Abstract
Background
Anthropogenic climate change affects the burden of infectious diseases via several interconnected mechanisms. In recent years, there has been greater awareness of the ways in which climate-sensitive infectious diseases pose a growing threat to global public health.
Objective
This study aimed to categorize and describe the effects of climate change on infectious diseases with skin manifestations.
Methods
A scoping review of the MEDLINE and PubMed online databases for climate-sensitive infections was performed in February and March 2020. A representative selection of conditions with skin manifestations was included in this review.
Results
Several representative climate-sensitive infectious diseases were identified in each of the following categories: vector-borne infectious diseases, infectious diseases associated with extreme weather events, and infectious diseases linked to human migration.
Conclusion
Climate variables directly influence the survival and reproduction of infectious microorganisms, their vectors, and their animal reservoirs. Due to sustained warmer temperatures at higher latitudes, climate change has expanded the geographic range of certain pathogenic microbes. More frequent climate change-related extreme weather events create circumstances where existing infectious microorganisms flourish and novel infections emerge. Climate instability is linked to increased human migration, which disrupts health care infrastructure as well as the habitats of microbes, vectors, and animal reservoirs and leads to widespread poverty and overcrowding. Dermatologists should understand that climate change will affect the burden and geographic distribution of infectious diseases, many of which have cutaneous signs and might be encountered in their regular practice.
Keywords: Vector-borne, Dermatology, Skin, Temperature, Extreme weather events, Migration
Introduction
According to the World Health Organization (WHO), the most significant effects of climate change on human health will be driven by infectious diseases and undernutrition (Hales et al., 2014). Climate variables affect all components of every ecosystem (Table 1). In the biomedical paradigm, this includes all microorganisms (beneficial and pathogenic, opportunistic and indifferent) that are involved in human health and affairs. The geographic expansion of microbes and vectors into new territories, made possible by sustained warmer temperatures at higher latitudes and increased human travel, exposes all planetary life to diseases previously unknown in particular habitats. More frequent extreme weather events (EWEs; National Climate Assessment, 2020), including heat waves, drought, and floods (Table 2), create circumstances where existing infectious microorganisms flourish and novel infections emerge. Furthermore, in many regions, the myriad consequences of climate change drive mass intranational and international human migration, which disturbs regional health care infrastructure and the habitats of microbes, vectors, and animal reservoirs.
Table 1.
Essential climate variables.
| Atmosphere | Ocean | Land |
|---|---|---|
Surface
|
Physics
|
|
Upper atmosphere
|
Biogeochemistry
|
|
Composition
|
Biology/ecosystems
|
Adapted from The Global Climate Observing System Essential Climate Variable Data Access Matrix (https://www.ncdc.noaa.gov/gosic/gcos-essential-climate-variable-ecv-data-access-matrix).
Table 2.
Extreme weather events.
| Extreme weather events |
|---|
| Heat waves |
| Drought |
| Heavy downpours |
| Floods |
| Hurricanes |
| Winter storms |
Climate-sensitive infections have various transmission patterns (Table 3), including vector-borne, water-borne without requiring vectors, and human-to-human. Physicians who were trained or practice where these diseases are rare may find them challenging to recognize and diagnose. Herein, we review the effects of climate change on infectious diseases with dermatologic manifestations.
Table 3.
Climate-sensitive infectious diseases with dermatologic manifestations.
| Mechanism of climate sensitivity | Specific disease examples |
|---|---|
| Infectious microbes are directly sensitive to climate variables (temperature, rainfall, humidity) |
Viruses: Enteroviruses (Hand, foot, and mouth disease), Chikungunya, Zika Bacteria: Vibrio vulnificus infection Fungi: coccidioidomycosis |
| Enhanced survival and expanded geographic range of climate-sensitive vectors and animal reservoirs |
Aedesmosquito species: Chikungunya, Dengue, yellow fever, Zika, lymphatic filariasis, Anophelesmosquito species: Lymphatic filariasis Culexmosquito species: West Nile fever, lymphatic filariasis Phlebotamine sandflies: Leishmaniasis variants Ixodid (hard) ticks: Lyme disease and other borrelial infections, Rickettsial diseases (spotted fever, Q fever), Tularemia Triatome bugs: Chagas disease (American trypanosomiasis) |
| Increased incidence during and after extreme weather events |
Flooding: Vibrio vulnificus infection, Mycobacterium marinum infection, melioidosis (Burkholderia pseudomallei infection), leptospirosis, Chromobacterium violaceum infection, chromoblastomycosis, blastomycosis, mucormycosis, dermatophytosis, immersion foot syndromes (polymicrobial infection) Drought: Coccidioidomycosis |
| Human migration, overcrowding, and poverty caused by climate change–related extreme weather events | Scabies infestation, body lice infestation (vector for epidemic typhus and louse-borne/epidemic relapsing fever), tuberculosis, human immunodeficiency virus, diarrheal diseases |
Climate-sensitive vector-borne diseases
Vector-borne diseases (VBDs) are infectious diseases transmitted by living organisms, most commonly blood-sucking arthropods such as mosquitoes, ticks, flies, and fleas (Table 4; Caminade et al., 2016). Globally, VBDs cause >700,000 deaths annually (WHO, 2020) and many lack vaccines, disease-specific treatments, or both. Over the last 150 years, major advances in public health, including sanitation, water supply safety, vector control, and vaccination, have driven a steady decline in the burden of VBDs. The overall prevalence of major, potentially life-threatening VBDs continued to decline from 2005 to 2015 (Wang et al., 2016), likely due to improved health care infrastructure and declining severe poverty worldwide. However, climate change threatens these gains and, in some cases, may reverse disease-specific trends.
Table 4.
Vector-borne diseases of human significance.
| Vector | Disease caused | Type of pathogen | |
|---|---|---|---|
| Mosquitoes | Aedes | Chikungunya Dengue Lymphatic filariasis Rift Valley fever Yellow fever Zika |
Virus Virus Parasite Virus Virus Virus |
| Anopheles | Lymphatic filariasis Malaria |
Parasite Parasite |
|
| Culex | Japanese encephalitis Lymphatic filariasis West Nile fever |
Virus Parasite Virus |
|
| Aquatic snails | Schistosomiasis | Parasite | |
| Blackflies | Onchocerciasis | Parasite | |
| Fleas | Plague Tungiasis |
Bacteria Ectoparasite |
|
| Lice | Typhus Louse-borne relapsing fever |
Bacteria Bacteria |
|
| Sandflies | Leishmaniasis Sandfly fever |
Bacteria Virus |
|
| Ticks | Crimean-Congo hemorrhagic fever Lyme disease Relapsing fever (borreliosis) Rickettsial diseases (spotted fever, Q fever) Tick-borne encephalitis Tularemia |
Virus Bacteria Bacteria Bacteria Virus Bacteria |
|
| Triatome bugs | Chagas disease (American trypanosomiasis) | Parasite | |
| Tsetse flies | Sleeping sickness (African trypanosomiasis) | Parasite | |
Adapted from the World Health Organization (https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases).
Climate variables affect the environmental suitability for VBD transmission in many ways. Warming promotes the expansion of geographic ranges of vector and reservoir populations, which establish themselves at higher latitudes and altitudes or across more seasons (i.e., during winter months when infections were previously rare). This is apparent in the increasing numbers of reported VBDs from arctic/subarctic regions, where warming is progressing more quickly and to a greater degree compared to lower latitudes (Waits et al., 2018). Warmer temperatures often shorten the pathogen development time in vectors and quicken vector life cycles (Greer et al., 2008) such that pathogen and vector populations may enter logarithmic growth. Changes in rainfall, including more frequent flooding, directly affect vectoral capacity (the efficiency with which vectors become infected with, carry, and transmit pathogens). Finally, environmental degradation, either manmade or due to EWEs, disrupts the habitats of disease vectors and animal reservoirs.
Mosquito-borne illnesses
Dengue
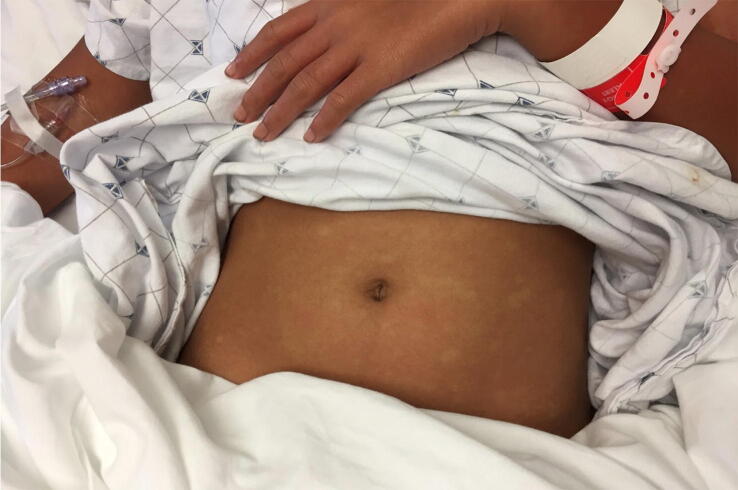
Dengue is a VBD caused by the dengue virus, a flavivirus transmitted to humans by Aedes aegypti and Aedes albopictus mosquitoes. As of 2019, approximately 3 billion people live in areas at risk for dengue, and an estimated 400 million dengue cases occur annually (Wilder-Smith et al., 2019b). Only 25% of infected persons develop symptoms, typically a mild, self-limited febrile illness (Wilder-Smith et al., 2019b). Facial flushing and blanching macular erythema with islands of sparing (Fig. 1) may appear during the acute phase (Wilder-Smith et al., 2019b). Rarely, severe and potentially fatal complications, including multisystem vascular leak syndrome and hemorrhagic disease, develop (Wilder-Smith et al., 2019b). Persons previously infected with a different dengue serotype, children, and pregnant women (especially during the third trimester) have a higher risk for severe disease (Wilder-Smith et al., 2019b).
Fig. 1.
Dengue. Morbilliform eruption with islands of sparing due to acute Dengue.
No disease-specific treatments for dengue are available. The first dengue vaccine, CYD-TDV (Dengvaxia, SanofiPasteur), is a recombinant, live-attenuated tetravalent vaccine approved by the U.S. Food and Drug Administration in 2019 (U.S. Food and Drug Administration, 2019). Its efficacy is influenced by viral serotype and host baseline serostatus. Seronegative individuals who receive the vaccine are more likely to develop severe disease with subsequent natural dengue infections (Hadinegoro et al., 2015). The WHO now recommends incorporating antibody screening into vaccine campaigns so that only seropositive individuals are vaccinated (Wilder-Smith et al., 2019a).
Although the incidence and mortality of other VBDs has declined recently, dengue-related mortality increased nearly 50% from 2005 to 2015 (Wang et al., 2016). Moreover, in the past half-century, dengue’s worldwide incidence increased 30-fold (Caminade et al., 2016). Approximately 75% of the global disease burden is in Asia (Wilder-Smith et al., 2019b), and Southeast Asia experienced the largest increase in recent dengue-related mortality (Watts et al., 2019). Indeed, dengue is now the leading cause of fever in travelers returning from Southeast Asia, surpassing malaria (Schwartz et al., 2008). These trends reflect a steady worldwide increase in vectoral capacity for both dengue vectors (Watts et al., 2019), which peaks near 29 °C (Liu-Helmersson et al., 2014).
Traditionally confined to the tropics, dengue’s geographic range is spreading because of warmer temperatures and increased human movement worldwide (Liu-Helmersson et al., 2014). Since 2010, sporadic autochthonous transmission has been reported in new regions, including Croatia (Gjenero-Margan et al., 2011), southern France (La Ruche et al., 2010), Portugal (Wilder-Smith et al., 2014), and the southern United States (Florida, Texas, and North Carolina; Centers for Disease Control and Prevention [CDC], 2020). Currently, approximately 30 U.S. states are in the likely or very likely range of both mosquito vectors (CDC, 2017). During this century, continued globalization and increasingly hospitable environments for Aedes spp. (due to global warming, forest destruction, disturbed urban and peri-urban environments, and human influence on land-use patterns) are projected to enable dengue’s geographic range to continue expanding (Liu-Helmersson et al., 2014). As the distribution of each serotype expands, more people are at risk for developing subsequent dengue infections, which are usually more severe; thus, the global burden of disease may increase substantially.
Chikungunya
The chikungunya virus, a togavirus, is also transmitted by Aedes (Morens and Fauci, 2014). Most infected patients (72%-95%) develop acute-phase symptoms (Staples et al., 2009), which include fever, severe symmetric polyarthralgias (typically fingers, wrists, elbows, knees, and ankles; Staples et al., 2009), and a morbilliform exanthem (Morens and Fauci, 2014). Despite low mortality, chikungunya has substantial acute and chronic morbidity; 48% of persons develop postchikungunya chronic inflammatory rheumatism, typically 20 months later (Yactayo et al., 2016). This causes economic losses, directly from health care costs and indirectly from impaired productivity (Yactayo et al., 2016). Currently, there is no preventive vaccine or disease-specific treatment for chikungunya.
Chikungunya outbreaks are climate sensitive. They occur mainly in rainy seasons, when mosquito density is maximal, and are rare at altitudes >2300 m (Yactayo et al., 2016). The first modern outbreak of chikungunya occurred in Tanzania (then Tanganyika) in the late 1950s. Since then, globalization has facilitated viral spread. In 2007, a single infected traveler arriving from South Asia led to a 200-person outbreak in Italy (Angelini et al., 2007). The first endemic cases in the Caribbean occurred in December 2013 (Morens and Fauci, 2014). Since then, multiple cases occur annually in the United States, mostly in returning travelers, except for occasional autochthonous cases in Florida and Texas (CDC, 2019a). Global spread is partly due to an adaptive viral mutation that improved its transmissibility by A. albopictus, which survives at cooler temperatures than A. aegypti (Weaver, 2014).
Zika
The Zika virus is another flavivirus transmitted by Aedes mosquitoes. Approximately 20% of infected persons are symptomatic (Nawas et al., 2016). Infection resembles mild dengue and may include fevers, rash, arthritis/arthralgia, and conjunctivitis (Nawas et al., 2016). Conjunctivitis (scleral erythema) may be more prominent with Zika than with other flavivirus infections (Koh et al., 2019). Dermatologic manifestations include a morbilliform exanthem, erythematous acral macules and papules, and postillness palmar desquamation (Nawas et al., 2016). Zika first reached the Americas (Brazil) in 2015 (Mayer et al., 2017) and was soon implicated in causing devastating neurological effects, including agyria and microcephaly, in utero. The WHO declared a public health emergency of international concern. Zika virus transmission occurs at 22.7 °C to 34.7 °C and is maximal at 29 °C (Tesla et al., 2018). Worldwide, as more regions approach this temperature and more immunologically naïve persons are exposed, additional outbreaks are likely to occur.
West Nile Virus
The West Nile Virus (WNV) is a flavivirus maintained in a bird–mosquito transmission cycle and has been identified in >65 mosquito species and >300 bird species in the United States alone (Petersen et al., 2013). However, only a few Culex species are competent at transmitting the virus to humans, who are typically dead-end viral hosts (Petersen et al., 2013). Clinical manifestations include an uncommon morbilliform rash, typically on days 5 to 12 of the illness, that affects the torso and extremities and spares the palms and soles (Ferguson et al., 2005, Tilley et al., 2007). No vaccines or disease-specific treatments are available.
WNV was introduced into the western hemisphere (New York) in 1999, spread to the Pacific Coast by 2003, and then to South America by 2005 (Petersen et al., 2013). WNV transmission is enhanced in warmer temperatures, which shorten the incubation time within mosquitoes and increase the efficiency of transmission to birds (Kilpatrick et al., 2008, Reisen et al., 2006). Outbreaks in Canada (Giordano et al., 2017), Israel (Paz, 2006), and Russia (Platonov et al., 2008) correlate with periods of high temperatures. In the United States, cases peak in late summer (ArboNET, 2019b). U.S. cases peaked at 9862 in 2003, but approximately 2000 cases still occur annually (ArboNET, 2019a).
Sandfly-borne illnesses
Leishmaniasis
The term “leishmaniasis” encompasses a variety of acute and chronic infections caused by several protozoa in the genus Leishmania and transmitted by phlebotomine sandflies. Cutaneous leishmaniasis is the most common form of the disease worldwide, but visceral disease (mainly found in the Old World) is the most virulent type. Cutaneous disease manifests with nonhealing ulcers, usually on exposed surfaces of the head, neck, forearms, and hands (Fig. 2).
Fig. 2.
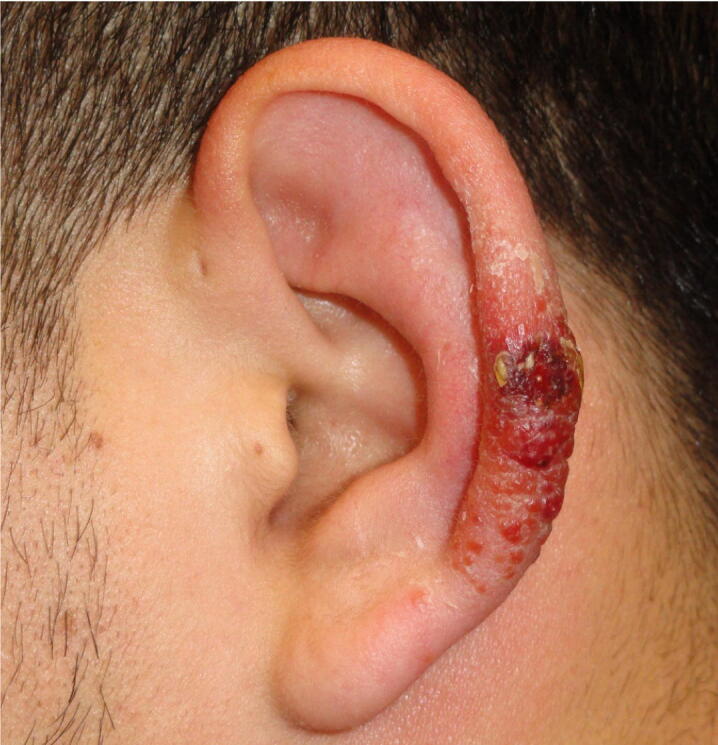
Cutaneous Leishmaniasis: Classic Chiclero’s ulcer on the ear, caused by Leishmania mexicana.
Temperature and humidity influence sandfly survival and reproduction (Negev et al., 2015). In the last several decades, rates of autochthonous cutaneous leishmaniasis in new areas have increased as environmental conditions become more favorable. In southern Europe, leishmaniasis case numbers are increasing as the sandfly’s geographic range expands northward (Maroli et al., 2008). In one series of cases diagnosed in Texas from 2007 to 2017, 59% of cutaneous leishmaniasis cases occurred in patients with no travel outside the United States in the prior decade, representing a shift away from mostly travel-related leishmaniasis in the United States (McIlwee et al., 2018).
Tick-borne illnesses
Lyme disease
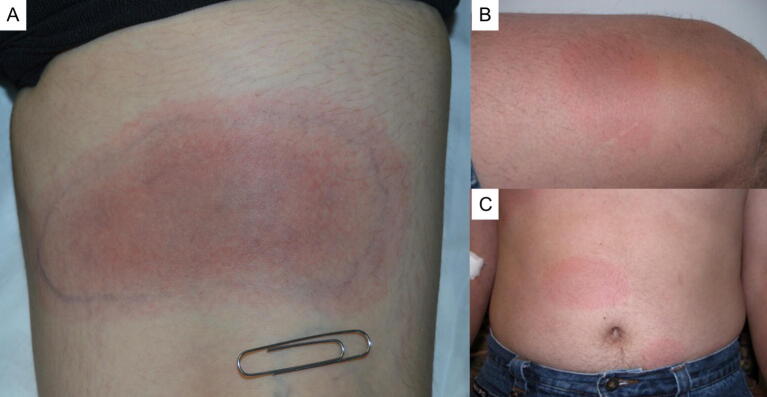
Lyme disease is a bacterial infection caused by several spirochetes, mainly Borrelia burgdorferi and Borrelia azfeli (Stanek et al., 2012). It is transmitted only by Ixodes ticks: Ixodes scapularis in eastern/central North America, Ixodes pacificus along North America’s West Coast, and Ixodes ricinus in Europe (Stanek et al., 2012). Important animal reservoirs include small mammals (e.g., mice, voles) and birds. Whitetail deer, which can support huge numbers of ticks, have an important role in maintaining tick populations (Stanek et al., 2012). Lyme disease manifests in three clinical stages, all with cutaneous manifestations, including localized erythema migrans (stage 1; Fig. 3A and B), disseminated erythema migrans (stage 2; Fig. 3C), and acrodermatitis chronica atrophicans and borrelial lymphocytoma (stage 3; both unique to Europe; Stanek et al., 2012).
Fig. 3.
Lyme disease. Clinical stages of Lyme disease. (A, B) Localized erythema migrans (stage 1), which may lack the classic bullseye appearance (C) Multiple red patches of disseminated Lyme disease, also called secondary erythema migrans (stage 2).
Lyme disease occurs at temperate latitudes and only in the Northern hemisphere. Disease risk has been linked to warm winters, high summer temperatures, and relatively low interseasonal temperature variation (Estrada-Peña et al., 2011). The overall Lyme disease case count is increasing; moreover, its geographic range is expanding northward in both North America and Europe (Jaenson et al., 2012) and will likely continue doing so due to the consequences of climate change on tick and reservoir populations (Roy-Dufresne et al., 2013, Simon et al., 2014).
Rocky Mountain spotted fever
Rocky Mountain spotted fever (RMSF) is a life-threatening condition caused by Rickettsia rickettsii, transmitted by American dog ticks (Dermacentor variabilis) and Rocky Mountain wood ticks (Dermacentor andersoni). Recently, an unrelated tick, the brown dog tick (Rhipicephalus sanguineus), was confirmed as a vector in eastern Arizona, discontinuous with areas of Dermacentor-borne RMSF (Demma et al., 2005, Nawas et al., 2016, Traeger et al., 2015). Clinical manifestations include flu-like symptoms with high fever, severe headache, malaise, and an acral, macular, erythematous rash that becomes petechial (Nawas et al., 2016). Early recognition is essential; diagnostic and treatment delays are associated with high mortality (Nawas et al., 2016).
The incidence of RMSF has increased steadily over the past 20 years (CDC, 2019). Although disease reporting practices and improved physician awareness are contributing factors (Openshaw et al., 2010), climate change may also play a role. Incidence is influenced by humidity and temperature, which affect the ranges of ticks and reservoirs and the amount of skin humans expose (Raghavan et al., 2016). RMSF has its highest incidence in June and July, when adult Dermacentor are the most active (CDC, 2019). Transmission via R. sanguineus also increases with warmer temperatures (Parola et al., 2008).
The geographic range of RMSF is expanding in latitude and altitude (Raghavan et al., 2016). Given the severity of untreated RMSF, dermatologists should be aware that RMSF may appear in previously nonendemic areas.
Chagas disease
Chagas disease (CD) is caused by the protozoan Trypanosoma cruzi, transmitted to humans through bites by triatomine insects (Triatoma, Panstrongylus, Rhodnius; Pérez-Molina and Molina, 2018). Acute phase symptoms include fever, inoculation site inflammation, unilateral eyelid edema (Romaña sign), lymphadenopathy, and hepatosplenomegaly. This phase lasts 4 to 8 weeks and resolves spontaneously. Approximately 30% to 40% later develop visceral involvement, which can cause substantial morbidity and death (Pérez-Molina and Molina, 2018).
Triatomids are climate-sensitive insects. To avoid dehydration, they feed more often during times of high temperature (>30 °C) and low humidity. Higher temperatures are associated with shorter life cycles (Carcavallo and de Casas, 1996) and faster parasite maturation (Asin and Catala, 1995).
Historically, CD has been endemic to rural Latin America, where poor housing conditions favor vector infestation. Most persons diagnosed in the United States acquired their infections in Latin America (Montgomery et al., 2016). However, CD’s geographic range may be expanding. In the United States, 28 autochthonous cases were reported from 1955 to 2015, but >75% of these cases were after 2011 (Bern et al., 2011, Montgomery et al., 2016). Since 2015, several additional autochthonous U.S. cases have been reported (Beatty et al., 2018, Gunter et al., 2017, Harris et al., 2017, Hernandez et al., 2016, Turabelidze et al., 2020, Webber et al., 2017;Jan-Jun(1–17):55–9.). In the United States, local transmission is typically sylvatic (related to outdoor exposures) rather than domestic (from infested human dwellings; Turabelidze et al., 2020). Models of future distribution predict further poleward expansion (Garza et al., 2014).
Climate-sensitive infectious diseases and extreme weather events
Many microorganisms have distinct geographic distributions based on the ecological niches of microbes, vectors, nonhuman reservoirs, and human populations. Climate and weather patterns generally and temperature patterns specifically are among the most important ecological variables that define distributions across latitude and altitude. Human populations living outside the historical thermal boundaries of particular microbes may not have been exposed to these organisms. Changes in global temperature patterns therefore expose new and vulnerable populations to unfamiliar infectious diseases.
Moreover, infectious disease outbreaks often cluster around EWEs, which are increasingly common because of climate change. For example, drought-associated water scarcity impairs personal and public sanitation and may lead to exposure to contaminated water. Conversely, severe flooding damages infrastructure, which can compromise water supply safety and expose humans to water-borne pathogens (Bandino et al., 2015, McMichael, 2015). Several outbreaks of infectious diseases, including hantavirus infection (Four Corners region of the southwestern United States) in the early 1990s (Wenzel, 1994), cryptosporidiosis (Milwaukee, Wisconsin, 1993; Mac Kenzie et al., 1994), and malaria (East Africa; Brown et al., 1998, Kilian et al., 1999, Lindsay et al., 2000, Loevinsohn, 1994) occurred after severe flooding. Given the skin barrier’s protective role, skin disease is especially common after flooding (Shabir, 2013). Below, we review specific EWE-associated infections with cutaneous manifestations.
Coccidioidomycosis
Coccidioidomycosis, or valley fever, is a systemic mycosis caused by the dimorphic organisms Coccidioides immitis and Coccidioides posadasii, which are endemic to arid regions of the Americas. Their confined geographic range reflects climate sensitivity. Their spores, found in soil approximately 10 cm below the surface, require moist periods to germinate, followed by dry spells to aerosolize (Matlock et al., 2019, Nguyen et al., 2013). Precipitation and wind are primary contributors to aerosolization. During heat waves, high temperatures deplete soil biota, reducing competition against Coccidioides (Maddy, 1957, Maddy, 1965). Up to 50% of people living in endemic areas have been exposed to some form of Coccidioides, mainly via spore inhalation (rarely via cutaneous inoculation), but most never display symptoms (Matlock et al., 2019). Approximately 40% of infected people are symptomatic, and only 1% develop disseminated disease (Sondermeyer et al., 2016). Transplant recipients, certain ethnic groups (e.g., Filipino-Americans), and patients with diabetes, malignancy, and HIV experience higher mortality (Sondermeyer et al., 2016).
In the last 2 decades, the incidence of coccidioidomycosis has increased steadily for several reasons. Better diagnostic tests improve case detection, and regional population growth increases the size of vulnerable populations. Climate change leads to edaphic (soil-related) changes that permit marked expansion of the fungus’ geographic range (CDC, 2019b). Spores have now been identified far north of the warm, arid Southwest, including Oregon (Hawryluk, 2016). Since 2010, at least 16 autochthonous cases have occurred in south-central Washington State (Washington State Department of Health, 2020). Heavy rainfall in 2016 after prolonged drought in California was associated with increased cutaneous coccidioidomycosis incidence (Coates and Fox, 2018, Shiu et al., 2018). More recently, California’s wildfires have been linked to an increased risk for coccidioidomycosis (Mulliken et al., 2019).
Vibrio species
Vibrio vulnificus is a gram-negative bacterium transmitted by exposure to marine and brackish waters (Park and Lee, 2018). Typical clinical manifestations include bullous hemorrhagic cellulitis of the distal extremities (Park and Lee, 2018). The risk for cutaneous Vibrio infections increases after coastal flooding, as seen after Hurricane Katrina (CDC, 2005). The worldwide environmental suitability for all pathogenic Vibrio species has steadily increased for several decades, particularly in the northern regions (Watts et al., 2019), and cases are now seen along the Northern European coastline (Baker-Austin et al., 2017, Huehn et al., 2014, Vezzulli et al., 2013).
Hand, foot, and mouth disease
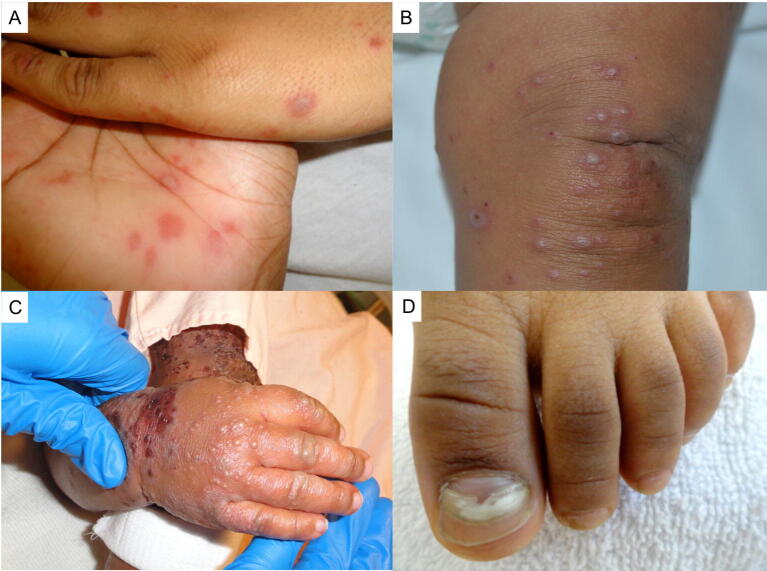
Hand, foot, and mouth disease (HFMD) is caused by various human enteroviruses. The condition typically affects children <5 years old and manifests with erythematous papules and vesicles distributed acrally and intraorally (Fig. 4A-C). Rarely, severe systemic manifestations occur. Onychomadesis may develop (Fig. 4D). Enteroviruses are sensitive to temperature and humidity (Coates et al., 2019). Climate change may have a role in HFMD epidemiology. In the past 30 years, HFMD has become a substantial public health burden in southeast Asia, where large, frequent, and virulent outbreaks have killed thousands of children (Coates et al., 2019).
Fig. 4.
Hand, foot, and mouth disease (HFMD) (A) Classic acral papules and vesicles in a patient with HFMD. (B) Papules and vesicles overlying the knee in a patient with HFMD. (C) Diffuse eczema coxsackium. (D) Onychomadesis following HFMD.
Future climate-sensitive microorganisms
Higher temperatures pose the threat of new, previously unidentified infectious diseases emerging as a consequence of progressive microbe adaptation. For example, most fungal species fare poorly at human core temperatures, which are far above average ambient temperatures anywhere in the world. However, many fungi have the capacity to develop thermotolerance (Casadevall et al., 2019, de Crecy et al., 2009), which may eventually enable them to defeat the human endothermy thermal barrier (Casadevall and Casadevall, 2020). Likewise, viruses that currently pose no threat to humans might spillover to humans if they acquire the capacity to replicate at higher temperatures. Additionally, organisms (including those that cause tularemia and anthrax) that have been frozen in thawing arctic permafrost may emerge to cause a greater disease burden (Waits et al., 2018).
Human migration and infectious disease
For millennia, humans have migrated for environmental, sociopolitical, and economic reasons. Climate change threatens already marginalized populations worldwide, especially in regions dependent on subsistence agriculture. Crop production is threatened by pests, pathogens, and EWEs. Globally, crop yields for staple products (e.g., maize, winter wheat, soybeans) have declined in concert with rising temperatures (Watts et al., 2019). Furthermore, the number of undernourished persons worldwide has been increasing since 2014 due to impaired access to and affordability of food (Watts et al., 2019). Undernutrition predisposes individuals to numerous infectious diseases (The Lancet Infectious Diseases, 2017).
When local environments are no longer salubrious, populations decline and humans migrate, often across international borders when home countries become inhospitable. Mass migration destabilizes health care infrastructure, weakening individual-based medical care and public health measures. This decreases access to health care, lowers vaccination rates, impairs food quality and quantity, limits clean water access, degrades hygiene, and leads to overcrowding. This further exposes migrants to communicable diseases associated with poverty, including tuberculosis, ectoparasite (scabies and lice) infestations, HIV infection, and diarrheal diseases (many of which have dermatologic manifestations). Disease vectors and animal reservoirs are also affected—increased cases of cutaneous leishmaniasis have been linked to conflict and mass human migration in the Middle East (Eroglu and Ozgoztasi, 2019, Muhjazi et al., 2019).
Conclusion
Climate change alters the epidemiology of infectious diseases. VBDs may emerge or re-emerge within populations when climate variables change significantly. Climate change has driven increased environmental suitability for infectious diseases, from changes in climate, soils, forest cover, and land use. The current COVID-19 pandemic illustrates the consequences of failing to respond to an infectious disease outbreak swiftly and in a manner based on expert recommendations for containing disease spread. As the climate crisis unfolds, humanity will likely be challenged by additional opportunities to respond to novel or emerging infectious diseases. This warrants a concerted effort to develop enhanced surveillance methods to detect emerging and re-emerging infectious diseases, particularly those with no disease-specific vaccinations or treatments. Climate-based warning systems that identify regions at particular risk for disease outbreaks are under development. Targeted vector-control efforts, which have successfully addressed certain arbovirus outbreaks in the past, may be used successfully in areas with emerging diseases. Given the concern for antibiotic resistance and waning supplies of next-generation antibiotics, good stewardship of existing antibiotics and continued research to develop new antibiotics is essential. Dermatologists should understand that climate change will affect the burden and geographic distribution of infectious diseases, many of which have cutaneous signs and might be encountered during regular practice.
Funding
Dr. Sarah J. Coates was supported by the Fogarty International Center of the National Institutes of Health under award number D43TW009343. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Study Approval
The author(s) confirm that any aspect of the work covered in this manuscript that has involved human patients has been conducted with the ethical approval of all relevant bodies.
Conflict of Interest
None.
References
- Angelini R., Finarelli A., Angelini P., Po C., Petropulacos K., Macini P. An outbreak of chikungunya fever in the province of Ravenna, Italy. Euro Surveill. 2007;12(9) doi: 10.2807/esw.12.36.03260-en. E070906.1. [DOI] [PubMed] [Google Scholar]
- ArboNET. West Nile virus disease cases reported to CDC by state of residence, 1999–2018 [Internet]. 2019a [cited March 20, 2020]. Available from: https://www.cdc.gov/westnile/resources/pdfs/data/West-Nile-virus-disease-cases-by-state_1999-2018-P.pdf.
- ArboNET. West Nile virus disease cases reported to CDC by week of illness onset, 1999–2018 [Internet]. 2019b [cited March 20, 2020]. Available from: https://www.cdc.gov/westnile/resources/pdfs/data/WNV-Week-Onset-1999–2018-P.pdf.
- Asin S., Catala S. Development of Trypanosoma cruzi in Triatoma infestans: Influence of temperature and blood consumption. J Parasitol. 1995;81(1):1–7. [PubMed] [Google Scholar]
- Baker-Austin C., Trinanes J., Gonzalez-Escalona N., Martinez-Urtaza J. Non-cholera Vibrios: The microbial barometer of climate change. Trends Microbiol. 2017;25(1):76–84. doi: 10.1016/j.tim.2016.09.008. [DOI] [PubMed] [Google Scholar]
- Bandino J., Hang A., Norton S. The infectious and noninfectious dermatological consequences of flooding: A field manual for the responding provider. Am J Clin Dermatol. 2015;16(5):399–424. doi: 10.1007/s40257-015-0138-4. [DOI] [PubMed] [Google Scholar]
- Beatty N.L., Perez-Velez C.M., Yaglom H.D., Carson S., Liu E., Khalpey Z.I. Evidence of likely autochthonous transmission of Chagas disease in Arizona. Am J Trop Med Hyg. 2018;99(6):1534–1536. doi: 10.4269/ajtmh.18-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bern C., Kjos S., Yabsley M.J., Montgomery S.P. Trypanosoma cruzi and Chagas disease in the United States. Clin Microbiol Rev. 2011;24(4):655–681. doi: 10.1128/CMR.00005-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown V., Issak M.A., Rossi M., Barboza P., Paugam A. Epidemic of malaria in north-eastern Kenya. Lancet. 1998;352(9137):1356–1357. doi: 10.1016/s0140-6736(05)60747-7. [DOI] [PubMed] [Google Scholar]
- Caminade C., McIntyre M.K., Jones A.E. Climate change and vector-borne diseases: Where are we next heading? J Infect Dis. 2016;214(9):1300–1301. doi: 10.1093/infdis/jiw368. [DOI] [PubMed] [Google Scholar]
- Carcavallo R.U., de Casas S.C. Some health impacts of global warming in South America: Vector-borne diseases. J Epidemiol. 1996;6(4 suppl):153–157. [Google Scholar]
- Casadevall A., Casadevall A. Climate change brings the specter of new infectious diseases. J Clin Invest. 2020;130(2):553–555. doi: 10.1172/JCI135003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadevall A., Kontoyiannis D., Robert V. On the emergence of Candida auris: Climate change, azoles, swamps, and birds. MBio. 2019;10(4):e01397–e1419. doi: 10.1128/mBio.01397-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Rocky Mountain spotted fever: Epidemiology and statistics [Internet]. 2019 [cited 2020 February 22]. Available from: https://www.cdc.gov/rmsf/stats/index.html#anchor_1531851146113.
- Centers for Disease Control and Prevention. Dengue cases in the US [Internet]. 2020 [cited 2020 February 6]. Available from: https://www.cdc.gov/dengue/statistics-maps/index.html.
- Centers for Disease Control and Prevention. Vibrio illnesses after Hurricane Katrina – multiple states – August-September 2005 [Internet]. 2005 [cited March 20, 2020]. Available from: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5437a5.htm.
- Centers for Disease Control and Prevention. Estimated potential range of Aedes aegypti and Aedes albopictus in the United States, 2017 [Internet]. 2017 [cited March 20, 2020]. Available from: https://www.cdc.gov/zika/pdfs/Zika-mosquito-maps.pdf.
- Centers for Disease Control and Prevention. Chikungunya virus in the United States [Internet]. 2019a [cited 2020 February 9]. Available from: https://www.cdc.gov/chikungunya/geo/united-states.html.
- Centers for Disease Control and Prevention. Valley fever (coccidioidomycosis) statistics [Internet]. 2019b [cited 2020 February 12]. Available from: https://www.cdc.gov/fungal/diseases/coccidioidomycosis/statistics.html.
- Coates S.J., Davis M.D.P., Andersen L.K. Temperature and humidity affect the incidence of hand, foot, and mouth disease: A systematic review of the literature – a report from the International Society of Dermatology Climate Change Committee. Int J Dermatol. 2019;58(4):388–399. doi: 10.1111/ijd.14188. [DOI] [PubMed] [Google Scholar]
- Coates S.J., Fox L.P. Disseminated coccidioidomycosis as a harbinger of climate change. JAAD Case Reports. 2018;4(5):424–425. doi: 10.1016/j.jdcr.2017.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Crecy E., Jaronski S., Lyons B., Lyons T., Keyhani N. Directed evolution of a filamentous fungus for thermotolerance. BMC Biotechnol. 2009;9(74) doi: 10.1186/1472-6750-9-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demma L.J., Traeger M.S., Nicholson W.L., Paddock C.D., Blau D.M., Eremeeva M.E. Rocky Mountain spotted fever from an unexpected tick vector in Arizona. N Engl J Med. 2005;353(6):587–594. doi: 10.1056/NEJMoa050043. [DOI] [PubMed] [Google Scholar]
- Eroglu F., Ozgoztasi O. The increase in neglected cutaneous leishmaniasis in Gaziantep province of Turkey after mass human migration. Acta Trop. 2019;(192):138–143. doi: 10.1016/j.actatropica.2019.01.026. [DOI] [PubMed] [Google Scholar]
- Estrada-Peña A., Ortega C., Sánchez N., DeSimone L., Sudre B., Suk J.E. Correlation of Borrelia burgdorferi sensu lato prevalence in questing Ixodes ricinus ticks with specific abiotic traits in the Western Palearctic. Appl Env Microbiol. 2011;77(11):3838–3845. doi: 10.1128/AEM.00067-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson D.D., Gershman K., LeBailly A., Petersen L.R. Characteristics of the rash associated with West Nile Virus fever. Clin Infect Dis. 2005;41(8):1204–1207. doi: 10.1086/444506. [DOI] [PubMed] [Google Scholar]
- Garza M., Feria Arroyo T.P., Casillas E.A., Sanchez-Cordero V., Rivaldi C.L., Sarkar S. Projected future distributions of vectors of Trypanosoma cruzi in North America under climate change scenarios. PLoS Negl Trop Dis. 2014;8(5):32818. doi: 10.1371/journal.pntd.0002818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano B.V., Kaur S., Hunter F.F. West Nile virus in Ontario, Canada: A twelve-year analysis of human case prevalence, mosquito surveillance, and climate data. PLoS One. 2017;12(8):1–15. doi: 10.1371/journal.pone.0183568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjenero-Margan I., Aleraj B., Krajcar D., Lesnikar V., Klobučar A., Pem-Novosel I. Autochthonous dengue fever in Croatia, August-September 2010. Euro Surveill. 2011;16(9) pii: 19805. [PubMed] [Google Scholar]
- Greer A., Ng V., Fisman D. Climate change and infectious diseases in North America: The road ahead. CMAJ. 2008;178(6):715–722. doi: 10.1503/cmaj.081325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunter S.M., Murray K.O., Gorchakov R., Beddard R., Rossmann S.N., Montgomery S.P. Likely autochthonous transmission of Trypanosoma cruzi to humans, South Central Texas, USA. Emerg Infect Dis. 2017;23(3):500–503. doi: 10.3201/eid2303.161157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadinegoro S.R., Arredondo-García J.L., Capeding M.R., Deseda C., Chotpitayasunondh T., Dietze R. Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. N Engl J Med. 2015;373(13):1195–1206. doi: 10.1056/NEJMoa1506223. [DOI] [PubMed] [Google Scholar]
- Hales S, Kovats S, Lloyd S, Campbell-Lendrum D. Quantitative risk assessment of the effects of climate change on selected causes of death, 2030s and 2050s [Internet]. 2014 [cited March 20, 2020]. Available from: http://www.who.int/globalchange/publications/quantitative-risk-assessment/en/.
- Harris N., Woc-Colburn L., Gunter S.M., Gorchakov R., Murray K.O., Rossmann S. Autochthonous Chagas disease in the southern United States: A case report of suspected residential and military exposures. Zoonoses Public Heal. 2017;64(6):491–493. doi: 10.1111/zph.12360. [DOI] [PubMed] [Google Scholar]
- Hawryluk M. Traces of valley fever fungus found in Central Oregon [Internet]. 2016 [cited March 20, 2020]. Available from: https://www.bendbulletin.com/lifestyle/health/traces-of-valley-fever-fungus-found-in-central-oregon/article_5c77c059-93ef-565c-b29b-3b7aee922a78.html.
- Hernandez S., Flores C.A., Viana G.M., Sanchez D.R., Traina M.I., Meymandi S.K. Autochthonous transmission of Trypanosoma cruzi in Southern California. Open Forum Infect Dis. 2016;3(4):32–34. doi: 10.1093/ofid/ofw227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huehn S., Eichhorn C., Urmersbach S., Breidenbach J., Bechlars S., Bier N. Pathogenic vibrios in environmental, seafood and clinical sources in Germany. Int J Med Microbiol. 2014;304(7):843–850. doi: 10.1016/j.ijmm.2014.07.010. [DOI] [PubMed] [Google Scholar]
- Jaenson T.G.T., Hjertqvist M., Bergström T., Lundkvist Å. Why is tick-borne encephalitis increasing? A review of the key factors causing the increasing incidence of human TBE in Sweden. Parasites Vectors. 2012;5(1):1–13. doi: 10.1186/1756-3305-5-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Kenzie W., Hoxie N., Proctor M., Gradus M., Blair K., Peterson D. A massive outbreak in Milwaukee of cryptosporidium infection transmitted through the public water supply. N Engl J Med. 1994;331(3):161–167. doi: 10.1056/NEJM199407213310304. [DOI] [PubMed] [Google Scholar]
- Kilian A., Langi P., Talisuna A., Kabagambe G. Rainfall pattern, El Niño and malaria in Uganda. Trans R Soc Trop Med Hyg. 1999;93(1):22–23. doi: 10.1016/s0035-9203(99)90165-7. [DOI] [PubMed] [Google Scholar]
- Kilpatrick A., Meola M., Moudy R., Kramer L. Temperature, viral genetics, and the transmission of West Nile virus by Culex pipiens mosquitoes. PLoS Pathog. 2008;4(6) doi: 10.1371/journal.ppat.1000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh X.Q., Chandran N.S., Tambyah P.A. Mucocutaneous features of Zika—a review. Curr Infect Dis Rep. 2019;21(5) doi: 10.1007/s11908-019-0671-z. [DOI] [PubMed] [Google Scholar]
- Lindsay S.W., Bødker R., Malima R., Msangeni H.A., Kisinza W. Effect of 1997–98 EI Niño on highland malaria in Tanzania. Lancet. 2000;355(9208):989–990. doi: 10.1016/s0140-6736(00)90022-9. [DOI] [PubMed] [Google Scholar]
- Liu-Helmersson J., Stenlund H., Wilder-Smith A., Rocklöv J. Vectorial capacity of Aedes aegypti: Effects of temperature and implications for global dengue epidemic potential. PLoS One. 2014;9(3) doi: 10.1371/journal.pone.0089783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loevinsohn M.E. Climatic warming and increased malaria incidence in Rwanda. Lancet. 1994;343(8899):714–718. doi: 10.1016/s0140-6736(94)91586-5. [DOI] [PubMed] [Google Scholar]
- Maddy K. Symposium on Coccidioidomycosis, Phoenix, AZ. Washington, DC. 1957. Ecological factors possibly relating to the geographic distribution of Coccidioides immitis; pp. 11–13. [Google Scholar]
- Maddy K. Observations on Coccidioides immitis found growing naturally in soil. Ariz Med. 1965;22:281–288. [PubMed] [Google Scholar]
- Maroli M., Rossi L., Baldelli R., Capelli G., Ferroglio E., Genchi C. The northward spread of leishmaniasis in Italy: Evidence from retrospective and ongoing studies on the canine reservoir and phlebotomine vectors. Trop Med Int Heal. 2008;13(2):256–264. doi: 10.1111/j.1365-3156.2007.01998.x. [DOI] [PubMed] [Google Scholar]
- Matlock M., Hopfer S., Ogunseitan O.A. Communicating risk for a climate-sensitive disease: A case study of valley fever in central California. Int J Env Res Public Heal. 2019;16(18):E3254. doi: 10.3390/ijerph16183254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer S., Tesh R., Vasilakis N. The emergence of arthropod-borne viral diseases: A global prospective on dengue, chikungunya and zika fevers. Acta Trop. 2017;166:155–163. doi: 10.1016/j.actatropica.2016.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlwee B.E., Weis S.E., Hosler G.A. Incidence of endemic human cutaneous leishmaniasis in the United States. JAMA Dermatol. 2018;154(9):1032–1039. doi: 10.1001/jamadermatol.2018.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael A.J. Extreme weather events and infectious disease outbreaks. Virulence. 2015;6(6):543–547. doi: 10.4161/21505594.2014.975022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery S.P., Parise M.E., Dotson E.M., Bialek S.R. What do we know about Chagas disease in the United States? Am J Trop Med Hyg. 2016;95(6):1225–1227. doi: 10.4269/ajtmh.16-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morens D., Fauci A. Chikungunya at the door–déjà vu all over again? N Engl J Med. 2014;371(10):885–887. doi: 10.1056/NEJMp1408509. [DOI] [PubMed] [Google Scholar]
- Muhjazi G., Gabrielli A.F., Ruiz-Postigo J.A., Atta H., Osman M., Bashour H. Cutaneous leishmaniasis in Syria: A review of available data during the war years: 2011–2018. PLoS Negl Trop Dis. 2019;13(12) doi: 10.1371/journal.pntd.0007827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulliken J, Rappold A, Fung M, Babik J, Doernberg S. Is exposure to wildfires associated with invasive fungal infections? [Internet]. 2019 [cited March 20, 2020]. Available from: https://atcmeetingabstracts.com/abstract/is-exposure-to-wildfires-associated-with-invasive-fungal-infections/.
- National Climate Assessment. Extreme weather [Internet]. 2014 [cited 2020 March 17]. Available from: https://nca2014.globalchange.gov/highlights/report-findings/extreme-weather.
- Nawas Z.Y., Tong Y., Kollipara R., Peranteau A.J., Woc-Colburn L., Yan A.C. Emerging infectious diseases with cutaneous manifestations: Viral and bacterial infections. J Am Acad Dermatol. 2016;75(1):1–16. doi: 10.1016/j.jaad.2016.04.033. [DOI] [PubMed] [Google Scholar]
- Negev M., Paz S., Clermont A., Pri-Or N.G., Shalom U., Yeger T. Impacts of climate change on vector borne diseases in the Mediterranean basin — implications for preparedness and adaptation policy. Int J Env Res Public Heal. 2015;12(6):6745–6770. doi: 10.3390/ijerph120606745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen C., Barker B.M., Hoover S., Nix D.E., Ampel N.M., Frelinger J.A. Recent advances in our understanding of the environmental, epidemiological, immunological, and clinical dimensions of coccidioidomycosis. Clin Microbiol Rev. 2013;26(3):505–525. doi: 10.1128/CMR.00005-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Openshaw J.J., Swerdlow D.L., Krebs J.W., Holman R.C., Mandel E., Harvey A. Rocky mountain spotted fever in the United States, 2000–2007: Interpreting contemporary increases in incidence. Am J Trop Med Hyg. 2010;83(1):174–182. doi: 10.4269/ajtmh.2010.09-0752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J., Lee C.S. Vibrio vulnificus infection. N Engl J Med. 2018;379(4):375. doi: 10.1056/NEJMicm1716464. [DOI] [PubMed] [Google Scholar]
- Parola P., Socolovschi C., Jeanjean L., Bitam I., Fournier P.E., Sotto A. Warmer weather linked to tick attack and emergence of severe rickettsioses. PLoS Negl Trop Dis. 2008;2(11) doi: 10.1371/journal.pntd.0000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz S. The West Nile virus outbreak in Israel (2000) from a new perspective: the regional impact of climate change. Int J Env Heal Res. 2006;16(1):1–13. doi: 10.1080/09603120500392400. [DOI] [PubMed] [Google Scholar]
- Pérez-Molina J.A., Molina I. Chagas disease. Lancet. 2018;391(10115):82–94. doi: 10.1016/S0140-6736(17)31612-4. [DOI] [PubMed] [Google Scholar]
- Petersen L.R., Brault A.C., Nasci R.S. West Nile virus: Review of the literature. JAMA. 2013;310(3):308–315. doi: 10.1001/jama.2013.8042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platonov A.E., Fedorova M.V., Karan L.S., Shopenskaya T.A., Platonova O.V., Zhuravlev V.I. Epidemiology of West Nile infection in Volgograd, Russia, in relation to climate change and mosquito (Diptera: Culicidae) bionomics. Parasitol Res. 2008;103(Suppl. 1):S45–S53. doi: 10.1007/s00436-008-1050-0. [DOI] [PubMed] [Google Scholar]
- Raghavan R.K., Goodin D.G., Neises D., Anderson G.A., Ganta R.R. Hierarchical Bayesian spatio-temporal analysis of climatic and socio-economic determinants of Rocky Mountain spotted fever. PLoS One. 2016;11(3) doi: 10.1371/journal.pone.0150180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisen W., Fang Y., Martinez V. Effects of temperature on the transmission of west Nile virus by Culex tarsalis (Diptera: Culicidae) J Med Entomol. 2006;43(2):309–317. doi: 10.1603/0022-2585(2006)043[0309:EOTOTT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Roy-Dufresne E., Logan T., Simon J.A., Chmura G.L., Millien V. Poleward expansion of the white-footed mouse (Peromyscus leucopus) under climate change: Implications for the spread of lyme disease. PLoS One. 2013;8(11) doi: 10.1371/journal.pone.0080724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Ruche G., Souarès Y., Armengaud A., Peloux-Petiot F., Delaunay P., Desprès P. First two autochthonous dengue virus infections in metropolitan France, September 2010. Euro Surveill. 2010;15(39):19676. [PubMed] [Google Scholar]
- Schwartz E., Weld L.H., Wilder-Smith A., Von Sonnenburg F., Keystone J.S., Kain K.C. Seasonality, annual trends, and characteristics of dengue among ill returned travelers, 1997–2006. Emerg Infect Dis. 2008;14(7):1081–1088. doi: 10.3201/eid1407.071412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabir O. A summary case report on the health impacts and response to the Pakistan floods of 2010. PLoS Curr. 2013;11(5):pii. doi: 10.1371/currents.dis.cc7bd532ce252c1b740c39a2a827993f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiu J., Thai M., Elsensohn A.N., Nguyen N.Q., Lin K.Y., Cassarino D.S. A case series of primary cutaneous coccidioidomycosis after a record-breaking rainy season. JAAD Case Rep. 2018;4(5):412–414. doi: 10.1016/j.jdcr.2017.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon J.A., Marrotte R.R., Desrosiers N., Fiset J., Gaitan J., Gonzalez A. Climate change and habitat fragmentation drive the occurrence of Borrelia burgdorferi, the agent of Lyme disease, at the northeastern limit of its distribution. Evol Appl. 2014;7(7):750–764. doi: 10.1111/eva.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondermeyer G., Lee L., Gilliss D., Vugia D. Coccidioidomycosis-associated deaths in California, 2000–2013. Public Heal Rep. 2016;131(4):531–535. doi: 10.1177/0033354916662210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanek G., Wormser G.P., Gray J., Strle F. Lyme borreliosis. Lancet. 2012;379(9814):461–473. doi: 10.1016/S0140-6736(11)60103-7. [DOI] [PubMed] [Google Scholar]
- Staples J.E., Breiman R.F., Powers A.M. Chikungunya fever: An epidemiological review of a re-emerging infectious disease. Clin Infect Dis. 2009;49(6):942–948. doi: 10.1086/605496. [DOI] [PubMed] [Google Scholar]
- Tesla B., Demakovsky L.R., Mordecai E.A., Ryan S.J., Bonds M.H., Ngonghala C.N. Temperature drives Zika virus transmission: Evidence from empirical and mathematical models. Proc R Soc B. 2014;1884(285):20180795. doi: 10.1098/rspb.2018.0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diseases T.L.I. Climate change: The role of the infectious disease community. Lancet Infect Dis. 2017;17(12):1219. doi: 10.1016/S1473-3099(17)30645-X. [DOI] [PubMed] [Google Scholar]
- Tilley P.A.G., Fox J.D., Jayaraman G.C., Preiksaitis J.K. Maculopapular rash and tremor are associated with West Nile fever and neurological syndromes. J Neurol Neurosurg Psychiatry. 2007;78(5):529–531. doi: 10.1136/jnnp.2006.107862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traeger M., Regan J., Humpherys D., Mahoney D., Martinez M., Emerson G. Rocky Mountain spotted fever characterization and comparison to similar illnesses in a highly endemic area, Arizona, 2002–2011. Clin Infect Dis. 2015;60(11):1650–1658. doi: 10.1093/cid/civ115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turabelidze G., Vasudevan A., Rojas-Moreno C., Montgomery S.P., Baker M., Pratt D. Autochthonous Chagas disease, Missouri, 2018. MMWR Morb Mortal Wkly Rep. 2020;69(7):193–195. doi: 10.15585/mmwr.mm6907a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration. Approval Letter - Dengvaxia [Internet]. 2019 [Cited March 20, 2020]. Available from: https://www.fda.gov/media/124402/download.
- Vezzulli L., Colwell R.R., Pruzzo C. Ocean warming and spread of pathogenic Vibrios in the aquatic environment. Microb Ecol. 2013;65(4):817–825. doi: 10.1007/s00248-012-0163-2. [DOI] [PubMed] [Google Scholar]
- Waits A., Emelyanova A., Oksanen A., Abass K., Rautio A. Human infectious diseases and the changing climate in the Arctic. Env Int. 2018;121(Pt 1):703–713. doi: 10.1016/j.envint.2018.09.042. [DOI] [PubMed] [Google Scholar]
- Wang H., Naghavi M., Allen C., Barber R.M., Bhutta Z.A., Carter A. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1459–1544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington State Department of Health. Coccidioidomycosis (Valley Fever) [Internet]. 2020 [cited 2020 February 12]. Available from: https://www.doh.wa.gov/ForPublicHealthandHealthcareProviders/NotifiableConditions/Coccidioidomycosis.
- Watts N., Amann M., Arnell N., Ayeb-Karlsson S., Belesova K., Boykoff M. The 2019 report of The Lancet Countdown on health and climate change: ensuring that the health of a child born today is not defined by a changing climate. Lancet. 2019;394(10211):1836–1878. doi: 10.1016/S0140-6736(19)32596-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver S.C. Arrival of chikungunya virus in the New World: Prospects for spread and impact on public health. PLoS Negl Trop Dis. 2014;8(6):6–9. doi: 10.1371/journal.pntd.0002921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber B., Wozniak E., Chang D., Bush K., Wilson M., Watts J. A case of Chagas cardiomyopathy following infection in south central Texas. US Army Med Dep J. 2017;(1–17):55–59. [PubMed] [Google Scholar]
- Wenzel R. A new Hantavirus infection in North America. N Engl J Med. 1994;330(14):1004–1005. doi: 10.1056/NEJM199404073301410. [DOI] [PubMed] [Google Scholar]
- Wilder-Smith A., Hombach J., Ferguson N., Selgelid M., O’Brien K., Vannice K. Deliberations of the strategic advisory group of experts on immunization on the use of CYD-TDV dengue vaccine. Lancet Infect Dis. 2019;19(1):e31–e38. doi: 10.1016/S1473-3099(18)30494-8. [DOI] [PubMed] [Google Scholar]
- Wilder-Smith A., Ooi E.E., Horstick O., Wills B. Dengue. Lancet. 2019;393(10169):350–363. doi: 10.1016/S0140-6736(18)32560-1. [DOI] [PubMed] [Google Scholar]
- Wilder-Smith A., Quam M., Sessions O., Rocklov J., Liu-Helmersson J., Franco L. The 2012 dengue outbreak in Madeira: Exploring the origins. Euro Surveill. 2014;19(8):20718. doi: 10.2807/1560-7917.es2014.19.8.20718. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Vector-borne diseases [Internet]. 2020 [cited 2020 March 22]. Available from: https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases.
- Yactayo S., Staples J.E., Millot V., Cibrelus L., Ramon-Pardo P. Epidemiology of chikungunya in the Americas. J Infect Dis. 2016;214(Suppl 5):441–445. doi: 10.1093/infdis/jiw390. [DOI] [PMC free article] [PubMed] [Google Scholar]