Abstract
In BRAF wild type advanced melanoma, immune checkpoint blockers such as anti-PD1 (anti-programmed cell death 1) are usually continued beyond progression for a hypothetical rare further response. Chemotherapy as a second-line option is considered ineffective by many practitioners based on historical data. Continuing anti-PD1 beyond progression has a high health-economic impact and is not recommended by the FDA. This study aimed to describe the efficacy and survival of advanced melanoma patients who received second-line (or more) chemotherapy after immunotherapy failure.
This was a retrospective single center study conducted in a French University Hospital during an 11-month period. All advanced melanoma patients treated with chemotherapy after immunotherapy failure were included.
Eighteen patients were analyzed. Therapeutic response to chemotherapy was evaluable in 16 patients: partial response was achieved in 3/16 (19%), stable disease in 1/16 (6%) and progressive disease in 12/16 (75%). Median overall survival from chemotherapy start was 12 months. Median progression-free survival was 5.4 months. The 6-month overall survival rate was 81% and the 6-month progression-free survival rate was 40%.
Although the disease control rate with chemotherapy was low (25%), survival data in our study are far superior to those previously published. This could be linked to a high proportion of patients treated with anti-PD1 just prior to chemotherapy, which may suggest a potential synergy between immunotherapy and chemotherapy.
Keywords: chemotherapy, immunotherapy, melanoma
1. Introduction
The treatment landscape of advanced melanoma has been revolutionized in recent years with the advent of several new approaches including immunotherapy and targeted therapy. Before these recent therapeutic advances, treatment was based on chemotherapy, mostly dacarbazine. In previous studies, the response rate to chemotherapy in advanced melanoma was very low, ranging from 5% to 28% with a median of 15%. In a meta-analysis of 42 phase II trials published in 2007, the median survival time of stage IV melanoma patients was 6.2 months (95% CI, 5.9–6.5 months) with 25.5% of patients alive at 1 year.[1]
Nowadays, in a BRAF wild type advanced melanoma patient, the standard of care is immunotherapy with a checkpoint blocker, specifically anti-programmed cell death 1 (anti-PD1) and/or anti-cytotoxic T-lymphocyte antigen 4 (anti-CTLA4). In case of “mild” disease progression (except the rare cases of hyperprogression), the anti-PD1 is usually continued because a further response can be achieved in some patients (phenomenon of pseudoprogression).[2]
In a US-FDA pooled analysis of 8 multicenter trials, 2624 melanoma patients treated with anti-PD1 were analyzed.[3] Among the 1361 patients who had disease progression, 51% continued anti-PD1 beyond progression and 49% did not. Those who continued beyond progression had a median overall survival (OS) of 24.4 months versus 11.2 months in those who stopped anti-PD1 at progression. However, after pooling the data, 19% of patients treated beyond progression had a late response to PD-1 blockade (Response Evaluation Criteria in Solid Tumors version 1.1-defined as a 30% or greater decrease in tumor burden). In another systematic review of unconventional patterns of benefit with PD-(L)1 inhibitors, 19 prospective trials were considered, including 8 in advanced melanoma patients: among the 5404 patients with solid tumors, rates of response beyond progression ranged from 4% to 10% regardless of the tumor type.[4]
In light of this, anti-PD1 therapy is sometimes continued beyond progression in the hope of a hypothetical further response, which corresponds to pseudoprogression and is found to occur in about 20% of patients or less. In this context, the appropriate time point to stop anti-PD1 is very controversial. Indeed, continuing anti-PD1 beyond progression has a high health economic impact. When compared to first-line dacarbazine, nivolumab (anti-PD1) has an incremental cost-effectiveness ratio (ICER) of $90,871 per quality-adjusted life-year.[5] The FDA decided not to recommend continuing anti-PD1 beyond progression based on the results of the randomized, double-blind CheckMate-066 trial in which the estimated 1-year OS in patients who received treatment beyond progression was 61% with anti-PD1 versus 53% with chemotherapy (dacarbazine). The follow-up of this trial showed a 3-year overall survival rate significantly higher for first-line nivolumab versus dacarbazine (respectively 51.2% and 21.6%).[6]
Another reason for continuing anti-PD1 beyond progression is the absence of an effective alternative treatment, since many practitioners consider that chemotherapy is ineffective based on historical data. There are a few published case reports of unexpected response to chemotherapy after immunotherapy failure but large studies on this topic are lacking. Here, we report the efficacy of chemotherapy after at least 1 prior immunotherapy in 18 advanced melanoma patients.
2. Materials and methods
2.1. Study design
This was a retrospective, single center study in patients who received chemotherapy as second-line treatment (or more) for advanced melanoma from January to November 2017 in the Oncodermatology Department of Nantes University Hospital. Patients were identified from the files of the hospital pharmacy which prepares chemotherapies.
2.2. Study population
Inclusion criteria were:
-
i.
histologically-proven advanced melanoma defined as unresectable stage III or stage IV according to the American Joint Committee on Cancer classification 8th edition;[7]
-
ii.
failure or limiting toxicity of a previous immunotherapy;
-
iii.
at least 1 chemotherapy infusion received during the 11-month period. Patients who received first-line chemotherapy were not included. For the included patients, the Research and Clinical Investigation on Melanoma database, a French prospective cohort of melanoma patients, was used to gather data (clinical trials.gov identifier NCT03315468). All patients signed an informed consent for the use of their clinical and biological data for scientific purpose at the time of inclusion in the Research and Clinical Investigation on Melanoma cohort, which was approved by an ethics committee CCTIRS (number 12.108).
2.3. Clinical data
Collected data included: baseline demographics (age, gender), BRAF and NRAS mutational status, previous treatment(s) received before chemotherapy [type of treatment, dose and number of infusions received, start, and end dates, ≥ grade 3 toxicity according to the CTCAE (Common Terminology Criteria for Adverse Events), efficacy], chemotherapy treatment (presence of brain metastasis or not at the beginning of chemotherapy, type of chemotherapy, dose and number of infusions received, start and end dates, ≥ grade 3 toxicity according to the CTCAE, efficacy) and if applicable, details of treatment(s) received after chemotherapy. The radiological follow-up was performed for each patient according to the standard of care of the center, that is, CT scan every 3 months. Concerning efficacy, Response Evaluation Criteria in Solid Tumors version 1.1 were considered to define the therapeutic response on CT scans for all patients as follows: progressive disease (PD), stable disease (SD), partial response (PR) and complete response.[8] Concerning prognosis, the date of progression, date of death, or latest news were collected.
2.4. Statistical analysis
Descriptive statistics were used to present epidemiological data. OS was defined as the time elapsed from the date of the first chemotherapy infusion to the date of death from any cause or the date of latest news. Progression-free survival (PFS) was defined as the time elapsed from the date of the first chemotherapy infusion to the date of progression. OS and PFS were estimated at the data cut-off (April 1, 2019).
3. Results
3.1. Demographic characteristics and treatments received by patients before chemotherapy
Eighteen patients (10 women, 8 men) were included and analyzed. The mean age was 63.5 years. Three patients had a BRAF V600E mutation, 7 had a NRAS mutation. The 18 patients had received an average of 2 previous lines (range: 1–4). The 3 BRAF-mutated patients had all previously received targeted therapies. Table 1 summarizes the demographic, molecular, and clinical data, and treatment sequences for the 18 patients.
Table 1.
Demographic, molecular, and clinical data and treatment sequences for the 18-patient cohort.
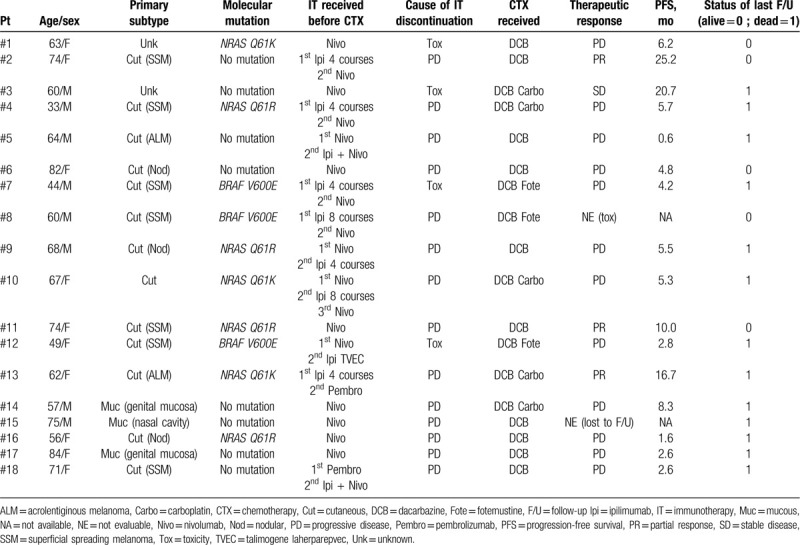
The PD1 inhibitor was either nivolumab 3 mg/kg every 2 weeks or pembrolizumab 2 mg/kg every 3 weeks in accordance with the European Medicine Agency marketing authorization at the time of the study. The CTLA4 inhibitor was ipilimumab 3 mg/kg every 3 weeks for 4 cycles. The combined anti-PD1 and anti-CTLA4 was nivolumab 1 mg/kg and ipilimumab 3 mg/kg every 3 weeks for 4 cycles followed by nivolumab 3 mg/kg every 2 weeks.
Immunotherapy was stopped because of CTCAE grade ≥ 3 toxicity in 4 cases (renal failure, myasthenic crisis, aseptic meningitis and colitis; 1 case of each) and for progression in the other 14 cases.
3.2. Chemotherapy treatment
After immunotherapy, the chemotherapy regimen was dacarbazine alone in 10 cases, in combination with carboplatin or fotemustine in 5 and 3 cases, respectively. The chemotherapy regimens were the standards used in Nantes University Hospital: dacarbazine alone 1 g/m2 on day 1 every 3 to 4 weeks; dacarbazine 400 mg/m2 plus carboplatin area under the curve (AUC) 4 or 5 every 4 weeks; dacarbazine 500 mg/m2 plus fotemustine 100 mg/m2 every 4 weeks (maintenance regimen).
One patient (#15) was lost to follow-up after 2 infusions of chemotherapy. Another patient (#8, with BRAF-mutated melanoma) had a grade 3 thrombocytopenia 2 weeks after the first chemotherapy infusion, requiring definitive discontinuation of chemotherapy.
Therapeutic response was therefore evaluable in 16 patients: 3/16 (19%) had a PR, 1/16 (6%) had SD, and 12/16 (75%) had PD. The 2 BRAF-mutated and evaluable patients progressed under chemotherapy and died within 7 months of chemotherapy start. The 4 patients with brain metastases (#4, 7, 8 and 12) progressed under chemotherapy. One patient (#14) had a SD after 6 cycles of dacarbazine and carboplatin (progression-free at 6 months) but then progressed after 3 cycles of dacarbazine. His final therapeutic response was therefore PD whereas he was progression-free at 6 months.
3.3. Survival analysis and profile of patients
The median OS from chemotherapy start was 12 months. The median OS of the 5 chemotherapy-controlled melanoma patients (PR and SD) was 26.6 months. The median PFS was 5.4 months. The 6-month OS was 81% and the 6-month PFS was 40%. OS and PFS survival curves are represented in Figures 2 and 3. Thirteen out of 18 patients died after an average of 10.8 months. As shown in Figure 1, 3 different profiles of BRAF wild type patients were identified after the start of chemotherapy:
Figure 2.
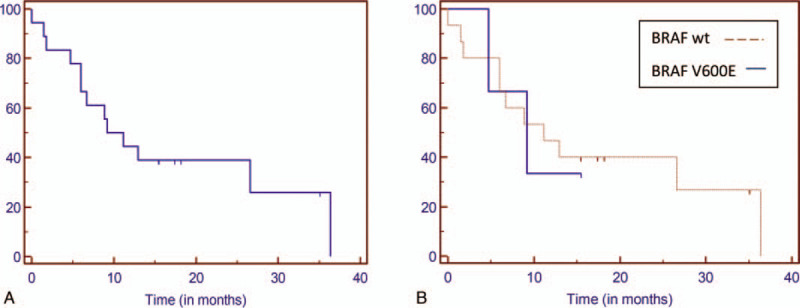
Overall survival Kaplan-Meier curves. A. In the whole cohort. B. According to BRAF status.
Figure 3.
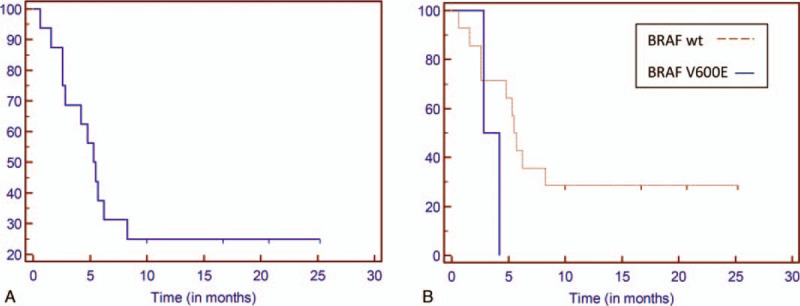
Progression-free survival Kaplan Meier curves. A. In the whole cohort. B. According to BRAF status.
Figure 1.
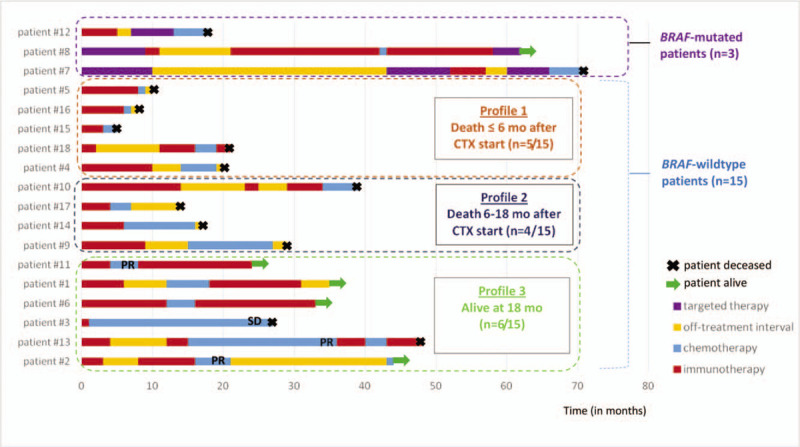
Patients’ swimmer plot. Each line corresponds to 1 patient. The duration of each treatment is represented with 1 color for each type of treatment. CTX = chemotherapy, Mo = months, PR = partial response, SD = stable disease.
-
i.
death in less than 6 months (n = 5),
-
ii.
death in 6 to 18 months (n = 4) and
-
iii.
patients still alive at 18 months (n = 6).
4. Discussion
In this retrospective study of 18 advanced melanoma patients who received chemotherapy after failure of immunotherapy, none of the patients had a complete response, 19% had a PR and 6% had SD. This disease control rate is consistent with historical data on first-line chemotherapy. In an exploratory analysis of digitized survival curves from 25 clinical trials, Uruguel et al showed that advanced melanoma patients treated with chemotherapy as a second-line or more had a 6-month PFS of 20.7% (95% CI, 13.1– 28.4).[9] Our results showed a 2 fold higher proportion (40%) of patients without PD at 6 months. The major difference between study by Uruguel et al and this study is the first-line treatment received before chemotherapy. Indeed, Uruguel et al pooled the results of 25 clinical trials, 4 of which included second-line patients (Checkmate-037, Keynote-002, Metric and NEMO studies). The large majority of these latter patients received anti-CLTA4 in first-line. In contrast, in our study, the previous immunotherapy line received by all the patients included anti-PD1.
In an abstract presented at the ASCO congress in 2018 by Goldinger et al, 463 consecutive patients treated between 2007 and 2017 with chemotherapy after failing immunotherapy were analyzed. The disease control rate across the entire cohort was 33.4% with a median PFS of 2.7 months (95% CI, 2.4–3 months). Median OS from chemotherapy start was 7.1 months (95% CI, 6.5–8 months), as compared to 12 months in our study.[10] What differentiates our study is the higher proportion of patients treated with an anti-PD1 just prior to chemotherapy (78% vs 42% in the study of Goldinger et al). Moreover, the patients in our study were treated recently (in 2017) and benefited from several treatments that were more effective and numerous than those before 2011.
Survival data in our study are far superior to those previously published but are comparable to the results of 2 recent studies. A recent American retrospective study compared the outcome of 11 advanced melanoma patients that received chemotherapy after immunotherapy (mainly anti-PD1 alone or combined with anti-CTLA4) vs 24 patients that received chemotherapy without previous immunotherapy (as first-line or second-line after targeted therapy). Median PFS was significantly longer in the group receiving chemotherapy after immunotherapy (respectively 5.2 vs 2.5 months, P = .046).[11] Another Japanese retrospective study of 9 patients treated with chemotherapy after immunotherapy showed comparable outcome results (mean PFS 5.0 months and mean OS 7.6 months).[12] Several hypotheses can be advanced to explain this better prognosis, including selection bias of long-responder patients, or continuation of the immunotherapy effect after its discontinuation. One hypothesis could be the importance of the treatment sequence in which anti-PD1 therapy is given just prior to chemotherapy.
According to our experience, some patients are still fit enough for further therapy when progressing under immunotherapy. Notably, in this study, patients received chemotherapy as last-line treatment. Indeed, 4 patients had brain metastases and all progressed under chemotherapy. Among them, 3 were BRAF-mutated and previously received targeted therapy. One patient (#7) received targeted therapy 3 times as reinduction before receiving last-line chemotherapy. According to the European guidelines, chemotherapy can be an option in patients with good performance status and with resistance to kinase inhibitors and checkpoint blockade. Moreover, patients should be screened for trial participation before chemotherapy is chosen.[13]
At the preclinical level, chemotherapy is known to have immunostimulatory properties such as changes in antigen presentation, tumor cell targeting and depletion of immunosuppressive cells. Some authors have previously described immunogenic cell death induced by several chemotherapeutic agents such as anthracyclines, cyclophosphamide, and oxaliplatin.[14] Chemotherapy, by inducing necrosis and tumor antigen reloading, could permit or enhance the effect of immunotherapy. For example, in a murine tumor model, the combination of gemcitabine chemotherapy and anti-CTLA4 resulted in the induction of an anti-tumor immune response.[15] On the basis of some significant clinical results, several combined immunotherapy and chemotherapy regimens have recently been approved by the FDA in different indications such as non-squamous cell lung carcinoma, triple negative breast cancer and head and neck squamous cell carcinoma. However, there is a clear gap between these promising preclinical (mouse-modeled) results and human clinical trials.[16] Indeed, in mouse models, chemotherapy is injected intra-tumorally whereas in human patients it is given by infusion.
A few published case reports suggest that chemotherapy may have greater efficacy than expected when used immediately after immunotherapy failure, as we observed in our study. In these cases, the response to chemotherapy was dramatic with a profile of response that is classically described with immunotherapy: up to 80% decrease in target lesions, occurring after only 1 cycle of chemotherapy with a durable response.[17–20] These response features are very different from those usually seen with chemotherapy alone, and could suggest a synergy between chemotherapy and immunotherapy at the clinical level.
This study has several limitations: small sample size, retrospective design, heterogeneity of previous treatments and of chemotherapy patterns.
In conclusion, in light of our results, in a BRAF wild type patient who fails to respond to immunotherapy, the question remains open: would it be better for this patient to continue anti-PD1 therapy or switch to chemotherapy? The answer should take into account efficacy data but also cost-effectiveness analysis. According to our results, chemotherapy seems to be a valid option. In this rapidly evolving therapeutic era, further prospective investigation of chemotherapy efficacy after immunotherapy failure, especially anti-PD1 failure, is needed.
Author contributions
Conceptualization: Mélanie Saint-Jean, Brigitte Dréno
Data curation: Mélanie Saint-Jean, Clémentine Fronteau, Emilie Varey
Investigation: Mélanie Saint-Jean, Lucie Peuvrel, Gaëlle Quéreux, Brigitte Dréno
Methodology: Emilie Varey, Amir Khammari
Project administration: Mélanie Saint-Jean, Lucie Peuvrel, Gaëlle Quéreux
Resources: Clémentine Fronteau, Emilie Varey
Supervision: Brigitte Dréno
Validation: Lucie Peuvrel, Amir Khammari, Gaëlle Quéreux, Brigitte Dréno
Writing – original draft: Mélanie Saint-Jean
Writing – review & editing: Lucie Peuvrel, Amir Khammari, Gaëlle Quéreux, Brigitte Dréno
Footnotes
Abbreviations: anti-CTLA4 = anti-cytotoxic T-lymphocyte antigen 4, anti-PD1 = anti-programmed cell death 1, CTCAE = Common Terminology Criteria for Adverse Events, OS = overall survival, PD = progressive disease, PFS = progression-free survival, PR = partial response, SD = stable disease.
How to cite this article: Saint-Jean M, Fronteau C, Peuvrel L, Khammari A, Varey E, Quéreux G, Dréno B. Chemotherapy efficacy after first-line immunotherapy in 18 advanced melanoma patients. Medicine. 2020;99:29(e21329).
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Korn EL, Liu P-Y, Lee SJ, et al. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol 2008;26:527–34. [DOI] [PubMed] [Google Scholar]
- [2].Chiou VL, Burotto M. Pseudoprogression and immune-related response in solid tumors. J Clin Oncol 2015;33:3541–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Beaver JA, Hazarika M, Mulkey F, et al. Patients with melanoma treated with an anti-PD-1 antibody beyond RECIST progression: a US food and drug administration pooled analysis. Lancet Oncol 2018;19:229–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Abdel-Rahman O. Nonconventional patterns of benefit of solid tumors treated with PD-(L)1 inhibitors: a systematic review. Immunotherapy 2017;9:995–1004. [DOI] [PubMed] [Google Scholar]
- [5].Kohn CG, Zeichner SB, Chen Q, et al. Cost-effectiveness of immune checkpoint inhibition in BRAF wild-type advanced melanoma. J Clin Oncol 2017;35:1194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ascierto PA, Long GV, Robert C, et al. Survival Outcomes in patients with previously untreated BRAF wild-type advanced melanoma treated with nivolumab therapy: three-year follow-up of a randomized phase 3 trial. JAMA Oncol 2019;5:187–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual: melanoma staging: AJCC 8th edition. CA Cancer J Clin 2017;67:472–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- [9].Ugurel S, Röhmel J, Ascierto PA, et al. Survival of patients with advanced metastatic melanoma: the impact of novel therapies-update 2017. Eur J Cancer 2017;83:247–57. [DOI] [PubMed] [Google Scholar]
- [10].Goldinger SM, Lo S, Hassel JC, et al. The utility of chemotherapy after immunotherapy failure in metastatic melanoma: a multicenter case series. J Clin Oncol 2018;36: suppl 15: e21588–121588. [Google Scholar]
- [11].Hadash-Bengad R, Hajaj E, Klein S, et al. Immunotherapy potentiates the effect of chemotherapy in metastatic melanoma–a retrospective study. Front Oncol 2020;10: article no. 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Maeda T, Yoshino K, Nagai K, et al. The efficacy of platinum-based chemotherapy for immune checkpoint inhibitor–resistant advanced melanoma. Acta Oncol 2019;58:379–81. [DOI] [PubMed] [Google Scholar]
- [13].Garbe C, Peris K, Hauschild A, et al. Diagnosis and treatment of melanoma. European consensus-based interdisciplinary guideline ––update 2016. Eur J Cancer 2016;63:201–17. [DOI] [PubMed] [Google Scholar]
- [14].Kroemer G, Galluzzi L, Kepp O, et al. Immunogenic cell death in cancer therapy. Annu Rev Immunol 2013;31:51–72. [DOI] [PubMed] [Google Scholar]
- [15].Lesterhuis WJ, Salmons J, Nowak AK, et al. Synergistic effect of CTLA-4 blockade and cancer chemotherapy in the induction of anti-tumor immunity. de Mello RA, ed. PLoS ONE 2013;8: article no. e61895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Cook AM, Lesterhuis WJ, Nowak AK, et al. Chemotherapy and immunotherapy: mapping the road ahead. Curr Opin Immunol 2016;39:23–9. [DOI] [PubMed] [Google Scholar]
- [17].Simon A, Kourie HR, Kerger J. Is there still a role for cytotoxic chemotherapy after targeted therapy and immunotherapy in metastatic melanoma? A case report and literature review. Chin J Cancer 2017;36: article no. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Saito R, Kawai M, Hirakawa K, et al. Case of malignant melanoma responding to dacarbazine following nivolumab. Dermatol Ther 2019;32: article no. e12791. [DOI] [PubMed] [Google Scholar]
- [19].Swami U, Monga V, Freesmeier M, et al. Exceptional responses with sequential metronomic temozolomide after pembrolizumab failure in patients with metastatic melanoma. Melanoma Res 2019;29:643–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Karachaliou GS, Ayvali F, Collichio FA, et al. Chemotherapy following PD-1 inhibitor blockade in patients with unresectable stage III/stage IV metastatic melanoma: a single academic institution experience. Oncology 2020;98:174–8. [DOI] [PubMed] [Google Scholar]


