Abstract
The aim of this study was to investigate the prognostic value of neutrophils-to-lymphocyte ratio in peripheral blood (NLR) and in cancer nest (iNLR) in patients with esophageal squamous cell carcinoma (ESCC).
Totally 103 patients with ESCC treated with surgical radical surgery in the Shuyang People's Hospital from February 2010 to November 2014 were collected retrospectively. Peripheral blood routine test and immunohistochemistry examination of carcinoma nest were mainly performed. Survival rates were analyzed with Kaplan–Meier curves. Univariate analysis and multivariate analysis were also performed to explore potential prognostic factors of ESCC.
The median survival time after surgery of low NLR group and high NLR group were 48 months and 30 months, respectively. The difference of overall survival between the 2 groups was statistically significant (χ2 = 7.435, P = .006). The median survival time after surgery of low iNLR group and high iNLR group were 37 months and 24.5 months, respectively. The difference between the 2 groups was also statistically significant (χ2 = 33.640, P = .000). Univariate analysis showed influence factors of postoperative survival in patients with ESCC included tumor-node-metastasis staging, NLR, iNLR, and grade of NLR score + iNLR score (P ≤ .05). Multivariate analysis confirmed NLR, iNLR, and tumor-node-metastasis staging were independent influence factors of postoperative survival in patients with ESCC (P ≤ .05).
High level of NLR and iNLR implies a poor prognosis of ESCC. The application of both NLR and iNLR could guide clinicians to take aggressive treatments for high risk population.
Keywords: blood routine, esophageal squamous cell carcinoma, immunohistochemistry, neutrophil-to-lymphocyte ratio, prognosis
1. Introduction
Esophageal cancer is placed among the most common malignancies all over the word with approximately 572,000 new cases and cause the cancer-related death of 509,000 patients every year.[1] The prognosis of esophageal cancer is poor with a 5-year overall survival (OS) rate as low as 15%.
It has been reported that immune reaction is closely associated with tumor progression and prognosis. Studies exploring the relationship between neutrophil-to-lymphocyte ratio (NLR) and tumor prognosis is notably increasing in recent years. Previous studies have shown that high NLR level before surgery may predict poor prognosis in oral squamous-cell carcinoma, colorectal cancer, and esophageal squamous cell carcinoma (ESCC).[2–4] Furthermore, in patients with locally advanced or metastatic ESCC, NLR has proved to be a potential marker of tumor response, immune suppression, malnutrition, and progression upon chemotherapy and radiotherapy.[5]
There are also a few studies about inflammatory cells in tumor microenvironment.[6–9] CD8+ lymphocytes, also known as cytotoxic lymphocytes, specifically kill target cells. CD66 protein is commonly used to label neutrophils.[10–12] Ilie et al[6] assessed the prognostic value of intratumoral cluster of neutrophils and lymphocytes in patients with resectable non-small cell lung cancer. The results identified the CD66b-positive neutrophil to CD8-positive T lymphocyte ratio in cancer nest (iNTR) as an independent prognostic predictor indicating tumor recurrence and poor long-term survival. However, there is few studies available on the prognostic value of peripheral and intratumoral NLR in patients with esophageal carcinoma. Herein, we designed the study to evaluate peripheral NLR and iNLR through blood routine test and immunohistochemistry (IHC) examination, and explore their prognostic role in patients with ESCC.
2. Material and methods
2.1. Patients
Patients who were diagnosed with ESCC and treated with radical operation in the Shuyang People's Hospital (Shuyang, Jiangsu, China) between February 2010 and November 2014 were screened for this observational study. The main inclusion criteria were as following:
-
(1)
Complete clinical, pathological, laboratory, and follow-up information were available.
-
(2)
Blood routine test was available within 1 week before surgery.
-
(3)
Diagnosis of ESCC was confirmed by postoperative pathological examination.
The main exclusion criteria were as following:
-
(1)
Other treatment (eg, radiotherapy and chemotherapy) were delivered before operation.
-
(2)
The operation patients received were nonradical or palliative.
-
(3)
Patients died during perioperative period or within 1 month after operation due to severe complications.
-
(4)
Patients also suffered from immunological disease or received immunotherapy.
-
(5)
Patients also suffered from systemic infection.
After screening, 103 patients were eligible for the study. Collected information included gender, age, maximum diameter of tumor, tumor location, differentiation, tumor-node metastasis (TNM) staging, history of smoking and drinking, and so on. Of 103 patients, 64 were males and 39 were females. The age ranged from 39 to 79 years with a mean age of 64.2 ± 8.4 years. Tumor staging included 15 cases in stage I, 67 cases in stage II, and 21 cases in stage III (according to the 7th edition of the American Joint Committee on Cancer Staging Manual).
The study was reviewed and approved by the Shuyang People's Hospital, The Affiliated Shuyang Hospital of Xuzhou Medical University Ethic Committee.
2.2. Blood routine test
One week before surgery, 3 mL of fasting blood was collected in the morning through the elbow vein. The automatic blood analyzer (Sysmex XE-2100) was used for testing. All operations were carried out in accordance with the instructions.
2.3. IHC test
Tissue specimens were formalin-fixed and paraffin-embedded as 3 μm tissue blocks. Paraffin sections of tumor tissues were dewaxed at 56°C for 4 hours. Antigen retrieval was conducted by heating the sections in citrate buffer for 30 minutes, natural cooling and washing with distilled water for 3 minutes. Intrinsic peroxidase activity was quenched by incubating the samples with 3% hydrogen peroxide for 10 minutes. The sections were then incubated overnight at 4°C with primary antibodies: anti-CD8 (Rabbit mAb, Gene Tech, Shanghai, China) and anti-CD66b (Rabbit mAb, Abcam, Cambridge, UK). Following incubation was performed with GTVision secondary antibody (GK600705, HRP-conjugated anti-rabbit/mice antibody). After treating the samples with 3,3-diaminobenzidine and counterstaining the nuclei with Mayer hematoxylin for 3 minutes, the sections were dehydrated and sealed with neutral balsam.
Images were obtained using a light microscope (Olympus BX41, Tokyo, Japan). Tumor nests staining and distribution of positive cells were observed at low power. Then 8 nonoverlapping views at high magnification were randomly selected for neutrophil count and lymphocyte count (Fig. 1). Cell counting was conducted by 2 experienced pathologists independently and took the average. Intravascular neutrophil and lymphocyte staining were set as internal control group.
Figure 1.
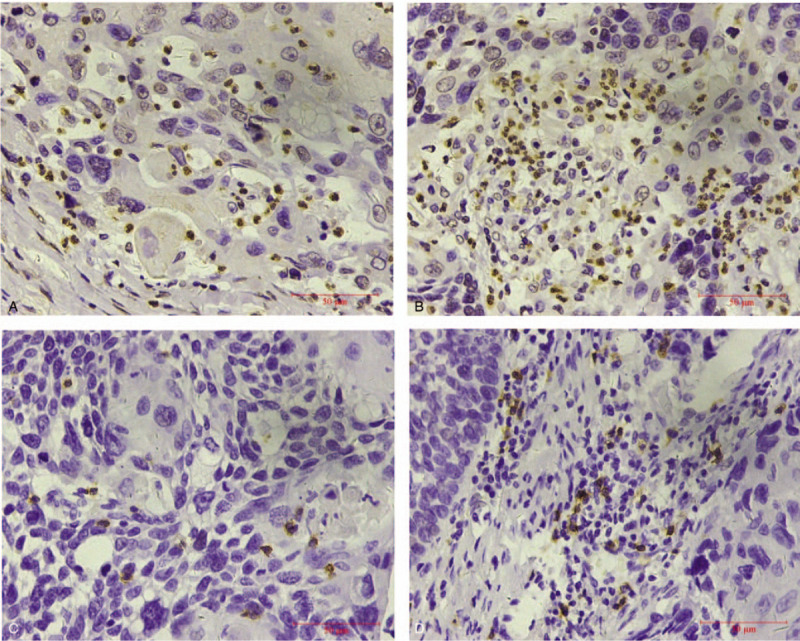
Immunohistochemistry (IHC) staining of neutrophils. (A) IHC staining ×400 times showed CD66b+ neutrophils in cancer nest; (B) IHC staining ×400 times showed intratumoral interstitial CD66b+ neutrophils; (C) IHC staining ×400 times showed CD8+ lymphocytes in cancer nest; (D) IHC staining ×400 times showed intratumoral interstitial CD8+ lymphocytes.
2.4. Statistical analysis
Statistical analysis was conducted with statistical software package SPSS 18.0 (SPSS Inc.: Chicago, IL). χ2 test was performed to compare the pathological feature between 2 groups. Receiver operating characteristics curve was applied to calculate the cut-off values. Kaplan–Meier survival and Cox regression analysis were conducted to identify the prognostic factors of ESCC. P ≤ .05 was considered statistically significant.
3. Results
3.1. Correlation between neutrophil-to-lymphocyte ratio in peripheral blood (NLR) and clinicopathologic parameters
The NLR fluctuated from 0.74 to 13.67 in 103 patients. The area under the curve of NLR was 0.732 (P ≤ .05). Accordingly, the optimal cutoff value of NLR was 2.5 in receiver operating characteristics curve analysis, and the sensitivity and specificity were 59.3% and 68.2%, respectively (Fig. 2).
Figure 2.
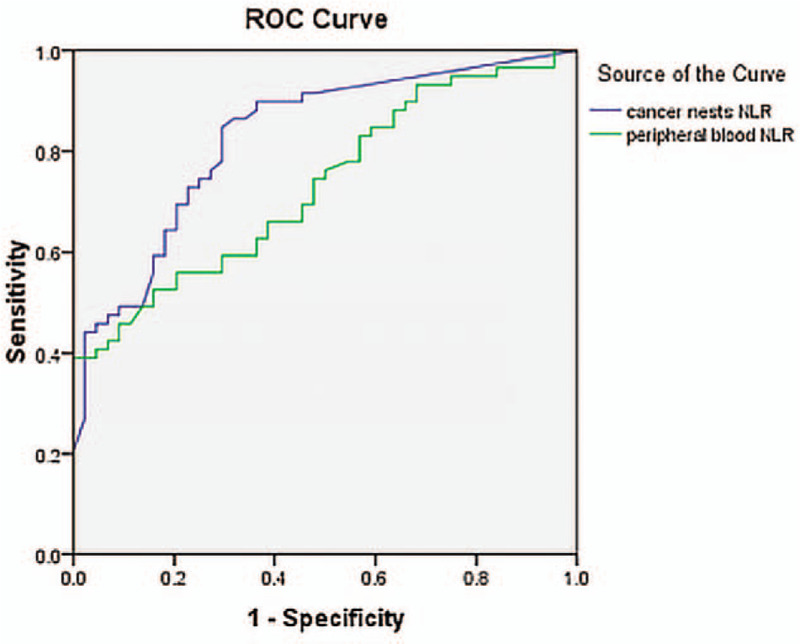
Receiver operating characteristics (ROC) curves of neutrophils-to-lymphocyte ratio in peripheral blood (NLR) and cancer nests (iNLR). Accordingly, the optimal cutoff value of NLR and iNLR was 2.5 and 0.2 respectively in ROC curve analysis.
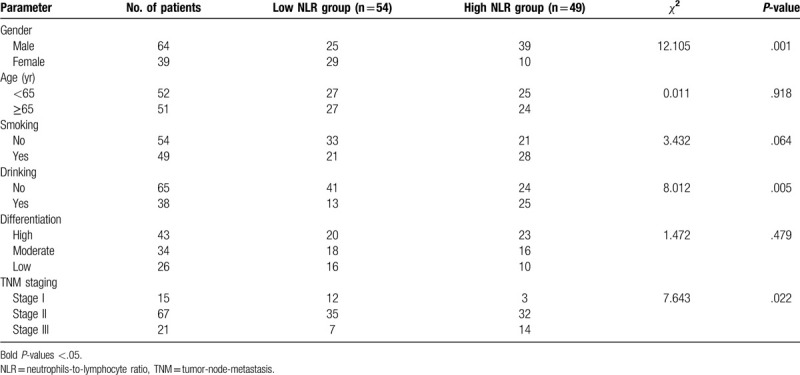
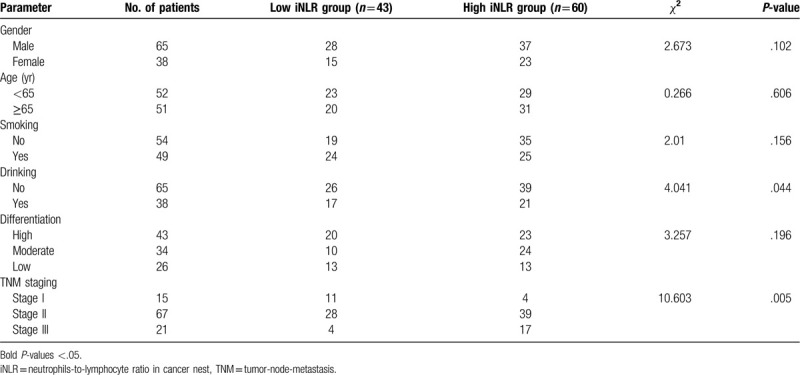
Therefore, low NLR group was identified as NLR ≤ 2.5 (54 cases) while high NLR group was identified as NLR ≤ 2.5 (49 cases). According to χ2 test of clinicopathologic parameters, NLR level was significantly correlated with gender, alcohol assumption, and TNM staging (P ≤ .05), while there was no statistical difference of age, smoking, and differentiation (P ≤ .05). Table 1 summarized the clinicopathologic characteristics of 2 groups.
Table 1.
Correlation between neutrophils-to-lymphocyte ratio in peripheral blood and clinicopathologic parameters in 103 patients with esophageal squamous cell carcinoma.
3.2. Correlation between neutrophil-to-lymphocyte ratio in cancer nests (iNLR) and clinicopathologic parameters
The iNLR fluctuated from 0 to 26 in 103 patients. Since the area under the curve of iNLR was 0.828 (P ≤ .05) and 0.608 for interstitial iNLR (P > .05), the optimal cutoff value of iNLR was 0.2 with relatively higher sensitivity (79.7%) and specificity (70.5%) (Fig. 3).
Figure 3.
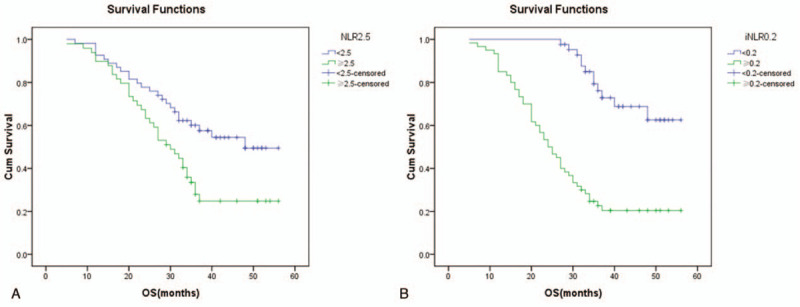
The Kaplan–Meier curves illustrate the cumulative survival time stratified according to (A) the level of neutrophils-to-lymphocyte ratio in peripheral blood (NLR) and the (B) level of intratumoral neutrophils-to-lymphocyte ratio (iNLR). In part A, the green curve represents patients with high NLR while blue curve represents patients with low NLR (P = .006). In part B, the green curve represents patients with high iNLR while blue curve represents patients with low iNLR (P = .000).
Therefore, low iNLR group was set as NLR ≤ 0.2 (43 cases) while high iNLR group was set as NLR ≤ 0.2 (60 cases). Chi-square analysis indicated significant difference of alcohol assumption and TNM staging between 2 groups (P ≤ .05), but no meaningful difference of gender, age, smoking habit, and tumor differentiation (P ≤ .05). Table 2 reviewed the clinicopathologic characteristics of 2 groups.
Table 2.
Correlation between neutrophils-to-lymphocyte ratio in cancer nests and clinicopathologic parameters in 103 patients with esophageal squamous cell carcinoma.
3.3. Survival analysis
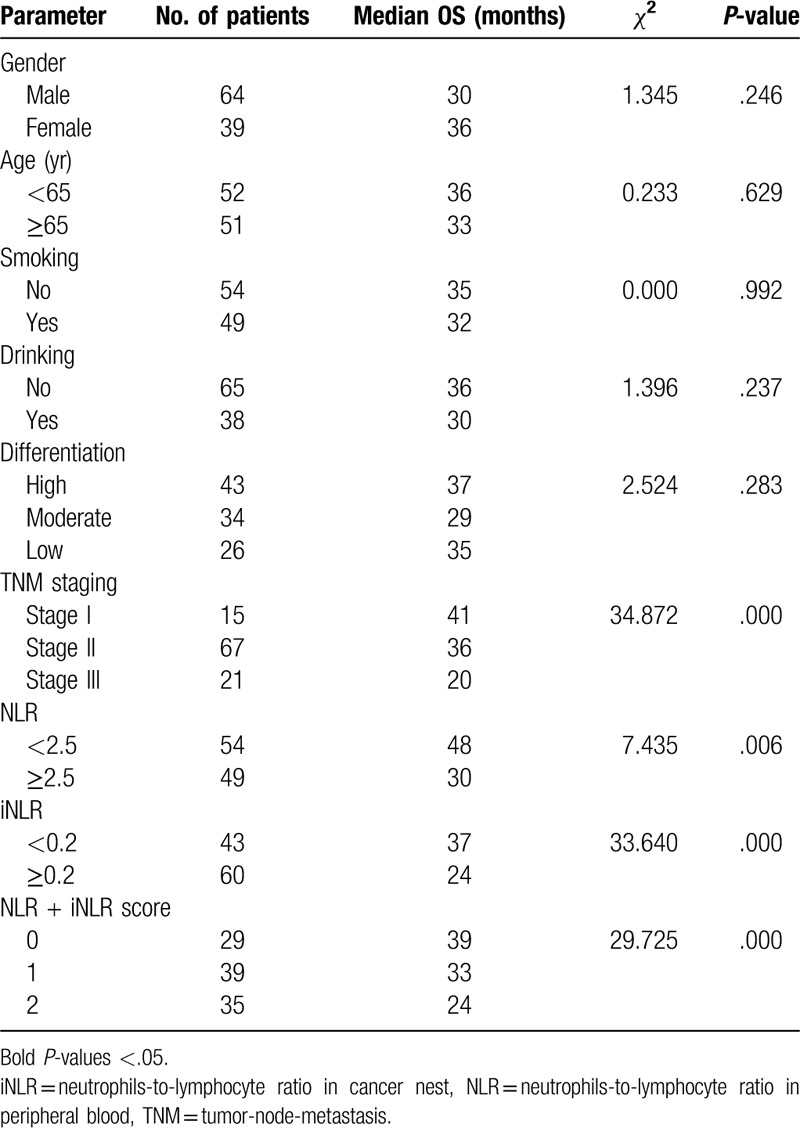
The median OS time of low NLR group was significantly longer than that of high NLR group as 48 months versus 30 months (χ2 = 7.435, P = .006) (Fig. 3A). Likewise, the distinction was also detected between low iNLR group and high iNLR group. The median survival time of 2 groups were 37 months and 24.5 months (χ2 = 33.640, P = .000) (Fig. 3B). Subgroup analysis of stage II disease further implied patients low iNLR had longer survival time compared with their high iNLR counterparts (37 months vs 28 months, χ2 = 16.150, P = .000). However, comparable survival benefit was not obtained in stage II patients with low NLR against high NLR (48 months vs 34 months, χ2 = 1.390, P = .238).
In univariate analysis, low NLR or iNLR level was assigned as 0 while high NLR or iNLR was 1, then the score of NLR + iNLR was calculated for further analysis. The results showed TNM staging, NLR, iNLR, and NLR + iNLR score were significantly associated with OS (P ≤ .05) (Table 3). More specifically, the median OS time was significantly declined with increasing NLR + iNLR score. When the score were 0, 1, and 2, the OS were 38 months, 36 months in and 27 months, respectively (χ2 = 12.822, P = .002).
Table 3.
Univariate analysis of overall survival in patients with esophageal squamous cell carcinoma after radical surgery.
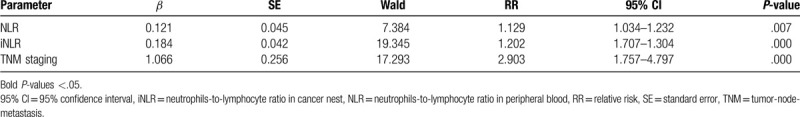
In multivariate analysis, NLR level, iNLR level, and TNM staging were introduced into Cox regression model. The results identified advanced TNM staging, high level of NLR and iNLR were independent factors implying worse OS (P ≤ .05) (Table 4).
Table 4.
Multivariate analysis of overall survival in patients with esophageal squamous cell carcinoma after radical surgery.
4. Discussion
In China, nearly 300,000 patients die from esophageal carcinoma every year, accounting for 50% of the total worldwide.[13]
Although great development has achieved in esophagectomy, long-term survival of patients with esophageal carcinoma after surgery has stayed poor.[5] Comprehensive treatment combining surgery with radiotherapy and chemotherapy may be the way to make improvement. However, unbridled generalization of comprehensive treatment without considering individual difference, on the other hand, causes overtreatment and increase patients’ physical and economic burden. Therefore, independent and effective prognostic factors are in urgent need to predict high risk group for aggressive treatment and intensive follow-up. As is well-known, neutrophils play an important role in non-specific immune response, but its antitumor mechanisms remain unclear.[14]
It was once believed neutrophils have a short lifespan. They only have a transitory stopover of 6 to 8 hours after migrating from bone marrow to peripheral blood, then transport to connected tissue and survive 2 to 3 days. Nevertheless, recent studies discovered neutrophils may live more than 4 days in peripheral circulation, which is much longer than expected.[15] Meanwhile, neutrophils have various functions involving in broad pathways of T cell migration, activation, differentiation, and receptor regulation.[16–18] A study of early-stage lung cancer found that tumor-associated neutrophil (TAN) revealed as an activated phenotype compared with neutrophils in peripheral blood. Its distinct chemokine receptor expression expanded immune function by upregulating CD54, CCR5, CCR7, CXCR3, and CXCR4, and downregulating CXCR1 and CXCR2. Meanwhile, TAN stimulated T cell to further regulate immune reaction.[19] TAN was also detected to encourage tumorigenesis by releasing vascular endothelial growth factor and supporting tumor angiogenesis.[20] Lymphocyte is also placed among critical inflammatory cells. Reduction of CD8+ lymphocytes will provide favorable condition for proliferation and metastasis of tumor cells. Hence, they have been widely recognized as important anti-tumor cells with specific killing activity to tumor cells.[7]
In recent years, cumulative studies have proved that NLR in peripheral blood can comprehensively reflect the inflammation and immune status of cancer patients. He et al [21] found that patients with esophageal carcinoma had worse OS if their NLR in peripheral blood was higher than 3.5. In multivariate analysis, age ≥65 years, nonsquamous cell type, high TNM staging, unresectable tumor, and NLR > 3.5 were independent risk factors of poor prognosis. In 2016, Kosumi et al[22] retrospectively analyze 283 ESCC patients undergoing resection in the period from 2005 to 2011. High NLR level was also detected to be related to shorter survival time (P = .0081). In the present study, NLR = 2.5 was set as the cut-off point to divide the patients into high NLR group and low NLR group. Survival analysis showed significant prolong of survival time as 18 months in low NLR group (χ2 = 7.435, P = .006). Both univariate and multivariate analysis identified high NLR level in peripheral blood as a predictor of poor prognosis. Nonetheless, subgroup analysis of stage II cases failed to confirm this finding, which might be related to the deficient number of stage II cases in our study.
NLR in tumor tissue can also function as peripheral NLR or even better. In 2012, Ilie et al[6] analyzed 632 non-small cell lung cancer cases by detecting the expression of CD66b and CD8 in tumor tissue. The results showed an elevated iNLR was strongly associated with high cumulative incidence of relapse, in other words, patients with high iNLR relapsed earlier than those with low iNLR (P < .0001). In 2016, an IHC study collected the clinicopathological information of 28 patients received surgical resection for pancreatic cancer to determine the value of NLR as a prognostic factor in patients with pancreatic cancer.[23] Researchers found iNLR ≥ 5 was an important risk factor of poor disease-free survival and OS. Nevertheless, few studies focusing on iNLR in ESCC are available. To our best knowledge, it was the first study combining NLR and iNLR to detect the prognosis of patients with ESCC.
We collected 103 ESCC cases and labeled neutrophils and lymphocytes by immunohistochemical staining with CD66b+ and CD8+ to investigate the prognostic value of iNLR. In this study, Kaplan–Meier method was used to compare the survival curves of patients with low iNLR and high iNLR. It was confirmed that patients with high iNLR had worse prognosis and the OS rate was lower than that of low iNLR group. In addition, univariate and multivariate analysis showed that iNLR is an independent factor affecting the survival of ESCC after surgery, high TNM staging, and iNLR indicated poor prognosis after surgery.
NLR + iNLR score was confirmed to be a novel and potential prognostic factor. Though concurrent increasing of NLR and iNLR evidently forecast poor long-term prognosis, it remains vague whether combining assessment is better than NLR or iNLR only. Multivariate analysis with COX model suggested that the relative risk of TNM staging was higher than iNLR and NLR. Large scale and retrospective studies are required.
There are also some limitations of this study. Firstly, there is no consensus of the cutoff value of NLR or iNLR, making it difficult for its application in clinical practice. Secondly, the role of postoperative chemotherapy and radiotherapy was not taken into consideration, which, on the other hand, may improve the prognosis and prolong survival time. There is a certain degree of bias in prognosis prediction with preoperative peripheral blood sample and pathological sections from surgery only. Third, very few studies on iNLR have been published, further mechanisms of its action remain unclear.
In conclusion, we demonstrated NLR and iNLR are trustworthy prognostic markers to predict patients with potential poor prognosis. The application of both NLR, iNLR, and NLR + iNLR could guide clinicians to take aggressive treatments for high risk population.
Author contributions
Quanquan Guo and Zhiying Shao designed the study, performed the analysis, and draft the manuscript; Dan Xu responded for all the pathological tests in this study; Lili Fan collected clinical information; Xin Ding and Huiru Xiong help to perform the statistical analysis. Longzhen Zhang and Chuanwen You instruct the study and manuscript writing.
Footnotes
Abbreviations: ESCC = esophageal squamous cell carcinoma, IHC = immunohistochemistry, iNLR = neutrophils-to-lymphocyte ratio in cancer nest, NLR = neutrophils-to-lymphocyte ratio in peripheral blood, OS = overall survival, TAN = tumor-associated neutrophil, TNM = tumor-node metastasis.
How to cite this article: Guo Q, Shao Z, Xu D, Fan L, Xiong H, Ding X, You C, Zhang L. Prognostic value of neutrophil-to-lymphocyte ratio in peripheral blood and pathological tissue in patients with esophageal squamous cell carcinoma. Medicine. 2020;99:29(e21306).
QG and ZS contributed equally to this study.
This work is supported by 2 funds from the National Natural Science Foundation of China (No. 81372424) and the Innovation Team Project of Jiangsu Province (No. CXTDA2017034).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [2].Chen S, Guo J, Feng C, et al. The preoperative platelet-lymphocyte ratio versus neutrophil-lymphocyte ratio: which is better as a prognostic factor in oral squamous cell carcinoma? Ther Adv Med Oncol 2016;8:160–7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [3].Walsh SR, Cook EJ, Goulder F, et al. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol 2005;91:181–4. [DOI] [PubMed] [Google Scholar]
- [4].Zhou SB, Xin-Wei G, Liang G, et al. Influential factors on radiotherapy efficacy and prognosis in patients with secondary lymph node metastasis after esophagectomy of thoracic esophageal squamous cell carcinoma. Cancer Manag Res 2018;10:217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sato Y, Gonda K, Harada M, et al. Increased neutrophil-to-lymphocyte ratio is a novel marker for nutrition, inflammation and chemotherapy outcome in patients with locally advanced and metastatic esophageal squamous cell carcinoma. Biomed Rep 2017;7:79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ilie M, Hofman V, Ortholan C, et al. Predictive clinical outcome of the intratumoral CD66b-positive neutrophil-to-CD8-positive T-cell ratio in patients with resectable nonsmall cell lung cancer. Cancer 2012;118:1726–37. [DOI] [PubMed] [Google Scholar]
- [7].Knocke S, Fleischmann-Mundt B, Saborowski M, et al. Tailored tumor immunogenicity reveals regulation of CD4 and CD8 T cell responses against cancer. Cell Rep 2016;17:2234–46. [DOI] [PubMed] [Google Scholar]
- [8].Caldeira PC, Vieira éLM, Sousa AA, et al. Immunophenotype of neutrophils in oral squamous cell carcinoma patients. J Oral Pathol Med 2017;46:703–9. [DOI] [PubMed] [Google Scholar]
- [9].Wikberg ML, Ling A, Li X, et al. Neutrophil infiltration is a favorable prognostic factor in early stages of colon cancer. Hum Pathol 2017;68:193–202. [DOI] [PubMed] [Google Scholar]
- [10].Silva KDD, Caldeira PC, Alves AM, et al. High CD3+ lymphocytes, low CD66b+ neutrophils, and scarce tumor budding in the invasive front of lip squamous cell carcinoma. Arch Oral Biol 2019;104:46–51. [DOI] [PubMed] [Google Scholar]
- [11].Huang X, Pan Y, Ma J, et al. Prognostic significance of the infiltration of CD163 + macrophages combined with CD66b + neutrophils in gastric cancer. Cancer Med 2018;7:1731–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yamada Y, Nakagawa T, Sugihara T, et al. Prognostic value of CD66b positive tumor-infiltrating neutrophils in testicular germ cell tumor. Bmc Cancer 2016;16:898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Qiao HLJ-HFY-L. Epidemiology, etiology, and prevention of esophageal squamous cell carcinoma in China. Cancer Biol Med 2017;14:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Vols S, Sionov RV, Granot Z. Always look on the bright side: anti-tumor functions of neutrophils. Curr Pharm Des 2017;23:4862–92. [DOI] [PubMed] [Google Scholar]
- [15].Pillay J, den Braber I, Vrisekoop N, et al. In vivo labeling with 2H2O reveals a human neutrophil lifespan of 5.4 days. Blood 2010;116:625–7. [DOI] [PubMed] [Google Scholar]
- [16].Moses K, Brandau S. Human neutrophils: Their role in cancer and relation to myeloid-derived suppressor cells. Sem Immunol 2016;28:187–96. [DOI] [PubMed] [Google Scholar]
- [17].Scapini P, Marini O, Tecchio C, et al. Human neutrophils in the saga of cellular heterogeneity: insights and open questions. Immunol Rev 2016;273:48–60. [DOI] [PubMed] [Google Scholar]
- [18].Mishalian I, Bayuh R, Eruslanov E, et al. Neutrophils recruit regulatory T-cells into tumors via secretion of CCL17 - a new mechanism of impaired antitumor immunity. Int J Cancer 2014;135:1178–86. [DOI] [PubMed] [Google Scholar]
- [19].Eruslanov EB, Bhojnagarwala PS, Quatromoni JG, et al. Tumor-associated neutrophils stimulate T cell responses in early-stage human lung cancer. J Clin Investg 2014;124:5466–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Winer AG, Motzer RJ, Hakimi AA. Prognostic biomarkers for response to vascular endothelial growth factor–targeted therapy for renal cell carcinoma. Urol Clin North Am 2016;43:95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yutong H, Xiaoli X, Shumei L, et al. Increased neutrophil-lymphocyte ratio is a poor prognostic factor in patients with esophageal cancer in a high incidence area in China. Arch Med Res 2015;46:557–63. [DOI] [PubMed] [Google Scholar]
- [22].Kosumi K, Baba Y, Ishimoto T, et al. Neutrophil/lymphocyte ratio predicts the prognosis in esophageal squamous cell carcinoma patients. Surg Today 2015;46:405–13. [DOI] [PubMed] [Google Scholar]
- [23].Takakura K, Ito Z, Suka M, et al. Comprehensive assessment of the prognosis of pancreatic cancer: peripheral blood neutrophil-lymphocyte ratio and immunohistochemical analyses of the tumour site. Scand J Gastroenterol 2015;51:1–8. [DOI] [PubMed] [Google Scholar]


