Abstract
Lefamulin is a novel pleuromutilin antibiotic with potent in vitro activity against key community-acquired bacterial pneumonia (CABP) pathogens. However, the clinical efficacy and safety of lefamulin for treating CABP remains unclear.
An integrated analysis of 2 phase III trials investigating the clinical efficacy and safety of lefamulin vs moxifloxacin in the treatment of CABP was conducted.
A total of 1289 patients (lefamulin group: 646 and moxifloxacin group: 643) were included in this analysis. The early clinical response rate was 89.3% and 90.5% among lefamulin and moxifloxacin group, respectively. Lefamulin was noninferior to moxifloxacin (89.3% vs 90.5%, RR: 0.99, 95% CI: 0.95–1.02, I2 = 0%). In terms of clinical response at test of cure, no significant difference was observed between the lefamulin and moxifloxacin groups (for modified intention to treat population, RR: 0.98, 95% CI: 0.94–1.02, I2 = 0%; for clinically evaluable population, RR: 0.96, 95% CI: 0.93–1.00, I2 = 0%). In the subgroup analysis, the early clinical response rate at early clinical assessment and clinical response rate at test of cure of lefamulin was similar to that of moxifloxacin across different subgpopulations and all baseline CABP pathogens. Lefamulin was associated with a similar risk of adverse events as moxifloxacin.
Clinical efficacy and tolerability for lefamulin in the treatment of CABP were similar to those for moxifloxacin.
Keywords: community-acquired bacterial pneumonia, lefamulin, moxifloxacin
1. Introduction
Community-acquired bacterial pneumonia (CABP) remains a global health threaten, and is a leading cause of hospitalization and infection-related mortality.[1] This type of infection is commonly caused by the typical pathogens - Streptococcus pneumoniae, Staphylococcus aureus, Haemophilus influenzae, Moraxella catarrhalis, and the atypical pathogens - Mycoplasma pneumoniae, Chlamydophila pneumoniae, and Legionella pneumophila.[2] Empirical treatment regimen of CABP typically involves either a respiratory fluoroquinolone or a combination of β-lactams and a macrolide.[2–4] However, like other types of infections, antimicrobial resistance has reduced the effectiveness of commonly used antibiotic and become another serious concern in the treatment of CABP. The emergence of antibiotic-resistant organism – penicillin-resistant S. pneumoniae, methicillin-resistant S. aureus, macrolide-resistance in M. pneumoniae, and levofloxacin-nonsusceptible S. pneumoniae makes the condition more complicated than before.[5–7] All of these issues triggered the urgent need of a newly effective antibiotic for treating CAPB.
Lefamulin is a promising novel pleuromutilin antibiotic, which exhibits a unique mechanism of action through inhibition of protein synthesis and further preventing the binding of transfer RNA for peptide transfer.[8] Several in vitro studies[9–11] have demonstrate its potent activity against commonly encountered CAPB pathogens, even for antibiotic-resistant bacteria. However, the clinical study investigating the usefulness of lefamulin in the treatment of infectious disease is limited. Until now, only two randomized clinical trials[12,13] assess the uses of lefamulin in the treatment of CABP. Both of these two studies[12,13] used moxifloxacin as comparator. Moxifloxacin is an extended-spectrum fluoroquinolone which exhibit potent activity against gram-positive cocci and atypical pathogen and recommended as a respiratory fluoroquinolone in the treatment of CABP.[14–16] To better understand the clinical efficacy and safety of lefamulin, we conducted this integrated analysis of two phase III studies[12,13] investigating the usefulness of lefamulin in the treatment of CABP.
2. Methods
2.1. The characteristics of the studies
The Lefamulin Evaluation Against Pneumonia (LEAP) program comprised 2 phased III randomized, multicenter, multinational studies – LEAP 1 (NCT02559310) and LEAP 2 (NCT02813694).[12,13] LEAP 1 included adult patients with CABP at pneumonia outcome research team (PORT) risk class ≥ III. This trial compared the clinical efficacy and tolerability of Lefamulin (150 mg intravenous [IV], every 12 hours) and moxifloxacin (400 mg IV every 24 hours). On or after 3 days (6 doses) of IV treatment, patients could be switched to oral lefamulin 600 mg q12 hours or moxifloxacin 400 mg q24 h and the total duration of antibiotic treatment ranged from 5 to 10 days.[13] LEAP 2 included adult patients with CABP at PORT risk class II, III or IV and compared the effect and safety of oral lefamulin (600 mg every 12 hours for 5 days) and oral moxifloxacin (400 mg every 24 hours for 7 days).[12] This study study was exempt from ethics approval of Chi Mei Medical center as the study authors just collected and synthesized data from previous clinical trials in which informed consent has already been obtained by the trial investigators.
2.2. Analysis population and outcome measurement
The intent-to-treat (ITT) population included all patients who were randomized and the modified intent-to-treat (MITT) population was the population to assess adverse events and comprised all patients who received 1 or more doses of study drug. The microbiologic (mITT) population included all patients in the ITT population had a baseline qualifying bacterial pathogen. The clinically evaluable (CE) population included patients who received study drug for a total duration of 48 hours or longer (unless patient died prior to 48 hours), and had assessable efficacy. Efficacy endpoints included: proportion of patients with early clinical response (ECR) at early clinical assessment and clinical response at the end of treatment (EOT) and test of cure (TOC) (5–10 days after EOT). ECR responders was defined as improvement in ≥2 of 4 CABP signs/symptoms, had no worsening in any CABP sign/symptom, and had not received other non-study antibiotic for CABP at 96 ± 24 hours after first study drug dose. Clinical responder was defined as successful if resolution or improvement of CABP signs/symptoms and no additional antibiotics was needed for CABP.
2.3. Statistical analysis
Categorical variables were reported as frequency counts with percentages. In addition, the differences of baseline characteristics between the lefamulin and moxifloxacin groups were evaluated using Pearson's chi-squared test for categorical variables. Treatment effects, including clinical response, and the risk of adverse event were calculated as risk ratio (RR) with 95% confidence interval (CI) for dichotomous data. A noninferiority margin of 10% was used for ECR, based on historical data comparing antibacterial drugs vs nonantibacterial treatments and current guidance from the FDA.[17] The 2-sided 95% confidence intervals (CI) for the differences in ECR and IACR clinical success rates were calculated using the Miettinen and Nurminen method, with stratification.[18] Noninferiority of lefamulin to moxifloxacin was concluded if the lower limit of the 95% CI for the treatment difference was >10%.
3. Results
3.1. The clinical manifestations of patients
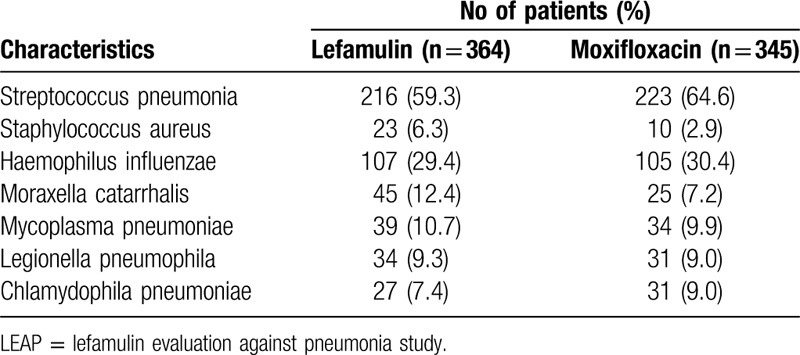
Overall, a total of 1289 patients (lefamulin group: 646 and moxifloxacin group: 643) were in this analysis (Table 1, Fig. 1). Their mean age was 58.7 years and 40.1% (n = 517) of patients were ≥ 65 years. 55.6% (n = 717) of patients were male and 79.3% (n = 1022) were white race. 1230 patients (95.4%) had systemic inflammation response syndrome and 25 patients (1.9%) had bacteremia. The distribution of PORT risk class was class I: 0.2% (n = 3), II: 28.9% (n – 373), III: 52.4% (n = 675), IV: 17.7% (n = 228) and V: 0.8% (n = 10). Among mITT population (n = 709), S. pneumoniae was the most common baseline pathogens (n = 439, 61.9%), followed, Haemophilus influenzae (n = 212, 29.9%), Moraxella catarrhalis (n = 70, 9.9%), Mycoplasma pneumoniae (n = 73, 10.3%), Legionella pneumophila (n = 65, 9.2%), Chlamydophila pneumoniae (n = 58, 8.2%) and S. aureus (n = 33, 4.7%)(Table 2). There was no significant difference in terms of the demographic and baseline characteristics between lefamulin and moxifloxacin group.
Table 1.
Demographic characteristics for patients in LEAP 1 and LEAP 2 studies in intention-to-treat population.
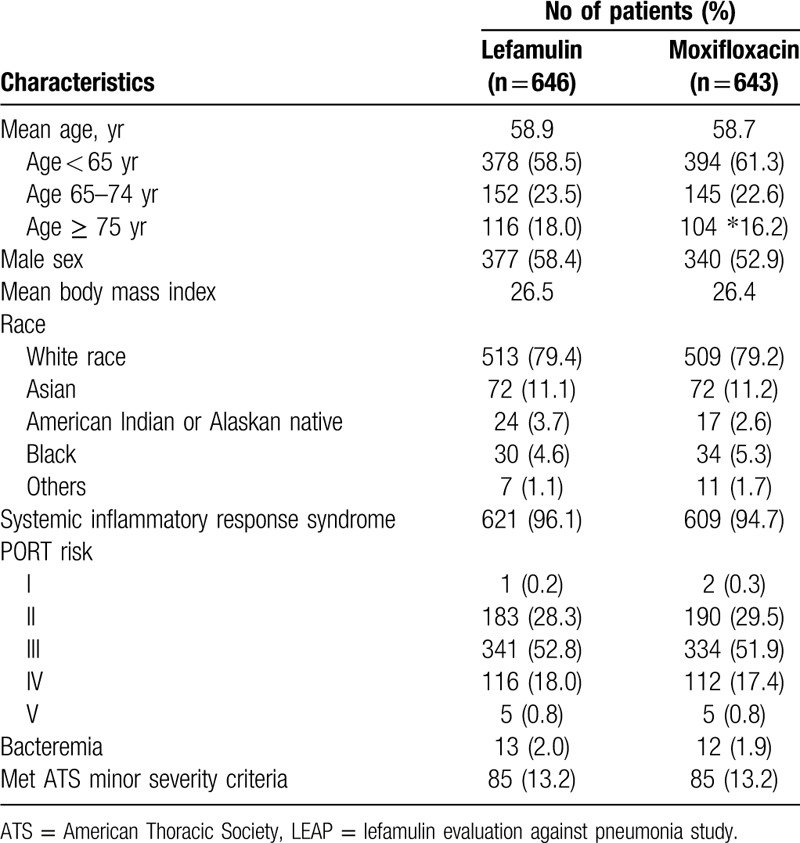
Figure 1.
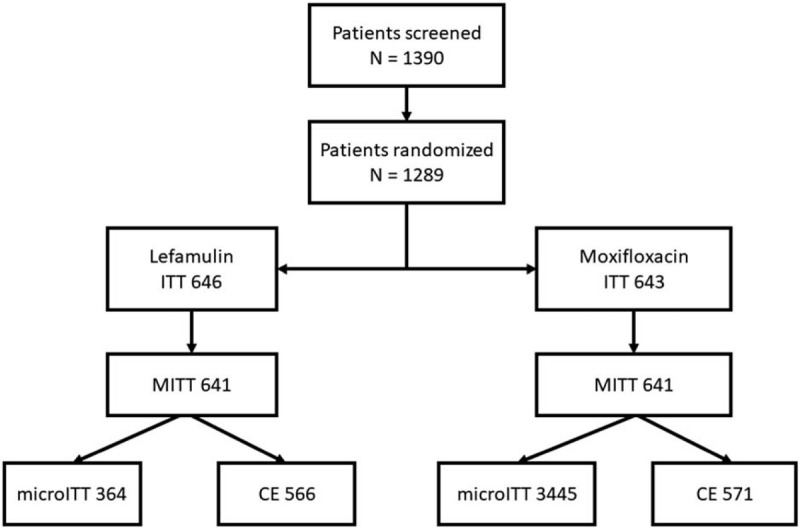
Disposition of patients enrolled in LEAP-1 and LEAP-2. CE = clinically evaluable; ITT = intent-to-treat; MITT = modified ITT; microITT = microbiologic ITT; LEAP = lefamulin evaluation against pneumonia study.
Table 2.
Common baseline pathogens for patients in LEAP 1 and LEAP 2 studies in microbiological intention-to-treat population.
3.2. Minimum inhibitory concentrations (MICs)
For S. pneumoniae isolates, MIC50/MIC90 of lefamulin were 0.25/0.5 μg/ml in both LEAP 1 trial (n = 50) and LEAP 2 trials (n = 80). For macrolide-resistant S. pneumoniae isolates, MIC50/MIC90 of lefamulin were 0.25/0.5 μg/ml in LEAP 1 trial (n = 12) and 0.25/0.25 μg/mL in LEAP 2 trial (n = 19). For multidrug-resistant S. pneumoniae isolates, MIC50/MIC90 of lefamulin were 0.25/0.5 μg/ml in LEAP 1 trial (n = 12) and 0.25/0.25 μg/mL in LEAP 2 trial (n = 20). For S aureus isolates, MIC50/MIC90 of lefamulin were 0.12/0.25 μg/ml in LEAP 1 trial (n = 10) and 0.12/0.12 μg/ml in LEAP 2 trial (n = 14). For 3 MRSA isolates in LEAP 2 trial, MIC of lefamulin was exclusively 0.12 μg/mL. For H influenzae isolates, MIC50/MIC90 of lefamulin were 1/2 μg/mL in both LEAP 1 trial (n = 11) and LEAP 2 trial (n = 24). For M catarrhalis isolates, MIC range of lefamulin were 0.12–0.12 μg/ml in LEAP 1 trial (n = 2) and 0.06 to 0.25 μg/mL in LEAP 2 trial (n = 5). The MIC range of lefamulin against M. pneumoniae was ≤ 0.001–≤ 0.001 μg/mL in LEAP 1 trial (n = 6) and MIC50/MIC90 were ≤ 0.001/≤ 0.001 μg/mL in in LEAP 2 trial (n = 11).
3.3. Clinical efficacy
Overall, the early clinical response rate was 89.3% and 90.5% among lefamulin and moxifloxacin group, respectively. Lefamulin was noninferior to moxifloxacin (RR: 0.99, 95% CI: 0.95–1.02, I2 = 0%; Fig. 2). Regarding nonresponder rate at early clinical assessment, no significant difference was observed between lefamulin and moxifloxacin (9.0% vs 8.1%; RR: 1.11, 95% CI: 0.76–1.63, I2 = 11%). In terms of clinical response at TOC, no significant difference was observed between the lefamulin and moxifloxacin groups (for MITT population, RR: 0.98, 95% CI: 0.94–1.02, I2 = 0%; for CE population, RR: 0.96, 95% CI: 0.93–1.00, I2 = 0%; Fig. 1). Furthermore, the clinical failure rate at TOC remained similar between the lefamulin and moxifloxacin groups (for MITT population, RR: 1.20, 95% CI: 0.90–1.61, I2 = 0% and for CE population, RR: 1.40, 95% CI: 0.98–1.99, I2 = 0%).
Figure 2.
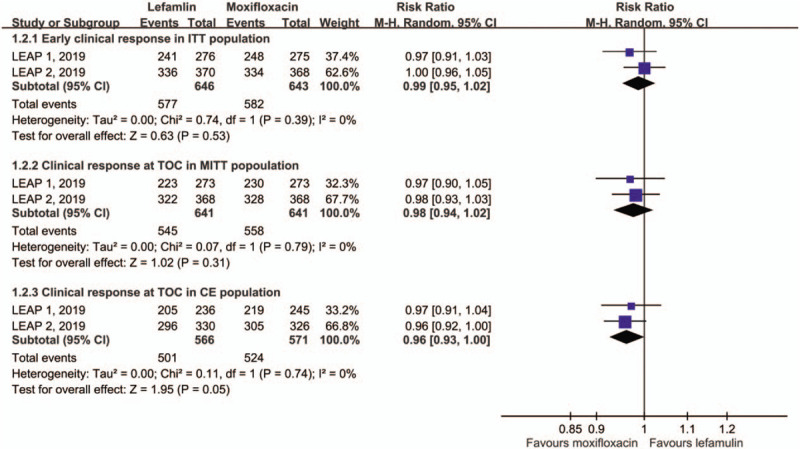
Early clinical response for the intent-to-treat (ITT) population and clinical response at test of cure (TOC) in modified ITT (MITT) and clinically evaluable (CE) populations.
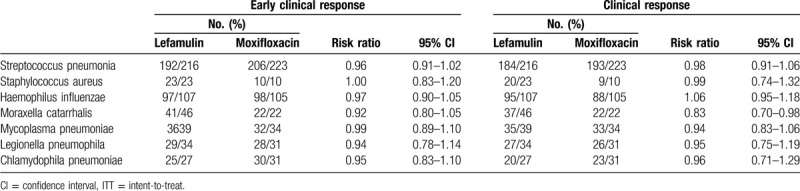
In the subgroup analysis, the early clinical response rate at early clinical assessment and clinical response rate at TOC of lefamulin was similar to that of moxifloxacin across different subgpopulations (Tables 3 and 4). Lefamulin and moxifloxacin demonstrated high rates of at early clinical assessment and clinical response rate at TOC across all baseline CABP pathogens (Table 5). Lefamulin exhibit similar clinical efficacy to moxifloxacin for most of CABP pathogens, except Moraxella catarrhalis (Table 5).
Table 3.
Early clinical response in intention-to-treat population by subgroup.
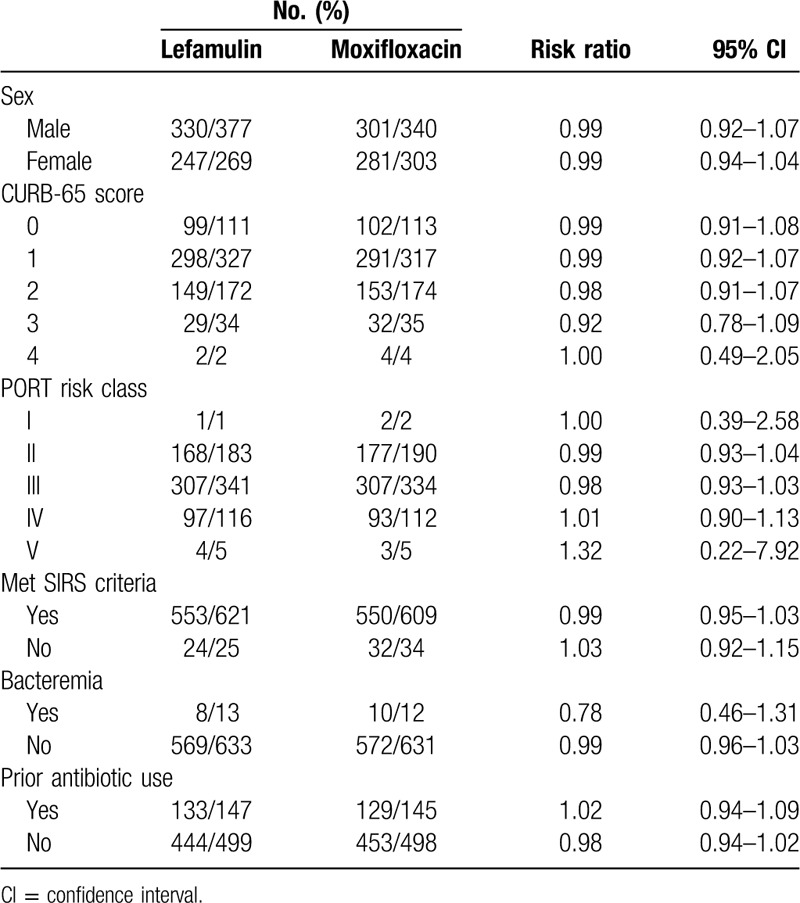
Table 4.
Clinical response in modified intention-to-treat population by subgroup.
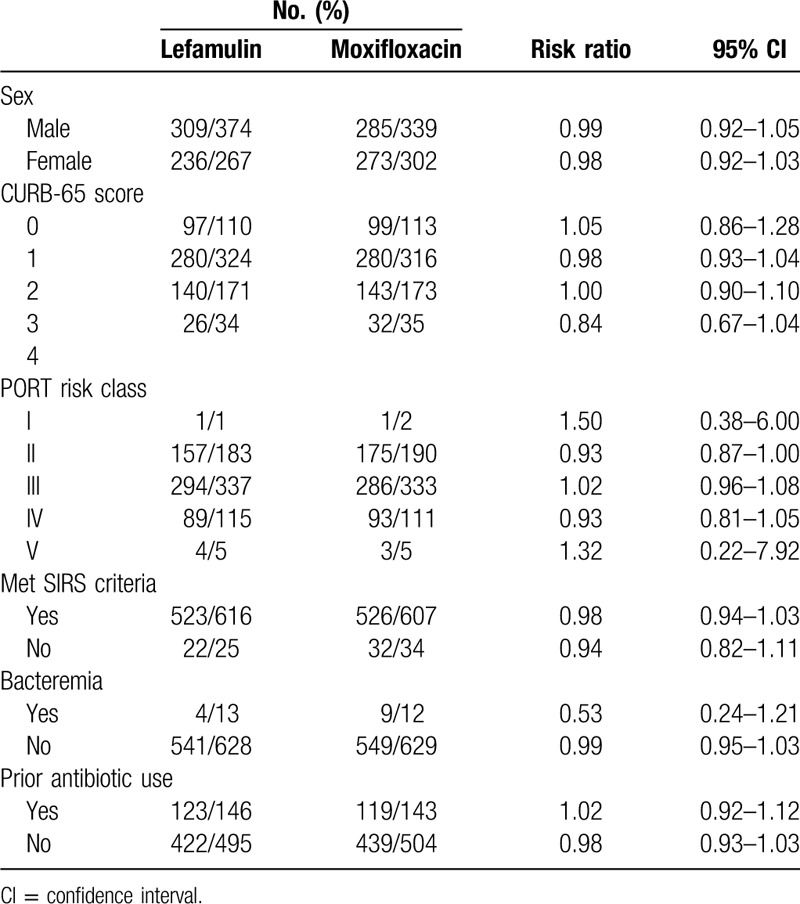
Table 5.
Early clinical response for the intent-to-treat (ITT) population and clinical response at test of cure in modified ITT by baseline pathogen.
3.4. Risk of adverse events
Overall, lefamulin was associated with a similar risk of AEs as moxifloxacin (TEAE, RR: 1.14, 95% CI: 0.89–1.47, I2 = 61%; serious AEs, RR: 1.16, 95% CI: 0.72–1.86, I2 = 0%; treatment related TEAEs, RR: 1.45, 95% CI: 0.77–2.72, I2 = 79%; treatment related serious AEs, RR: 1.35, 95% CI: 0.17–0.74, I2 = 17%; treatment discontinuation due to TEAE, RR: 0.95, 95% CI: 0.45–1.88, I2 = 19%; treatment withdraw due to TEAE, RR:0.63, 95% CI: 0.29–1.40, I2 = 0%; and treatment leading to death, RR: 1.37, 95% CI: 0.55–3.39, I2 = 0%; Fig. 3). However, lefamulin was associated with a higher risk of TEAE in moderate severity than moxifloxacin (RR: 1.41, 95% CI: 1.02–1.96, I2 = 79%).
Figure 3.
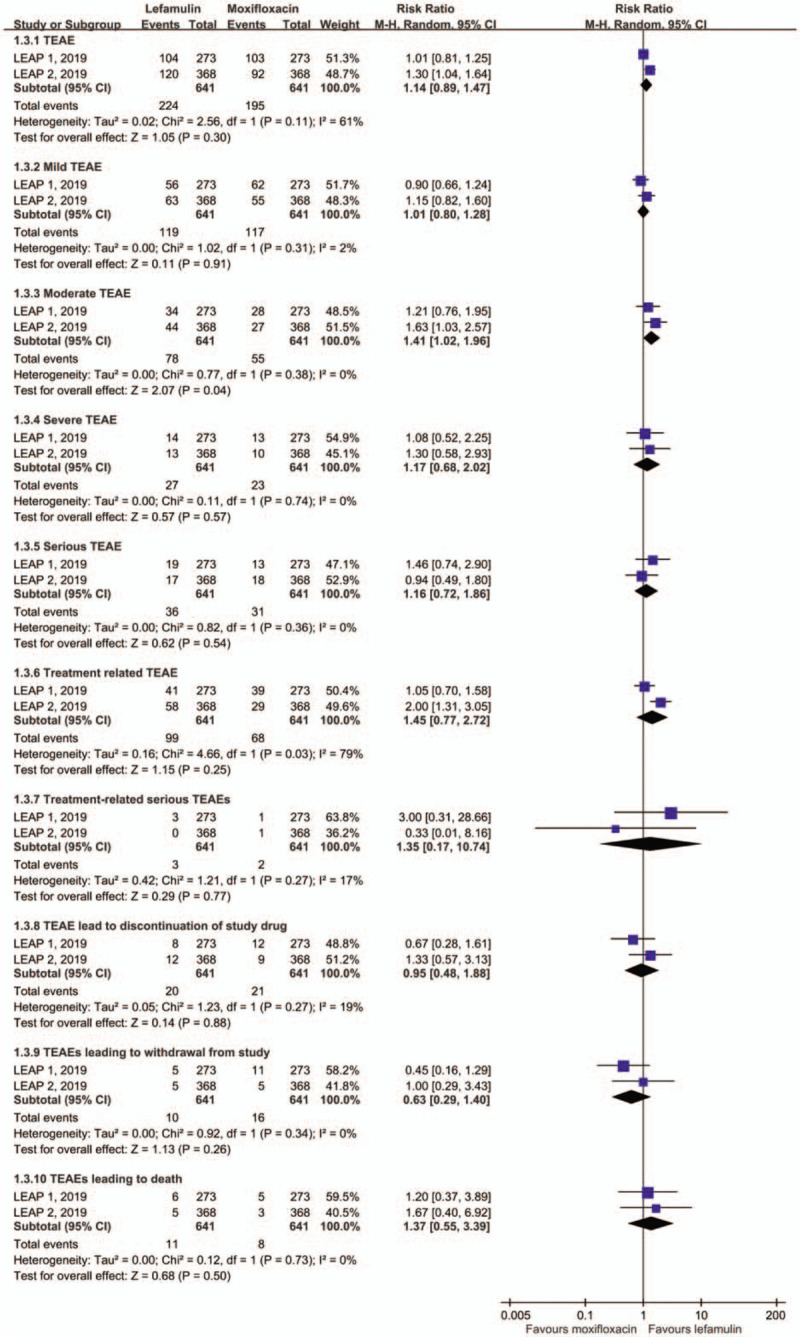
Risk of adverse event.
For common gastrointestinal adverse event, the risk of nausea and diarrhea was 4.2% and 7.3% among lefamulin group in the pooled analysis. Both were higher than those of moxifloxacin (nausea, RR: 2.03, 95% CI: 1.02–4.03, I2 = 6%; diarrhea, RR: 1.06, 95% CI: 0. 01–116. 69, I2 = 96%).
4. Discussion
The integrated analysis of data from 2 RCTs[12,13] with 1289 patients were collated to compare the efficacy and safety of lefamulin and moxifloxacin for treating CABP. In the present study, lefamulin could achieve a similar clinical response as moxifloxacin, which is supported by the following evidence. First, the early clinical response rate for lefamulin was similar to moxifloxacin in the pooled analysis of ITT population. This similarity between lefamulin and moxifloxacin was observed in terms of clinical response rate at TOC among both MITT, and CE population. Second, lefamulin exhibited similar clinical efficacy than moxifloxacin across different age, severity, and various subgroups. Third, the clinical efficacies of lefamulin were similar to those of moxifloxacin across infections caused by different pathogens, including S pneumoniae, S aureus. H inlfuenzae, M pneumoniae, L pneumophila and C pneumoniae. The only exception was M catarrhalis, in which lefamulin exhibit a lower clinical response at TOC than moxifloxacin. In summary, all these findings indicate that lefamulin can be as effective as moxifloxacin for treating CABP.
In this study, we also found the potent in vitro activity of lefamulin against CABP pathogens according to the MIC tests. For key CABP pathogens, including S pneumonia, S aureus, H influenzae, M catarrhalis, and Mycoplasma pneumoniae, the MIC value of lefamulin remain low in both LEAP 1 and LEAP 2 trials.[12,13] Even for antibiotic-resistant organisms, such as MDR S. pneumoniae and MRSA, lefamulin still exhibit good in vitro activity. In fact, several global investigations have revealed that lefamulin exhibited potent in vitro activity against key CABP pathogens as well as was active against antibiotic-resistant organisms.[10,11,19] For S pneumoniae isolates, lefamulin exhibited MIC50 and MIC90 values of 0.12 and 0.25 μg/mL, respectively, against a total of 822 strains collected in United State.[19] Even for MDR S. pneumoniae isolates, the MIC50 and MIC90 of lefamulin was only 0.12 and 0.25 μg/mL, respectively.[19] For Mycoplasma pneumoniae isolates, all MICs of lefamulin against 18 macrolide-susceptible and 42 macrolide-resistant strains were ≤ 0.008 μg/mL and lefamulin had lowest MIC90 (0.002 μg/mL) for macrolide-resistant strains among all tested agents including azithromycin, erythromycin, tetracycline, doxycycline, and moxifloxacin.[11] In a global surveillance of 8595 commonly encountered pathogens causing CABP, lefamulin can inhibited 99.2% of all isolates tested, including 100% of S pneumoniae isolates, 99.8% of S aureus isolates, 93.8% of H influenzae isolates, and 100% of M catarrhalis isolates, using the susceptible breakpoint of MIC ≤1 μg/mL.[10] For multidrug-resistant and extensively drug-resistant S pneumoniae strains, all MIC50/90 values were only 0.06/0.12 μg/mL. The MIC50/90 value of lefamulin against MRSA were 0.06/0.12 μg/mL. Therefore, these findings can help support the use of lefamulin for treating CABP.
Finally, the risk of AEs for lefamulin was assessed. Lefamulin was associated with higher risk of gastrointestinal AE than moxifloxacin, especially for oral form. In LEAP 2 trial, only oral lefamulin was used and the gastrointestinal-related AE – mostly diarrhea, occurred in 17.9% of patients. In LEAP 1 trial, gastrointestinal events more developed during oral than IV treatment with lefamulin (7.7% vs 3.7%). Thus, it reminds us the importance of closely monitoring gastrointestinal intolerance during the use of oral lefamulin. Although lefamulin carried higher risk of moderate TEAE than moxifloxacin, lefamulin had a similar risk of AEs in TEAEs, serious AEs, treatment related TEAE, treatment related serious AE, treatment discontinuation/withdraw due to TEAEs, and treatment leading to death when compared with moxifloxacin. All these findings indicated that lefamulin was found to be as tolerable as moxifloxacin.
This study has some limitations. First, we could not assess the association between in vitro activity and clinical response for each specific pathogen due to the unavailability of data. Second, we did not evaluate the cost-effectiveness of lefamulin in this study. The cost of lefamulin is several-fold more than moxifloxacin.[20] Third, neutrophil biology and inflammation plays important role in pneumonia,[21] however, these 2 studies did not assess this issue. Finally, all of the data were obtained from the published articles only and that participant level data were not available. Although this issue could limit the findings of this integrated analysis, this study combined LEAP 1 and LEAP 2 to allow subgroup analysis and confirm the results of each individual study in the combined analysis. However, further study is warranted to clarify these above issues.
In conclusion, clinical efficacy and tolerability for lefamulin in the treatment of CABP were similar to those for moxifloxacin.
Author contributions
Conceptualization: Hung-Jen Tang, Chih-Cheng Lai.
Data curation: Hung-Jen Tang, Jui-Hsiang Wang.
Formal analysis: Jui-Hsiang Wang.
Writing – original draft: Hung-Jen Tang, Chih-Cheng Lai.
Footnotes
Abbreviations: CABP = community-acquired bacterial pneumonia, PORT = pneumonia outcome research team, TOC = test of cure.
How to cite this article: Tang HJ, Wang JH, Lai CC. Lefamulin vs moxifloxacin for community-acquired bacterial pneumonia. Medicine. 2020;99:29(e21223).
Ethical approval was not required.
Informed consent was not required.
The authors have no funding and conflicts of interest to disclose.
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
- [1].Peyrani P, Mandell L, Torres A, et al. The burden of community-acquired bacterial pneumonia in the era of antibiotic resistance. Expert Rev Respir Med 2019;13:139–52. [DOI] [PubMed] [Google Scholar]
- [2].Wunderink RG, Waterer GW. Clinical practice. Community-acquired pneumonia. N Engl J Med 2014;370:543–51. [DOI] [PubMed] [Google Scholar]
- [3].Lim WS, Smith DL, Wise MP, et al. British Thoracic Society community acquired pneumonia guideline and the NICE pneumonia guideline: how they fit together. Thorax 2015;70:698–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chou CC, Shen CF, Chen SJ, et al. Recommendations and guidelines for the treatment of pneumonia in Taiwan. J Microbiol Immunol Infect 2019;52:172–99. [DOI] [PubMed] [Google Scholar]
- [5].Song JH, Jung SI, Ki HK, et al. Clinical outcomes of pneumococcal pneumonia caused by antibiotic-resistant strains in asian countries: a study by the Asian Network for Surveillance of Resistant Pathogens. Clin Infect Dis 2004;38:1570–8. [DOI] [PubMed] [Google Scholar]
- [6].Kang CI, Song JH, Kim SH, et al. Association of levofloxacin resistance with mortality in adult patients with invasive pneumococcal diseases: a post hoc analysis of a prospective cohort. Infection 2013;41:151–7. [DOI] [PubMed] [Google Scholar]
- [7].Wunderink RG, Yin Y. Antibiotic resistance in community-acquired Pneumonia pathogens. Semin Respir Crit Care Med 2016;37:829–38. [DOI] [PubMed] [Google Scholar]
- [8].Veve MP, Wagner JL. Lefamulin: review of a promising novel pleuromutilin antibiotic. Pharmacotherapy 2018;38:935–46. [DOI] [PubMed] [Google Scholar]
- [9].Paukner S, Sader HS, Ivezic-Schoenfeld Z, et al. Antimicrobial activity of the pleuromutilin antibiotic BC-3781 against bacterial pathogens isolated in the SENTRY antimicrobial surveillance program in 2010. Antimicrob Agents Chemother 2013;57:4489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Paukner S, Gelone SP, Arends SJR, et al. Antibacterial activity of lefamulin against pathogens most commonly causing community-acquired bacterial pneumonia: SENTRY antimicrobial surveillance program (2015-2016). Antimicrob Agents Chemother 2019;63(4.pii:):e02161–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Waites KB, Crabb DM, Duffy LB, et al. In vitro activities of lefamulin and other antimicrobial agents against macrolide-susceptible and macrolide-resistant Mycoplasma pneumoniae from the United States, Europe, and China. Antimicrob Agents Chemother 2017;61(2.): [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Alexander EGL, Das A, et al. Oral lefamulin vs moxifloxacin for early clinical response among adults with community-acquired bacterial pneumonia: the LEAP 2 randomized clinical trial. JAMA 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].File TM, Jr, Goldberg L, Das A, et al. Efficacy and safety of iv-to-oral lefamulin, a pleuromutilin antibiotic, for treatment of community-acquired bacterial pneumonia: the phase 3 LEAP 1 Trial. Clin Infect Dis 2019;69:1856–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Balfour JA, Lamb HM. Moxifloxacin: a review of its clinical potential in the management of community-acquired respiratory tract infections. Drugs 2000;59:115–39. [DOI] [PubMed] [Google Scholar]
- [15].Chou CC, Shen CF, Chen SJ, et al. Infectious Diseases Society of Taiwan; Taiwan Society of Pulmonary and Critical Care Medicine,; Medical Foundation in Memory of Dr. Deh-Lin Cheng; Foundation of Professor Wei-Chuan Hsieh for Infectious Diseases Research and Education; CY Lee's Research Foundation for Pediatric Infectious Diseases and Vaccines,; 4th Guidelines Recommendations for Evidence-based Antimicrobial agents use in Taiwan (GREAT) working group. Recommendations and guidelines for the treatment of pneumonia in Taiwan. J Microbiol Immunol Infect 2019;52:172–99.30612923 [Google Scholar]
- [16].Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med 2019;200:e45–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Center for Drug Evaluation, Research,. Guidance for industry: acute bacterial skin and skin structure infections: developing drugs for treatment. Silver Spring, Maryland: US Department of Health and Human Services, Food and Drug Administration; 2013. [Google Scholar]
- [18].Miettinen O, Nurminen M. Comparative analysis of two rates. Stat Med 1985;4:213–26. [DOI] [PubMed] [Google Scholar]
- [19].Mendes RE, Farrell DJ, Flamm RK, et al. In vitro activity of lefamulin tested against Streptococcus pneumoniae with defined serotypes, including multidrug-resistant isolates causing lower respiratory tract infections in the United States. Antimicrob Agents Chemother 2016;60:4407–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Malani PN. Lefamulin-a new antibiotic for community-acquired pneumonia. JAMA 2019;322:1671–2. [DOI] [PubMed] [Google Scholar]
- [21].Dwivedi P, Greis KD. Granulocyte colony-stimulating factor receptor signaling in severe congenital neutropenia, chronic neutrophilic leukemia, and related malignancies. Exp Hematol 2017;46:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]


