Abstract
Objective
To investigate the characteristics of lymphocytes in type 2 diabetic patients with coronavirus disease (COVID-19).
Methods
Patients with COVID-19 admitted to hospital in Wuxi, China from January 29 to March 15 were included in the study. Lymphocytes were measured and recorded at admission and during treatment. Hospitalization days, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) nucleic acid positive days, minimal lymphocyte count, and occurrence time were collected and comparatively analyzed. Correlations between minimal lymphocyte count and hospitalization days as well as SARS-CoV-2 nucleic acid positive days were analyzed.
Results
A total of 63 patients were included in the study, with 16 in the diabetic group and 47 in the non-diabetic group. After adjusting for potential confounding factors, we observed lower minimal lymphocyte count (0.67 ± 0.36 * 109/L vs. 1.30 ± 0.54 * 109/L, adjusted P = 0.001), earlier occurrence of the minimal lymphocyte count (2.68 ± 2.33 days vs. 5.29 ± 4.95 days, adjusted P = 0.042), and longer hospitalization time (20.44 ± 5.24 days vs. 17.11 ± 4.78 days, adjusted P = 0.047) in the diabetic group than in the non-diabetic group. There was a negative correlation between minimal lymphocyte count and hospitalization days (R = −0.600, P < 0.05) as well as SARS-CoV-2 nucleic acid positive days (R = −0.420, P < 0.05).
Conclusions
The diabetic group with COVID-19 had lower lymphocyte count, reached the minimal count faster, and had longer hospital stays than the non-diabetic group. Hospitalization days and SARS-CoV-2 nucleic acid positive days were negatively correlated with the minimal lymphocyte count.
Keywords: Type 2 diabetes mellitus, COVID-19, Lymphocyte count, SARS-CoV-2
1. Introduction
The coronavirus disease (COVID-19) that erupted in China since December last year has gradually spread throughout the world. At present, it has caused tens of thousands of deaths, and there are no special antiviral drugs to treat it. Depending on the region, 20–50% of patients affected by the COVID-19 pandemic had diabetes [1]. According to related reports, patients with diabetes are more prone to disease progression than those without diabetes [2], [3]; however, the mechanism is not yet clear. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes a reduction in the lymphocyte count of COVID-19 patients [4], [5]. This study aims to analyze the characteristics of lymphocyte count in COVID-19 patients with type 2 diabetes mellitus (T2DM).
2. Materials and methods
2.1. Patients
Adult COVID-19 patients admitted to the Fifth People's Hospital of Wuxi City in China from January 29 to March 15 were included.
2.1.1. Inclusion criteria
Age > 14 years and diagnosis of COVID-19 (for the criteria, refer to the diagnosis and treatment plan for the COVID-19 in China – Trial Version 7; see the supplementary file for details).
2.1.2. Exclusion criteria
Patients with blood diseases, AIDS, and T1DM; patients without incomplete data.
2.2. Data collection
Data pertaining to gender, age, T2DM status, body mass index (BMI), systolic blood pressure (SBP), and diastolic blood pressure (DBP) were obtained at the time of admission. Data on alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine (Cr), and fasting glucose were obtained the next morning after admission. White blood cell count (WBC), C-reactive protein (CRP) and Lymphocytes were measured at admission and during treatment. Data on maximal and minimal WBC, maximal CRP, minimal lymphocyte count and emergence time, hospitalization days, and SARS-CoV-2 nucleic acid positive days were recorded during hospitalization. According to our medical practice, each patient’s WBC count, lymphocyte count, and CRP were measured and recorded at admission and during treatment. SARS-CoV-2 nucleic acid tests with nose and throat swabs were conducted daily 7 days after admission. Viral nucleic acid tests were stopped after detection of negative results on 2 consecutive days. SARS-CoV-2 nucleic acid positive days refers to the time from the first to the last positive results. This study used retrospective case notes, so the requirement for ethical approval was deemed unnecessary.
2.3. Groups and observation indicators
Patients were divided into diabetes and non-diabetes groups. The differences in general conditions such as gender, age, BMI, SBP, DBP, ALT, AST, and Cr were observed between the two groups. The differences in maximal and minimal WBC count, maximal CRP level, time and value of the minimal lymphocyte count, hospitalization days, and SARS-CoV-2 nucleic acid positive days between the two groups were analyzed. Correlations between minimal lymphocyte count and hospitalization days as well as SARS-CoV-2 nucleic acid positive days were analyzed.
2.4. Statistical analysis
IBM SPSS statistics 23 software was used for statistical analysis. Continuous variables with normal distribution were presented as mean ± standard deviation (M ± SD), and differences between the two groups were determined by one-way ANOVA test. Continuous variables with non-normal distribution were presented as median with inter-quartile range (IQR), and differences between the two groups were determined by a rank test. In addition, a multiple linear regression model was used to analyze the effect of diabetes on minimal lymphocyte count and its emergence time, patient's hospitalization days, and SARS-CoV-2 nucleic acid positive days by adjusting for potential confounding factors including age; gender; BMI; SBP; DBP; and ALT, AST, and Cr levels. Categorical variables were described as number or rate, and differences between the two groups were determined by a Chi-square test. The correlation between the two groups was observed using a scatter chart. When linear correlation between two continuous variables was observed, Pearson’s product-moment correlation coefficient was used. P < 0.05 was considered to be statistically significant for all tests.
3. Results
Of the 66 patients reviewed, three patients were excluded from the study due to lack of data. No patient was excluded from the study due to blood disease, AIDS, or T1DM. Thus, the final study population included 63 people, of whom 16 were in the diabetes group and 47 in the non-diabetes group. There was no significant difference between the two groups in terms of gender; age; BMI; SBP; DBP; and ALT, AST, and Cr levels. There was no significant difference between the two groups in maximal and minimal WBC count. Patients in the diabetes group had higher maximal CRP [91.75 (9.13, 170.33) mg/L vs. 17.0 (4.20, 28.00) mg/L, P = 0.004] and higher fasting glucose (8.81 ± 2.42 mmol/L vs. 6.01 ± 1.89 mmol/L, P = 0.000) than those in the non-diabetes group. The minimal lymphocyte count of diabetic patients was lower than that of non-diabetic patients (0.67 ± 0.36 * 109/L vs. 1.30 ± 0.54 * 109/L, P = 0.000, adjusted P = 0.001). The minimal lymphocyte count occurred earlier in the diabetic group than in the non-diabetic group (2.68 ± 2.33 days vs. 5.29 ± 4.95 days, P = 0.047, adjusted P = 0.042); diabetic patients had longer hospitalization time than non-diabetic patients (20.44 ± 5.24 days vs. 17.11 ± 4.78 days, P = 0.022, adjusted P = 0.047). SARS-CoV-2 nucleic acid positive days were in 14.80 ± 4.85 days and 12.50 ± 4.55 days in patients with and without diabetes, respectively (P = 0.126, adjusted P = 0.094). When adjusting for confounding factors, we found that age and lymphocyte count were negatively correlated (B = −0.011, P = 0.006). The impact of other confounding factors, such as gender; BMI; SBP; DBP; and ALT, AST, and Cr levels was not statistically significant. Baseline characteristics and clinical characteristics of the two groups are described in Table 1 .
Table 1.
Analysis of clinical indicators in diabetic and non-diabetic groups.
| Diabetic group n = 16 |
Non-diabetic group n = 47 |
F/U/χ2 value | P value | |
|---|---|---|---|---|
| Male/% | 8(50%) | 25/53.1% | 0.825 | 0.526 |
| Age, years | 43.5 ± 17.8 | 51.0 ± 12.6 | 1.545 | 0.127 |
| BMI, kg/m2 | 23.79 ± 2.46 | 23.89 ± 3.59 | 0.100 | 0.921 |
| SBP, mmHg | 130.0 ± 10.2 | 128.2 ± 8.9 | 0.673 | 0.503 |
| DBP, mmHg | 80.2 ± 6.7 | 77.8 ± 6.2 | 0.194 | 0.694 |
| Fasting Glucose, mmol/L | 8.81 ± 2.42 | 6.01 ± 1.89 | 4.757 | 0.000 |
| ALT, U/L | 39.2 ± 32.9 | 36.6 ± 27.2 | 0.308 | 0.759 |
| AST, U/L | 35.1 ± 29.6 | 32.1 ± 25.5 | 0.392 | 0.693 |
| Cr, umol/L | 69.12 ± 77.85 | 53.23 ± 11.94 | 1.886 | 0.175 |
| Maximal CRP, mg/L | 91.75(9.13,170.33) | 17.0(4.20,28.00) | 560.00 | 0.004 |
| Maximal WBC, *109/L | 8.90 ± 5.42 | 7.79 ± 3.55 | 0.503 | 0.620 |
| Minimal WBC, *109/L | 5.00 ± 1.61 | 4.78 ± 1.24 | 0.762 | 0.455 |
| Minimal lymphocyte count (*109/L) | 0.67 ± 0.36 | 1.30 ± 0.54 | 5.262(3.585**) | 0.000(0.001**) |
| Minimal lymphocyte count time, days | 2.68 ± 2.33 | 5.29 ± 4.95 | 2.026(1.732*) | 0.047(0.042*) |
| Hospitalization days | 20.44 ± 5.24 | 17.11 ± 4.78 | 2.349(2.032*) | 0.022(0.047*) |
| SARS-CoV-2 nucleic acid positive days | 14.80 ± 4.85 | 12.50 ± 4.55 | 1.562(1.714*) | 0.126(0.094*) |
Data are reported as mean ± SD, median (IQR) or number and percentage. BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; WBC, white blood cell; ALT, Alanine aminotransferase; AST, aspartate aminotransferase; Cr, creatinine; CRP, C-reactive protein.
Multivariable regression model was used to adjust for potential confounding factors including age, gender, BMI, SBP, DBP, ALT, AST, Cr. The confounding factors showed no statistical significance.
Multivariable regression model was used to adjust for potential confounding factors including age, gender, BMI, SBP, DBP, ALT, AST, Cr. Among the confounding factors, age was observed to be statistically significant, P = 0.006. Other confounding factors showed no statistical significance.
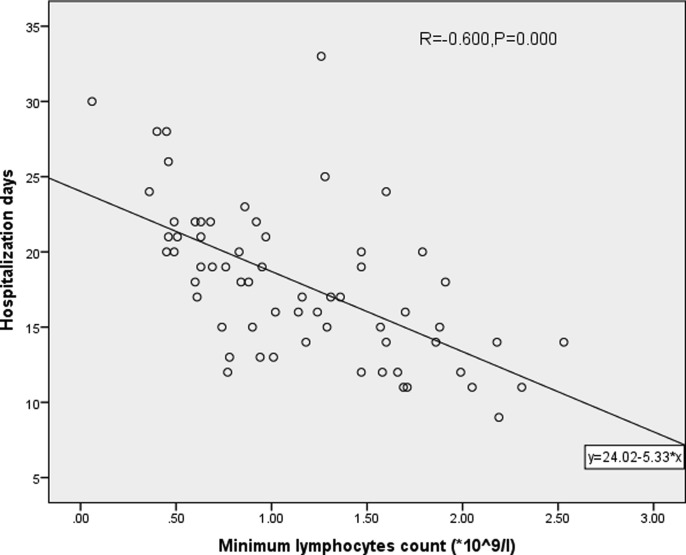
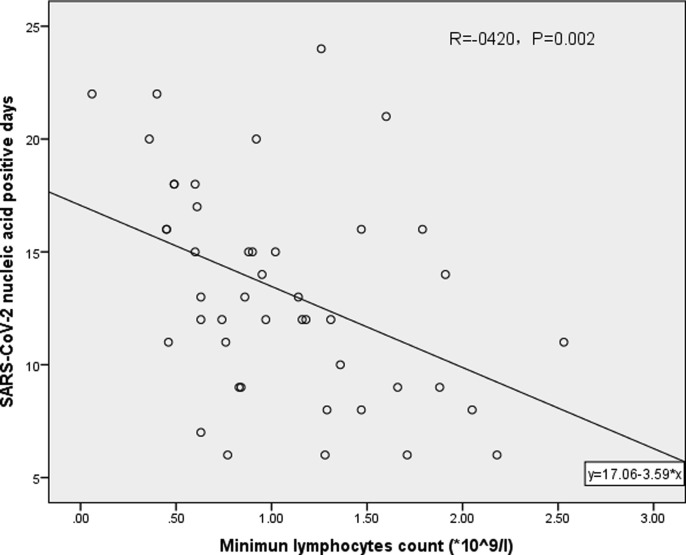
The scatter plot shows that hospitalization days (R = −0.600, P < 0.05, Fig. 1 ) and SARS-CoV-2 nucleic acid positive days (R = −0.420, P < 0.05, Fig. 2 ) are correlated with minimal lymphocyte count.
Fig. 1.
Correlation analysis of minimal lymphocyte count and hospitalization days.
Fig. 2.
Correlation analysis of the minimal lymphocyte count and SARS-CoV-2 nucleic acid positive days.
4. Discussion
This single center, observational, retrospective study of patients with COVID-19 showed that, patients with T2DM have higher CRP, lower level and more rapid decline in lymphocyte count, and longer hospitalization time than those without T2DM. Patients' age and lymphocyte count were negatively correlated. Hospitalization days and SARS-CoV-2 nucleic acid positive days were negatively correlated with minimal lymphocyte count.
Lymphocytes are divided into T lymphocytes, B lymphocytes, and natural killer lymphocytes. When a virus infects the human body, it binds to the receptor, enters the cell through the ligand, replicates the itself, and produces MHC-I molecules and MHC-II molecules in the cell; it is recognized by CD4+ T cells and CD8+ T cells after presenting the antigen, and this activates cellular immunity and humoral immunity, which help eliminate the virus[6], [7]. Virus clearance depends on the body's normal immune function, and the patient's lymphocyte count and function are critical factors. However, after viral infections, lymphocyte counts often decline. The possible causes include the consumption of lymphocytes after virus invasion, the direct killing of lymphocytes by the virus, apoptosis of lymphocytes [8], and the suppression of immune function by the virus. Bo Diao et al. reported a decrease in T-lymphocyte count in patients with COVID-19 [9]. Li Xinmin's research on patients with SARS showed that the absolute counts of T lymphocytes, T4 cells, and T8 cells were significantly reduced, suggesting that the T cell immune function of patients with SARS is weakened [10]. Liu et al. found [16] that lymphocyte counts were below the normal range in 29 (76.3%) patients, and no patient showed an increase. The median lymphocyte count was 0.73 × 109/L (IQR, 0.56–1.07). Lymphocyte subset counts of all subsets decreased in more than half of the patients on admission. T cell counts decreased in 24 (61.5%) patients, CD4+ T cell count decreased in 22 (56.4%) patients, CD8+ T cell count decreased in 28 (71.8%) patients, B cell count decreased in 27 (69.2%) patients, and NK cell count decreased in 30 (76.9%) patients. No patient showed an increase in the subset counts. Fan Wang [11] observed that patients with COVID-19 had significantly low counts of total lymphocytes (P < 0.0001), CD4+ T cells, CD8+ T cells, B cells, and NK cells. Thus, the T cell immune function of patients with COVID-19 is weakened too. Several recent studies have also found that peripheral blood lymphocyte count in patients with COVID-19 is reduced [4], [5], [9]. Pathological examination of the spleen showed degeneration and necrosis, and reduction in lymphocytes [12], which indirectly reflected lymphocyte depletion or immunosuppression. Our study found a decrease in lymphocyte count in patients with COVID-19, and the lower the lymphocyte count, the longer SARS-CoV-2 nucleic acid positive days and hospitalization days. It is suggested that the decrease in lymphocytes is one of the characteristics of COVID-19, and lymphocytes play an important role in the virus clearance in COVID-19 patients. A meta-analysis [13] showed that lymphopenia on admission was associated with poor outcomes in patients with COVID-19. According to some studies [14], [8], [15], decreased lymphocyte count is a characteristic of severe COVID-19. Among the patients enrolled, the median time of onset to RT-PCR turning negative was 14 (IQR, 10–20) days. In groups with rapid virus clearance, high counts of T cells, CD4+ T cells, CD8+ T cells, and B cells were observed. In our study, we found that SARS-CoV-2 nucleic acid positive days were negative correlated with the minimal lymphocyte count.
Patients with T2DM often experience immune impairment. Bailin et al. found that CD4+ and CD8+ T lymphocyte counts are lower in diabetic patients than in non-diabetic patients [17]. According to a Japanese research [18], CD3+CD56+ T cell% (4.1 ± 1.9%) of patients with pancreatic DM was lower than that (6.0 ± 3.0%) of normal subjects (p < 0.05). CD3+CD56+ T cells have cytotoxic activity and it is likely that this activity is similar to that of NK cells. These results suggest that a decrease in peripheral CD3+CD56+ T cell% is a factor reflecting weak host defense to infectious diseases in cases of pancreatic DM. The SARS-CoV-2 merges with target cells with the help of angiotensin converting enzyme 2 (ACE2), which is expressed by epithelial cells of the lung, intestine, and kidney [19]. Studies have shown that the expression of ACE2 in diabetic patients is significantly increased, and it seems that they are more likely to be infected with COVID-19 [20]. Yoshikawa et al. found through animal experiments that the overexpression of ACE2 reduces the activity of the T lymphocytes against SARS-CoV and impairs the immune function [21]. Gupta et al. found that diabetes enhances the severity of COVID-19 and several factors may be responsible for this [22]: (1) Hyperglycemia and possibly hypoglycemia; (2) immune defects especially impaired T-cell response; and (3) associated comorbidities like obesity, heart diseases, and kidney diseases. According to Muniyappa et al., diminished T cell function could be one of the reasons [8]. In our study, we found that diabetic patients showed a faster decline in lymphocyte count, lower minimal lymphocyte count, higher maximal CRP, and longer hospital stay than non-diabetic patients. Although there were no deaths in our study, many other studies have confirmed that diabetes is a risk factor for COVID-19 complications and increased mortality [2], [3], [5]. There is no specific treatment for COVID-19 now, and virus clearance is related to lymphocytes and immune function, so we can provide patients with symptomatic and support treatment, wait for the recovery of their lymphocyte count and immune function, and wait for virus clearance. Avoiding drugs that impair lymphocyte and immune function may be beneficial.
Our study has some limitations. The number of COVID-19 cases was small. There were no deaths in our study. The relationship between lymphocyte count and mortality was not observed. We did not obtain data of lymphocyte subsets and could not analyze the decline in specific types of lymphocytes. This research was a retrospective study, and there may be some unknown confounding factors in the study. Basic research and further clinical research are still needed to determine whether the mechanism of lymphocyte count decline in patients with COVID-19 has a synergistic effect with immune function abnormalities caused by diabetes.
In summary, COVID-19 patients with T2DM experience a faster decline in lymphocyte count, have lower lymphocyte count, and longer hospital stay than non-diabetic patients. The lower the lymphocyte count, the longer the hospitalization time and viral nucleic acid positive days.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.diabres.2020.108340.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Bornstein S.R., Rubino F., Khunti K., et al. Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol. 2020;8(6):546–550. doi: 10.1016/S2213-8587(20)30152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hill M.A., Mantzoros C., Sowers J.R. Commentary: COVID-19 in patients with diabetes. Metabolism. 2020:154217. doi: 10.1016/j.metabol.2020.154217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo W., Li M., Dong Y., et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020 doi: 10.1002/dmrr.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan W.J., Ni Z.Y., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang J., Natarajan K., Margulies D.H. MHC molecules, T cell receptors, natural killer cell receptors, and viral immunoevasins-key elements of adaptive and innate immunity. Adv Exp Med Biol. 2019;1172:21–62. doi: 10.1007/978-981-13-9367-9_2. [DOI] [PubMed] [Google Scholar]
- 7.Perot B.P., Ingersoll M.A., Albert M.L. The impact of macroautophagy on CD8(+) T-cell-mediated antiviral immunity. Immunol Rev. 2013;255(1):40–56. doi: 10.1111/imr.12096. [DOI] [PubMed] [Google Scholar]
- 8.Muniyappa R., Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab. 2020;318(5):E736–E741. doi: 10.1152/ajpendo.00124.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diao B., Wang C., Tan Y., et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19) Front Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xinmin L.i. The diagnostic signifcance of T lymphocyte in the early diagnosis of SARS. China Health Stand Manage. 2017;8(16):115–116. [Google Scholar]
- 11.Wang F., Nie J., Wang H., et al. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J Infect Dis. 2020;221(11):1762–1769. doi: 10.1093/infdis/jiaa150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao X.H., Li T.Y., He Z.C., et al. A pathological report of three COVID-19 cases by minimal invasive autopsies. Zhonghua Bing Li Xue Za Zhi. 2020;49(5):411–417. doi: 10.3760/cma.j.cn112151-20200312-00193. [DOI] [PubMed] [Google Scholar]
- 13.Huang I., Pranata R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis. J Intensive Care. 2020;8:36. doi: 10.1186/s40560-020-00453-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang Q., Xie L., Zhang W., et al. Analysis of the clinical characteristics, drug treatments and prognoses of 136 patients with coronavirus disease 2019. J Clin Pharm Ther. 2020 doi: 10.1111/jcpt.13170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao Q., Meng M., Kumar R., et al. Lymphopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a systemic review and meta-analysis. Int J Infect Dis. 2020;96:131–135. doi: 10.1016/j.ijid.2020.04.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Z., Long W., Tu M., et al. Lymphocyte subset (CD4+, CD8+) counts reflect the severity of infection and predict the clinical outcomes in patients with COVID-19. J Infect. 2020 doi: 10.1016/j.jinf.2020.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bailin S.S., McGinnis K.A., McDonnell W.J., et al. T lymphocyte subsets associated with prevalent diabetes in veterans with and without HIV. J Infect Dis. 2020 doi: 10.1093/infdis/jiaa069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsujino M., Kinpara I., Nakamura T., et al. Peripheral lymphocyte subset of patients with pancreatic diabetes mellitus–about a decreased ratio of T-LGL and ability of host defense. Kansenshogaku Zasshi. 1996;70(4):325–330. doi: 10.11150/kansenshogakuzasshi1970.70.325. [DOI] [PubMed] [Google Scholar]
- 19.Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020;94(7) doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection. Lancet Respir Med. 2020;8(4) doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshikawa N., Yoshikawa T., Hill T., et al. Differential virological and immunological outcome of severe acute respiratory syndrome coronavirus infection in susceptible and resistant transgenic mice expressing human angiotensin-converting enzyme 2. J Virol. 2009;83(11):5451–5465. doi: 10.1128/JVI.02272-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta R., Hussain A., Misra A. Diabetes and COVID-19: evidence, current status and unanswered research questions. Eur J Clin Nutr. 2020;74(6):864–870. doi: 10.1038/s41430-020-0652-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.