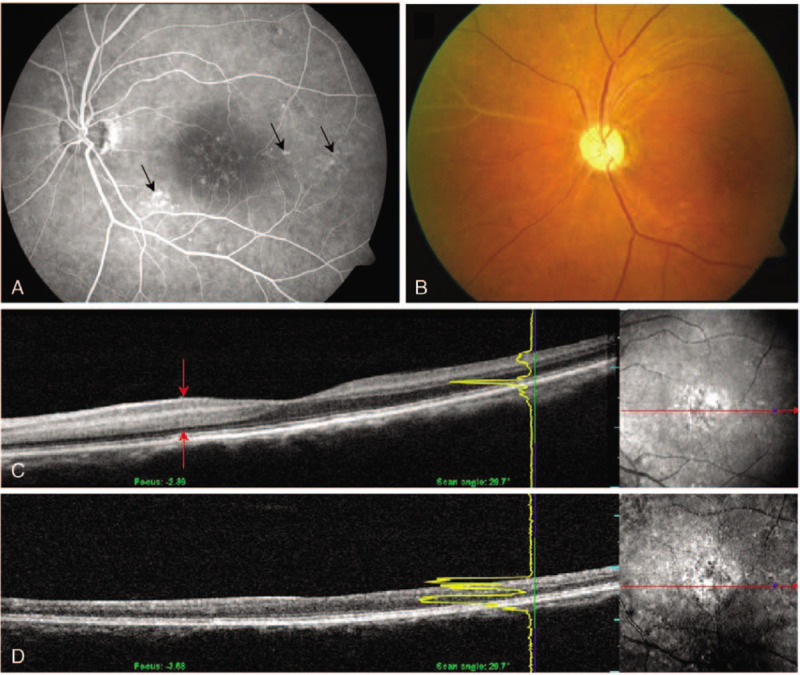
Fluorescein angiography, fundus photography and optical coherence tomography (OCT) of case 4. (A) Fluorescein angiography shows scattered dot retinal leakages around the posterior pole (arrows). (B) Fundus photography at the 1-mo follow-up shows sheathing of the arteriolar vasculature at the nasal vascular arcade. (C) An initial OCT shows hyperreflective and edematous inner retina (red arrows). (D) Repeat OCT obtained 7 mo later shows the atrophic and thinned inner retina layers.
Additionally, she was treated with oral steroids at an initial dose of 50 mg with gradual tapering. The daily dose was then reduced by 10 mg weekly until 10 mg was reached. One month after the steroid treatment, her VA improved to 20/100. However, inferonasal retinal vascular atrophy was noticed (Fig. 5 B). At 7 months after surgery, VA deteriorated to 20/400. OCT image showed inner retinal thinning and indistinct retinal layer boundaries (Fig. 5D). On final VA testing, she could count fingers at a distance of 10 cm.
5. Discussion
Orbital infarction syndrome is caused by varying etiologies involving the whole orbit and presents with ocular and extraocular dysfunction. Visual loss after brain aneurysm surgery is rare, but this devastating complication results from retinal infarction. Its incidence is reportedly between 0.01% and 1% depending on the surgery type.[8]
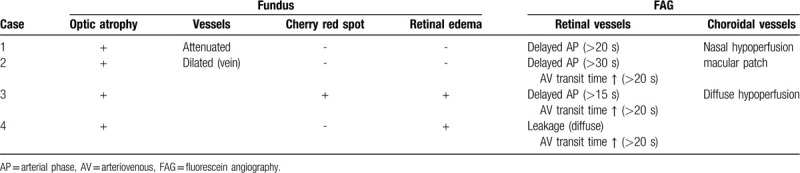
In our case series, we observed the diverse courses and outcomes of orbital infarction syndrome after cerebrovascular surgery. Using various ocular imaging techniques, we were able to distinguish several features (Table 2). In case 1, FA showed hypofluorescence more in the nasal than in the temporal area, which signifies a reduction in choroidal blood flow through the medial posterior ciliary artery. Similarly, the choroidal vasculature was not uniformly fluorescent in case 2; however, the unevenness was more evident in case 1. These findings imply disturbed choroidal hemodynamics, and the difference is presumed to stem from different intraorbital pressures when the ischemia occurred. In case 3, fundus photography showed a cherry-red spot. OCT showed an opaque inner retina in the acute phase and an atrophied retina 1 month later, with relative preservation of the outer retina. These findings are consistent with central retinal artery occlusion, although FA did not reveal any thrombogenic characteristics and only showed delayed AVTT. The retinal arterial disturbances and subsequent ischemia may be attributed to the pressure applied intraoperatively. In case 4, OCT confirmed that the changes to the inner retinal layers were concomitant with VA reduction. As VA deteriorated during follow-up, OCT showed progressive thinning and disruption of the inner retinal layers accordingly. It is plausible that hemodynamic alteration continued after the occurrence of ischemia, which progressed to irreversible damage in the inner retinal layers.
Table 2.
Initial findings of fundoscopy and fluorescein angiography.
The CT images of cases 1 and 2 show protrusion of the affected eyes and enlargement of the extraocular muscles at postoperative days 2 and 3. However, the serial elapsed CT images in cases 3 and 4 showed no such abnormalities. These differences might arise from difference in impaired blood supply via the long ciliary arteries to the extraocular muscles and ocular motor nerves. In cases 1 and 2, the SAH might have accelerated the elevation of intracranial pressure, thereby elevating intraorbital pressure and decreasing the perfusion pressure of the ophthalmic artery. The sudden elevation of intracranial pressure also increases the intraocular venous pressure. This pressure is transmitted along the optic nerve sheath and retinal vessel space, thus occluding the retinal-choroidal anastomosis.[9–12] Following surgery, an abrupt reduction or fluctuation of intracranial pressure can lead to dysfunctional vascular autoregulation and impaired ophthalmic perfusion.[13] Similar to glaucoma, the intracranial pressure fluctuation is a risk factor for optic nerve damage.[14] However, it is difficult to distinguish between damage caused by high intracranial pressure or fluctuation. A disturbance in the posterior ciliary artery can lead to either ophthalmoplegia or choroidal filling defects. Because of the rich collateral circulation between the branches of the external carotid and ophthalmic arteries, ophthalmic artery occlusion alone does not cause this syndrome. However, if collateral blood flow is compromised, orbital infarction can develop.
Cases 3 and 4 did not show proptosis or eyelid swelling following the uncomplicated surgeries. They showed retinal circulatory disturbances only, without choroidal filling abnormalities. Based on these findings, we believe that cases 3 and 4 share a diverse mechanism compared to cases 1 and 2. In cases 3 and 4, the impairment of visual and pupillary responses was recognized immediately after surgery. The POVL is usually caused by prolonged prone positioning during surgery or direct ocular compression.[15] Given that case 4 had an ipsilateral central corneal epithelial defect, we believe that either the surgical procedure or posturing was the most probable cause; especially a direct compression of the eyeball that decreased central retinal artery perfusion to the inner retina.[16] Ocular trauma, embolic and vasospastic episodes in the perioperative period with improper patient positioning, and external ocular compression can affect the central retinal artery leading to retinal ischemia.[17–19] Several perioperative factors like low blood pressure, direct orbital compression, preoperative anemia, obesity, tobacco use, prolonged surgical duration, and massive blood loss have all been associated with the development of POVL and orbital infarction syndrome.[20,21]
Brain surgery performed in the prone position elevates the venous pressure causing interstitial edema.[22] In our cases, the surgeries were performed with the patient in the supine position, with a head-fixating instrument. However, the weight of the craniotomy skin flap that was bent back with an underlying fat pad in the direction of the face may have compressed the periorbital tissues. Moreover, the surgeons had secured the skin flap using a relatively forceful retractor, and piled gauzes between the flap and the eyelid, without any eyeball protection, which might have also contributed to the distortion of vascular channels further.[23,24] Furthermore, surgical decompression techniques could have been used to prevent ischemia and irreversible vision loss when symptoms of compression-induced visual disturbances develop.[25,26] Surgical decompression was not performed in any of the cases presented here, because the IOP was within the normal range, and the symptoms had manifested relatively late during the postoperative period. The steroid pulse therapy administered subsequently did not have a positive effect on any of our 4 cases.[27]
Our patients had different aspects of the similar disease spectrum, namely orbital compartment syndrome (OCS), ocular ischemic syndrome and vasospastic disorder, resulting in retinal ischemia. In the literature, the terms OCS and orbital infarction syndrome are used interchangeably, which is confusing to the reader.[28] OCS is similar to a compartment syndrome in which increased pressure within 1 of the body's enclosed compartments results in insufficient blood flow to that area, potentially damaging the muscles and nerves. OCS is caused by an acute rise in volume within the confined orbital space, such as retrobulbar hematoma, intraorbital abscess, and tumors, which results in an acute increase in orbital tension.[29–31] There were no precipitating factors, such as retrobulbar hemorrhage in our cases.[31,32] Also, there was no internal carotid artery stenosis, which is a potential cause of ocular ischemic syndrome. Our cases show partial similarity to vasospastic disorders, especially retinal vasospasm.[33] In those with a diathesis to cold or emotional stress, vasospasm in the ciliary or choroidal circulation and probably also in the optic nerve head occurs.[34,35] In general, their VA is normal, and visual field defects are diffuse.
The differences in the clinical aspects between cases 1 and 2, and cases 3 and 4 appear to be due to whether the aneurysm was ruptured or not and which artery was affected, such as the posterior ciliary artery or the central retinal artery.
6. Conclusion
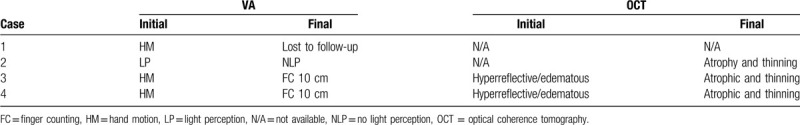
In our case series, cases 1 and 2 had combined choroidal and retinal circulatory impairment, which was evident clinically as optic atrophy, vascular narrowing, whole retinal layer atrophy, and choroidal hypoperfusion. In cases 3 and 4 only the retinal circulation was disturbed and presented with cherry-red spots or marked retinal edema and a relatively conserved choroidal circulation. With progression, retinal edema gradually subsided, and the optic disc showed progressive blurring or atrophy (Table 3). Considering that brain aneurysmal clipping takes several hours, the ischemic insults caused by the factors above could have accumulated over the long surgical period.[5] Indeed, the duration of the operation was shorter in case 4 than in case 3, and the ischemic damage varied.
Table 3.
Follow-up visual acuity (VA) and optical coherence tomography (OCT) findings.
Although the number of cases in this study is small, and the time of ocular examinations was different, the study showed that ocular infarction is associated with ischemia of not only the retinal vessels but also the choroidal circulation. Even though this catastrophic vision-threatening condition is extremely rare, this case series can aid its prevention and also provides useful diagnostic clinical and imaging information.
Author contributions
Conceptualization: Yu Cheol Kim.
Data curation: Sung Won Choi, Jong Hwa Jun.
Formal analysis: Sung Won Choi, Jong Hwa Jun.
Funding acquisition: Yu Cheol Kim.
Investigation: Sung Won Choi, Jong Hwa Jun.
Methodology: Yu Cheol Kim, Jong Hwa Jun, Ji Hye Jang.
Project administration: Sung Won Choi, Yu Cheol Kim.
Resources: Sung Won Choi, Yu Cheol Kim.
Software: Sung Won Choi, Jong Hwa Jun.
Supervision: Yu Cheol Kim.
Validation: Sung Won Choi, Kyung Tae Kang, Jong Hwa Jun. Ji Hye Jang.
Visualization: Sung Won Choi, Jong Hwa Jun.
Writing – original draft: Sung Won Choi, Jong Hwa Jun.
Writing – review & editing: Kyung Tae Kang, Ji Hye Jang, Yu Cheol Kim.
Footnotes
Abbreviations: AVTT = arteriovenous transit time, CT = computed tomography, FA = fluorescein angiography, IOP = intraocular pressure, LP = light perception, OCS = orbital compartment syndrome, OCT = optical coherence tomography, POVL = perioperative visual loss, VA = visual acuity.
How to cite this article: Choi SW, Kang KT, Jun JH, Jang JH, Kim YC. Orbital infarction syndrome after cerebral aneurysm surgery: a case series and literature review. Medicine. 2020;99:29(e21277).
This work was supported by the research promoting grant from the Keimyung University Dongsan Medical Center in 2019.
The study concept was presented in part as a poster at the 108th Annual Meeting of Korean Ophthalmological Society, KINTEX, Seoul, Korea.
Financial disclosure: Kim YC reports honorarium from Allergan, Bayer, Novartis and Santen, and a research grant from Bayer, outside the submitted work; Jang JH reports honorarium from Bayer and Novartis outside the submitted work; the others have no financial or non-financial disclosure.
The authors have no conflicts of interest to disclose.
References
- [1].American Society of Anesthesiologists Task Force on Perioperative Visual L.. Practice advisory for perioperative visual loss associated with spine surgery: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Visual Loss. Anesthesiology 2012;116:274–85. [DOI] [PubMed] [Google Scholar]
- [2].Stevens WR, Glazer PA, Kelley SD, et al. Ophthalmic complications after spinal surgery. Spine (Phila Pa 1976) 1997;22:1319–24. [DOI] [PubMed] [Google Scholar]
- [3].Leibovitch I, Casson R, Laforest C, et al. Ischemic orbital compartment syndrome as a complication of spinal surgery in the prone position. Ophthalmology 2006;113:105–8. [DOI] [PubMed] [Google Scholar]
- [4].Tachfouti S, Karmane A, El Moussaif H, et al. [Orbital ischemia syndrome after surgical intervention of the spine. A case report] [Article in French] Bull Soc Belge Ophtalmol 2007;305:27–30. [PubMed] [Google Scholar]
- [5].Newman NJ. Perioperative visual loss after nonocular surgeries. Am J Ophthalmol 2008;145:604–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Borruat FX, Bogousslavsky J, Uffer S, et al. Orbital infarction syndrome. Ophthalmology 1993;100:562–8. [DOI] [PubMed] [Google Scholar]
- [7].Bogousslavsky J, Pedrazzi PL, Borruat FX, et al. Isolated complete orbital infarction: a common carotid artery occlusion syndrome. Eur Neurol 1991;31:72–6. [DOI] [PubMed] [Google Scholar]
- [8].Lee LA, Newman NJ, Wagner TA, et al. Postoperative ischemic optic neuropathy. Spine (Phila Pa 1976) 2010;35: 9 Suppl: S105–116. [DOI] [PubMed] [Google Scholar]
- [9].Medele RJ, Stummer W, Mueller AJ, et al. Terson's syndrome in subarachnoid hemorrhage and severe brain injury accompanied by acutely raised intracranial pressure. J Neurosurg 1998;88:851–4. [DOI] [PubMed] [Google Scholar]
- [10].Muller PJ, Deck JH. Intraocular and optic nerve sheath hemorrhage in cases of sudden intracranial hypertension. J Neurosurg 1974;41:160–6. [DOI] [PubMed] [Google Scholar]
- [11].Moraru A, Mihailovici R, Costin D, et al. Terson's Syndrome - case report. Rom J Ophthalmol 2017;61:44–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Skevas C, Czorlich P, Knospe V, et al. Terson's syndrome--rate and surgical approach in patients with subarachnoid hemorrhage: a prospective interdisciplinary study. Ophthalmology 2014;121:1628–33. [DOI] [PubMed] [Google Scholar]
- [13].Zimmerman CF, Van Patten PD, Golnik KC, et al. Orbital infarction syndrome after surgery for intracranial aneurysms. Ophthalmology 1995;102:594–8. [DOI] [PubMed] [Google Scholar]
- [14].Martin-Du Pan RC, Benoit R, Girardier L. The role of body position and gravity in the symptoms and treatment of various medical diseases. Swiss Med Wkly 2004;134:543–51. [DOI] [PubMed] [Google Scholar]
- [15].Berg KT, Harrison AR, Lee MS. Perioperative visual loss in ocular and nonocular surgery. Clin Ophthalmol 2010;4:531–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hayreh SS. The blood supply of the optic nerve head and the evaluation of it - myth and reality. Prog Retin Eye Res 2001;20:563–93. [DOI] [PubMed] [Google Scholar]
- [17].Noble MJ, Alvarez EV. Combined occlusion of the central retinal artery and central retinal vein following blunt ocular trauma: a case report. Br J Ophthalmol 1987;71:834–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kollarits CR, Lubow M, Hissong SL. Retinal strokes. I. Incidence of carotid atheromata. JAMA 1972;222:1273–5. [DOI] [PubMed] [Google Scholar]
- [19].Brown GC, Magargal LE, Shields JA, et al. Retinal arterial obstruction in children and young adults. Ophthalmology 1981;88:18–25. [DOI] [PubMed] [Google Scholar]
- [20].Cheng MA, Todorov A, Tempelhoff R, et al. The effect of prone positioning on intraocular pressure in anesthetized patients. Anesthesiology 2001;95:1351–5. [DOI] [PubMed] [Google Scholar]
- [21].Ho VT, Newman NJ, Song S, et al. Ischemic optic neuropathy following spine surgery. J Neurosurg Anesthesiol 2005;17:38–44. [PMC free article] [PubMed] [Google Scholar]
- [22].Postoperative Visual Loss Study G.. Risk factors associated with ischemic optic neuropathy after spinal fusion surgery. Anesthesiology 2012;116:15–24. [DOI] [PubMed] [Google Scholar]
- [23].Hollenhorst RW, Svien HJ, Benoit CF. Unilateral blindness occurring during anesthesia for neurosurgical operations. AMA Arch Ophthalmol 1954;52:819–30. [DOI] [PubMed] [Google Scholar]
- [24].Takahashi Y, Kakizaki H, Selva D, et al. Bilateral orbital compartment syndrome and blindness after cerebral aneurysm repair surgery. Ophthalmic Plast Reconstr Surg 2010;26:299–301. [DOI] [PubMed] [Google Scholar]
- [25].Goodall KL, Brahma A, Bates A, et al. Lateral canthotomy and inferior cantholysis: an effective method of urgent orbital decompression for sight threatening acute retrobulbar haemorrhage. Injury 1999;30:485–90. [DOI] [PubMed] [Google Scholar]
- [26].Saussez S, Choufani G, Brutus JP, et al. Lateral canthotomy: a simple and safe procedure for orbital haemorrhage secondary to endoscopic sinus surgery. Rhinology 1998;36:37–9. [PubMed] [Google Scholar]
- [27].Braughler JM, Hall ED. Effects of multi-dose methylprednisolone sodium succinate administration on injured cat spinal cord neurofilament degradation and energy metabolism. J Neurosurg 1984;61:290–5. [DOI] [PubMed] [Google Scholar]
- [28].Gauden AJ, Hardy T, Mack HG, et al. Orbital compartment syndrome following aneurysm surgery. J Clin Neurosci 2012;19:1032–6. [DOI] [PubMed] [Google Scholar]
- [29].Curran EL, Fleming JC, Rice K, et al. Orbital compression syndrome in sickle cell disease. Ophthalmology 1997;104:1610–5. [DOI] [PubMed] [Google Scholar]
- [30].Hayreh SS, Kolder HE, Weingeist TA. Central retinal artery occlusion and retinal tolerance time. Ophthalmology 1980;87:75–8. [DOI] [PubMed] [Google Scholar]
- [31].Lima V, Burt B, Leibovitch I, et al. Orbital compartment syndrome: the ophthalmic surgical emergency. Surv Ophthalmol 2009;54:441–9. [DOI] [PubMed] [Google Scholar]
- [32].Sundu C, Dinc E, Sari A, et al. Bilateral subperiosteal hematoma and orbital compression syndrome in sickle cell disease. J Craniofac Surg 2017;28:e775–6. [DOI] [PubMed] [Google Scholar]
- [33].Flammer J, Prunte C. [Ocular vasospasm. 1: functional circulatory disorders in the visual system, a working hypothesis] [Article in German]. Klin Monbl Augenheilkd 1991;198:411–2. [DOI] [PubMed] [Google Scholar]
- [34].Guthauser U, Flammer J, Mahler F. The relationship between digital and ocular vasospasm. Graefes Arch Clin Exp Ophthalmol 1988;226:224–6. [DOI] [PubMed] [Google Scholar]
- [35].Flammer J. Psychophysical mechanisms and treatment of vasospastic disorders in normal-tension glaucoma. Bull Soc Belge Ophtalmol 1992;244:129–34. [PubMed] [Google Scholar]


