Abstract
Background:
The purpose of the current meta-analysis was to compare the oncological outcomes of pemetrexed versus gefitinib in pre-treated advanced or metastatic non-small cell lung cancer (NSCLC) patients.
Methods:
Search the online electronic databases on comparison the effectiveness and adverse effects of pemetrexed versus gefitinib in therapy outcomes of pre-treated NSCLC to September 2019. All studies analyzed the summary odds ratios (ORs) of the main outcomes, including survival efficacy and toxicity complications.
Results:
In all, 5 trials involving 676 subjects were included, with 332 receiving pemetrexed and 344 using gefitinib. The pooled analysis of overall survival (OS) (OR = 0.97, 95%CI = 0.77–1.21, P = .76) and progression-free survival (PFS) (OR = 1.17, 95%CI = 0.60–2.30, P = .65) showed that pemetrexed did not achieve benefit when compared with gefitinib. In the results of subgroup analysis among the EGFR mutation-positive patients, the comparison of gefitinib therapy versus pemetrexed did show PFS benefit 0.35 (95%CI 0.12–1.01; P = .05). In terms of grade 3 or 4 side effects, a similar toxicity profile of both pemetrexed and gefitinib was shown in the incidence rate of rash (P = .045), fatigue (P = .97), thrombocytopenia (P = .68) and anemia (P = .21) between the 2 groups.
Conclusion:
Pemetrexed was not associated with survival benefit than gefitinib therapy among pre-treated NSCLC patients. While, gefitinib showed superior PFS efficacy than pemetrexed for patients with EGFR mutation-type. Future investigations are required to identify relevant biomarkers in selected patients that would most likely benefit from pemetrexed or gefitinib treatment in pre-treated advanced NSCLC patients.
Keywords: gefitinib, meta-analysis, pemetrexed, pretreated non-small cell lung cancer patients
1. Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide, and about 80% to 85% are diagnosed as non-small cell lung cancer (NSCLC).[1,2] Nearly, 30% to 40% of NSCLC patients are diagnosed with advanced or distant metastases at the time of diagnosis.[3] A combination of platinum-based anticancer therapy is the standard regimen for these patients, while its response rate is only 30% to 40%.[4] The resistance to standard first-line treatment is quickly developed,[5] most patients finally develop into progressive-stage that result in requiring further therapies after the initial treatment.
Pemetrexed as a multi- targeted antifolate cytotoxic agent has reported superiority in targeting a variety of enzymes in the progression of pyrimidine and purine synthesis, which was also accepted its use in the therapy of advanced NSCLC who has failed the prior platinum-based anticancer therapy because of its favorable safety profile and relatively good efficacy.[6]
In addition, NSCLC is considered as a highly heterogeneous disease on gene level, within the various subtypes,[7] introducing molecular targeted drugs could be approved to treat the subgroup of cancer patients for whom develop progressive after the failure of initial therapy.[5] Developments in genetic discoveries have proved that epidermal growth factor receptor (EGFR) -dependent pathway is effective in most of NSCLC patients and it has important effect on in the progression of epithelial cells.[8]
Gefitinib, one of the first-generation epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs), has been accepted by National Comprehensive Cancer Network (NCCN) guidelines to treat NSCLC.[9] Relevant articles have been designed to explore its safety and effectiveness, and reported a superiority for second-line therapy.[8]
Previously, there are randomized controlled trials assessing the effect of pemetrexed compared with gefitinib in a second-line therapy setting with different results. There are still conflicting results because of their side effects or lack of therapeutic effectiveness or both pemetrexed and gefitinib are limited to tumors with specific genetic alterations.[10] Our meta-analysis aims to assess the effectiveness and safety of pemetrexed versus gefitinib in pre-treated NSCLC.
2. Methods and materials
2.1. Search strategy
Pubmed, Embase, Cochrane library were performed to identify the eligible studies up to September 2019. The literature search process was established with the keywords and relevant Medical Subject Heading (MeSH) terms: “non small cell lung cancer” AND “pemetrexed” AND “gefitinib”, AND “pretreated patients.” The reference lists were also hand-searched to check for additional relevant articles.
2.2. Eligibility criteria
Researches were included in the current study should meet the following criteria:
-
(1)
the studies are designed as random control trials (RCTs);
-
(2)
all patients are NSCLC patients treated with pemetrexed therapy as compared to gefitinib;
-
(3)
patient harboring treatment-refractory who fail the prior therapy;
-
(4)
the interested outcomes were survival efficacy and adverse effects;
-
(5)
the full-text papers were only included.
Publications with the following exclusion criteria were excluded:
-
1)
articles are not designed as RCT;
-
2)
the reported data was insufficient;
-
3)
case reports, or observational literature;
-
4)
duplicated previous researches.
2.3. Quality assessment and data extraction
Two authors independently assessed the quality of the retrieved trials. Study quality was justified using the Jadad scale.[11] Two investigators separately carried out the relevant data from each article independently. Disagreement was revolved by consensus. We extracted the main contents based on the following: family name of the first author, the year of the publication, country, number of patients, mean age, and end-point of interested.
2.4. Statistical analysis
We extracted the corresponding odds ratios (ORs) to describe the interested outcomes, including survival and dichotomous data, respectively, with corresponding 95% confidence intervals (CIs). The statistical analyses were conducted using Review Manager version 5.3 software (Revman; The Cochrane collaboration Oxford, United Kingdom). The heterogeneity across studies was using the I2 statistic.[12] I2 value >50% recognized as the high degree of heterogeneity.[13] When there was high degree of heterogeneity among articles, the random-effects model was used. Otherwise, we choose the fixed effects model. A P value less than .05 was suggested as statistically significant difference. Results of our meta-analysis were shown in forest plots.
3. Results
3.1. Overview of literature search and general features of the studies
In all, 267 studies were included originally. Based on the review of the abstracts and titles, 10 publications were evaluated in more detail, but some did not provide enough detail of results of 2 groups. Finally, a total of 5 RCTs[14–18] assess the impact of pemetrexed versus gefitinib. The search process is described in Figure 1.
Figure 1.

PRISMA flow chart of selection process to identify studies eligible for pooling.
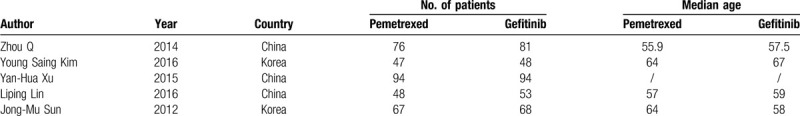
All included trials in our analysis were based on moderate to high quality evidence. Table 1 provided a brief description of these 5 studies.
Table 1.
Pemetrexed vs Gefitinib NSCLC.
3.2. Clinical and methodological heterogeneity
Pooled analysis of PFS comparing pemetrexed versus gefitinib
The pooled data showed that there is no benefit between pemetrexed and gefitinib in terms of the PFS (OR = 1.17, 95%CI = 0.60–2.30, I2 = 93%, P = .65) (Fig. 2). While, subgroup analyses by the EGFR mutation-type indicated that the comparison of gefitinib therapy versus pemetrexed did show PFS benefit 0.35 (95% CI 0.12–1.01, I2 = 81%, P = .05) (Fig. 3) among EGFR mutation-positive patients, whereas in EGFR wild-type subgroup the PFS was similar between 2 groups (OR = 0.83, 95%CI = 0.31–2.24, I2 = 84%,P = .72) (Fig. 4).
Figure 2.

Pooled analysis of progression-free survival comparing pemetrexed versus gefitinib.
Figure 3.

Pooled analysis of progression-free survival comparing pemetrexed versus gefitinib in epidermal growth factor receptor mutation-positive subgroup.
Figure 4.
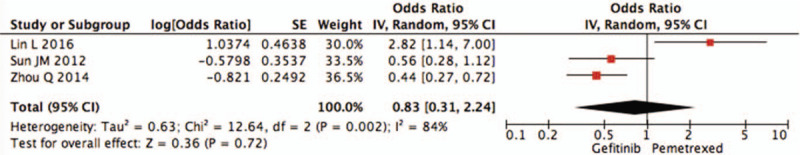
Pooled analysis of progression-free survival comparing pemetrexed versus gefitinib in epidermal growth factor receptor wild-type subgroup.
Pooled analysis of OS comparing pemetrexed versus gefitinib.
Pooling the OS data from 4 articles revealed that pemetrexed did not prolong the OS (OR = 0.97, 95%CI = 0.77–1.21, I2 = 17%, P = .76) than the gefitinib group (Fig. 5).
Figure 5.

Pooled analysis of overall survival comparing pemetrexed versus gefitinib.
Pooled analysis of objective response rate (ORR) comparing pemetrexed versus gefitinib.
The pooled data showed that there is no advantage of ORR between 2 arms (OR = 0.84, 95%CI = 0.34–2.05, I2 = 80%, P = .70). In other words, neither pemetrexed nor gefitinib increased the rate of ORR (Fig. 6).
Figure 6.

Pooled analysis of objective response rate comparing pemetrexed versus gefitinib.
Pooled analysis of SAE comparing pemetrexed versus gefitinib.
We define the grade 3/4 toxicities as severe adverse effects (SAEs). In the analysis of thrombocytopenia (OR = 1.34, 95%CI = 0.33–5.43, I2 = 25%,P = .68), anemia (OR = 2.25, 95%CI = 0.63–8.09, I2 = 11%,P = .21), fatigue (OR = 1.02, 95%CI = 0.38–2.69, I2 = 0%,P = .97) and rash (OR = 0.42, 95%CI = 0.04–4.05, I2 = 66%,P = .45) were included, and the data are shown in Figures 7–10. However, all above pooling analysis does not reach a statistically significant level (P > .05).
Figure 7.
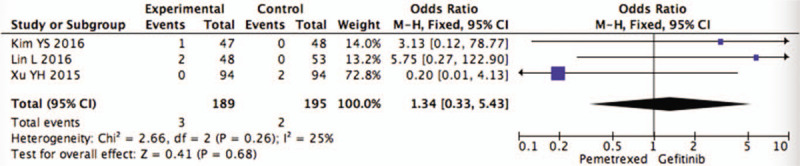
Pooled analysis of thrombocytopenia comparing pemetrexed versus gefitinib.
Figure 10.

Pooled analysis of rash comparing pemetrexed versus gefitinib.
Figure 8.

Pooled analysis of anemia comparing pemetrexed versus gefitinib.
Figure 9.

Pooled analysis of fatigue comparing pemetrexed versus gefitinib.
4. Discussion
NSCLC is the common malignancy worldwide. Despite new therapies improved the survival of advanced NSCLC patients, few effective treatment options have emerged to treat NSCLC, especially for patients who failed the initial therapy.[19]
Pemetrexed is a multi-targeted antifolate (MTA) and exerts its antitumor activity by inhibiting the replication process folate metabolism. The pemetrexed can suppress several key enzymes result in reducing the rate of drug- resistant tumors, which makes it achieve superior efficacy than traditional antifolate chemotherapy. Pemetrexed has been accepted using as a second-line treatment for advanced NSCLC by FDA.[17] Gefitinib is an EGFR-targeted agent. Previous articles have reported that gefitinib has selected as therapeutic option in pre-treated NSCLC.
Recent clinical trials respectively assess the effect of gefitinib compared with pemetrexed as a second-line therapy setting for NSCLC patients. However, the conclusions remain controversial. Thus, it is very necessary to identify an efficient and tolerable adverse effects maintenance therapy option to achieve survival and toxicity profile benefit for advanced lung cancer patients.[17]
In this study, our analysis demonstrated the maintenance effectiveness and toxicity profile of pemetrexed versus gefitinib. However, subgroups analysis revealed that EGFR mutation-positive subgroup did improved PFS from gefitinib than pemetrexed. It demonstrated that the superiority of gefitinib was associated with genetic characteristics between arms.
The evidences demonstrating survival efficacy of gefitinib treatment in a subgroup analysis of INFORM and SATURN study was better than placebo with EGFR mutation-positive patients, while similar survival efficacy compared with placebo for EGFR wild-type patients in maintenance treatment.[20,21] Previous phase III trials have demonstrated that gefitinib was the favored choice in second-line therapy for patients with mutation-positive when compared with pemetrexed single agent;[16] in contrast, EGFR wild-type patients achieved better efficacy with pemetrexed.[14,18] Even though, the prolongation of PFS did not associate with the difference of OS, which was probably due to treatment crossover at the progression.
These findings suggest that decisions making for the second-line therapy should be done with the knowledge of genotype mutational status. In our meta-analysis, there is no significant difference of efficacy between pemetrexed and gefitinib for EGFR wild-type patients. This result might be derived from a small number of enrolled patients in EGFR wild-type subgroup, which can be considered statistical significance when clinically sample size is sufficient.
In terms of the toxicities, previous studies have indicated that the adverse effects of the 2 groups were somewhat different, but were all mild and well tolerated, which was consistent with our finding.[20,22,23] This result demonstrated that the systematically established management of adverse events used in this therapy could be accepted as an effective treatment for patients on therapy to achieve the maximum benefit from drugs. Given the different toxicity profiles of pemetrexed or gefitinib, a key factor to select therapy should be patients’ comorbidities and tolerance of expected tumor-related side effects.
Even though, all included studies are designed as RCTs, imbalance in experimental design and clinical characteristics, bias still exist, and this may have effect on the findings of our analysis. So, it indicated that more large-scale, high-quality studies with greater statistical power are required to verify the clinical efficacy of pemetrexed and gefitinib.
5. Conclusion
Our study showed that pemetrexed was not associated with survival benefit than gefitinib therapy among pre-treated NSCLC patients. However, gefitinib showed superior PFS efficacy than pemetrexed for EGFR mutation-type patients. Moreover, pemetrexed had no significantly favorable safety profile, as compared with gefitinib. Future investigations are required to identify relevant biomarkers in selected patients that would most likely benefit from pemetrexed or gefitinib treatment in pre-treated advanced NSCLC patients.
Author contributions
Xiaoxin Lu and Shengshu Li designed experiments on articles; conducted research; collected data; analyzed data and wrote articles with the same contribution. Weizong Chen and Dongyang Zheng carried out the literature research. Yuzhu Li and Fang Li focused on the critical review, revision and partial financial support of the intellectual content of the article.
Footnotes
Abbreviations: CIs = confidence intervals, EGFR = epidermal growth factor receptor, EGFR-TKI = epidermal growth factor receptor tyrosine kinase inhibitor, MTA = multi-targeted antifolate, NCCN = National Comprehensive Cancer Network, NSCLC = non-small cell lung cancer, ORR = objective response rate, ORs = odds ratios, OS = overall survival, PFS = progression-free survival, RCTs = random control trials, SAEs = severe adverse effects.
How to cite this article: Lu X, Li S, Chen W, Zheng D, Li Y, Li F. A meta-analysis of the safety and effectiveness of pemetrexed compared with gefitinib for pre-treated advanced or metastatic NSCLC. Medicine. 2020;99:29(e21170).
XL and SL are the co-first authors.
The authors declare that ethical approval was not necessary for meta-analysis
The authors have no funding and conflicts of interest to disclose.
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
- [1].Park S, Keam B, Kim SH, et al. Pemetrexed singlet versus nonpemetrexed-based platinum doublet as second-line chemotherapy after first-line epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor failure in non-small cell lung cancer patients with EGFR mutations. Cancer Res Treat 2015;47:630–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Masuda T, Imai H, Kuwako T, et al. Efficacy of platinum combination chemotherapy after first-line gefitinib treatment in non-small cell lung cancer patients harboring sensitive EGFR mutations. Clin Transl Oncol 2015;17:702–9. [DOI] [PubMed] [Google Scholar]
- [3].Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest 1997;111:1710–7. [DOI] [PubMed] [Google Scholar]
- [4].Cimino GD, Pan CX, Henderson PT. Personalized medicine for targeted and platinum-based chemotherapy of lung and bladder cancer. Bioanalysis 2013;5:369–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Dempke WCM. Targeted therapy for NSCLC–a double-edged sword? Anticancer Res 2015;35:2503–12. [PubMed] [Google Scholar]
- [6].Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 2004;22:1589–97. [DOI] [PubMed] [Google Scholar]
- [7].Chang JT, Lee YM, Huang RS. The impact of the Cancer Genome Atlas on lung cancer. Transl Res 2015;166:568–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wo H, He J, Zhao Y, et al. The efficacy and toxicity of gefitinib in treating non-small cell lung cancer: a meta-analysis of 19 randomized clinical trials. J Cancer 2018;9:1455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ettinger DS, Wood DE, Akerley W, et al. NCCN Guidelines insights: non-small cell lung cancer, version 4.2016. J Natl Compr Canc Netw 2016;14:255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tomasello C, Baldessari C, Napolitano M, et al. Resistance to EGFR inhibitors in non-small cell lung cancer: clinical management and future perspectives. Crit Rev Oncol Hematol 2018;123:149–61. [DOI] [PubMed] [Google Scholar]
- [11].Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controlled Clin Trials 1996;17:1–2. [DOI] [PubMed] [Google Scholar]
- [12].Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- [13].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kim YS, Cho EK, Woo HS, et al. Randomized phase ii study of pemetrexed versus gefitinib in previously treated patients with advanced non-small cell lung cancer. Cancer Res Treat 2016;48:80–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lin L, Zhao J, Hu J, et al. Comparison of the efficacy and tolerability of gefitinib with pemetrexed maintenance after first-line platinum-based doublet chemotherapy in advanced lung adenocarcinoma: single-center experience. Onco Targets Ther 2016;9:6305–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sun JM, Lee KH, Kim SW, et al. Gefitinib versus pemetrexed as second-line treatment in patients with nonsmall cell lung cancer previously treated with platinum-based chemotherapy (KCSG-LU08-01): an open-label, phase 3 trial. Cancer 2012;118:6234–42. [DOI] [PubMed] [Google Scholar]
- [17].Xu YH, Mei JS, Zhou J. Randomized study of gefitinib versus pemetrexed as maintenance treatment in patients with advanced glandular non-small cell lung cancer. Int J Clin Exp Med 2015;8:6242–6. [PMC free article] [PubMed] [Google Scholar]
- [18].Zhou Q, Cheng Y, Yang JJ, et al. Pemetrexed versus gefitinib as a second-line treatment in advanced nonsquamous nonsmall-cell lung cancer patients harboring wild-type EGFR (CTONG0806): a multicenter randomized trial. Ann Oncol 2014;25:2385–91. [DOI] [PubMed] [Google Scholar]
- [19].Garon EB, Ciuleanu TE, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet 2014;384:665–73. [DOI] [PubMed] [Google Scholar]
- [20].Zhang L, Ma S, Song X, et al. Gefitinib versus placebo as maintenance therapy in patients with locally advanced or metastatic non-small-cell lung cancer (INFORM; C-TONG 0804): a multicentre, double-blind randomised phase 3 trial. Lancet Oncol 2012;13:466–75. [DOI] [PubMed] [Google Scholar]
- [21].Cappuzzo F, Ciuleanu T, Stelmakh L, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multi-centre, randomised, placebo-controlled phase 3 study. Lancet Oncol 2010;11:521–9. [DOI] [PubMed] [Google Scholar]
- [22].Paz-Ares LG, de Marinis F, Dediu M, et al. PARAMOUNT: final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Clin Oncol 2013;31:2895–902. [DOI] [PubMed] [Google Scholar]
- [23].Pujol JL, Paz-Ares L, de Marinis F, et al. Long-term and low-grade safety results of a phase III study (PARAMOUNT): maintenance pemetrexed plus best supportive care versus placebo plus best supportive care immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. Clin Lung Cancer 2014;15:418–25. [DOI] [PubMed] [Google Scholar]


