Abstract
Developing and/or tailoring psychological interventions to align with patient preferences is a critical component of patient-centered care and has the potential to improve patient engagement and treatment outcomes. Discrete choice experiments (DCEs) are a quantitative method of assessing patient preferences that offer numerous strengths (i.e., ability to account for trade-offs), but are not routinely incorporated into health psychology coursework, likely leaving many unaware of the potential benefits of this methodology. To highlight the potential applications of DCEs within health psychology, this systematic review synthesizes previous efforts to utilize DCEs to inform the design of patient-centered psychological care, defined as interventions targeting psychological (e.g., depression, anxiety) or behavioral health (e.g., pain management, adherence) concerns. Literature searches were conducted in March 2017 and November 2019 for articles reporting on DCEs using the terms “discrete choice,” “conjoint,” or “stated preference.” Thirty-nine articles met all inclusion criteria and used DCEs to understand patient preferences regarding psychosocial clinical services (n = 12), lifestyle behavior change interventions (n = 11), HIV prevention and/or intervention services (n = 10), disease self-management programs (n = 4), or other interventions (n = 2). Clinical implications as well as limitations and directions for future research are discussed.
Keywords: psychological intervention, behavioral intervention, discrete choice experiment
Since being included as one of the six aims for high-quality healthcare in the Institute of Medicine (IOM) report Crossing the Quality Chasm, patient-centered care has received significant and growing attention, with references in PubMed-indexed articles increasing by 475% since 2001 (Committee on Quality of Health Care in America and Institute of Medicine, 2001; Epstein, Fiscella, Lesser, & Stange, 2010). Per the IOM, patient-centered care refers to “care that is respectful of and responsive to individual patient preferences, needs, and values” (page 6) (Committee on Quality of Health Care in America and Institute of Medicine, 2001). Patient-centered psychological care aligns with medical ethical principles, has been linked to improved patient engagement (Crits-Christoph, Gallop, Diehl, Yin, & Gibbons, 2017; Swift, Callahan, Cooper, & Parkin, 2018; Swift, Callahan, & Vollmer, 2011) and treatment-related outcomes (Swift et al., 2011; Swift et al., 2018), and holds promise as a means of improving the value of health care (Benning, Kimman, Dirksen, Boersma, & Dellaert, 2012; Epstein et al., 2010).
Patient-centered care requires providers who are “receptive and responsive” to the preferences, needs, and values of the patient and family (page 1492) (Epstein et al., 2010). Thus, a critical first step in providing patient-centered psychological care is to develop interventions that align with patient and family preferences (Fraenkel, 2013). To date, patient preferences for psychological care have primarily been assessed using qualitative methods or quantitative measures of beliefs, acceptability, or attitudes (Frewer, Salter, & Lambert, 2001). These strategies provide some insight into desirable intervention characteristics, but do not assess the relative importance of each characteristic (e.g., “Is the intervention setting, duration, or frequency most important?”) or the degree to which patients would be willing to trade-off one characteristic for another (e.g., “Would patients be willing to receive care via telehealth as opposed to in-person if it reduced their wait time?”). Without this information, psychologists have limited guidance on how best to design care that aligns with patient and family preferences.
One patient preference assessment method that can answer these questions is a discrete choice experiment (DCE) (Bridges, Onukwugha, Johnson, & Hauber, 2007). DCEs originated in mathematical psychology and are grounded in random utility theory (Lancsar & Louviere, 2008). Random utility theory posits that individuals choose (or prefer) options whose characteristics maximize their utility or provide the greatest value (Thurstone, 1927). Patient preferences, thus, can be quantified as the degree to which each intervention characteristic influences an individual’s intervention choice. To elicit this information, DCEs ask individuals to complete a series of hypothetical choice tasks. In each choice task, the individual is asked to select their preferred option from two or more alternatives (e.g., psychological interventions) with differing intervention characteristics.
For example, assume a health psychologist is designing an intervention to promote physical activity among college students. The psychologist has selected three behavior change techniques (BCTs) to deliver as part of the intervention: “provide information on consequences”, “provide information about behavior-health link”, and “use follow-up prompts” (Michie, Abraham, Whittington, McAteer, & Gupta, 2009). The psychologist now wants to develop an intervention package that delivers these BCTs in a manner that aligns with the preferences of college students. To inform the intervention design, the psychologist could use a DCE to ask: “Which intervention characteristics impact the preferences of college students for a physical activity intervention?”
Developing a DCE to answer a research question such as the one above begins by identifying intervention characteristics or “attributes” (e.g., intervention frequency) likely to impact a patient’s choice (Lancsar & Louviere, 2008). Attributes can be identified using qualitative research, literature reviews, and/or professional recommendations (Coast et al., 2011; Lancsar & Louviere, 2008). While the list of attributes need not encompass all possible intervention characteristics, the selected attributes should be important to patients and able to be modified independently (without altering another attribute) (Ryan, Gerard, & Amaya-Amaya, 2008).
For illustrative purposes, assume that four characteristics were identified as likely to influence a student’s choice of a physical activity intervention: intervention delivery format, intervention setting, prompt frequency, and prompt modality. Creating a DCE with these four attributes (as depicted in Figure 1) allows several questions to be explored. Specifically, including the first two attributes (intervention delivery format and intervention setting) could elucidate how college students would prefer to receive psychoeducational intervention content (i.e., first two BCTs: information about consequences and information about the health-behavior link). Including the prompt frequency and prompt modality attributes could identify the manner of implementing the third BCT (use follow-up prompts) that aligns with students’ preferences.
Figure 1.
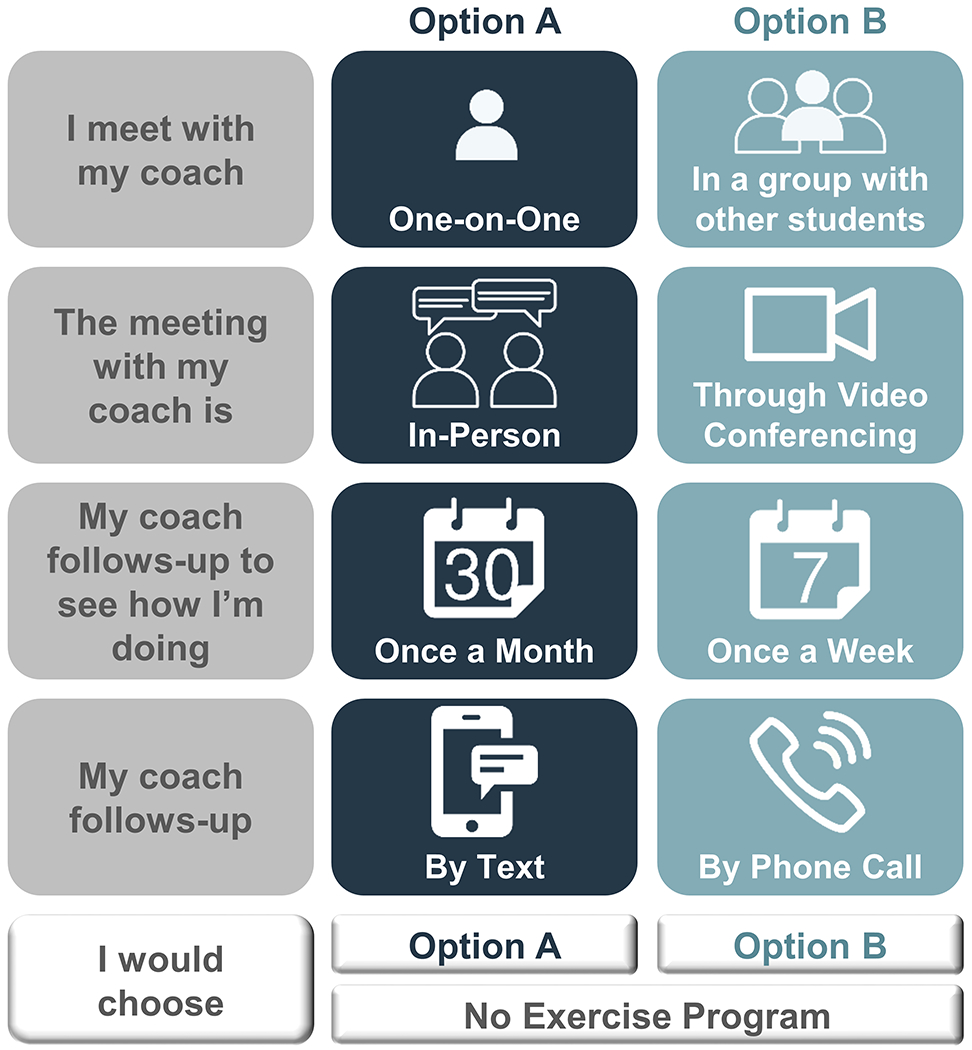
Sample DCE choice task.
After attributes are selected, levels, or potential attribute values, are defined. As with attributes, levels can be developed using qualitative research, literature reviews, and/or professional recommendations (Coast et al., 2011; Lancsar & Louviere, 2008). There is no rule for the number of levels as the goal is to represent real-world choices (Bridges et al., 2011). In practice, the number of levels is typically between two and five. More levels may be included for attributes such as cost. Depending on the question of interest, attribute levels may include available or hypothetical/not yet available clinical practices (Lancsar & Louviere, 2008). For example, assume the psychologist can deliver interventions in an individual or group format but does not have telehealth capabilities. The psychologist could select the levels “one-on-one” or “in a group with other students” for the attribute “intervention delivery format” to understand which of these two currently available delivery formats students prefer. Selecting the levels “in-person” and “through videoconferencing” for the “intervention setting” attribute could help the psychologist determine if adding telehealth services may increase the uptake of the physical activity program.
Following the selection of attributes and levels, an experimental design is used to generate a series of “choice tasks” (see Johnson et al., 2013). In each choice task, two or more options (hypothetical interventions) are presented. An “opt-out” or “none” option may also be included (see Johnson et al., 2013). While some studies generate every possible combination of levels and present each of these options in a choice task, this full factorial design typically results in a DCE with a large and unfeasible number of choice tasks. Instead, an experimental design (fractional factorial design) is generally used to generate a subset of choice tasks that maximizes statistical and response efficiency (Johnson et al., 2013). When the DCE includes a large number of attributes, partial profile designs in which each option is described by a subset of attributes can also be used (see Chrzan, 2010).
Figure 1 includes a sample choice task for the hypothetical example. In this choice task, two options are presented as well as a “none” option. Each option is described in terms of the four attributes (intervention delivery format, intervention setting, prompt frequency, and prompt modality). In this example, the levels for Options A and B differ on all four attributes (e.g., Option A is delivered one-on-one while Option B is delivered in a group format).
When completing a choice task, individuals are asked to consider all levels and weigh the pros and cons of each option. In most instances, no option will include all of an individual’s preferred levels. For example, a student completing the choice task in Figure 1 may prefer a one-on-one format (Option A) held through video conferencing (Option B) with monthly (Option A) follow-ups via phone (Option B). Thus, determining their preferred intervention requires the student to weigh the relative importance of each attribute/level. Because DCEs require individuals to make trade-offs like these, the cognitive heuristics that simplify real-world decision-making are activated, resulting in responses that more accurately predict actual behavior than alternative preference elicitation methods (e.g., Likert-type scales) (Cunningham et al., 2008; Phillips, Johnson, & Maddala, 2002).
DCE responses can be analyzed individually or at a population-level to identify the intervention characteristics preferred by a patient or population, respectively (Hauber et al., 2016; Powell et al., 2017). When DCE results are analyzed using a conditional logit model, a coefficient, standard error, t value, and p value are generated for each attribute and level (Hauber et al., 2016). Higher coefficients represent a more preferred attribute/level and comparing each coefficient elucidates the relative importance of each attribute/level. Unlike other measures of preferences, DCEs can also predict trade-offs patients would be willing to make (see Hauber et al., 2016). Data from the hypothetical DCE, thus, could answer questions such as “What is more important - the frequency or modality of follow-up prompts?” and “Would students be willing to receive more frequent follow-up prompts if they were delivered via text instead of via phone?”
DCEs have the potential to transform patient-centered psychological intervention development efforts and are increasingly being used to design health care services and products that align with patient preferences (Reed & Lavezzari, 2016). Since formally integrating patient preference information in their premarket approval and de novo classification decisions for medical products, the United States Food and Drug Administration (FDA) has endorsed DCEs as the “favored” method for assessing patient preferences (Johnson & Zhou, 2016; US Food and Drug Administration, 2016; Vass & Payne, 2017). Specifically, developers may use DCEs to understand the relative importance of medical product attributes (e.g., effectiveness, safety, and effect duration) and develop products that align with these preferences (US Food and Drug Administration, 2016). Once a product is developed, DCEs can be used to understand patients’ willingness to accept the product’s benefit-risk profile and developers may submit this data for consideration as part of the review (US Food and Drug Administration, 2016). As an example, the FDA’s Center for Devices and Radiological Health sponsored a study in which a DCE was used to quantify the relative importance of eight weight-loss device attributes among adults with obesity (Ho et al., 2015; Johnson & Zhou, 2016). These data informed the creation of a decision-aid calculator which FDA reviewers can use to evaluate the degree to which new products align with the preferences of adults with obesity. In 2015, after using the decision-aid calculator, the FDA approved EnteroMedics’ Maestro Rechargeable System due, in part, to the fact that the device aligned with patient preferences elicited via the DCE (Johnson & Zhou, 2016). Data derived from DCEs also play a significant role in health care decision making across Europe and the ongoing PREFER project is developing best-practice recommendations for using DCEs and other patient preference elicitation methods to inform health care decision making and regulation (Mühlbacher, Juhnke, Beyer, & Garner, 2016).
Applications of DCEs to health care and health economics such as those detailed above have been summarized in previous reviews (Clark, Determann, Petrou, Moro, & de Bekker-Grob, 2014; Ryan & Gerard, 2003). Means by which DCEs can inform the design of patient-centered interventions delivered by psychologists, however, have not yet been articulated. As psychology training does not routinely include instruction in DCEs, this gap may prevent health psychologists from capitalizing on the advantages of DCEs. The purpose of this manuscript is to summarize the applications of DCEs to patient-centered psychological care development by conducting the first systematic review of studies using DCEs to understand patient preferences regarding interventions targeting psychological (e.g., depression, anxiety) or behavioral health (e.g., pain management, adherence) concerns. Future directions and relevant resources are also discussed.
Methods
Methods and results are reported in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines (Moher, Liberati, Tetzlaff, Altman, & The, 2009). In March 2017, PubMed (i.e., MEDLINE), Academic Search Complete, PsycINFO, CINAHL, and SocINDEX databases were searched for articles reporting on DCEs using the terms “discrete choice” or “conjoint.” As DCEs are a type of stated preference method, the term “stated preference” was also included (Louviere, Flynn, & Carson, 2010). No restrictions were placed on publication language or date. An additional search was conducted in November 2019 to identify relevant manuscripts published since the original search.
Following the removal of duplicates, all records were screened by the first author and articles published in a language other than English, applying a DCE to a question unrelated to psychology (e.g., drug development), and/or including a systematic review were excluded. The full text articles for the remaining records were obtained and assessed for adherence to inclusion criteria as summarized below. Although discrete choice experiments are considered to be a stated preference method, the focus of this review was on DCEs and manuscripts including other stated preference methods (e.g., best-worst scaling exercise) were excluded. In addition, manuscripts describing the use of a DCE without mention of the implications for behavioral or psychological intervention development (e.g., DCE to evaluate preferences for non-modifiable flu vaccine attributes [effectiveness, side effects, duration, absorption time], de Bekker-Grob et al., 2018) were excluded. Finally, manuscripts of a format other than an original research article (e.g., study protocols) were excluded. Data regarding the study sample, type of intervention, DCE design, DCE administration, DCE analysis, and study results were extracted by the first author using a standardized data collection form. Data were checked for accuracy by a second individual and discrepancies were resolved via discussion and consultation with the original article.
Consideration of Key Criteria.
To guide good DCE research practices, the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) Good Research Practices for Conjoint Analysis Task Force created a checklist of 10 items (each with three sub-domains) that should be considered during the development, analysis, and publication of DCEs (Bridges et al., 2011). Because the field of stated preference assessment methods is rapidly evolving and there remain many unanswered questions regarding “best” practices, the checklist evaluates the degree to which studies consider multiple important issues rather than degree to which studies adhere to specific research practices (Bridges et al., 2011). The first author rated the degree to which each article reported on the sub-domains of all 10 items as “no sub-domains addressed,” “some sub-domains addressed,” or “all sub-domains addressed.”
Results
The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) four-phase flow diagram is included in Figure 2 (Moher et al., 2009). The searches resulted in 13,178 records (first search = 11,501; second search = 1,677) representing 10,890 unique articles. Of the 10,890 unique articles, 180 were published in English and mentioned the assessment of patient preferences. A total of 141 articles did not meet at least one of the inclusion criteria (DCE [n = 38], DCE informing psychological intervention [n = 92], and original research article [n = 11]) and were excluded, resulting in a final sample of 39 articles.
Figure 2.
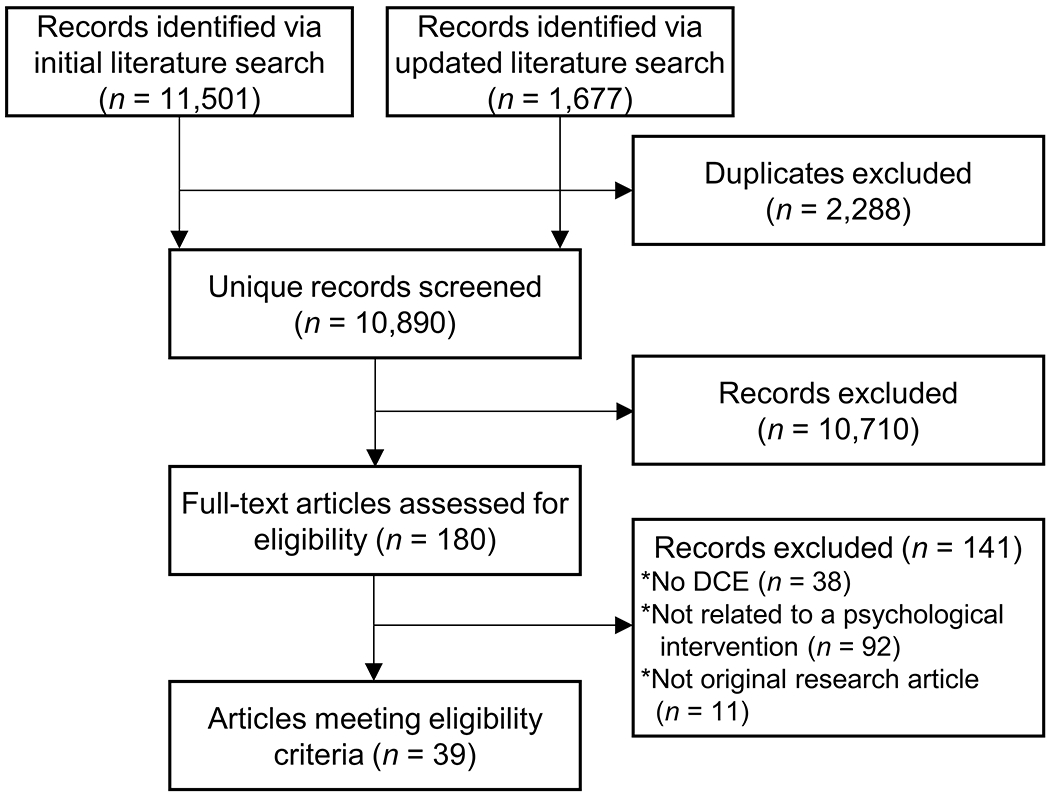
Study flow diagram.
In the 39 articles meeting inclusion criteria, DCEs were used to understand patient preferences for lifestyle behavior change interventions (i.e., physical activity, dietary modifications, smoking cessation) (n = 11), disease self-management programs (n = 4), HIV prevention and/or intervention services (n = 10), psychosocial clinical services (n = 12), or other interventions (n = 2). Article characteristics are summarized in Table 1. Of the 39 articles, 30 used DCEs to identify intervention attributes and levels preferred by a given population. In two of these 30 articles and nine additional articles, DCE data were analyzed using latent class analysis to identify groups of patients with similar preferences.
Table 1.
Characteristics of Included Manuscripts
| First Author (Year) | Sample |
DCE |
Intervention | |||||
|---|---|---|---|---|---|---|---|---|
| n | Population | Age Range | Country | Format | Choice Tasks* | Options | ||
| Lifestyle Behavior Change Interventions (i.e., Physical Activity, Dietary Modifications, Smoking Cessation) | ||||||||
| Aboagye (2017) | 112 | Adults consulted for non-specific low back pain | 18-65 years | Sweden | Computer-based | 10 | 2 | Physical exercise for secondary prevention of non-specific low back pain |
| Farooqui (2014) | 1000 | Adults | ≥ 50 years | Singapore | Paper and pencil | 10 | 2 | Walking program |
| Geidl (2018) | 104 | Adults post-stroke receiving neurological inpatient rehabilitation | ≥ 25 years | Germany | Face-to-face | 8 | 2 | Exercise during neurological rehabilitation for patients who have survived a stroke |
| Grisolía (2013) | 493 | Adults | 40-65 years | Northern Ireland | Computer-based | 10 | 2 and “current lifestyle” | Dietary and physical activity intervention to reduce risk of cardiovascular disease |
| Katz (2019) | 61 | Veteran’s Health Administration primary care outpatients who planned to quit smoking within the next 6 months | NR | United States | Face-to-face | 14 | 2 and “opt-out” | Smoking cessation counseling |
| Molema (2019) | 290 | Adults with Type 2 diabetes and/or cardiovascular disease | 38-92 years | Netherlands | Paper and pencil | 9 | 2 and “opt-out” | Financial incentives for promoting lifestyle behavior change |
| Owen (2010) | 57 | Adults with symptoms of metabolic syndrome participating in a trial of a community-based lifestyle intervention | NR | Australia | Computer-based | 16 | 3 | Community-based lifestyle intervention |
| Promberger (2012) | 450 | Adults | ≥ 18 years | United Kingdom | Paper and pencil | 22 | 2 | Incentive-based health behavior change (smoking cessation, weight loss) interventions |
| Ramirez (2016) | 125 | Latino adults with diabetes | ≥ 18 years | United States | Face-to-face | 7 | 2 | Text message intervention for promoting physical activity behavior change |
| Veldwijk (2013) | 391 | Adults with Type 2 diabetes | 35-65 years | Netherlands | Paper and pencil | 9 | 2 and “opt-out” | Lifestyle intervention for patients with Type 2 diabetes |
| Wright (2019) | 304 | Parents of children ages 6-17 years | ≥ 18 years | United States | Computer-based | 10 | 2 | Incentives for promoting participation in family-based treatment (weight management approach for children with obesity) |
| Disease Self-Management Programs | ||||||||
| Atkinson-Clark (2018) | 299 | Adults with epilepsy | ≥ 18 years | France, Germany, and the Netherlands | Computer-based | 15 | 2 and “neither” | Self-management program for patients with epilepsy |
| Burton (2017) | 717 | Patients with one or more self-reported conditions associated with chronic pain or breathlessness | ≥ 16 years | United Kingdom | Computer-based | 12 | 3 | Self-management program for patients with long-term conditions |
| Hertroijs (2019) | 288 | Patients with Type 2 diabetes | NR | Netherlands | Paper and pencil | 10 | 2 | Non-pharmaceutical aspects of diabetes care |
| McMorrow (2018) | 105 | Young adults with Type 1 diabetes | NR | Ireland | Paper and pencil | 12 | 2 and “opt-out” | Diabetes clinic-related services |
| HIV Prevention and/or Intervention Services | ||||||||
| Dubov (2018) | 1,184 | Men who were HIV negative and had sexual contact with another man in the past 6 months | ≥ 18 years | Ukraine | Computer-based | 14 | 2 and “none” | Pre-exposure prophylaxis (PrEP) program |
| Dubov (2019) | 554 | Men self-identifying as men who have sex with men and HIV negative | 21-53 years | United States | Computer-based | 14 | 2 and “none” | Pre-exposure prophylaxis (PrEP) program |
| Eshun-Wilson (2019) | 486 | Adults | ≥ 18 years | Zambia | Computer-based | 7 | 2 | Differentiated service delivery models for clinically stable patients on antiretroviral therapy |
| Kim (2018) | 424 | Adults newly diagnosed with HIV | ≥ 18 years | South Africa | NR | 8 | 2 | Motivation to take medication to prevent transmission of HIV and/or tuberculosis |
| Kim (2019) | 65 | Women who were newly diagnosed with HIV and currently pregnant | ≥ 18 years | South Africa | NR | 8 | 2 | Motivation to take medication to prevent mother-to-child transmission of HIV and/or tuberculosis |
| Michaels-Igbokwe (2015) | 537 | Youth | 15-24 years | Malawi | Face-to-face | 12 | 2 and “neither” | Family planning and HIV service delivery |
| Strauss (2018a)** | 305 | Male truck drivers self-reporting HIV-negative or unknown HIV status | ≥ 18 years | Kenya | Face-to-face | 8 | 2 | HIV testing and counseling service delivery models |
| Strauss (2018b)** | 150 | Male truck drivers self-reporting HIV-negative or unknown HIV status | ≥ 18 years | Kenya | Face-to-face | 8 | 2 | HIV testing and counseling service delivery models |
| Terris-Prestholt (2019) | 325 | Adult males | ≥ 18 years | Tanzania | NR | 9 | 2 and “neither” | Voluntary medical male circumcision to prevent HIV |
| Zanolini (2018) | 1,617 | Adolescents and adults | 16-49 years | Zambia | Computer-based | 9 | 2 and “standard” | HIV self-testing |
| Psychosocial Clinical Services | ||||||||
| Becker (2016) | 562 | Patients, family members, and mental health professionals | ≥ 16 years | Canada | Computer-based | 18 | 3 | Early intervention services for patients with mental illnesses |
| Becker (2019) | 516 | Patients, family members, and mental health professionals | ≥ 16 years | Canada | Computer-based | 18 | 3 | Sustained engagement in early intervention mental health treatment |
| Cunningham (2008) | 1,194 | Parents seeking mental health services for their children (ages 6-18 years) | NR | Canada | Computer-based or Paper and pencil | 30 | 3 | Mental health services for children |
| Cunningham (2013) | 1,059 | Parents seeking mental health services for their children (ages 4-16 years) | NR | Canada | Computer-based | 25 | 3 | Interim services for parents of children waiting for formal mental health assessment and treatment |
| Cunningham (2014) | 1,068 | Young adults | 18-35 years | Canada | Computer-based | 17 | 3 | Information strategies detailing mental health services targeting young adults |
| Cunningham (2015) | 1,036 | Parents seeking mental health services for their children (ages 4-16 years) | NR | Canada | Computer-based | 25 | 3 | Interim services for parents of children waiting for formal mental health assessment and treatment |
| Cunningham (2017) | 909 | University students | ≥ 16 years | Canada | Computer-based | 17 | 3 | University mental health services |
| Goodall (2012) | 83 | Adolescents and young adults with cancer or a blood disorder and caregivers | 16-32 years (for patients) | Australia | Paper and pencil | 16 | 2 | Psychosocial support services for adolescents and young adults with cancer or a blood disorder and their families |
| Herman (2016) | 604 | Adult patients receiving care at a federally-qualified migrant health center | ≥ 18 years | United States | Computer-based | 15 | 3 | Patient-centered mental health services in a federally-qualified health center serving a low-income Hispanic population |
| Lokkerbol (2019a) | 165 | Adults receiving treatment for depression in the past 12 months | ≥ 18 years | Netherlands | Computer-based | 12 | 2 | Psychotherapy for depression |
| Lokkerbol (2019b) | 126 | Adults receiving treatment for anxiety in the past 12 months | ≥ 18 years | Netherlands | Computer-based | 12 | 2 | Psychotherapy for anxiety |
| Muntingh (2019) | 109 | Adults with a partially or fully remitted anxiety or depressive disorder | 21-69 years | Netherlands | Paper and pencil | 20 | 2 and “opt-out” | Relapse prevention program for anxiety and depression |
| Other Interventions | ||||||||
| Crutchfield (2017) | 251 | Adult patients | 18-83 years | United States | Computer-based | 16 | 3 | Medical appointment reminders |
| Yi (2011) | 124 | Adults with chronic low back pain | ≥ 20 years | Scotland | Paper and pencil | 16 | 2 and “neither” | Pain management program for patients with chronic low back pain |
Number of unique choice tasks, excluding choice tasks repeated to examine response quality;
Manuscripts include subsets of the same sample;
NR = not reported
DCEs Identifying Preferred Intervention Attributes and Level
In the 30 articles using DCEs to identify intervention attributes and levels preferred by a given population, DCEs were used to understand preferences related to lifestyle behavior change interventions (n = 11), disease self-management programs (n = 4), HIV prevention and/or intervention services (n = 8), psychosocial clinical services (n = 5), or other interventions (n = 2). By identifying the attributes and levels most preferred by a given population (see Supplemental Table 1), each manuscript was able to conclude with recommendations regarding intervention content and format that most closely aligns with patient preferences.
Lifestyle behavior change interventions.
Eleven articles used DCEs to understand preferences related to lifestyle behavior change interventions among adults surveyed in the United States (n = 3), the Netherlands (n = 2), Australia (n = 1), Germany (n = 1), Northern Ireland (n = 1), Singapore (n = 1), Sweden (n = 1), and the United Kingdom (n = 1). Ten studies assessed preferences for interventions targeting adults, with three studies including adults with type 2 diabetes (Molema et al., 2019; Ramirez, Shinyi, Beale, & Wu, 2016; Veldwijk et al., 2013), one including adults with symptoms of metabolic syndrome (Owen, Pettman, Haas, Viney, & Misan, 2010), one including adults with low back pain (Aboagye et al., 2017), one including adults post-stroke (Geidl, Knocke, Schupp, & Pfeifer, 2018), one including adults who planned to quit smoking (Katz et al., 2019), and three including generally healthy adults (Farooqui, Tan, Bilger, & Finkelstein, 2014; Grisolía, Longo, Boeri, Hutchinson, & Kee, 2013; Promberger, Dolan, & Marteau, 2012). Wright et al. (2019) assessed parent preferences for incentives to promote participation in a family-based weight management intervention for children (ages 6-17) with obesity.
While the specific attributes included in each DCE differed by study, the preference weights for intervention dose (n = 6 articles), intervention efficacy (n = 4), and incentive type or value (n = 4) were significant in multiple studies and these attributes may be particularly relevant in adults’ decisions to pursue lifestyle behavior change interventions (Supplemental Table 1). By calculating marginal willingness-to-pay, a measure of the amount of out-of-pocket costs that a potential patient would be willing to pay for a change in an attribute level, Veldwijk et al. (2013) used DCE data to quantify the importance of intervention efficacy. Specifically, Veldwijk et al. (2013) found that adults with type 2 diabetes in the Netherlands were willing to pay €96.8 per year for 10 kg anticipated weight loss. The types of preferred incentives differed by groups, with adults in Singapore preferring cash to vouchers for participation in a physical activity intervention (Farooqui et al., 2014) and adults in the United Kingdom preferring grocery vouchers to cash incentives for a weight-loss intervention (Promberger et al., 2012). This difference between populations highlights the utility of DCEs in developing interventions to match the specific preferences of a given population.
In addition to understanding the intervention characteristics preferred by individuals considering lifestyle behavior change interventions, DCEs can be used to assess changes in preferences over time. Owen et al. (2010) administered a DCE to adults with symptoms of metabolic syndrome throughout their participation in a community-based trial of a lifestyle intervention. Patient preferences changed over time, with cost emerging as a significant attribute at subsequent assessment points (but not baseline). This finding suggests that engaging individuals throughout an intervention may require attention to different attributes (e.g., cost) than those relevant at enrollment. In addition, as the significant attributes differed based on an individual’s weight loss during the intervention, findings such as these may begin to illuminate how intervention components could be modified in response to treatment success.
Disease self-management programs.
Four studies used DCEs to understand preferences of adults with epilepsy (n = 1), chronic pain or breathlessness (n = 1), Type 2 diabetes (n = 1), or Type 1 diabetes (n = 1) for disease self-management programs in Europe (Atkinson-Clark, Charokopou, van Osselaer, Hiligsmann, 2018; Burton et al., 2017; Hertroijs et al., 2019; McMorrow et al., 2018). Each article included different attributes, but cost (n = 3), program content (n = 3), and provider type (n = 3) significantly influenced patient’s preferences for disease self-management programs in multiple populations (Supplemental Table 1). With an understanding of significant attributes, these teams are now equipped with information to design self-management programs that align with the preferences of their patient populations.
HIV prevention and/or intervention services.
The eight studies using DCEs to understand patient preferences for HIV prevention and/or intervention services included adolescents and/or adults in Kenya (n = 2), South Africa (n = 2), Zambia (n = 2), Malawi (n = 1), and Tanzania (n = 1). Five studies included an attribute representing counseling availability and/or format (Supplemental Table 1). In each of these studies, the counseling attribute was significant, with adolescents and young adults preferring HIV prevention and/or intervention services including counseling for patients and/or their partners (Eshun-Wilson et al., 2019; Strauss et al., 2018a; Strauss et al., 2018b; Terris-Prestholt et al., 2019; Zanolini et al., 2018). Once counseling is identified as a preferred component of HIV prevention and/or intervention, DCEs can also be used to understand preferences for counseling content. In studies of adults (Kim et al., 2018) and pregnant women with HIV (Kim et al., 2019), Kim and colleagues used DCEs to identify factors likely to motivate adherence to preventative therapy. Attributes related to transmission prevention and impact on family were significant in both studies, suggesting that health promotion messages focusing on these benefits align with patients’ preferences and ultimately have the potential to increase medication adherence (Kim et al., 2018; Kim et al., 2019).
Psychosocial clinical services.
Five studies used DCEs to understand patient preferences for psychosocial clinical services. Goodall et al. (2012) administered a DCE to adolescents and young adults with cancer or a blood disorder and their caregivers to determine the preferred features of psychosocial support services. Not all attributes included in the DCE were significant, with adolescents and young adults and their caregivers preferring psychosocial services including patient emotional support, family emotional support, work/study support, and financial support, but not spiritual support and/or cultural/ethnic support (Goodall, King, Ewing, Smith, & Kenny, 2012). Based on this information, Goodall et al. (2012) were able to prioritize the allocation of limited resources to meet the most pressing psychosocial needs of their patients. While Goodall et al. (2012) used a DCE to understand patient preferences for psychosocial clinical service content, the other four DCEs examined patient preferences for the logistical and structural features of care. The significant attributes in these DCEs including low-income Hispanic families (n = 1) (Herman et al., 2016) or adults with depression and/or anxiety (n = 3) (Lokkerbol et al., 2019a; Lokkerbol et al., 2019b; Muntingh et al., 2019) represent aspects of psychosocial clinical service format (e.g., digitization), location, frequency, and duration that can be modified to align with patient preferences.
Other services.
The remaining two studies applied DCEs to other interventions. To inform the development of an appointment reminder system to increase patient attendance, Crutchfield et al. (2017) administered a DCE to adults in the United States. Patients in this study preferred a single reminder via email, phone, or text that arrives within 14 days of the scheduled appointment and includes information about the location. Yi and colleagues (2011) administered a DCE to adults with chronic low back pain in Scotland and determined that patients preferred individual or small group pain management programs that are delivered less frequently but for a longer duration and in a location closer to home.
DCEs Identifying Groups of Patients with Similar Preferences
Eleven articles, including two articles using DCEs to identify preferred intervention attributes and levels (Burton et al., 2017; Katz et al., 2019), analyzed DCE data using latent class analysis to identify groups of patients with similar preferences (Hauber et al., 2016; Zhou, Thayer, & Bridges, 2018) (see Supplemental Table 2).
Psychosocial clinical services.
The majority of these articles (n = 7) used a DCE to identify groups of patients, family members, and/or mental health professionals with similar preferences for psychosocial clinical services. For example, Becker et al. (2016) used a DCE to determine groups of patients, family members, and mental health professionals with similar preferences for early intervention services for patients with a psychiatric illness. Results indicated a two class solution, suggesting that patients could be categorized into one of two groups based on their preferences (Becker et al., 2016). Of note, the three most important attributes differed across groups with no overlap. The preferred attributes and levels of the first group (43% of sample) indicated that these patients would be most interested in face-to-face evidence-based early intervention services provided by psychiatrists or psychologists. In the second group, the method of service delivery (face-to-face versus phone, etc.), evidence-base of the intervention content, and training level of the provider were less important. Instead, this group (57% of sample) preferred a service with minimal wait times that accepted self-referrals or walk-in patients and involved families in treatment as appropriate. These findings suggest that teams developing early intervention services should consider providing multiple treatment options to ensure services align with the preferences of different subgroups of patients.
Once patient groups are identified using latent class models, it is possible to simulate the uptake of various treatment packages. In a study of the interim service preferences of parents waiting for mental health treatment for their children, Cunningham et al. (2013) found four groups of parents with similar preferences. Based on the attributes preferred by each group, Cunningham et al. (2013) created four hypothetical interim service options: group parenting, telephone-supported parenting, internet parenting, and wait list as usual. Randomized First Choice simulation was then used to generate the probability that each parent would select each of the four interim service options. This was completed by combining the results of 200,000 simulations in which choice probabilities were computed following the addition of random variance (Orme & Huber, 2000). Simulation results suggested that most parents would prefer group parenting (42%) followed by telephone-supported parenting (41%), internet parenting (14%), and wait list (3%). Simulations like these are useful as they can elucidate the potential implications of offering various combinations of treatment packages when data on actual/observed choices are not available. Similar efforts were undertaken in five other articles to simulate the uptake of various information programs for parents of children with mental health problems (Cunningham et al., 2008), programs for parents awaiting mental health treatment for their child (Cunningham et al., 2015), informational strategies describing mental health services (Cunningham et al., 2014), mental health services for university students (Cunningham et al., 2017), and efforts to sustain engagement in early intervention mental health treatment (Becker et al., 2019).
Lifestyle behavior change interventions and disease self-management programs.
In addition to using DCEs to identify preferred intervention attributes and levels, Burton et al. (2017) and Katz et al. (2019) used DCEs to identify groups of patients with similar preferences for self-management programs and smoking cessation counseling, respectively. Analyzing DCE data using both of these methods enabled the authors to understand patient preferences at the group level while also identifying clusters of patients with a unique preference “phenotype” (Katz et al., 2019).
HIV prevention and/or intervention services.
Two articles administered the same DCE to men in Ukraine (Dubov, Fraenkel, Yorick, Ogunbajo, & Altice, 2018) and the United States (Dubov, Ogunbajo, Altice, & Fraenkel, 2019) who were HIV negative and engaged in sexual contact with other men to identify groups with similar preferences for pre-exposure prophylaxis (PrEP) programs. While each latent class analysis identified five distinct segments of patients with similar preferences, only two of the five segments were similar in both studies. The majority of men from Ukraine fell into a group whose preferences did not align with those of any of the groups of men from the United States (Dubov et al., 2018; Dubov et al., 2019).
Consideration of Key Criteria
The degree to which each study considered the key criteria in DCE development, analysis, and reporting as defined by the International Society for Pharmacoeconomics and Outcomes Research are presented in Supplemental Table 3. All studies (n = 39, 100%) adequately addressed criteria relevant to the research question, preference elicitation, results and conclusions, and study presentation. The majority of studies (n = 26, 67%) adequately detailed their use of qualitative methods, literature reviews, or other scientific evidence to develop and provide justification for DCE attributes and levels (checklist item 2). For items related to DCE construction and study design (checklist items 3, 4, 6), themes in reporting emerged across studies. Specifically, many studies did not provide information on their choice task option selection (checklist item 3), experimental design evaluation (checklist item 4), and/or DCE instructions and descriptions provided to participants (checklist item 6). All 21 articles with insufficient information regarding data collection (checklist item 7) did not provide a sample size justification and all 25 articles with insufficient information regarding statistical analysis (checklist item 8) did not report on the examinations of DCE quality (e.g., reliability, validity, rationality).
Discussion
This systematic review identified 39 articles which used DCEs to inform the development of patient-centered interventions. Together, these articles not only enhance our understanding of the preferences of multiple patient populations for a variety of interventions, but also highlight the range of applications of DCEs to health psychology. Based on the included articles and the larger DCE literature, we propose that DCEs can facilitate patient-centered psychological care by enabling health psychologists to: 1) design patient-centered interventions; 2) adapt existing interventions; 3) prioritize patient-centered services; 4) develop multiple intervention packages; and 5) simulate uptake. Table 2 includes an exemplar question relevant to each application as well as the DCE data required to explore this question and resulting implications. Below, we elaborate on these five potential DCE applications, using relevant studies included in this review as examples.
Table 2.
Exemplar Applications of DCEs to Health Psychology
| Aim | Example Question | DCE Data of Interest | Potential Implications |
|---|---|---|---|
| Design Patient-Centered Interventions | “Which intervention attributes/levels impact the preferences of my patient population for a physical activity intervention?” | Attributes/levels with statistically significant coefficients | Design the intervention to include significant attributes/levels |
| “What is the relative importance of HIV prevention program attributes/levels among my patient population?” | Relative magnitude of coefficients for each attribute/level | Deliver HIV prevention program in a manner that aligns with attributes/levels with the largest coefficients | |
| Adapt Existing Interventions | “Do the attributes/levels of an established smoking cessation intervention align with the preferences of my patient population?” | Alignment of statistically significant attributes/levels with those of established intervention | Modify established intervention as necessary to align with significant attributes/levels |
| Prioritize Patient-Centered Services | “What is the relative importance of psychosocial service attributes/levels among my patient population?” | Relative magnitude of coefficients for each attribute/level | Prioritize efforts to deliver psychosocial services reflecting the attributes/levels with the largest coefficients |
| Develop Multiple Intervention Packages | “Are there segments of my patient population with different preferences for an intervention targeting depressive symptoms?” | Preferred attributes/levels for each segment | Develop multiple intervention package options that align with the attributes/levels of each segment |
| Simulate Intervention Uptake | “Would adding telehealth services have the potential to increase the uptake of individual therapy service in my patient population?” | Percentage of patients preferring telehealth over in-person individual therapy | Add telehealth services if simulated uptake is deemed a significant improvement over current uptake |
Designing Patient-Centered Interventions
First, as illustrated by the first 30 articles highlighted in this review, DCEs can be used to identify the attributes/levels of a psychological intervention preferred by a given patient or patient population. To date, researchers have used DCEs to identify attributes/levels that impact patient preferences for lifestyle behavior change interventions, disease self-management programs, HIV prevention and/or intervention services, psychosocial clinical services, pain management programs, and appointment reminders. Patient-centered interventions for these populations can now be developed by designing interventions that include the significant attributes/levels in each study. As an example, after using a DCE to learn that the frequency of text messaging and physical activity behavior-change education were significant drivers of Latino adults with Type 2 diabetes’ physical activity intervention preferences, Ramirez et al. (2016) designed an intervention including these components. Potentially due to its alignment with patient preferences, results of a pilot randomized clinical trial (RCT) indicated that participants found the intervention feasible and useful (Ramirez & Wu, 2017).
Designing an intervention that aligns with patient preferences elicited via DCEs is not only consistent with patient-centered psychological care, but also has the potential to increase patient engagement in psychological care (Crits-Christoph, Gallop, Diehl, Yin, & Gibbons, 2017; Swift, Callahan, Cooper, & Parkin, 2018; Swift, Callahan, & Vollmer, 2011) and adherence to medical treatment. Motivated by the finding that sub-optimal adherence is a primary reason for failure to detect an effect in RCTs of complex HIV-prevention interventions, Terris-Prestholt et al. (2019) used the results of a DCE to design a voluntary medical male circumcision intervention whose features (e.g., availability of partner counseling) aligned with the preferences of the target population (adult males in Tanzania). This patient-centered intervention development approach improved engagement and the rates and proportions of young adult enrollment were higher in the DCE-informed intervention than in the standard of care intervention (Wambura et al., 2017).
Adapting Existing Interventions to Align with Patient Preferences
Second, as articles included in this review found differences in patient preferences by demographic (i.e., age [Aboagye et al., 2017, Lokkerbol et al., 2019b; Michaels-Igbokwe, Lagarde, Cairns, & Terris-Prestholt, 2015; Muntingh et al., 2019], gender [Hertroijs et al., 2019], education [Hertroijs et al., 2019; Lokkerbol et al., 2019a; Lokkerbol et al., 2019b]), clinical (e.g., medication regimen [Hertroijs et al., 2019]), and geographic (e.g., country of residence [Atkinson-Clark et al., 2018; Dubov et al., 2018; Dubov et al., 2019], population density [Eshun-Wilson et al., 2019]) characteristics, DCEs may be useful when considering how to adapt or spread an existing intervention to another population. As an example, assume a health psychologist has a colleague who is implementing a smoking cessation intervention with great success at another institution. By administering a DCE to their patients including the attributes/levels of the colleague’s intervention, the health psychologist could determine if the attributes/levels preferred by their patients align with those included in the intervention. If the colleague’s intervention is not well-aligned with the preferences of the patient population, the health psychologist could use DCE data to inform potential modifications.
Prioritizing Patient-Centered Services
DCEs may also prove helpful in informing efforts to advocate for patient-centered modifications when resources are limited. Herman et al. (2016) administered a DCE to understand preferences for mental health services among low-income Hispanic adults receiving care at a federally-qualified health center. When the results were presented to the center’s leadership, it was noted that it was not possible to implement all of the changes necessary to align mental health services with patient preferences (Herman et al., 2016). Because DCE results quantify the relative importance of each attribute and level, however, leadership were provided with the data necessary to prioritize changes relevant to the most important attribute/levels. In the same way, DCEs may help health psychologists prioritize their efforts. For example, assume a health psychologist is currently conducting regular psychosocial assessments with cancer survivors and is hoping to expand their practice to include intervention services at the institution. In this instance, a DCE could be used to identify which types of interventions (e.g., individual therapy, couples counseling) should be prioritized as they begin to develop additional patient-centered services (see Goodall et al., 2012 for an example).
Developing Multiple Intervention Packages
Fourth, DCEs can be analyzed using latent class analysis to identify groups of patients with similar preferences as in 11 articles included in this review. These methods may be most relevant in populations where it is expected that patient preferences are heterogeneous. For example, a psychologist designing a therapy-based intervention to target depression among adults being treated by their primary care physician may recognize that there is unlikely to be an applicable “one size fits all” approach. By administering a DCE with varied intervention attributes (e.g., therapy frequency, therapy location, therapist) and using latent class analysis, the psychologist could develop possible intervention packages that could be matched to different cohorts of patients.
Simulating Intervention Uptake
Once hypothetical intervention packages are designed, DCE data can be used to simulate uptake. This method enables the psychologist to estimate how many patients would be expected to participate in each type of intervention. Simulations have applications for both clinical and research health psychologists. A health psychologist interested in adding a telehealth weight-management program to an ongoing in-person weight management service, for example, could administer a DCE and use simulations to estimate the percentage of patients who would seek telehealth but not in-person services. These data could then be used to advocate for coverage of materials/staffing to support the new telehealth program as appropriate. From a research perspective, simulations could be used to estimate the potential uptake of behavioral interventions to be tested in a research protocol and support the recruitment feasibility in a grant application.
Limitations and Future Directions
In addition to the numerous potential applications of DCEs to health psychology, this review identified multiple remaining gaps in the literature. First, few articles explored the degree to which DCE data predicted actual choices and the validity of DCEs remains largely unknown (Janssen, Marshall, Hauber, & Bridges, 2017). In addition, while authors of included manuscripts cited intervention design as an aim or implication, the degree to which DCE results informed future research and/or clinical initiatives was unclear in many articles. Finally, no articles reported on the sub-domains of all 10 criteria for DCE good research practices (Bridges et al., 2011) and incomplete data limited our ability to report on attribute/level significance in multiple instances.
To address the remaining gaps in the literature and ensure that the data resulting from DCEs are of the highest quality, psychologists interested in developing and administering DCEs are encouraged to seek specialized training and/or consult with researchers (i.e., health economists, marketer researchers) specializing in DCEs. A number of institutions, companies, and organizations including the Health Economics Research Unit (University of Aberdeen), the University of Sydney, the Massachusetts Institute of Technology, Sawtooth Software, the International Society for Pharamcoeconomics and Outcomes Research (ISPOR), and the Society for Medical Decision Making offer courses or workshops on DCEs. In addition, ISPOR has published several best practice guidelines on DCE design (Johnson et al., 2013) and analysis (Hauber et al., 2016) directly relevant to the criteria for DCE good research practices (Bridges et al., 2011).
Conclusions
In conclusion, DCEs offer an innovative approach to understanding patient preferences for psychological and behavioral interventions. The studies included in this review highlight how DCEs can aid health psychologists in developing interventions that align with patient preferences. As the analytic methods for handling DCE data continue to advance, it is likely that even more applications for the field will emerge (Hauber et al., 2016). Psychologists interested in using DCEs are encouraged to obtain additional training and partner with DCE experts to capitalize on this novel methodology.
Supplementary Material
Acknowledgements:
Gabriella Breen and Monica Kruse are gratefully acknowledged for their assistance with manuscript formatting and proofreading.
Role of Funding Sources: M.E.M. was supported by the National Cancer Institute of the National Institutes of Health under Award Number K07CA200668. The National Institutes of Health had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosure Statement:The authors have no conflicts of interest to disclose.
References
- Aboagye E, Hagberg J Axén I, Kwak L, Lohela-Karlsson M, Skillgate E, … Jensen I(2017). Individual preferences for physical exercise as secondary prevention for non-specific low back pain: A discrete choice experiment. PLoS One, 12(12): e0187709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson-Clark E, Charokopou M, Van Osselaer N, & Hiligsmann M (2018). A discrete-choice experiment to elicit preferences of patients with epilepsy for self-management programs. Epilepsy and Behavior, 79, 58–67. doi: 10.1016/j.yebeh.2017.11.015 [DOI] [PubMed] [Google Scholar]
- Becker MPE, Christensen BK, Cunningham CE, Furimsky I, Rimas H, Wilson F, … Zipursky RB (2016). Preferences for early intervention mental health services: A discrete-choice conjoint experiment. Psychiatric Services, 67(2), 184–191. doi: 10.1176/appi.ps.201400306 [DOI] [PubMed] [Google Scholar]
- Becker M, Cunningham CE, Christensen BK, Furimsky I, Rimas H, Wilson F, … Zipursky RB (2019). Investigating service features to sustain engagement in early intervention mental health services. Early Intervention in Psychiatry, 13(2), 241–250. doi: 10.1111/eip.12470 [DOI] [PubMed] [Google Scholar]
- Benning TM, Kimman ML, Dirksen CD, Boersma LJ, & Dellaert BGC (2012). Combining individual-level discrete choice experiment estimates and costs to inform health care management decisions about customized care: The case of follow-up strategies after breast cancer treatment. Value in Health, 15(5), 680–689. doi: 10.1016/j.jval.2012.04.007 [DOI] [PubMed] [Google Scholar]
- Bridges JF, Hauber AB, Marshall D, Lloyd A, Prosser LA, Regier DA, … Mauskopf J (2011). Conjoint analysis applications in health--a checklist: A report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value in Health, 14(4), 403–413. doi: 10.1016/j.jval.2010.11.013 [DOI] [PubMed] [Google Scholar]
- Bridges JF, Onukwugha E, Johnson FR, & Hauber AB (2007). Patient preference methods: A patient centered evaluation paradigm. ISPOR Connections, 13, 4–7. [Google Scholar]
- Burton CD, Entwistle VA, Elliott AM, Krucien N, Porteous T, & Ryan M (2017). The value of different aspects of person-centred care: a series of discrete choice experiments in people with long-term conditions. BMJ Open, 7(4), e015689. doi: 10.1136/bmjopen-2016-015689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrzan K (2010). Using partial profile choice experiments to handle large numbers of attributes. International Journal of Market Research, 52, 827–840. [Google Scholar]
- Clark M, Determann D, Petrou S, Moro D, & de Bekker-Grob E (2014). Discrete choice experiments in health economics: A review of the literature. Pharmacoeconomics, 32(9), 883–902. doi: 10.1007/s40273-014-0170-x [DOI] [PubMed] [Google Scholar]
- Coast J, Al-Janabi H, Sutton EJ, Horrocks SA, Vosper AJ, Swancutt DR, & Flynn TN (2012). Using qualitative methods for attribute development for discrete choice experiments: Issues and recommendations. Health Economics, 21, 730–741. [DOI] [PubMed] [Google Scholar]
- Committee on Quality of Health Care in America and Institute of Medicine. (2001). Crossing the Quality Chasm: A New Health System for the 21st Century. Washington DC: National Academies Press. [Google Scholar]
- Crits-Christoph P, Gallop R, Diehl CK, Yin S, & Gibbons MBC (2017). Methods for incorporating patient preferences for treatments of depression in community mental health settings. Administration and Policy in Mental Health and Mental Health Services Research, 44, 735–746. doi: 10.1007/s10488-016-0746-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crutchfield TM, & Kistler CE (2017). Getting patients in the door: Medical appointment reminder preferences. Patient Preference and Adherence, 11, 141–150. doi: 10.2147/ppa.s117396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CE, Chen Y, Deal K, Rimas H, McGrath P, Reid G, … Corkum P (2013). The interim service preferences of parents waiting for children’s mental health treatment: A discrete choice conjoint experiment. Journal of Abnormal Child Psychology, 41, 865–877. doi: 10.1007/s10802-013-9728-x [DOI] [PubMed] [Google Scholar]
- Cunningham CE, Deal K, Rimas H, Buchanan DH, Gold M, Sdao-Jarvie K, & Boyle M (2008). Modeling the information preferences of parents of children with mental health problems: A discrete choice conjoint experiment. Journal of Abnormal Child Psychology, 36(7), 1123–1138. doi: 10.1007/s10802-008-9238-4 [DOI] [PubMed] [Google Scholar]
- Cunningham CE, Rimas H, Chen Y, Deal K, McGrath P, Lingley-Pottie P, … Corkum P (2015). Modeling parenting programs as an interim service for families waiting for children’s mental health treatment. Journal of Clinical Child and Adolescent Psychology, 44(4), 616–629. doi: 10.1080/15374416.2014.888666 [DOI] [PubMed] [Google Scholar]
- Cunningham CE, Walker JR, Eastwood JD, Westra H, Rimas H, Chen Y, … The Mobilizing Minds Research, G. (2014). Modeling mental health information preferences during the early adult years: A discrete choice conjoint experiment. Journal of Health Communication, 19(4), 413–440. doi: 10.1080/10810730.2013.811324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CE, Zipursky RB, Christensen BK, Bieling PJ, Madsen V, Rimas H, … Munn, C. (2017). Modeling the mental health service utilization decisions of university undergraduates: A discrete choice conjoint experiment. Journal of American College Health, 65(6), 389–399. doi: 10.1080/07448481.2017.1322090 [DOI] [PubMed] [Google Scholar]
- de Bekker-Grob EW, Veldwijk J, Jonker M, Donkers B, Huisman J, Buis S, … Bindels P (2018). The impact of vaccination and patient characteristics on influenza vaccination uptake of elderly people: A discrete choice experiment. Vaccine, 36, 1467–1476. doi: 10.1016/j.vaccine.2018.01.054 [DOI] [PubMed] [Google Scholar]
- Dubov A, Fraenkel L, Yorick R, Ogunbajo A, & Altice FL (2018). Strategies to implement pre-exposure prophylaxis with men who have sex with men in Ukraine. AIDS and Behavior, 22(4), 1100–1112. doi: 10.1007/s10461-017-1996-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubov A, Ogunbajo A, Altice FL, & Fraenkel L (2019). Optimizing access to PrEP based on MSM preferences: results of a discrete choice experiment. AIDS Care, 31(5), 545–553. doi: 10.1080/09540121.2018.1557590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein RM, Fiscella K, Lesser CS, & Stange KC (2010). Why the nation needs a policy push on patient-centered health care. Health Affairs, 29(8), 1489–1495. doi: 10.1377/hlthaff.2009.0888 [DOI] [PubMed] [Google Scholar]
- Eshun-Wilson I, Mukumbwa-Mwenechanya M, Kim HY, Zannolini A, Mwamba CP, Dowdy D, … Geng EH (2019). Differentiated care preferences of stable patients on antiretroviral therapy in Zambia: A discrete choice experiment. Journal of Acquired Immune Deficiency Syndromes, 81(5), 540–546. doi: 10.1097/qai.0000000000002070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqui MA, Tan Y-T, Bilger M, & Finkelstein EA (2014). Effects of financial incentives on motivating physical activity among older adults: Results from a discrete choice experiment. BMC Public Health, 14, 141. doi: 10.1186/1471-2458-14-141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraenkel L (2013). Incorporating patients’ preferences into medical decision making. Medical Care Research and Review, 70(1_suppl), 80S–93S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frewer LJ, Salter B, & Lambert N (2001). Understanding patients’ preferences for treatment: The need for innovative methodologies. Quality in Health Care, 10(suppl 1), i50–i54. doi: 10.1136/qhc.0100050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geidl W, Knocke K, Schupp W, & Pfeifer K (2018). Measuring stroke patients’ exercise preferences using a discrete choice experiment. Neurology International, 10(1), 6993. doi: 10.4081/ni.2018.6993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodall S, King M, Ewing J, Smith N, & Kenny P (2012). Preferences for support services among adolescents and young adults with cancer or a blood disorder: A discrete choice experiment. Health Policy, 107(2–3), 304–311. doi: 10.1016/j.healthpol.2012.07.004 [DOI] [PubMed] [Google Scholar]
- Grisolía JM, Longo A, Boeri M, Hutchinson G, & Kee F (2013). Trading off dietary choices, physical exercise and cardiovascular disease risks. Social Science & Medicine, 93, 130–138. doi: 10.1016/j.socscimed.2013.05.031 [DOI] [PubMed] [Google Scholar]
- Hauber AB, González JM, Groothuis-Oudshoorn CGM, Prior T, Marshall DA, Cunningham C, … Bridges JFP (2016). Statistical methods for the analysis of discrete choice experiments: A report of the ISPOR Conjoint Analysis Good Research Practices Task Force. Value in Health, 19(4), 300–315. 10.1016/j.jval.2016.04.004 [DOI] [PubMed] [Google Scholar]
- Herman PM, Ingram M, Cunningham CE, Rimas H, Murrieta L, Schachter K, … Carvajal SC (2016). A comparison of methods for capturing patient preferences for delivery of mental health services to low-income Hispanics engaged in primary care. Patient, 9(4), 293–301. doi: 10.1007/s40271-015-0155-7 [DOI] [PubMed] [Google Scholar]
- Hertroijs DFL, Elissen AMJ, Brouwers M, Hiligsmann M, Schaper NC, & Ruwaard D (2019). Preferences of people with Type 2 diabetes for diabetes care: A discrete choice experiment. Diabetic Medicine. doi: 10.1111/dme.13969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho MP, Gonzalez JM, Lerner HP, Neuland CY, Whang JM, McMurry-Heath M, … Irony T (2015). Incorporating patient-preference evidence into regulatory decision making. Surgical Endoscopy, 29, 2984–2993. doi: 10.1007/s00464-014-4044-2 [DOI] [PubMed] [Google Scholar]
- Hofman R, de Bekker-Grob EW, Richardus JH, de Koning HJ, van Ballegooijen M, & Korfage IJ (2014). Have preferences of girls changed almost 3 years after the much debated start of the HPV vaccination program in The Netherlands? A discrete choice experiment. PLoS One, 9(8), e104772. doi: 10.1371/journal.pone.0104772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen EM, Marshall DA, Hauber AB, & Bridges JFP (2017) Improving the quality of discrete-choice experiments in health: how can we assess validity and reliability?. Expert Review of Pharmacoeconomics & Outcomes Research, 17, 531–542, doi: 10.1080/14737167.2017.1389648 [DOI] [PubMed] [Google Scholar]
- Johnson FR, Lancsar E, Marshall D, Kilambi V, Mühlbacher A, Regier DA, … Bridges JFP (2013). Constructing experimental designs for discrete-choice experiments: Report of the ISPOR Conjoint Analysis Experimental Design Good Research Practices Task Force. Value in Health, 16(1), 3–13. 10.1016/j.jval.2012.08.2223 [DOI] [PubMed] [Google Scholar]
- Johnson FR, & Zhou M (2016). Patient preferences in regulatory benefit-risk assessments: A US perspective. Value in Health, 19, 741–745. 10.1016/j.jval.2016.04.008 [DOI] [PubMed] [Google Scholar]
- Katz DA, Hamlin C, Vander Weg MW, Grant KM, Stewart Steffensmeier KR, Paez M, … Gaeth G (2019). Veterans’ preferences for tobacco treatment in primary care: A discrete choice experiment. Patient Education and Counseling. doi: 10.1016/j.pec.2019.10.002 [DOI] [PubMed] [Google Scholar]
- Kim HY, Hanrahan CF, Dowdy DW, Martinson N, Golub J, & Bridges JFP (2018). The effect of partner HIV status on motivation to take antiretroviral and isoniazid preventive therapies: A conjoint analysis. AIDS Care, 30, 1298–1305. doi: 10.1080/09540121.2018.1455958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HY, Dowdy DW, Martinson NA, Kerrigan D, Tudor C, Golub J, … Hanrahan CF (2019). Maternal motivation to take preventive therapy in antepartum and postpartum among HIV-positive pregnant women in South Africa: A choice experiment. AIDS and Behavior, 23(7), 1689–1697. doi: 10.1007/s10461-018-2324-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancsar E, & Louviere J (2008). Conducting discrete choice experiments to inform healthcare decision making: A user’s guide. Pharmacoeconomics, 26(8), 661–677. [DOI] [PubMed] [Google Scholar]
- Lokkerbol J, Geomini A, van Voorthuijsen J, van Straten A, Tiemens B, Smit F, … Hiligsmann M (2019a). A discrete-choice experiment to assess treatment modality preferences of patients with depression. Journal of Medical Economics, 22(2), 178–186. doi: 10.1080/13696998.2018.1555404 [DOI] [PubMed] [Google Scholar]
- Lokkerbol J, van Voorthuijsen JM, Geomini A, Tiemens B, van Straten A, Smit F, … Hiligsmann M (2019b). A discrete-choice experiment to assess treatment modality preferences of patients with anxiety disorder. Journal of Medical Economics, 22(2), 169–177. doi: 10.1080/13696998.2018.1555403 [DOI] [PubMed] [Google Scholar]
- Louviere JJ, Flynn TN, & Carson RT (2010). Discrete choice experiments are not conjoint analysis. Journal of Choice Modelling, 3(3), 57–72. 10.1016/S1755-5345(13)70014-9 [DOI] [Google Scholar]
- McMorrow L, O’Hara MC, Hynes L, Cunningham A, Caulfield A, Duffy C, … Doherty E (2018). The preferences of young adults with Type 1 diabetes at clinics using a discrete choice experiment approach: the D1 Now Study. Diabetic Medicine, 35(12), 1686–1692. doi: 10.1111/dme.13809 [DOI] [PubMed] [Google Scholar]
- Michaels-Igbokwe C, Lagarde M, Cairns J, & Terris-Prestholt F (2015). Designing a package of sexual and reproductive health and HIV outreach services to meet the heterogeneous preferences of young people in Malawi: Results from a discrete choice experiment. Health Economics Review, 5, 9. doi: 10.1186/s13561-015-0046-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, & The PRISMA Group. (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA statement. PLoS Medicine, 6(7), e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molema C, Veldwijk J, Wendel-Vos W, de Wit A, van de Goor I, & Schuit J (2019). Chronically ill patients’ preferences for a financial incentive in a lifestyle intervention. Results of a discrete choice experiment. PLoS One, 14(7), e0219112. doi: 10.1371/journal.pone.0219112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlbacher AC, Juhnke C, Beyer AR, & Garner S (2016). Patient-focused benefit-risk analysis to inform regulatory decisions: The European Union perspective. Value in Health, 19, 734–740. 10.1016/j.jval.2016.04.006 [DOI] [PubMed] [Google Scholar]
- Muntingh ADT, Hoogendoorn AW, Van Schaik DJF, Van Straten A, Stolk EA, Van Balkom A, & Batelaan NM (2019). Patient preferences for a guided self-help programme to prevent relapse in anxiety or depression: A discrete choice experiment. PLoS One, 14(7), e0219588. doi: 10.1371/journal.pone.0219588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme B, & Huber J (2000). Improving the value of conjoint simulations. Marketing Research, 12, 12–20. [Google Scholar]
- Owen K, Pettman T, Haas M, Viney R, & Misan G (2010). Individual preferences for diet and exercise programmes: Changes over a lifestyle intervention and their link with outcomes. Public Health Nutrition, 13(2), 245–252. doi: 10.1017/s1368980009990784 [DOI] [PubMed] [Google Scholar]
- Phillips KA, Johnson FR, & Maddala T (2002). Measuring what people value: A comparison of “attitude” and “preference” surveys. Health Services Research, 37(6), 1659–1679. doi: 10.1111/1475-6773.01116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell BJ, Beidas RS, Lewis CC, Aarons GA, McMillen JC, Proctor EK, & Mandell DS (2017). Methods to improve the selection and tailoring of implementation strategies. Journal of Behavioral Health Services and Research, 44(2), 177–194. doi: 10.1007/s11414-015-9475-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Promberger M, Dolan P, & Marteau TM (2012). “Pay them if it works”: Discrete choice experiments on the acceptability of financial incentives to change health related behaviour. Social Science and Medicine, 75(12), 2509–2514. doi: 10.1016/j.socscimed.2012.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez M, Shinyi W, Beale E, & Wu S (2016). Designing a text messaging intervention to improve physical activity behavior among low-income Latino patients with diabetes: A discrete-choice experiment, Los Angeles, 2014-2015. Preventing Chronic Disease, 13, 1–9. doi: 10.5888/pcd13.160035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez M, & Wu S (2017). Phone messaging to prompt physical activity and social support among low-income Latino patients with type 2 diabetes: A randomized pilot study. JMIR Diabetes, 2, e8. doi: 10.2196/diabetes.7063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed SD, & Lavezzari G (2016). International experiences in quantitative benefit-risk analysis to support regulatory decisions. Value in Health, 19, 727–729. 10.1016/j.jval.2016.06.004 [DOI] [PubMed] [Google Scholar]
- Ryan M, & Gerard K (2003). Using discrete choice experiments to value health care programmes: Current practice and future research reflections. Applied Health Economics and Health Policy, 2(1), 55–64. [PubMed] [Google Scholar]
- Ryan M, Gerard K, & Amaya-Amaya M (2008). Using discrete choice experiments to value health and health care. The Netherlands: Springer. [Google Scholar]
- Strauss M, George G, Lansdell E, Mantell JE, Govender K, Romo M, … Kelvin EA (2018a). HIV testing preferences among long distance truck drivers in Kenya: A discrete choice experiment. AIDS Care, 30(1), 72–80. doi: 10.1080/09540121.2017.1367086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss M, George G, Mantell JE, Romo ML, Mwai E, Nyaga EN, … Kelvin EA (2018b). Stated and revealed preferences for HIV testing: Can oral self-testing help to increase uptake amongst truck drivers in Kenya? BMC Public Health, 18(1), 1231. doi: 10.1186/s12889-018-6122-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift JK, Callahan JL, & Vollmer BM (2011). Preferences. Journal of Clinical Psychology, 67(2), 155–165. doi: 10.1002/jclp.20759 [DOI] [PubMed] [Google Scholar]
- Swift JK, Callahan JL, Cooper M, & Parkin SR (2018). The impact of accommodating client preference in psychotherapy: A meta-analysis. Journal of Clinical Psychology, 74, 1924–1937. doi: 10.1002/jclp.22680 [DOI] [PubMed] [Google Scholar]
- Terris-Prestholt F, Neke N, Grund JM, Plotkin M, Kuringe E, Osaki H, … Wambura M (2019). Using discrete choice experiments to inform the design of complex interventions. Trials, 20(1), 157. doi: 10.1186/s13063-019-3186-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurstone LL (1927). A law of comparative judgment. Psychological Review, 34, 273–286. [Google Scholar]
- US Food and Drug Administration. Patient preference information - voluntary submission, review in premarket approval applications, humanitarian device exemption applications and de novo requests, and inclusion in decision summaries and device labeling. Retrieved from https://www.fda.gov/media/92593/download
- van Gils PF, Lambooij MS, Flanderijn MH, van den Berg M, de Wit GA, Schuit AJ, … van den Berg B (2011). Willingness to participate in a lifestyle intervention program of patients with type 2 diabetes mellitus: A conjoint analysis. Patient Preference and Adherence, 5, 537–546. doi: 10.2147/PPA.S16854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vass CM, & Payne K (2017). Using discrete choice experiments to inform the benefit-risk assessment of medicines: Are we ready yet? Pharmacoeocnomics, 35, 859–866. doi: 10.1007/s40273-017-0518-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldwijk J, Lambooij MS, van Gils PF, Struijs JN, Smit HA, & de Wit GA (2013). Type 2 diabetes patients’ preferences and willingness to pay for lifestyle programs: A discrete choice experiment. BMC Public Health, 13, 1099. doi: 10.1186/1471-2458-13-1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wambura M, Mahler H, Grund JM, Larke N, Mshana G, Kuringe E, … Weiss HA (2017). Increasing voluntary medical male circumcision uptake among adult men in Tanzania. AIDS, 31, 1025–1034. doi: 10.1097/QAD.0000000000001440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright DR, Saelens BE, Fontes A, & Lavelle TA (2019). Assessment of parents’ preferences for incentives to promote engagement in family-based childhood obesity treatment. JAMA Network Open, 2, e191490. doi: 10.1001/jamanetworkopen.2019.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi D, Ryan M, Campbell S, Elliott A, Torrance N, Chambers A, … Smith BH (2011). Using discrete choice experiments to inform randomised controlled trials: An application to chronic low back pain management in primary care. European Journal of Pain, 15(5), 531.e531–510. doi: 10.1016/j.ejpain.2010.10.008 [DOI] [PubMed] [Google Scholar]
- Zanolini A, Chipungu J, Vinikoor MJ, Bosomprah S, Mafwenko M, Holmes CB, & Thirumurthy H (2018). HIV self-testing in Lusaka province, Zambia: Acceptability, comprehension of testing instructions, and individual preferences for self-test kit distribution in a population-based sample of adolescents and adults. AIDS Research and Human Retroviruses, 34(3), 254–260. doi: 10.1089/aid.2017.0156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Thayer WM, & Bridges JFP (2018). Using latent class analysis to model preference heterogeneity in health: a systematic review. PharmacoEconomics, 36(2), 175–187. doi: 10.1007/s40273-017-0575-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


