Abstract
Background
Host dysregulation of immune response was highly involved in the pathological process of Coronavirus disease 2019 (COVID-19), especially COVID-19 severe cases with DM.
Aim
In this study we aimed at the dynamic change of peripheral lymphocyte and subsets during COVID-19 covery.
Methods
The peripheral lymphocyte and subsets of 95 confirmed cases with COVID-19 from baseline to four weeks were compared between critical illness and non-critical illness cases with or without DM.
Results
The dynamic characteristics of lymphocyte and subsets in COVID-19 patients was that it reduced significantly at one week, rapidly elevated to the peak at two weeks after onset, then gradually declined during recovery. The COVID-19 critical illness patients with DM had the lowest decline at one week and the slow lowest rise at two weeks after onset, while COVID-19 non-critical illness patients with DM had the rapid highest rise at two weeks after onset, both of them had similar lymphocyte and subsets at five weeks after onset and lower than those patients without DM.
Conclusions
These findings provide a reference for clinicians that for COVID-19 patients with DM and the lowest decline of lymphocyte and subsets, immunomodulatory therapy as soon as possible might avoid or slow down disease progression; moreover for COVID-19 critical illness patients with or without DM and non-critical illness patients with DM, continuous immunomodulatory therapy in later stages of disease might speed up virus clearance, shorten hospital stay, improve disease prognosis in COVID-19 critical illness patients with DM.
Keywords: Coronavirus disease 2019 (COVID-19), Diabetes mellitus, Lymphocyte subsets, Dynamic change
1. Introduction
The outbreak of novel coronavirus (2019-nCOV) infection named as coronavirus disease 2019 (COVID-19) is wide spread in the world [1], [2], [3], [4].As of April 18, 2020, cases were reported in China and the whole world, a total of cumulative confirmed and death cases were 2,160,207 and 146,088 cases in the whole world [5], 82,735 and 4632 cases in China [6], respectively.
Those COVID-19 patients who is the elderly and those with chronic underlying disease have a poor prognosis. Diabetes mellitus is one of the common underlying diseases [7]. Host dysregulation of immune response was highly involved in the pathological process of COVID-19 [8].Our previous research found that the COVID-19severe cases with diabetes mellitus (DM) had overall decreased lymphocytes and subsets which can affect the diseasese verity, disease progression, viral negative conversion and prognosis [9]. The dynamic changes of lymphocyte and subsets between critical illness case and non-critical illness case of COVID-19 with or without DM are unknown and worth studying in this article.
2. Methods
2.1. Objects
95 patients with COVID-19 was retrospectively recruited from January 16, 2020 to March 16, 2020in hospital isolation ward of the Public and Health Clinic Centre of Chengdu,“ the specific hospital for the treatment of severe patients with COVID-19 in Chengdu” designated by the government. The study was approved by the Public and Health Clinic Centre of Chengdu Ethics Committee (PJ-K2020-06-01). For emerging infectious diseases the Ethics Commission of the designated hospital agreed to waive written informed consent [9].
The diagnosis criteria, the clinical typing criteria of COVID-19 was judged according to the seventh Trial Version of the Novel Coronavirus Pneumonia Diagnosis and Treatment Guidance [7].
DM diagnostic criteria was judged according to Guidelines for the Prevention and Treatment of Type 2 Diabetes in China (2017 edition) [10].
The participants were divided in two subgroups according to clinical typing: the non-critical illness subgroup (including light and common type) and critical illness subgroup (including severe and critically illness type).
2.2. Clinical data collection
Data including underline disease history, demographic information (age and sex), lymphocyte subsets at baseline, one week and 4 weeks, clinical data and glucose metabolic parameters [FPG levels and hemoglobin A1c (HbA1c) levels] were obtained from the hospital electronic medical record system of the Public and Health Clinic Centre of Chengdu [9].
According to the needs of the research databases were established by two researchers simultaneously collecting and entering, 30% of that data was randomly selected by the researchers to assess data integrity, authenticity, and accuracy.
2.3. Statistical analyses
The Statistical Package for the Social Sciences software version 17.0 (IBM Inc., Armonk, NY, the USA) and GraphPad Prism 8 (GraphPad, CA, the USA) software were used for statistical analysis. The measurement data were expressed as x ± SD, and a multigroup comparison was performed using ANOVA. Further comparison between the two groups was conducted using Student-Newman-Keuls (SNK) analysis. The two groups were compared using an independent-sample t-test. Chi-square test was used for the enumeration data. A p value of <0.05 was considered statistically significant.
3. Results
3.1. Similar baseline conditions except glucose metabolic parameters between four subgroups
Patients in non-critical illness non-DM subgroup were significantly younger than those in the other three subgroups, but similar age was found in latter three subgroups and similar male percentage was found in four subgroups (Table 1 ). FPG levels and HbA1c level in DM group were obviously higher than that in non-DM group (Table 1). But there was no significant difference between each intra-group (Table 1) [9].
Table 1.
Comparison of baseline conditions and glucose metabolic parameters between four subgroups (n = 95).
| Variable | Non-DM group (n = 76) |
DM group(n = 19) |
x2or F score | P score | ||
|---|---|---|---|---|---|---|
| Non-critical illness subgroup(n = 57) | Critical illness subgroup(n = 19) | Non-critical illness subgroup(n = 8) | Critical illness subgroup(n = 11) | |||
| Age (year) | 42.67 ± 14.71 | 58.00 ± 19.24*** | 61.57 ± 12.01*** | 59.36 ± 12.31*** | 8.914 | 0.000 |
| Male (case, %) | 25(43.86) | 11(57.89) | 3(37.50) | 7(63.64) | 2.532 | 0.469 |
| Duration (day) | 7.54 ± 6.01 | 6.16 ± 3.98 | 8.45 ± 5.53 | 7.14 ± 5.11 | 0.423 | 0.738 |
| FPG (mmol/L) | 5.35 ± 0.65 | 5.81 ± 0.91** | 7.80 ± 4.91**** | 7.35 ± 1.19**** | 10.02 | 0.000 |
| HbA1c (%) | 5.46 ± 0.73 | 5.58 ± 0.48 | 7.49 ± 2.65** | 6.89 ± 1.18**** | 6.380 | 0.001 |
| Virus negative conversion time | 18.02 ± 8.66 | 19.26 ± 6.84 | 24.86 ± 11.50* | 26.36 ± 8.44***## | 4.490 | 0.006 |
| Prognosis | −3.394 | 0.001 | ||||
| Cured (case, %) | 53 (71.05) | 5(26.32) | ||||
| Unhealed | 21(26.32) | 13(68.42) | ||||
| Death | 2(2.63) | 1(5.26) | ||||
Abbreviations: FPG, fasting plasma glucose; HbA1c, glycated hemoglobin A1c; DM, diabetes mellitus. Compared with the non-critical illness non-DM subgroup, **P < 0.01, ***P < 0.001, ****P < 0.0001. Comparison of age between the latter three subgroups, P > 0.05.
3.2. The lowest lymphocyte and subsets at baseline in COVID-19 critical illness patients with DM
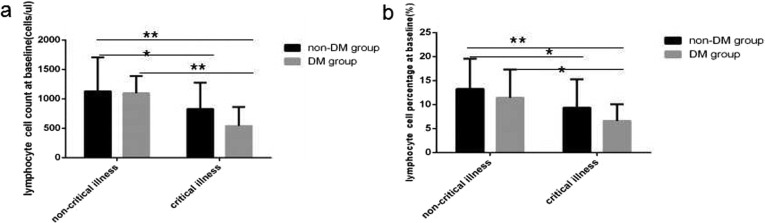
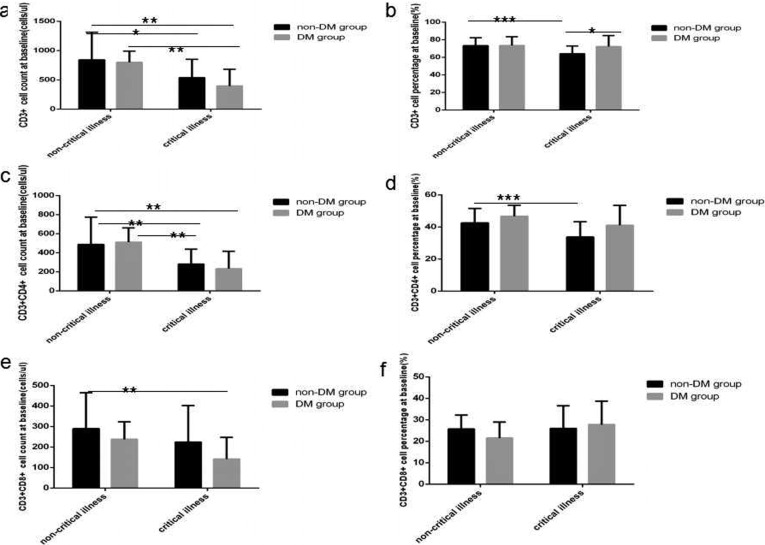
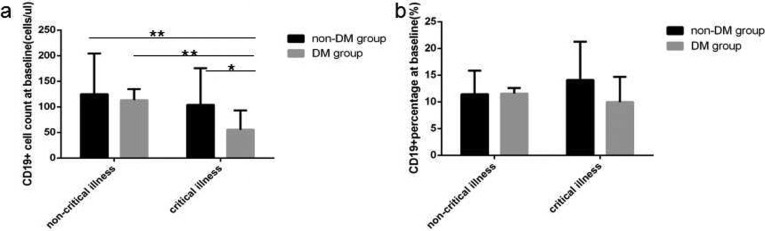
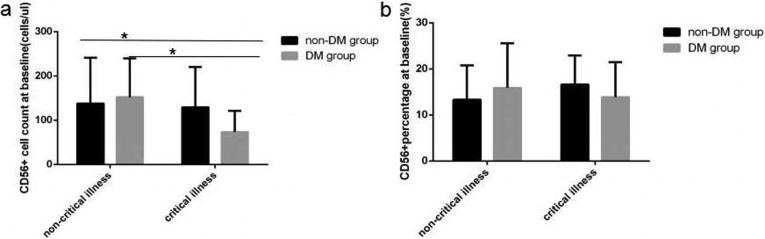
At baseline in COVID-19critical illness cases with DMlymphocyte count level and percentage value (Fig. 1 a and b), CD3+ count level (Fig. 2 a), CD3 + CD4+ count level (Fig. 2c), CD3 + CD8+ count level (Fig. 2e), B(CD19+) count level (Fig. 3 a) and NK (CD56+) count level (Fig. 4 a) were the lowest decline compared with that in critical illness cases without DM and non-critical illness cases with or without DM, all of significant differences were found.
Fig. 1.
Comparisonof lymphocyte count levels and percentage values between four subgroup. Abbreviations: DM, diabetesmellitus. a. lymphocytecount. b. lymphocyte percentage. Unpaired one ANOVA were used for intergroup comparison (a,b, p all < 0.01). Unpaired t-tests were used for the intra-group comparison.*P < 0.05,**P < 0.01.
Fig. 2.
Comparison of T lymphocytes and subsets count levels and percentage values between four subgroup. Abbreviations: DM, diabetes mellitus; non-DM, without diabetes mellitus. a. CD3 + cell count. b. CD3 + cell percentage. c. CD3 + CD4 + cell count. d. CD3 + CD4 + cell percentage. e. CD3 + CD8 + cell count. f. CD3 + CD8 + cell percentage. Unpaired one ANOVA were used for intergroup comparison (a,b,c,d,e,f, P < 0.01, 0.05, 0.001, 0.01, >0.05, 0.05, respectively). Unpaired t-tests were used for the intra-group comparison. *P < 0.05, **P < 0.01, ***P < 0.001.
Fig. 3.
Comparison of B lymphocytes count levels and percentage values between four subgroup. Abbreviations: DM, diabetes mellitus; non-DM, without diabetes mellitus. a. B lymphocytes count. b. B lymphocytes percentage. Unpaired one ANOVA were used for intergroup comparison (a,b, P < 0.05, >0.05, respectively). Unpaired t-tests were used for the intra-group comparison. *P < 0.05, **P < 0.01.
Fig. 4.
Comparison of NK lymphocytes count levels and percentage values between four subgroup. Abbreviations: DM, diabetes mellitus; non-DM, without diabetes mellitus. a. NK lymphocyte count. b. NK lymphocytes percentage. Unpaired one ANOVA were used for intergroup comparison ((a,b, P all > 0.05). Unpaired t-tests were used for the intra-group comparison. *P < 0.05.
At baseline in COVID-19 critical illness patients lymphocytes count level and percentage value (Fig. 1a and b), CD3+ count level (Fig. 2a), CD3+ CD4+ count level (Fig. 2c), B(CD19+) count level (Fig. 3a) were obviously lower than that in COVID-19 non-critical illness patients, the difference was significant. But no significantly difference of NK (CD56+) count level (Fig. 3a) and all lymphocyte subsets percentage value were found between critical illness and non-critical illness patients whether with or without DM.
At baseline in COVID-19 critical illness patients with DM except B (CD19+) count level (Fig. 3a) was obviously lower than that in COVID-19 critical illness patient without DM, the difference was significant, no statistical difference of the other lymphocytes subsets was found between the same disease severity with and without DM subgroups.
3.3. The slow lowest rise of lymphocyte and subsets within four weeks in COVID-19 critical illness patients with DM
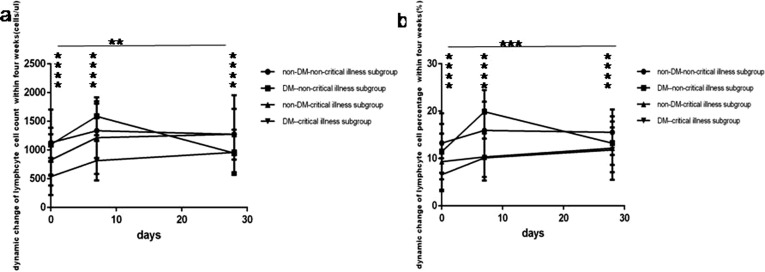
During recovery in COVID-19 patients whether critical illness or non-critical illness, whether with or without DM, lymphocyte count level and percentage value (Fig. 5 a and b), CD3 + CD4+ count level (Fig. 6 c) all rapidly raised to the peak at one week, then gradually declined, but still above baseline value at four weeks; simultaneously B (CD19 + ) count level (Fig. 7 a) also rapidly raised to the peak at one week, then gradually declined under baseline value at four weeks; while CD3 + CD8+ count level (Fig. 6e) gradually raised to the peak at fours week, there were all significant differences (p all < 0.05). In spite of NK (CD56+) count level (Fig. 8 a) also rapidly elevated up to the peak at one week then continuously maintained this level within four weeks, but no statistical difference was found between different time points.
Fig. 5.
Comparison of dynamic change of lymphocytes count levels and percentage values between four subgroup within four weeks. Abbreviations: DM, diabetes mellitus; non-DM, without diabetes mellitus. a. lymphocytes count. b. lymphocytes percentage. Unpaired one ANOVA were used for intergroup comparison. Unpaired t-tests were used for the intra-group comparison. **P < 0.01, ***P < 0.001, ****P < 0.0001.
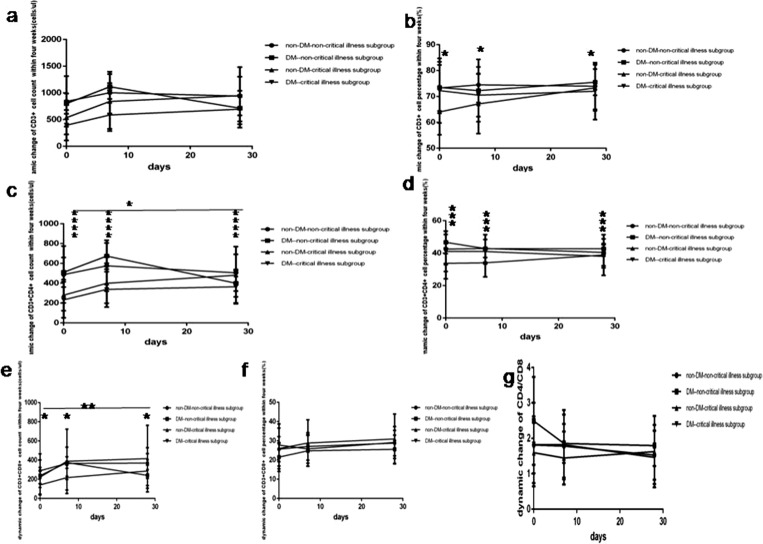
Fig. 6.
Comparison of the dynamic change of T lymphocytes count levels and percentage values between four subgroup within four weeks. Abbreviations: DM, diabetes mellitus; non-DM, without diabetes mellitus. a. CD3 + cell count. b. CD3 + cell percentage. c. CD3 + CD4 + cell count. d. CD3 + CD4 + cell percentage. e. CD3 + CD8 + cell count. f. CD3 + CD8 + cell percentage. g. CD4/CD8. Unpaired two ANOVA were used for intergroup comparison. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
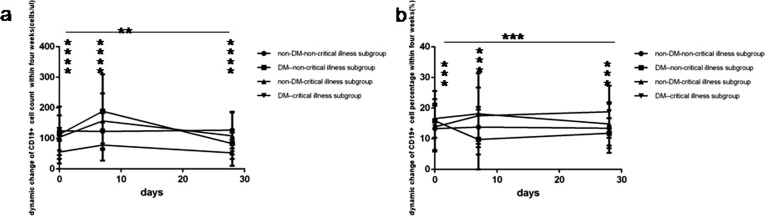
Fig. 7.
Comparison of the dynamic change of B lymphocytes count levels and percentage values between four subgroup within four weeks. Abbreviations: DM, diabetes mellitus; non-DM, without diabetes mellitus. a. B lymphocytes count. b. B lymphocyte percentage. Unpaired two ANOVA were used for intergroup comparison. **P < 0.01, ***P < 0.001, ****P < 0.0001.
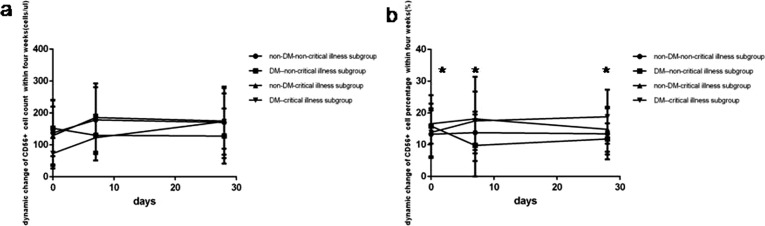
Fig. 8.
Comparison of the dynamic change of NK lymphocytes count levels and percentage values between four subgroup within four weeks. Abbreviations: DM, diabetes mellitus; non-DM, without diabetes mellitus. a. NK lymphocyte count. b. NK lymphocyte percentage. Unpaired two ANOVA were used for intergroup comparison. **P < 0.01, ***P < 0.001, ****P < 0.0001.
At one week COVID-19non-critical illness cases with DM had the rapid highest rise of lymphocyte count level and percentage value (Fig. 5a and b), CD3+ count level (Fig. 6a), CD3 + CD4+ count level (Fig. 6c), CD3 + CD8+ count level (Fig. 6e), B (CD19 + )count level (Fig. 7a), NK (CD56+) count level (Fig. 8a). On the contrary COVID-19 critical illness cases with DM had the slow lowest rise of corresponding lymphocyte and subsets. Simultaneously COVID-19 non-critical illness cases without DM had higher lymphocyte and subsets than COVID-19critical illness cases without DM.
At 4 weeks COVID-19 patients without DM had higher lymphocyte and subsets than those with DM, but the lymphocyte and subsets was similar between critical illness cases and non-critical illness cases whether with or without DM.
3.4. Dynamic lymphocyte and subsets influencing on the virus negative conversion time and the prognosis in COVID-19 patients
Pearson correlation analysisshowed that B (CD19+) count leveland percentage value at five weeks were negatively related to viral negative conversion time (Table 2 ). The important factors influencing the viral negative conversion time by multiple stepwise regression analysis was only B (CD19+) count level or percentage value at five weeks (Table 3 ).
Table 2.
Pearson correlation analysis between the virus negative conversion time and lymphocyte subsets at two weeks and five weeks after onset (n = 95).
| Time point | Variable | Virus negative conversion time (days) |
Prognosis (1 = cured, 2 = unhealed, 3 = death |
||
|---|---|---|---|---|---|
| r | p | r | p | ||
| At two weeks | CD3+(cells/ul) | −0.324 | 0.016 | ||
| CD3 + CD8+(cells/ul) | −0.288 | 0.033 | |||
| LY(cells/ul) | −0.326 | 0.015 | |||
| At five weeks | CD3+(cells/ul) | −0.400 | 0.005 | ||
| CD3 + CD4+(cells/ul) | −0.300 | 0.038 | |||
| CD3 + CD8+(cells/ul) | −0.398 | 0.005 | |||
| LY(cells/ul) | −0.448 | 0.001 | |||
| B(CD19 + ) | −0.322 | 0.015 | −0.404 | 0.005 | |
| B(CD19 + )% | −0.337 | 0.010 | −0.296 | 0.046 | |
Table 3.
Multiple stepwise regression analysis of influencing factors of the coronavirus negative conversion time (n = 95).
| Independent variable | B | Std. Error | Beta | t | p | |
|---|---|---|---|---|---|---|
| The coronavirus negative conversion time | constant | 24.703 | 2.146 | – | 11.514 | 0.000 |
| B (CD19 + ) (%) | −0.044 | 0.180 | −0.322 | −2.533 | 0.015 | |
| Prognosis | constant | 2.134 | 0.207 | 10.333 | 0.000 | |
| CD3 + CD8+(cells/ul) | −0.021 | 0.000 | −0.373 | −2.780 | 0.008 | |
| B(CD19 + )% | −0.037 | 0.017 | −0.286 | −2.128 | 0.039 |
Related to theprognosis negative factorswerelymphocytecount level, CD3+ count level, CD3 + CD4+ count level, CD3 + CD8+ count levelat two weeks after onset, and lymphocytecount level, CD3+ count level, CD3 + CD4+ count level, CD3 + CD8+ count level, B (CD19+)count level and percentage value at five weeks (Table 2). Influencing factors of the prognosis by multiple stepwise regression analysis was CD3 + CD8+ count level and B (CD19+) percentage value at five weeks (Table 3).
4. Discussion
Our previous research found that the COVID-19 severe cases with DM had the lowest lymphocytes, especially T lymphocytes and B lymphocytes. Overall decreased lymphocytes subsets and DM maybe aggravated the prognosis by aggravating the disease severity and prolonging the viral negative conversion time [9]. In this study we analyzed the dynamic characteristics of lymphocyte and subsets in coronavirus disease 2019 (COVID-19) patients, found that lymphocyte and subsets reduced significantly at baseline, rapidly rised to the peak at one week of hospitalization, then gradually declined during recovery, at four weeks of hospitalization B lymphocyte subset even below baseline, while NK lymphocyte subset continuously maintained the peak level. The average disease course from onset to admission of COVID-19 patients in this study was one week, that is to say the lymphocyte and subsets were decreased to the lowest decline at one week, and rised to the highest topat two weeks after the onset of disease, then gradually decreased again, this dynamic characteristics is inconsistent with that in severe acute respiratory syndrome (SARS) patients. Literature report that in SARS patients the CD3+, CD4 + and CD8 + T cells, especially CD4 + T cells decreased at the first two-week of the disease course and reached to the lowest level at the second week, and began to increase from the third week in all patients, the change pattern of CD3+, CD4+ and CD8 + T cells in the severe type of SARS was the same as that of the mild type of SARS, but with more serious extent and longer time [11]. 2019-nCOV infection affects host immune function earlier and faster than SARS coronavirus infection.
In this study we also found that the COVID-19 critical illness patients with DM had the lowest decline at baseline and the slow lowest rise of lymphocyte and subsets at one week after admission, while COVID-19 non-critical illness patients with DM had the highest rise of lymphocyte and subsets at one week after admission, both of them had similar lymphocyte and subsets at 4 weeks of hospitalization, all lower than those patients without DM whose also had similar lymphocyte and subsets between non-critical illness and critical illness cases. Important antiviral effects of T cells, CD4 + T cells, and CD8 + T cells are achieved by balancing the risk of fighting pathogens with the risk of developing autoimmunity or excessive inflammation [12]. CD4 + T cells activate T-dependent B cells to produce virus-specific antibodies. However, CD8 + T cells can kill virus-infected cells by cytotoxicity. CD8 + T cells account for about 80% of pulmonary stromal inflammatory cells in SARS-COV infected patients and play an important role in clearing COVs from infected cells and inducing immune damage [13], [14]. T cells rather than B cells play an important role in the control of pathogenesis of MERS-COV infection. A cross-reactive T cell response leads to a decrease in MERS-CoV [15]. CD4 + T cells are more susceptible to SARS-COV infection, resulting in itself reduction or even depletion, which reduces the recruitment of lymphocytes in the lungs and the neutralization of antibody and cytokine production, resulting in strong immune-mediated interstitial pneumonia and delay the clearance of SARS-COV in the lungs. But the depletion of CD8 + T cells does not affect or delay viral replication [16], [17], [18]. From this we speculate that for COVID-19 patients with DM in early stage of disease, the rapid highest rise of lymphocyte and subsets in some patients avoid disease progression to critical illness cases or slow down disease progression, while the slow lowest rise of lymphocyte and subsets in the others promote disease progression to critical illness cases.
Additionally, T helper cells produce proinflammatory cytokines via the NF-kB signalling pathway [19]. IL-17 cytokines recruit monocytes and neutrophils to the site of infection with inflammation and activate other downstream cytokine and chemokine cascades, such as IL‐1, LL‐6, IL‐8, IL‐21, TNF‐β, and MCP-1 [20], [21]. On the other hand, MERS-COV induces T cell apoptosis by activating the intrinsic and extrinsic apoptosis pathways. A novel BH3-like region located in the C-terminal cytosolic domain of SARS-COV protein mediates its binding to Bcl-xL and induced T-cell apoptosis [22].
In this study we also found that only B cell subset at five weeks after onset can influence the virus negative conversion time, CD3 + CD8 + T cell and B cell subsets at five weeks after onset can influence the prognosis. This is inconsistent with that in literature that during the later stage of infection, depletion of T cells having antiviral effects may prolong the infection and promote viral survival [23]. This cannot explain our previous research findings why the virus negative conversion time and the in hospital time of COVID-19 patients with DM were longer than that of those without DM.
5. Conclusions
Lymphocyte and subsets in COVID-19 patients reduced the lowest decline at one week, rapidly elevated up to the peak at two weeks after onset, then gradually declined during recovery. The COVID-19 critical illness patients with DM had the lowest decline at one week and the slow lowest rise at two weeks after onset, while COVID-19 non-critical illness patients with DM had the rapid highest rise at two weeks after onset, both of them had similar lymphocyte and subsets at five weeks after onset, all lower than those patients without DM whose also had similar lymphocyte and subsets between non-critical illness and critical illness cases. These findings provide a reference for clinicians that for patients with COVID-19 and DM coexistence and the lowest decline of lymphocyte and subsets at one week after onset early application of immunomodulatory therapy as soon as possible might avoid or slow down disease progression, moreover for COVID-19 patients with DM whether critical illness or not, and critical illness cases without DM, then continuous application of immunomodulatory therapy in later stages of disease might speed up virus clearance, shorten hospital stay, improve disease prognosis in COVID-19 critical illness patients with DM.
Acknowledgments
Acknowledgments
Thanks to Dr. Hong Chen, Ling Zhang, Min yang, Xiu Li (the Public and Health Clinic Centre of Chengdu, one ward, two ward of hospital isolation ward, respectively).
Declaration of Competing Interest
The authors declare that they have no competing interests.
Funding
This research was supported by the Thirteenth Five-Year Project on Tackling Key Problems of National Science and Technology (2017ZX10305501008), Sichuan Science and Technology Program (2020YFS0564), Chengdu Municipal Science and Technology Bureau Science and Technology Huimin Major Demonstration Project (00092), the Sichuan Province Health Commission (17PJ070), Chengdu Municipal Health Commission (2019079).
Authors’ contributions
Concept and design: Dafeng Liu, Lijuan Lan, Dongxia Luo, Bennan Zhao,Guo Wei, Yinsheng He; Data acquisition: Dafeng Liu, Lijuan Lan, Dongxia Luo, Bennan Zhao,Guo Wei, Yinsheng He; data analysis and interpretation: Dafeng Liu, Lijuan Lan, Dongxia Luo, Bennan Zhao; Drafting the manuscript: Dafeng Liu, Lijuan Lan, Dongxia Luo, Bennan Zhao; administrative, technical, or material support: Dafeng Liu, Lijuan Lan, Dongxia Luo, Bennan Zhao; study supervision: Renqing Zhang, Yalin Liu.
Data availability statement
All data, models, or code generated or used during the study are available from the corresponding author by request: Dafeng Liu, E-mail:liudf312@126.com
References
- 1.Wu G., Gao G.F., Tan W. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu H., Stratton C.W., Tang Y.W. Outbreak of Pneumonia of Unknown Etiology in Wuhan China: the Mystery and the Miracle. J Med Virol. 2020;92(4):401–402. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ji W., Wang W., Zhao X. Cross-species transmission of the newly identified coronavirus 2019-nCoV. J Med Virol. 2020;92(4):433–440. doi: 10.1002/jmv.25682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gates B. Responding to COVID-19 - A Once-in-a-Century Pandemic? N Engl J Med. 2020 Feb 28. doi: 10.1056/NEJMp2003762. Online ahead of print. [DOI] [PubMed]
- 5.World Health Organization.Coronavirus disease 2019 (COVID-19) situation report—89, 18April 2020 [cited 2020 Apr18]. Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200418-sitrep-89-covid-19.pdf[External Link].
- 6.National Health Commission of the People's Republic of China. Update on the epidemic situation of new coronavirus pneumonia as of 24:00 on April18, 2020. Available at: http://www.nhc.gov.cn/xcs/yqfkdt/202004/2d391a171acc4624a50a1188c8de7361.shtml[External Link].
- 7.National Health Commission of the People's Republic of China. The seventh Trial Version of the Novel Coronavirus Pneumonia Diagnosis and Treatment Guidance. Available at: http://medjournals.cn/2019NCP/index.do;jsessionid=F12B0B0FEBD6E6193A01B01FEA4E8109][External Link].
- 8.Qin C, Zhou L, Hu Z,et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020 Mar 12:ciaa248. http://doi.org/10.1093/cid/ciaa248. Online ahead of print.
- 9.Liu DF, Wang Y, Lan LJ, Liu YL, Zhao BN, et al. Overall reduced lymphocyte subsets worsening disease severity and prognosis in COVID-19 severe cases with diabetes mellitus in Chengdu, China. BMC infectious diseases, 2020. Research Square preprint http://doi.org/10.21203/rs.3.rs-20385/v1.Online ahead of print.
- 10.Chinese Diabetes Society. Guidelines for the prevention and control of type 2 diabetes in China (2017 Edition). Chinese J Pract Internal Med. 2018;38(4):292–344.
- 11.WAMG Xiao-mei, SHI Ling-fang, LIU Yi, Wang Li-ping, Zhang Jian-ping, et al. Analysis of Relationship Between Dynamic Change of T-lymphocyte Subpopulations and Clinical Progression in SARS Patients. J Chin Phys. (Chin) 2003;5(10):1308–10.
- 12.Cecere T.E., Todd S.M., Leroith T. Regulatory T cells in arterivirus and coronavirus infections: do they protect against disease or enhance it? Viruses. 2012;4(5):833–846. doi: 10.3390/v4050833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maloir Q., Ghysen K., von Frenckell C., Louis R., Guiot J. Acute respiratory distress revealing antisynthetase syndrome. Rev Med Liege. 2018;73(7–8):370–375. [PubMed] [Google Scholar]
- 14.Zhao J., Li K., Wohlford-Lenane C. Rapid generation of a mouse model for Middle East respiratory syndrome. Proc Natl Acad Sci USA. 2014;111(13):4970–4975. doi: 10.1073/pnas.1323279111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pascal K.E., Coleman C.M., Mujica A.O. Pre-and postexposure efficacy of fully human antibodies against Spike protein in a novel humanized mouse model of MERS-CoV infection. Proc Natl Acad Sci USA. 2015;112(28):8738–8743. doi: 10.1073/pnas.1510830112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Channappanavar R., Fett C., Zhao J., Meyerholz D.K., Perlman S. Virus-specific memory CD8 T cells provide substantial protection from lethal severe acute respiratory syndrome coronavirus infection. J Virol. 2014;88(19):11034–11044. doi: 10.1128/JVI.01505-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen J., Lau Y.F., Lamirande E.W. Cellular immune responses to severe acute respiratory syndrome coronavirus (SARS-CoV) infection in senescent BALB/c mice: CD4+ T cells are important in control of SARS-CoV infection. J Virol. 2010;84(3):1289–1301. doi: 10.1128/JVI.01281-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ng O.W., Chia A., Tan A.T. Memory T cell responses targeting the SARS coronavirus persist up to 11 years post-infection. Vaccine. 2016;34(17):2008–2014. doi: 10.1016/j.vaccine.2016.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manni M.L., Robinson K.M., Alcorn J.F. A tale of two cytokines: IL-17 and IL-22 in asthma and infection. Expert Rev Respiratory Med. 2014;8(1):25–42. doi: 10.1586/17476348.2014.854167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bunte K., Beikler T. Th17 Cells and the IL-23/IL-17 axis in the pathogenesis of periodontitis and immune-mediated inflammatory diseases. Int J Mol Sci. 2019;20(14):3394. doi: 10.3390/ijms20143394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dutzan N., Abusleme L. T helper 17 cells as pathogenic drivers of periodontitis. Adv Exp Med Biol. 2019;1197:107–117. doi: 10.1007/978-3-030-28524-1_9. [DOI] [PubMed] [Google Scholar]
- 22.Yang Y., Xiong Z., Zhang S. Bcl-xL inhibits T-cell apoptosis induced by expression of SARS coronavirus E protein in the absence of growth factors. Biochem J. 2005;392(Pt 1):135–143. doi: 10.1042/BJ20050698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mubarak A., Alturaiki W., Hemida M.G. Middle East respiratory syndrome coronavirus (MERS-CoV): infection, immunological response, and vaccine development. J Immunol Res. 2019;2019 doi: 10.1155/2019/6491738. 6491738-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data, models, or code generated or used during the study are available from the corresponding author by request: Dafeng Liu, E-mail:liudf312@126.com