Abstract
Aims
Remimazolam is a new, ultra‐short‐acting benzodiazepine developed for intravenous (IV) use during procedural sedation and in general anaesthesia. Two trials were conducted to characterize its effects on cardiac repolarization.
Methods
A thorough QT/QTc (TQT) study assessed electrocardiography effects of therapeutic and supratherapeutic doses of remimazolam and midazolam. To investigate whether RR‐QT hysteresis effects due to rapid heart rate changes might have confounded the QTc assessments in the TQT trial, a second trial used continuous IV remimazolam infusion to achieve stable heart rates during periods of stable remimazolam plasma concentration.
Results
During the TQT, both compounds produced a 10–20‐beats/min increase in heart rate within 30 seconds, persisting for 5–10 minutes. Within 30 seconds, the upper bound of the 2‐sided 90% confidence interval for the placebo‐corrected change from baseline for QTcI (ΔΔQTcI) exceeded 10 ms for both doses of remimazolam (ΔΔQTcI 7.2 [3.2, 11.2] ms for the 10 mg dose and 10.4 [6.5, 14.3] ms for the 20 mg dose) as well as for the 7.5‐mg dose of midazolam (8.2 [4.4, 12.1] ms). At 2 minutes after IV bolus, the upper bound of the 2‐sided 90% confidence interval for ΔΔQTcI exceeded 10 ms only for the remimazolam 20‐mg dose (6.3 [2.3, 10.2] ms). During the second study, during periods of stable heart rate, remimazolam had no clinically significant effect on QTc (peak ΔΔQTcI 3.4 [−1.1, 7.6] ms).
Conclusion
Remimazolam does not prolong cardiac repolarization (QTc). The methods reported here may allow assessment of the QTc effects of other drugs given by IV bolus.
Keywords: intravenous drug administration, QT interval, QT‐RR hysteresis
What is already known about this subject
Parenterally administered drugs may produce rapid changes in both heart rate and QTc.
Due to RR‐QT hysteresis, it may be difficult to determine if this represents a true effect on cardiac repolarization.
Methods for mathematical correction for RR‐QT hysteresis have been proposed but have not been widely validated.
What this study adds
Demonstration that the new chemical entity remimazolam does not produce clinically significant prolongation of cardiac repolarization (QTc).
Demonstration of a method for assessing the effects on cardiac repolarization of drugs that produce rapid changes in both heart rate and QTc without relying on attempts to mathematically correct for RR‐QT hysteresis.
1. INTRODUCTION
Remimazolam (CNS 7056) is a new benzodiazepine for intravenous use that is being developed as an ultra‐short‐acting agent for induction and maintenance of general anaesthesia and for procedural sedation. Remimazolam is an ester‐based drug with molecular weight 439 D that is rapidly hydrolysed, primarily in the liver, by carboxylesterase‐1 to an inactive metabolite, CNS 7054. Protein binding of remimazolam to human serum is 92%. Remimazolam inhibited hERG tail current in a concentration‐dependent manner with estimated 25 and 50% inhibitory concentration values of 62 and 207 μM, respectively (data on file, Paion). This compares favourably to plasma concentrations of remimazolam required for procedural sedation of 0.25–0.50 μg/mL (0.6–1.1 μM). CNS 7054 produced no inhibition of hERG tail current at concentrations up to 100 μM. During cardiovascular safety studies conducted in rats, pigs, sheep and monkeys, remimazolam produced a dose‐related transient drop in mean arterial blood pressure (maximum reduction 12%) and a compensatory increase in heart rate (HR; maximum increase 25%), but no effects on QTc (data on file, Paion).
In humans, remimazolam is rapidly cleared from plasma, with mean initial half‐life (t½α) of 1.3 (±0.3) min and rapid and extensive formation of CNS 7054. 1
During the clinical development of a new chemical entity, assessment of its effects on cardiac repolarization is required. The principles are outlined in the ICH E14 Guidance for Industry, which describes the rationale and design for a so‐called thorough QT/QTc trial, referred to nowadays as a TQT trial. 2 Study CNS7056–005 was therefore designed as a standard single‐dose, placebo and active‐controlled (moxifloxacin) 6‐period cross‐over TQT trial to assess the effects of remimazolam and midazolam, each at therapeutic and supratherapeutic doses, on the QTc intervals in healthy subjects. In this study, a small QTc (ΔΔQTcI) effect was observed for both remimazolam groups as well as for the supratherapeutic midazolam dose; however, the results may have been biased by the observed, rapid changes in HR. Intense electrocardiography (ECG) monitoring was subsequently incorporated into a second study utilizing infusion of remimazolam at constant rates (Study CNS7056–017), in which ECG effects could be studied during periods of stable HRs.
2. METHODS
2.1. Study population and study design
The TQT study was a blinded (except for moxifloxacin), randomized, single‐site, 6‐arm crossover design in healthy male and female subjects. Fifty‐seven healthy subjects were randomized to receive all 6 treatment regimens for 1 day with a minimum 3 day washout period. Subjects were nonsmokers, age 18–45 years, with body mass index 18–28 kg/m2. Standard inclusion/exclusion criteria for a healthy volunteer QT study were used.
The 6 treatment sequences included placebo, moxifloxacin 400 mg, remimazolam 10 mg (therapeutic dose), remimazolam 20 mg (supratherapeutic dose), midazolam 2.5 mg (therapeutic dose) and midazolam 7.5 mg (supratherapeutic dose). Placebo, remimazolam and midazolam were administered by intravenous (IV) bolus over 60 seconds, and time zero for assessments started at the end of the bolus. Moxifloxacin was administered as a single oral dose.
The second infusion study was a prospective, placebo‐controlled, open‐label, randomized, 2‐arm, single‐centre, cross‐over phase 1 clinical trial primarily designed to allow pharmacokinetic (PK)/pharmacodynamic modelling of the hypnotic effect of remimazolam. The trial was also designed to evaluate the effect of steady state plasma concentrations of remimazolam and metabolite CNS 7054 on cardiac repolarization during constant infusion of remimazolam. Blinding of the subject and the trial site staff were not feasible due to the sedation produced by remimazolam, but the technician and cardiologist analysing the ECGs were blinded to the treatment sequence.
Twenty male subjects aged 18–40 years, with body mass index 20–30 kg/m2, were enrolled into the trial. The inclusion and exclusion criteria were similar to those used for the TQT. In a cross‐over design, subjects received remimazolam or placebo in randomized sequence with a 10‐day washout between treatments. Remimazolam was administered at an initial infusion rate of 5 mg/min for 5 minutes, followed by 3 mg/min for the next 15 minutes and 1 mg/min for a final 15 minutes. The initial infusion rate of 5 mg/min for 5 minutes was selected to provide the best conditions for characterization of the different stages of sedation and hypnosis prior to loss of consciousness. The infusion rates of 3 and 1 mg/min over 15 minutes each were selected to produce 2 plateaus in plasma remimazolam levels of approximately 1.9 and 1.0 μg/mL, matching the mean maximum plasma concentration (Cmax) values for the supratherapeutic and therapeutic doses of remimazolam evaluated in the TQT study. It was expected that each plateau would last for at least 5–10 minutes, allowing evaluation of the effects of remimazolam on cardiac conduction during periods of stable plasma concentration and HR, eliminating any confounding effects of QT‐RR hysteresis. Placebo was administered as an IV infusion of normal saline without active medication.
2.2. ECG collection and processing
During the TQT, ECGs were collected digitally using a Mortara Instrument (Milwaukee, WI, USA) H‐12+ ECG continuous 12‐lead digital recorder, on Day −1 (predose baseline day) and Day 1 (dosing day) during each period. The central ECG laboratory (eResearch Technology; ERT) extracted triplicate 12‐lead ECGs approximately 1 minute apart at the following timepoints on Day −1 and Day 1 of each treatment period: 15 minutes prior to dose, and at 0.5, 2, 5, 10, 30 and 60 minutes, and 2, 3, 4, 6, 8, 12, 18 and 23 hours postdose.
The digital 10‐second 12‐lead ECGs extracted from the continuous recordings were processed in ERT's Expert system. Each subject's ECGs were processed by the same analyst. ECG readers were blinded to subject identifiers, treatment and visit. Interval duration measurements were collected using computer assisted calliper placements on 3 consecutive beats on a single lead with Lead II as the primary lead, as previously described. 3 A trained analyst reviewed all ECGs for correct lead and beat placement and adjudicated the preplaced algorithm callipers as necessary. A cardiologist verified the interval duration measurements and performed the morphology analysis.
During the second infusion study, ECGs were recorded using the same recording device and analysed by ERT with the same methodology. Triplicate 12‐lead ECGs were extracted from the recordings prior to each dose at −45, −30 and − 15 minutes, and 5, 10, 15, 20, 30, 40, 65, 110, 120, 180, 240 and 360 minutes postdose in each treatment period.
2.3. PK sampling and processing
In the TQT study, venous PK samples were collected to be time‐matched to the ECG timepoints: predose (at −15 minutes), and postdose at 0.5, 2, 5, 10, 30 and 60 minutes, and 2, 3, 4, 6, 8, 12, 18 and 23 hours postdose. In the infusion study, PK samples were drawn from an indwelling radial artery catheter prior to start of IMP administration and at approximately 1, 3, 5 (first reduction of rate of IMP administration), 7, 10, 15, 20 (second reduction of rate of IMP administration), 25, 30, 35 (end of IMP administration), 36, 37, 39, 42, 45, 50, 55, 65, 80, 95, 125, 155, 185, 215, 275 and 395 minutes thereafter. One PK sample was collected from the venous cannula in the forearm during the control visit (to confirm absence of remimazolam and metabolite).
During both trials, ECG extractions were performed before the actual plasma sampling to avoid changes in autonomic tone from bloodletting.
3. STATISTICAL METHODS
3.1. Trial endpoints
In the TQT study, the primary cardiac endpoint was the time‐matched (Day 1 vs Day −1) placebo‐corrected, change from baseline QT interval corrected for HR based on an individual correction method (QTcI; ΔΔQTcI).
In the infusion study, the primary cardiac endpoint was also (ΔΔQTcI), using a predose baseline. In both studies, secondary endpoints included placebo corrected change from baseline for PR, QRS, QT and QTcF, as well as assessment of ECG morphological changes.
3.2. TQT study
The sample size for the TQT study (n = 44) was calculated using an assumed underlying effect on ΔΔQTcI of 3 ms and a SD of ΔQTcI of 8 ms, which would provide over 80% power to show that the upper limit of the 90% 2‐sided confidence interval (UCI) for the comparison of remimazolam to placebo fell below 10 ms.
Moxifloxacin was included to allow demonstration of assay sensitivity. 4 Demonstration of assay sensitivity required that for at least 1 prespecified time point (hours 1, 2, 3 and 4), the lower confidence bound of the moxifloxacin ΔΔQTcI exceeded 5 ms (Bonferroni adjusted for α error level of 0.05/4 = 0.0125).
The primary QTc correction formula (individual QT correction method, QTcI) was determined for each subject by iterating the QT‐RR relationship using the ECGs from Day −1 of each treatment period. To determine QTcI, the goal was to determine the exponent β such that QTcI was a constant, where: QTcI = QT/ (RR)β. 5 , 6 , 7
The primary analysis for QTc was based on the time‐matched analysis for each treatment group, evaluating the placebo adjusted change from baseline for QTc. Specifically, for each individual subject, the baseline value for a timepoint was subtracted from the time‐matched value on Day 1. The measurements from the 3 ECGs collected at a time point were averaged to produce a single value for each ECG interval for that time point. Baseline was defined separately for each period for each subject. These data were then subjected to a mixed effects model (using the SAS Procedure PROC MIXED) with the following covariates: time (categorical), treatment, time by treatment interaction, sex and the baseline value of the parameter. Since this was a crossover design, period and sequence terms were also included in the model. Subject was included as a random effect. The estimate of the delta delta and its CIs were performed using a LSMEAN statement within PROC MIXED and a diff option. For this analysis 90% 2‐sided confidence intervals were calculated. Hypotheses were based upon the Intersection Union Test as specified below.
To evaluate the drug effect, the statistical hypotheses was stated as follows:
HO: ∪ {μremimazolam(i) – μplacebo(i)} ≥ 10, i = 1, 2, …, k and.
HA: ∩ {μremimazolam(i) – μplacebo(i)} < 10, i = 1, 2, …, k.
where μremimazolam(i) and μplacebo(i) are the mean change from baseline of QTc for the drug and placebo at time point i for k time points, respectively. Since the intersection–union test can be applied here, no multiple endpoint adjustment was needed. Based on the ICH E14 guidance, this hypothesis was evaluated by observing if any of the time points had a 2‐sided 90% upper confidence bound (i.e. 1‐sided 95%) ≥10 ms.
A similar analysis was performed for the supratherapeutic and therapeutic doses of midazolam.
3.3. Infusion study
The sample size chosen for the infusion study was primarily driven by the requirements of the EEG based PK‐PD modelling objectives.
The primary endpoint for QTc was based on the by timepoint analysis of the placebo adjusted change from baseline for QTcI. QTcI was derived from the ECGs collected during the placebo treatment sequence, and baseline was defined as the mean of the interval duration measurements of the 3 sets of triplicate ECGs collected prior to dosing for each treatment sequence. For the time point analysis, baseline measurements were subtracted from the mean of the triplicate ECGs obtained at each of the following time points on during the placebo and remimazolam treatment periods: 5, 10, 15, 20, 30, 40, 65, 110, 120, 180, 240 and 360 minutes postdose using a mixed effects model. The UCI on treatment was compared to the 10 ms bound for remimazolam vs placebo. If the UCI for remimazolam vs placebo (at timepoint corresponding to the mean therapeutic and supratherapeutic Cmax of 0.9–1.0 and 1.9 μg/mL, respectively) fell below 10 ms, it was concluded that the therapeutic dose and therapeutic exposure did not prolong the QTc interval to a clinically significant degree. Standard analyses of categorical outlier measurements and ECG morphological changes were also performed.
4. RESULTS
4.1. TQT study
In the TQT study, 57 subjects were randomized and 47 completed all treatment sequences. A consort cohort chart describing the number of subjects completing each treatment sequence is shown in Figure S1. The geometric mean Cmax for remimazolam 10‐ and 20‐mg doses were roughly 1 and 2 μg/mL, respectively, and were reached about 2 minutes after infusion (Figure S2). Remimazolam remained detectable for 1 and 2 hours, following 10 and 20 mg, respectively. The inactive metabolite CNS 7054 was formed rapidly, with a median time to Cmax (Tmax) of 0.617 hours. Cmax for CNS 7054 was 647 and 1264 ng/mL for the 2 dose levels. The half‐life of CNS 7054 was 2.3 hours, nearly 5 times longer than that of remimazolam.
A bolus infusion of remimazolam caused a very rapid increase in HR (Figure 1). Simultaneous with the increase in HR, absolute QT interval decreased by up to 30 ms. At the 30‐second postdose timepoint, mean placebo‐corrected changes‐from‐baseline HR (ΔΔHR) were 14.5 beats/min (bpm) for the remimazolam 10 mg dose group, 18.8 bpm for the remimazolam 20 mg dose group, 12.8 bpm for the midazolam 7.5 mg dose group and 8.6 bpm for the midazolam 2.5 mg dose group. These HR increases persisted for 5–10 minutes before resolving by 30–60 minutes after dosing.
FIGURE 1.
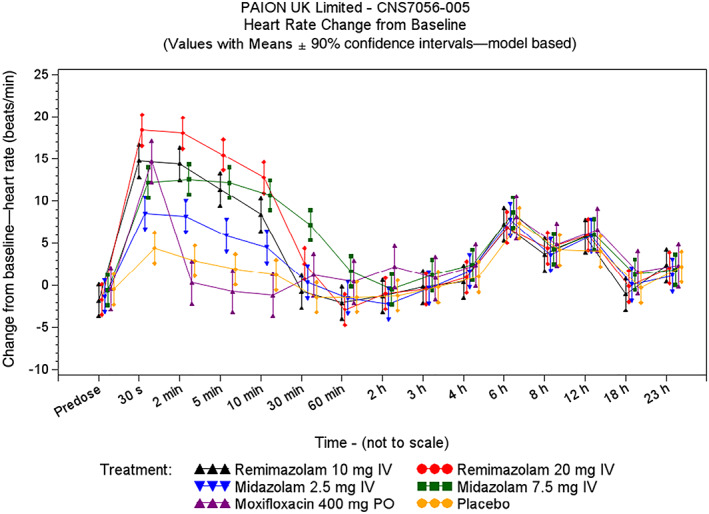
Heart rate: change from baseline (thorough QT/QTc study)
A small effect on QTcI was observed immediately after the bolus infusion of remimazolam and midazolam (Figure 2). The largest mean ΔΔQTcI was seen 30 seconds after dosing and reached 7.2 (2‐sided 90% UCI 11.2) and 10.4 (2‐sided 90% UCI 14.2) ms on remimazolam 10 and 20 mg, and 5.4 (2‐sided 90% UCI 9.4) and 8.2 (2‐sided 90% UCI 12.1) ms on 2.5 and 7.5 mg midazolam, respectively. Following the 2‐minute postdose timepoint, when HRs were more stable, the ΔΔQTcI upper 2‐sided 90% boundary was <10 ms for all timepoints in the remimazolam and midazolam treatment groups. QTcI and QTcF performed similarly in correcting QT for HR, with slight superiority for QTcI (Figure S3). The results of the by timepoint analysis using QTcF were very similar (Table S1).
FIGURE 2.
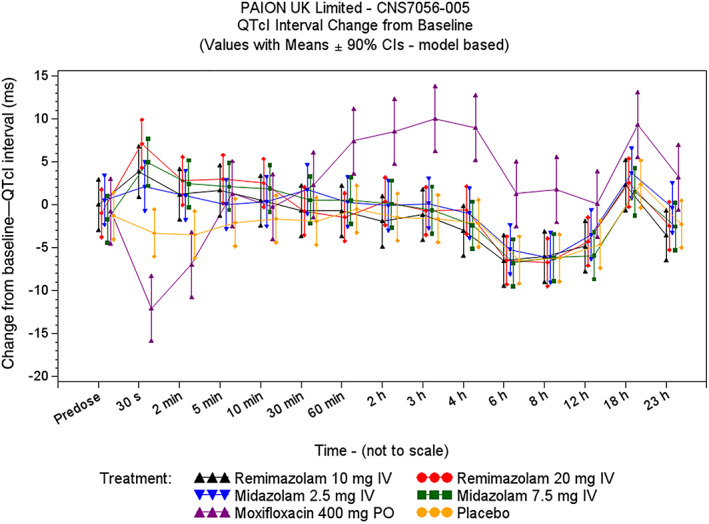
QTcI change from baseline vs time (thorough QT/QTc study)
The study's ability to demonstrate small QTc changes was confirmed by moxifloxacin with largest mean ΔΔQTcI of 11.6 ms observed at 3 hours and the lower bound of the 90% CI above 5 ms at 3 and 4 hours. (Figure 2 and Table 1). Overall, QTcI corrected very well for HR, as shown in Supplementary Figure S3. No clinically significant effects on PR, QRS or ECG morphology were detected.
TABLE 1.
Placebo‐adjusted change from baseline QTcI (TQT study)
| Time | Remimazolam 10 mg (n = 48) | Remimazolam 20 mg (n = 52) | Midazolam 2.5 mg (n = 49) | Midazolam 7.5 mg (n = 53) | Moxifloxacin 400 mg (n = 52) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate a | Lower bound b | Upper bound b | Estimate a | Lower bound b | Upper bound b | Estimate a | Lower bound b | Upper bound b | Estimate a | Lower bound b | Upper bound b | Estimate a | Lower bound b | Upper bound b | |
| Predose | 1.3 | −2.7 | 5.3 | 0.3 | −3.6 | 4.2 | 1.8 | −2.2 | 5.8 | −0.4 | −4.3 | 3.5 | 0.6 | −4.7 | 5.9 |
| 0.5 min | 7.2 | 3.2 | 11.2 | 10.4 | 6.5 | 14.3 | 5.4 | 1.4 | 9.4 | 8.2 | 4.4 | 12.1 | −8.8 | −14.1 | −3.5 |
| 2 min | 4.7 | 0.7 | 8.7 | 6.3 | 2.3 | 10.2 | 4.5 | 0.6 | 8.5 | 5.9 | 2.0 | 9.8 | −3.5 | −8.8 | 1.8 |
| 5 min | 3.7 | −0.3 | 7.7 | 5.0 | 1.1 | 8.9 | 2.1 | −1.9 | 6.0 | 4.2 | 0.3 | 8.1 | 3.4 | −1.9 | 8.7 |
| 10 min | 2.1 | −1.9 | 6.1 | 4.2 | 0.3 | 8.1 | 2.0 | −2.0 | 5.9 | 3.5 | −0.3 | 7.4 | 1.4 | −3.9 | 6.7 |
| 30 min | 1.2 | −2.8 | 5.2 | 1.2 | −2.7 | 5.1 | 3.7 | −0.3 | 7.6 | 2.5 | −1.4 | 6.3 | 4.2 | −1.1 | 9.5 |
| 60 min | −0.2 | −4.2 | 3.8 | −1.0 | −4.9 | 2.9 | 0.8 | −3.2 | 4.8 | 1.0 | −2.9 | 4.9 | 7.9 | 2.6 | 13.2 |
| 2 h | −0.5 | −4.5 | 3.5 | 1.8 | −2.1 | 5.7 | 1.3 | −2.6 | 5.3 | 1.5 | −2.3 | 5.4 | 10.0 | 4.7 | 15.3 |
| 3 h | 0.5 | −3.5 | 4.5 | 0.9 | −3.0 | 4.8 | 1.7 | −2.2 | 5.7 | 1.0 | −2.9 | 4.9 | 11.6 | 6.3 | 16.9 |
| 4 h | −0.8 | −4.8 | 3.2 | 1.5 | −2.4 | 5.4 | 1.2 | −2.8 | 5.1 | −0.2 | −4.1 | 3.7 | 11.2 | 5.8 | 16.5 |
| 6 h | −0.1 | −4.1 | 3.9 | −0.0 | −3.9 | 3.9 | 1.2 | −2.8 | 5.2 | −0.3 | −4.2 | 3.5 | 7.7 | 2.4 | 13.0 |
| 8 h | 0.2 | −3.8 | 4.2 | −0.5 | −4.4 | 3.4 | 0.0 | −3.9 | 4.0 | 0.0 | −3.8 | 3.9 | 8.0 | 2.7 | 13.3 |
| 12 h | −0.2 | −4.2 | 3.8 | 0.3 | −3.6 | 4.2 | 1.1 | −2.9 | 5.1 | −1.3 | −5.2 | 2.5 | 4.7 | −0.6 | 10.0 |
| 18 h | −0.1 | −4.1 | 3.9 | 0.2 | −3.7 | 4.1 | 1.3 | −2.7 | 5.2 | −0.9 | −4.8 | 3.0 | 6.9 | 1.6 | 12.2 |
| 23 h | −1.3 | −5.3 | 2.7 | −0.2 | −4.1 | 3.7 | 1.9 | −2.1 | 5.9 | −0.3 | −4.1 | 3.6 | 5.5 | 0.2 | 10.8 |
TQT = thorough QT/QTc. All estimates and confidence bounds are in ms.
Mixed effect general linear model is fit for time‐matched changes from baseline and includes terms for treatment, time and interactions: treatment by time and treatment by sex.
Lower/upper bound = lower or upper 2‐sided 90% (i.e. 1‐sided 95%) confidence limits. Only moxifloxacin confidence bounds are multiplicity adjusted.
4.2. Infusion study
In the infusion study, 20 subjects were randomized and completed both treatment sequences. Figure 3 illustrates the plasma concentrations of remimazolam plotted against time. The remimazolam plasma concentration was between 1.63 and 1.96 μg/mL 5–20 minutes postdose, and between 0.58 and 1.06 μg/mL 30–40 minutes postdose (Figure 3 and Table 2).
FIGURE 3.
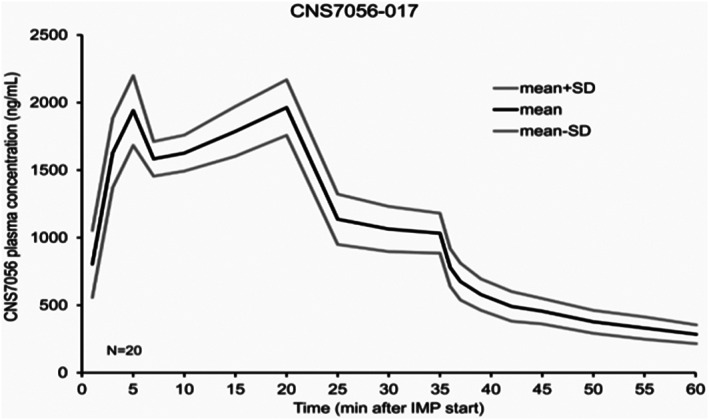
Remimazolam plasma concentration vs time (infusion study)
TABLE 2.
Placebo‐corrected QTcI change from baseline (infusion study)
| Time following start of infusion | Mean remimazolam plasma concentration (μg/mL) | Remimazolam (n = 20) | ||
|---|---|---|---|---|
| Mean placebo‐corrected QTcI change from baseline (ms) a | Lower bound (ms) b | Upper bound (ms) b | ||
| 5 min | 1.94 | −1.6 | −5.7 | 2.6 |
| 10 min | 1.63 | 3.1 | −1.1 | 7.3 |
| 15 min | 1.79 | 3.4 | −0.7 | 7.6 |
| 20 min | 1.96 | 0.8 | −3.4 | 4.9 |
| 30 min | 1.06 | −1.0 | −5.2 | 3.1 |
| 40 min | 0.58 | −4.7 | −8.8 | −0.5 |
| 65 min | 0.24 | −6.6 | −10.8 | −2.4 |
| 110 min | 0.11 | −1.3 | −5.5 | 2.8 |
| 120 min | 0.07 c | −2.4 | −6.6 | 1.7 |
| 180 min | 0.03 c | 0.5 | −3.7 | 4.6 |
| 240 min | 0.01 c | −1.4 | −5.5 | 2.8 |
| 360 min | 0.003 c | 0.8 | −3.3 | 5.0 |
The mean estimate and upper and lower confidence intervals are model‐based estimates.
Lower/upper bound = lower/upper 2‐sided 90% (1‐sided 95%) model‐based confidence limit.
Electrocardiograms were extracted at listed times, but closest corresponding plasma samples were collected at 125, 155, 275 and 395 minutes.
Five minutes after the start of the remimazolam infusion, mean ΔΔHR increased by 15 bpm, then remained stable for the next 60 minutes (Figure 4).
FIGURE 4.
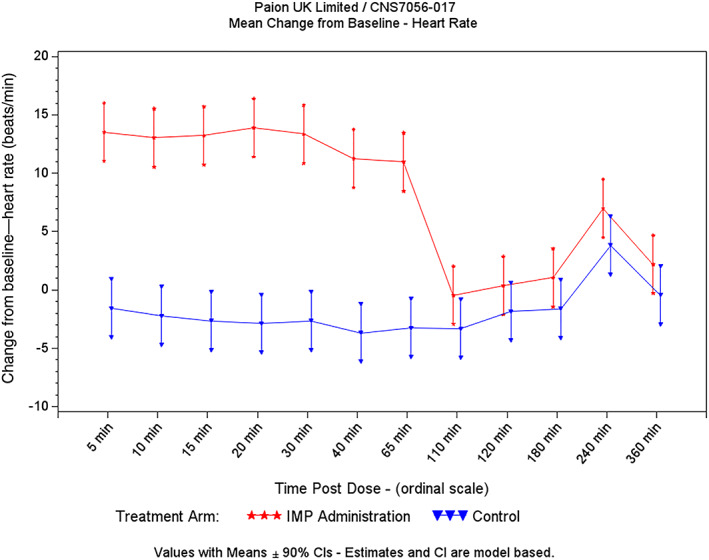
Heart rate change from baseline (infusion study)
Figure 5 details ΔQTcI vs time for the placebo and remimazolam treatment sequences, and Table 2 details ΔΔQTcI and mean remimazolam plasma concentrations by time point. The largest mean ΔΔQTcI was observed after 15 minutes infusion and reached 3.4 ms (2‐sided 90% UCI 7.6 ms). None of the remimazolam time points demonstrated an upper bound that approached or exceeded 10 ms. The mean remimazolam plasma concentration peaked at 1.96 μg/mL at 20 minutes after start of infusion, with corresponding placebo‐corrected change from baseline for QTcI of 0.8 ms (2‐sided 90% UCI 4.9 ms). Thirty minutes after the start of infusion, the mean remimazolam plasma concentration was 1.06 μg/mL, with corresponding placebo‐corrected change from baseline for QTcI of −1.0 ms (2‐sided 90% UCI 3.1 ms). The 5‐ and 10‐ms ΔΔQTcI bounds of regulatory interest are shown in grey in Figure 5. QTc vs RR plots demonstrated that QTcI performed significantly better than QTcF at correcting QT for RR (Figure S4), and therefore QTcF data are not presented here.
FIGURE 5.
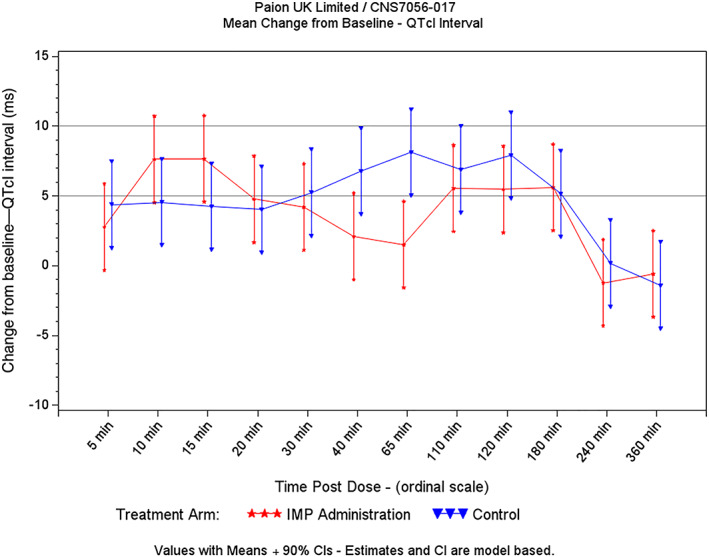
QTcI interval: change from baseline (infusion study)
No clinically significant effects on PR, QRS or ECG morphology were detected.
5. DISCUSSION
5.1. Effects of remimazolam on cardiac repolarization
An important part of the development of any new drug is the assessment of its risk of producing ventricular arrhythmias, which is currently evaluated by determining if the drug produces QTc prolongation. For most new small molecules that can safely be administered to healthy volunteers, QTc liability is assessed in a dedicated TQT study, or, more recently, using extensive ECG and PK data collected during phase I. For drugs that do not produce large changes in HR (or autonomic tone), QT correction for HR may be achieved with the use of the Fridericia QT correction method (QTcF). For drugs that do produce changes in HR exceeding 10 bpm, a variety of methods for minimizing the contribution of HR changes to QT assessment have been proposed; foremost among these is the use of the individual QT correction method (QTcI). 5 , 6 , 7 Provided a sufficiently wide range of HRs can be captured at baseline, an individual QT correction method is preferred to the Fridericia correction formula in cases where there is a substantial change in HR. 8 However, all standard QT correction methods, including QTcI, are effective only if the HR is relatively stable at the time that an ECG is recorded. It has been estimated that following a change in HR, it takes 90–180 seconds for QT to reach a new steady state value. 9 , 10 If an ECG is recorded when the preceding HR has not been stable, correction of QT for HR with standard methods (correction based on the preceding RR interval, or the average of 3 beats or 10 seconds of preceding RR intervals) will not adequately account for QT/RR hysteresis. QTc changes that are detected at the time of rapid changes in HR may represent effects of QT/RR hysteresis rather than true effects on cardiac repolarization, and thus may not be a valid biomarker for increased risk of drug induced ventricular arrhythmias.
Methods for performing hysteresis correction for calculation of QTc, using either a weighted or unweighted average of the RR intervals from 30–300 seconds prior to the measured QT interval have been proposed. 7 , 10 However, they have not been extensively validated in scenarios involving rapid HR changes, and their appropriateness for regulatory decision making in such cases is uncertain.
The QT assessment of remimazolam demonstrates the difficulty of assessing the QTc effects of a drug that is administered by IV bolus and that produces very rapid changes in HR. When a drug is administered by IV bolus, Cmax occurs very shortly after the injection. Since QTc prolongation by drugs which block cardiac ion channels is generally related to plasma concentration, the largest QTc effects therefore tend to occur around Tmax. As a result, when an IV drug produces a rapid change in HR, this will occur coincident with Tmax and may result in QT‐RR hysteresis, interfering with the ability to use changes in QTc to accurately reflect true changes in cardiac repolarization.
During the CNS7056–005 TQT study, this exact scenario was encountered for both remimazolam and midazolam. Each drug produced a large change in HR (10–20 bpm) immediately after injection. Simultaneously, the timepoint analysis demonstrated an increase in QTcI, which slightly exceeded the regulatory threshold of concern (2‐sided 90% CI >10 ms) as cited in the ICH E14 guidance. It was unclear if the increase in QTc was caused by inability of QTcI to appropriately correct for the rapid HR changes or to true changes in repolarization.
Rather than attempting mathematical hysteresis correction, an alternative approach was taken. In the infusion study, remimazolam was administered as a continuous infusion, with dosing that produced plateaus in remimazolam concentration of approximately 2.0 and 0.9 μg/mL, the observed Cmax values for the supratherapeutic and therapeutic doses of remimazolam in the TQT. Immediately after dosing, mean HR increased by 15 bpm, but HR subsequently remained stable, allowing the collection of ECGs and PK samples during periods of stable HR. Under these conditions, remimazolam plasma concentrations of up to 2.0 μg/mL did not produce clinically significant QTc prolongation, demonstrating that the effects on QTc observed in the TQT study were probably related to QT‐RR hysteresis effects and were not evidence of a true effect of remimazolam on cardiac repolarization.
5.2. Implications for QTc assessment in the setting of rapid changes in HR
The trial design used for the infusion study has proven to be valuable for assessing the QTc effects of compounds that produce rapid changes in HR at or around Tmax. This is particularly an issue for drugs administered by IV bolus that produce vasodilatation, sedation or direct increases in sympathetic tone. For such drugs, Tmax occurs within the first minutes following dosing. Since drug induced QTc prolongation (if present) generally also peaks near Tmax, both HR changes and QTc prolongation may occur simultaneously, making it difficult to distinguish whether QTc prolongation is simply an artefact of QT/RR hysteresis or represents a true drug‐related effect on cardiac repolarization. The trial design proposed here may eliminate this concern.
This trial design may, furthermore, be appropriate for other drugs whose PK parameters are similar to remimazolam (i.e. IV bolus dosing, short half‐life of parent and metabolites, and amenable to continuous infusion permitting stable plasma concentrations). This may be the preferred method for assessing the QTc effects of drugs that produce rapid changes in HR following IV dosing. Achieving periods of stable plasma concentration and HR allows for control of the potential confounding influence of QT‐RR hysteresis. This permits the generation of true measurements of the QT‐interval followed by the use of standard QTcF or QTcI calculations without need for mathematical manipulations of RR data for hysteresis correction. In general, we believe that it is preferable to use time‐tested QT correction methods rather than to perform data manipulations that are based on unproven assumptions and which have not been fully validated.
6. CONCLUSIONS
Remimazolam produces a relevant increase in HR immediately after bolus infusion, which led to a false positive result during a standard TQT trial. Administration of remimazolam as a continuous infusion allowed analysis of its effects on QTc during periods of HR stability, and demonstrated no clinically relevant effects on QTc. This demonstrates that remimazolam does not alter cardiac repolarization, and that the QTc increase noted in the presence of a large, rapid HR increase is probably related to QT‐RR hysteresis. Furthermore, this novel trial design may offer a useful, or even superior, paradigm for assessing a drug's effects on cardiac repolarization in the face of large, rapid changes in HR, but without requirement attempts to mathematically correct for the effects of QT‐RR hysteresis.
7. LIMITATIONS
The 2 studies presented in this paper studied the effects of remimazolam at similar plasma concentrations, but at different times after initial exposure. It is therefore possible that the small QTc increase noted very early after IV bolus administration was due to a very transient direct effect of remimazolam directly on cardiac ion currents, or a very transient indirect effect of remimazolam rather than to QT‐RR hysteresis effects. Such effects might theoretically have dissipated by the time of the QTc measurements in CNS7056–017, but are considered very unlikely when taking the absolute shortening of the QT interval into consideration. In general, however, drugs that prolong QTc due to direct ion channel effects do so in an exposure dependent fashion, and QTc prolongation does not dissipate while plasma concentrations remain constant. Another limitation of CNS7056–017 is that a positive control was not included to demonstrate assay sensitivity. However, assay sensitivity was demonstrated in CNS7056–005, which used the same ECG analysis methods as CNS7056–017. Finally, the difference in the sex mix between the 2 studies may be viewed as a limitation; the infusion study included only males, while the TQT study included a nearly equal mix of males (n = 30) and females (n = 27). In the TQT study, however, there was no evidence of a sex effect. Even though there is a sex difference in terms of the absolute QTc interval (females typically having 10–15 ms longer QTc interval), sex has been shown to have an impact on the level of QTc prolongation only for potent QT‐prolonging drugs, such as quinidine and sotalol. 8 It therefore seems very unlikely that the sex difference across the 2 studies can explain the observed differences in results.
COMPETING INTERESTS
The authors have not received funding from any sources other than their employing institutions, identified with the author's affiliations. The authors declare no other relationships or activities that might appear to have influenced the submitted work.
CONTRIBUTORS
R.B.K. designed the cardiac safety portions of the 2 trials, and oversaw their statistical analysis and interpretation. B.D. assisted with interpretation of the data. M.T. performed the statistical analysis of the cardiac safety data. T.S. and F.S, were responsible for the overall conduct and interpretation of the 2 trials.
Supporting information
FIGURE S1 Consort chart for thorough QT/QTc (CNS7056–005)
FIGURE S2 Remimazolam plasma concentration vs time, linear scale (thorough QT/QTc study, CNS7056–005)
FIGURE S3 QTc vs RR Interval, CNS7056–005
FIGURE S4 QTc vs RR Interval, CNS7056–017
TABLE S1 QTcF placebo‐adjusted change from baseline—estimates from mixed effects general linear model (thorough QT/QTc study, CNS7056–005)
ACKNOWLEDGEMENTS
The authors would like to acknowledge the contributions of Dr Daniel Dickerson and Professor Dr med. Jürgen Schüttler, the primary investigators respectively for clinical trials CNS7056‐005 and CNS7056‐017. Although they did not participate in the analysis of the cardiac safety data and did not contribute to this manuscript, we wish to acknowledge their invaluable contributions to ensuring that the clinical trials proceeded smoothly.
Kleiman RB, Darpo B, Thorn M, Stoehr T, Schippers F. Potential strategy for assessing QT/QTc interval for drugs that produce rapid changes in heart rate: Electrocardiographic assessment of the effects of intravenous remimazolam on cardiac repolarization. Br J Clin Pharmacol. 2020;86:1600–1609. 10.1111/bcp.14270
The authors confirm that the Principal Investigators for the 2 trials described in this paper were Dr Daniel Dickerson and Professor Dr med. Jürgen Schüttler (please see acknowledgments) and that they had direct clinical responsibility for patients.
DATA AVAILABILITY STATEMENT
Research data are not shared.
REFERENCES
- 1. Ihmsen H, Eisenried A, Lerch M, Fechner J, Jeleazcov C, Schuettler J. Population pharmacokinetics of remimazolam after continuous infusion in volunteers. Eur J Anaesthesiol. 2018;35(e‐Supplement 56):43.28937531 [Google Scholar]
- 2. ICH Guideline E14 . May 2005. Available at: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E14/E14_Guideline.pdf.
- 3. Morganroth J, Wang Y, Thorn M, et al. Moxifloxacin‐induced QTc interval prolongations in healthy male japanese and caucasian volunteers: a direct comparison in a thorough QT trial. Br J Clin Pharmacol. 2015;80(3):446‐459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Florian JA, Tornøe CW, Brundage R, Parekh A, Garnett CE. Population pharmacokinetic and concentration‐‐QTc models for moxifloxacin: pooled analysis of 20 thorough QT studies. J Clin Pharmacol. 2011;51(8):1152‐1162. [DOI] [PubMed] [Google Scholar]
- 5. Garnett C, Zhu H, Malik M, et al. Methodologies to characterize the QT/corrected QT interval in the presence of drug‐induced heart rate changes or other autonomic effects. Am Heart J. 2012;163(6):912‐930. [DOI] [PubMed] [Google Scholar]
- 6. Batchvarov VN, Ghuran A, Smetana P, et al. QT‐RR relationship in healthy subjects exhibits substantial inter‐subject variability and high intra‐subject stability. Am J Physiol Heart Circ Physiol. 2002;282(6):H2356‐H2363. [DOI] [PubMed] [Google Scholar]
- 7. Malik M, Hnatkova K, Novotny T, Schmidt G. Subject‐specific profiles of QT/RR hysteresis. Am J Physiol Heart Circ Physiol. 2008;295(6):H2356‐H2363. [DOI] [PubMed] [Google Scholar]
- 8. Darpo B, Karnad DR, Badilini F, et al. Are women more susceptible than men to drug‐induced QT prolongation? Concentration‐QTc modelling in a phase 1 study with oral rac‐sotalol. Br J Clin Pharmacol. 2014;77(3):522‐531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Franz MR, Swerdlow CD, Liem LB, Schaefer J. Cycle length dependence of human action potential duration in vivo. Effects of single extrastimuli, sudden sustained rate acceleration and deceleration, and different steady‐state frequencies. J Clin Invest. 1988;82(3):972‐979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Malik M, Johannesen L, Hnatkova K, Stockbridge N. Universal correction for QT/TT hysteresis. Drug Saf. 2016;39(6):577‐588. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1 Consort chart for thorough QT/QTc (CNS7056–005)
FIGURE S2 Remimazolam plasma concentration vs time, linear scale (thorough QT/QTc study, CNS7056–005)
FIGURE S3 QTc vs RR Interval, CNS7056–005
FIGURE S4 QTc vs RR Interval, CNS7056–017
TABLE S1 QTcF placebo‐adjusted change from baseline—estimates from mixed effects general linear model (thorough QT/QTc study, CNS7056–005)
Data Availability Statement
Research data are not shared.


